Adopted Factorial and New In-Situ Micro-Designs for Stimulation of Matrix Acidizing of Carbonate Reservoir Rocks
Abstract
1. Introduction
2. The Experimental Part
2.1. Methods and Materials
2.1.1. A New Experimental In-Situ Acidizing Micro-Model Setup
2.1.2. Experimental Macro-Design
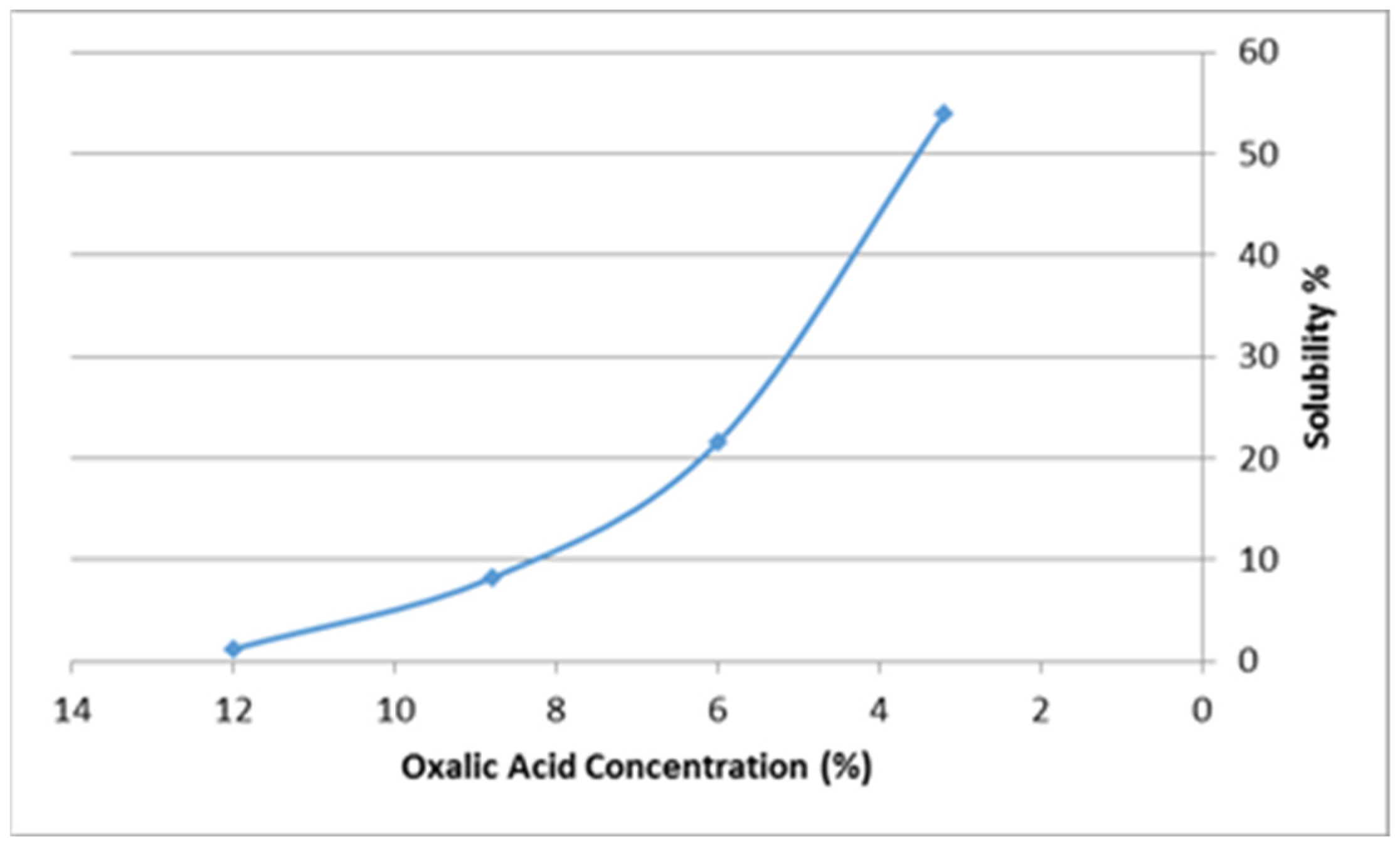
2.1.3. Solubility Tests
3. Results and Discussion
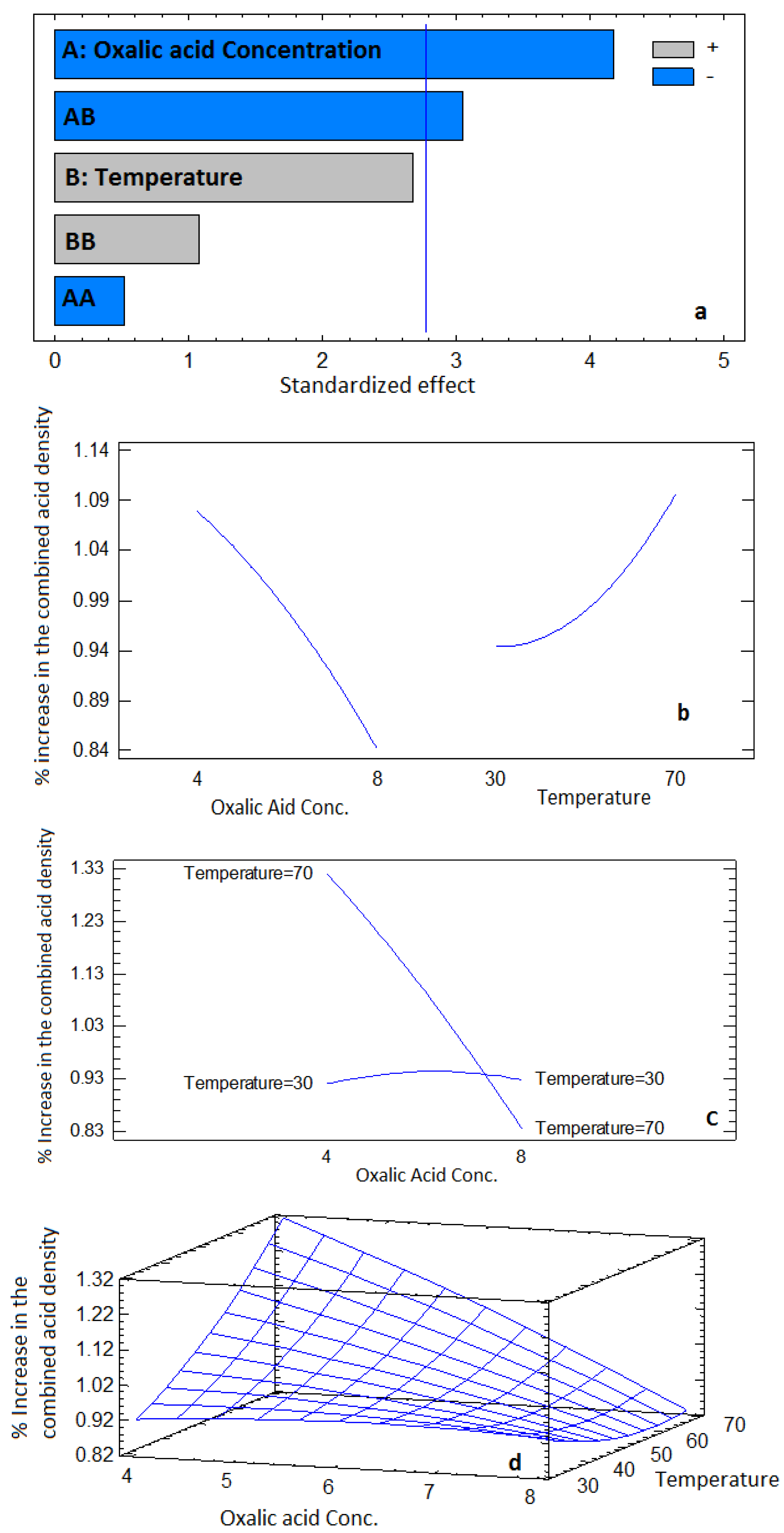
3.1. Experimental and ANOVA Results
3.2. In-Situ Acidizing Micro-Model and Acid–Rock Interaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| ANOVA | Analyses of variance |
| RSM | Response surface methodology |
| X | Acidizing temperature |
| Y | Pore area (%) |
References
- Shafiq, M.U.; Ben Mahmud, H. Sandstone matrix acidizing knowledge and future development. J. Pet. Explor. Prod. Technol. 2017, 7, 1205–1216. [Google Scholar] [CrossRef]
- Khormali, A.; Sharifov, A.R.; Torba, D.I. The control of asphaltene precipitation in oil wells. Pet. Sci. Technol. 2018, 36, 443–449. [Google Scholar] [CrossRef]
