Membrane Technology as a Strategy for Improving β-Galactosidase Concentration Processes: The Influence of the pH, Membrane Molecular Weight, Pressure, and Ionic Strength in the Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism, Inoculum, and Submerged Cultivation
2.3. Enzyme Extraction
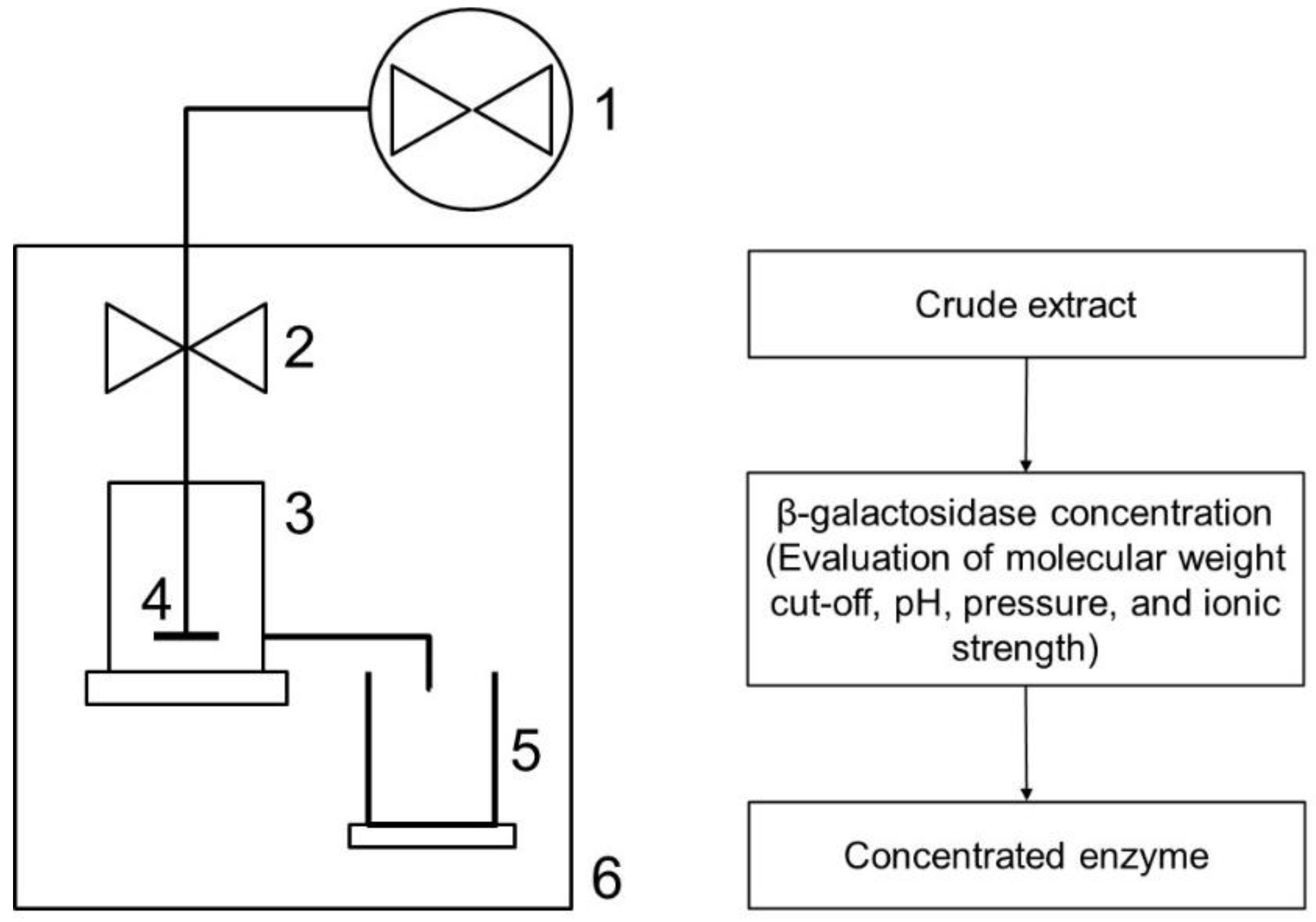