- Chacon, O.G.; Pournik, M. Matrix Acidizing in Carbonate Formations. Processes 2022, 10, 174. [Google Scholar] [CrossRef]
- Robertson, J.O., Jr.; Chilingarian, G.V. Acidizing Oilwells. In Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 1989; Volume 19, pp. 161–190. [Google Scholar]
- Economides, M.J.; Hill, A.D.; Ehlig-Economides, C.; Zhu, D. Petroleum Production Systems; Pearson Education: New York, NY, USA, 2012. [Google Scholar]
- Muecke, T.W. Principles of Acid Stimulation. Presented at the International Petroleum Exhibition and Technical Symposium, Beijing, China, 17–24 March 1982; OnePetro: Richardson, TX, USA, 1982. [Google Scholar] [CrossRef]
- Chang, F.F.; Nasr-El-Din, H.A.; Lindvig, T.; Qiu, X.W. Matrix Acidizing of Carbonate Reservoirs Using Organic Acids and Mixture of HCl and Organic Acids. Presented at the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008; OnePetro: Richardson, TX, USA, 2008. [Google Scholar] [CrossRef]
- Leong, V.H.; Ben Mahmud, H. A preliminary screening and characterization of suitable acids for sandstone matrix acidizing technique: A comprehensive review. J. Pet. Explor. Prod. Technol. 2018, 9, 753–778. [Google Scholar] [CrossRef]
- Lake, L.W.; Clegg, J.D. Petroleum Engineering Handbook; Society of Petroleum Engineers: Richardson, TX, USA, 2007; Volume IV. [Google Scholar]
- Zhu, D.; Hill, D.; Ugursal, A.; Shuchart, C.; Purdy, C.; Weissenberger, M.; Uribe, J. A modified acid system to enhance carbonate matrix acid stimulation: An experimental study. Can. J. Chem. Eng. 2021, 100, 1187–1201. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. Alternative Stimulation Fluids and Their Impact on Carbonate Acidizing. SPE J. 1998, 3, 34–41. [Google Scholar] [CrossRef]
- Tambini, M. Beyond Acidizing and Fracturing. Presented at the SPE European Formation Damage Conference, The Hague, The Netherlands, 13–14 May 2003; OnePetro: Richardson, TX, USA, 2003. [Google Scholar] [CrossRef]
- Houseworth, J. Advanced Well Stimulation Technologies. In Chapter, 2013, Volume 2, pp. 47–88. Available online: http://www.t.ccst.us/publications/2014/2014wst2.pdf (accessed on 30 July 2016).
- Kartini, R.; Kim, Y.; Lee, W. Evaluation of Surfactant Mixture for Supercritical Carbon Dioxide Foamed Acid in Car-bonate Matrix Acidizing. Energies 2021, 14, 6567. [Google Scholar] [CrossRef]
- Frenier, W.W.; Hill, D.G. Effect of acidizing additives on formation permeability during matrix treatments. Presented at the International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 20–21 February 2002; OnePetro: Richardson, TX, USA, 2002. [Google Scholar]
- King, G.E. Acidizing Concepts Matrix vs. Fracture Acidizing. J. Pet. Technol. 1986, 38, 507–508. [Google Scholar] [CrossRef]
- Shokry, A.F.; Tawfik, O.M. Acid Stimulation Results of Arab Carbonate Reservoirs in Offshore Field, U.A.E.: 10 Years of Review, How to Prepare the Future Challenges Linked to Stimulation. Presented at the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 1–4 November 2010; OnePetro: Richardson, TX, USA, 2010. [Google Scholar] [CrossRef]
- Garrouch, A.A.; Jennings, A.R. A contemporary approach to carbonate matrix acidizing. J. Pet. Sci. Eng. 2017, 158, 129–143. [Google Scholar] [CrossRef]
- Reyes, E.A.; Smith, A.L.; Beuterbaugh, A.; Calabrese, T. GLDA/HF facilitates high temperature acidizing and coiled tubing corrosion inhibition. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Budapest, Hungary, 3–5 June 2015. [Google Scholar]
- Hendrickson, A.R.; Thomas, R.L.; Economides, M.J. Stimulation of carbonate reservoirs. Dev. Pet. Sci. 1992, 30, 589–625. [Google Scholar] [CrossRef]
- Ortega, A. Acidizing High-Temperature Carbonate Formations Using Methanesulfonic Acid. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2015. Available online: https://hdl.handle.net/1969.1/155026 (accessed on 25 March 2015).
- Al Rbeawi, S.; Kadhim, F.S.; Farman, G.M. Optimum matrix acidizing: How much does it impact the productivity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012105. [Google Scholar] [CrossRef]
- Bellen, R.C.; Dunnington, H.V.; Wetzel, R.; Morton, D.M. Lexique Stratigraphique International; Centre National de la Recherche Scientifique: Paris, France, 1959; Volume 3, p. 333. [Google Scholar]
- Jassim, S.Z.; Goff, J.C. (Eds.) Geology of Iraq; Dolin, Prague and Moravian Museum: Brno, Czech Republic, 2006; p. 355. [Google Scholar]
- Salih, N.M. Scanning the Lower Cretaceous carbonate rocks utilizing stable isotopes and petrographic records. J. Earth Sci. Environ. Stud. 2022, 6, 148–163. [Google Scholar]
- Al-Peryadi, K. Sedimentological Study and Reservoir Characterizations for the Upper Qamchuqa and Jawan formations to Determine the Effective Porosity in Bai-Hassan Field. Unpublished Master’s Thesis, University of Baghdad, Baghdad, Iraq, 2002; p. 178. [Google Scholar]
- Al-Qayim, B.; Qadir, F.M.; Albeyati, F. Dolomitization and porosity evaluation of the Cretaceous Upper Qamchuqa (Mauddud) Formation, Khabbaz oil field, Kirkuk area, northern Iraq. Geoarabia 2010, 15, 49–76. [Google Scholar] [CrossRef]
- Ghafur, A.A.; Hasan, D.A. Petrophysical Properties of the Upper Qamchuqa Carbonate Reservoir through Well Log Evaluation in the Iraqi Khabbaz Oilfield. UKH J. Sci. Eng. 2017, 1, 72–88. [Google Scholar] [CrossRef]
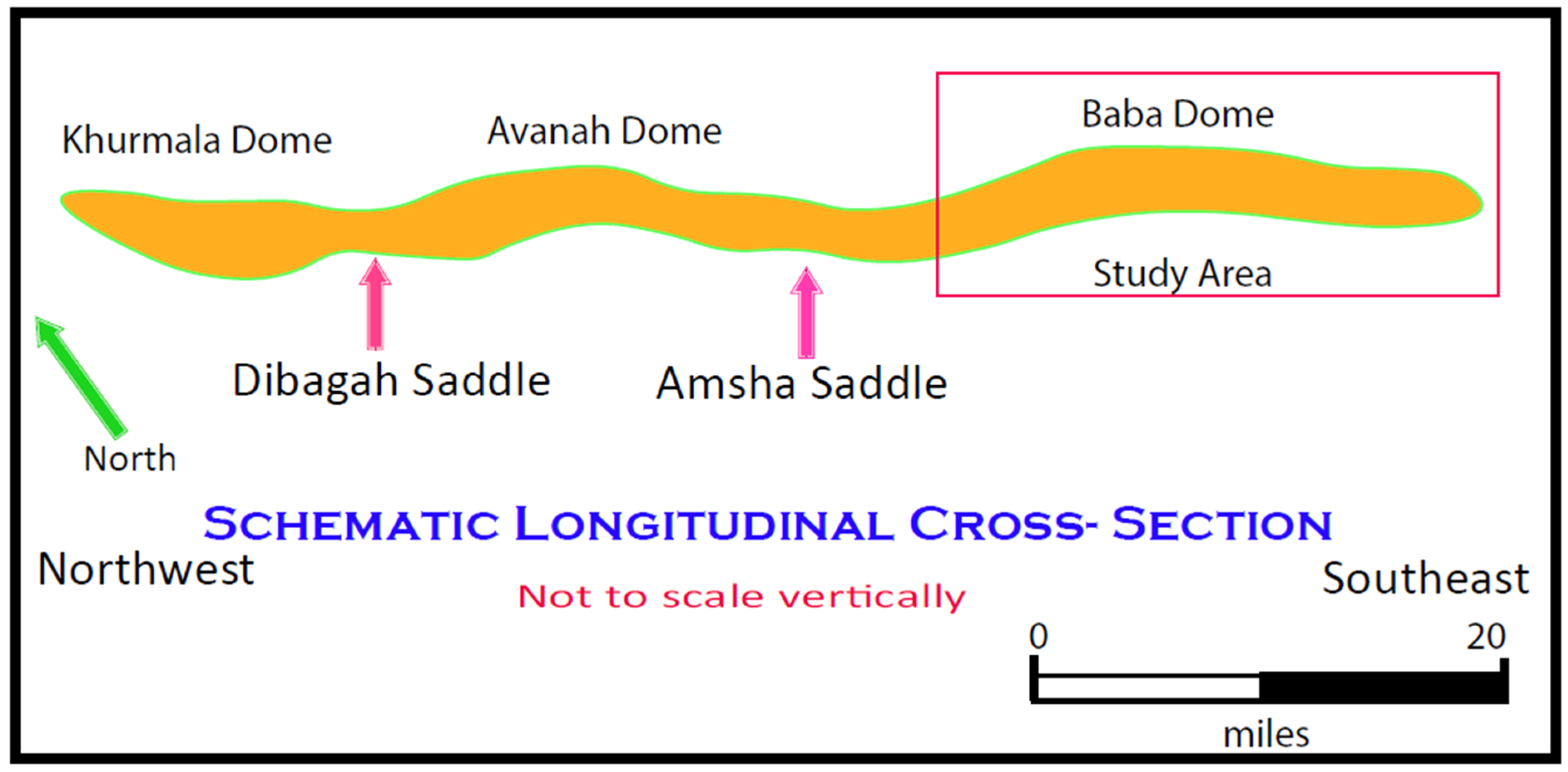
- Deikran, D.B.; AL-Lehaibe, A.M. Study of Joints in Baba Dome-NE Iraq. Kirkuk Univ. J.-Sci. Stud. 2016, 11, 26–37. [Google Scholar]
- Abdulraheem, A. Impact of HCl Acidizing Treatment on Mechanical Integrity of Carbonaceous Shale. ACS Omega 2022, 7, 13629–13643. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Hart, B. Explaining surface interactions for common associated gangues of rare earth minerals in response to the oxalic acid. Int. J. Min. Sci. Technol. 2018, 28, 343–346. [Google Scholar] [CrossRef]
- Dazem, C.L.; Noa, F.M.; Nenwa, J.; Öhrström, L. Natural and synthetic metal oxalates—A topology approach. CrystEngComm 2019, 21, 6156–6164. [Google Scholar] [CrossRef]
- Ahmadi, S.; Khormali, A.; Khoutoriansky, F.M. Optimization of the demulsification of water-in-heavy crude oil emulsions using response surface methodology. Fuel 2022, 323, 124270. [Google Scholar] [CrossRef]
- Salih, N.; Mansurbeg, H.; Préat, A. Geochemical and dynamic model of repeated hydrothermal injections in two Mesozoic successions, Provencal Domain, Maritime Alps, SE-France. Minerals 2020, 10, 775. [Google Scholar] [CrossRef]
- Salih, N.; Mansurbeg, H.; Muchez, P.; Gerdes, A.; Préat, A. Hydrothermal Fluids and Cold Meteoric Waters along Tectonic-Controlled Open Spaces in Upper Cretaceous Carbonate Rocks, NE-Iraq: Scanning Data from In Situ U-Pb Geochronology and Microthermometry. Water 2021, 13, 3559. [Google Scholar] [CrossRef]
- Isah, A.; Arif, M.; Hassan, A.; Mahmoud, M.; Iglauer, S. Fluid–rock interactions and its implications on EOR: Critical analysis, experimental techniques and knowledge gaps. Energy Rep. 2022, 8, 6355–6395. [Google Scholar] [CrossRef]
- Salih, N.; Mansurbeg, H.; Kolo, K.; Gerdes, A.; Préat, A. In situ U-Pb dating of hydrothermal diagenesis in tectonically controlled fracturing in the Upper Cretaceous Bekhme Formation, Kurdistan Region-Iraq. Int. Geol. Rev. 2019, 62, 2261–2279. [Google Scholar] [CrossRef]






| Experiment No. | 1 * | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | a | b | c | d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxalic acid % | 6 | 8 | 3.2 | 6 | 8.8 | 8 | 6 | 4 | 4 | 12 | 4 | 4 | 4 |
| Temp.°C | 50 | 70 | 50 | 21.7 | 50 | 30 | 78.3 | 70 | 30 | 50 | 50 | 40 | Room Temp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulrahman, A.; Salih, N.; Kamal, I.; Préat, A. Adopted Factorial and New In-Situ Micro-Designs for Stimulation of Matrix Acidizing of Carbonate Reservoir Rocks. Appl. Sci. 2023, 13, 1752. https://doi.org/10.3390/app13031752
Abdulrahman A, Salih N, Kamal I, Préat A. Adopted Factorial and New In-Situ Micro-Designs for Stimulation of Matrix Acidizing of Carbonate Reservoir Rocks. Applied Sciences. 2023; 13(3):1752. https://doi.org/10.3390/app13031752
Chicago/Turabian StyleAbdulrahman, Aram, Namam Salih, Ibtisam Kamal, and Alain Préat. 2023. "Adopted Factorial and New In-Situ Micro-Designs for Stimulation of Matrix Acidizing of Carbonate Reservoir Rocks" Applied Sciences 13, no. 3: 1752. https://doi.org/10.3390/app13031752
APA StyleAbdulrahman, A., Salih, N., Kamal, I., & Préat, A. (2023). Adopted Factorial and New In-Situ Micro-Designs for Stimulation of Matrix Acidizing of Carbonate Reservoir Rocks. Applied Sciences, 13(3), 1752. https://doi.org/10.3390/app13031752