2.4. β-Galactosidase Concentration Using Ultrafiltration Step
2.4.1. Assessment of Menbrane Molecular Weight Cut-Off and Operating pH and Pressure
2.4.2. Ionic Strength Influence
2.4.3. Diafiltration
2.5. Ultrafiltration Performance
2.6. Analytical Methods
Enzyme Activity and Total Protein
2.7. Statistical Analysis
3. Results and Discussion
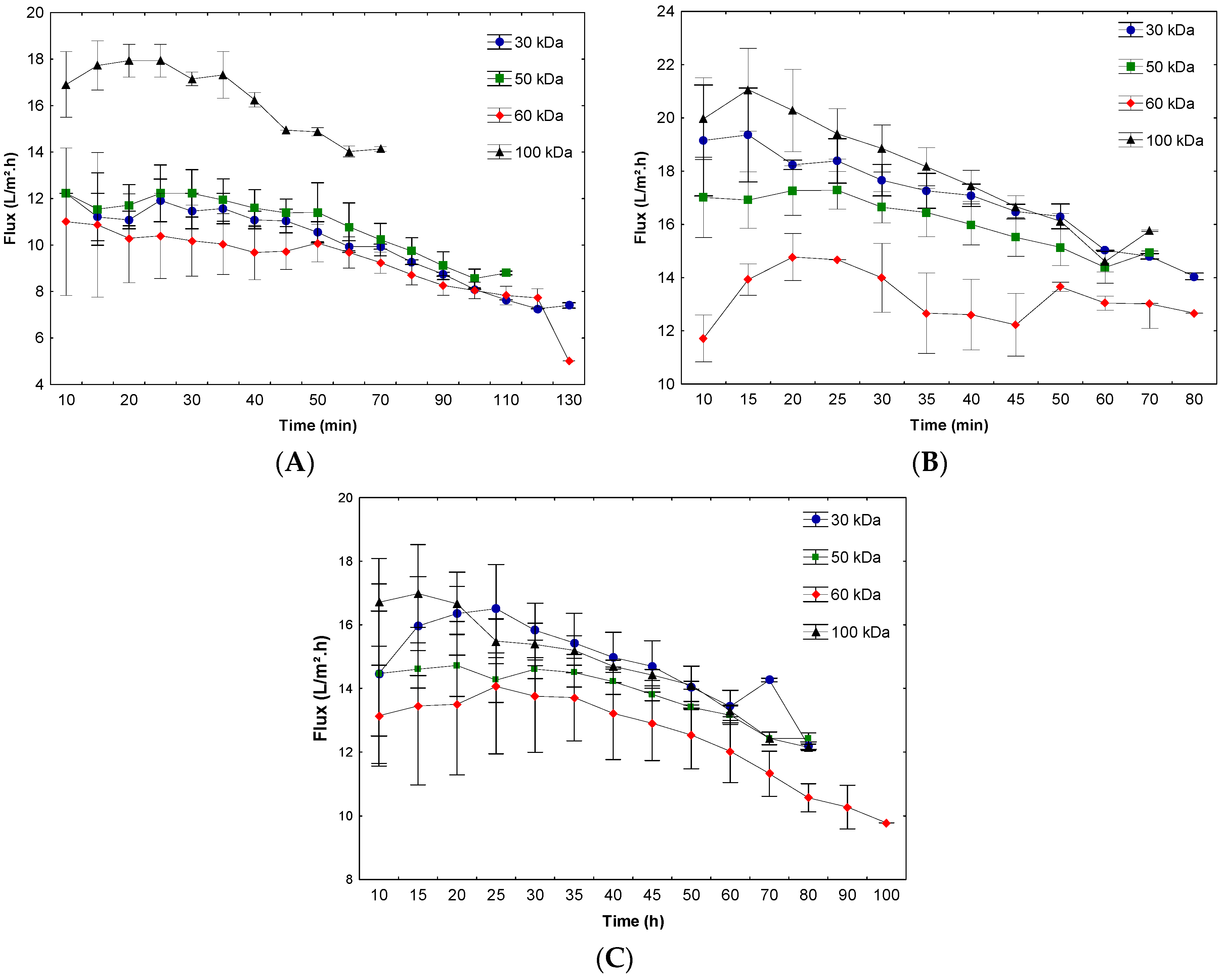
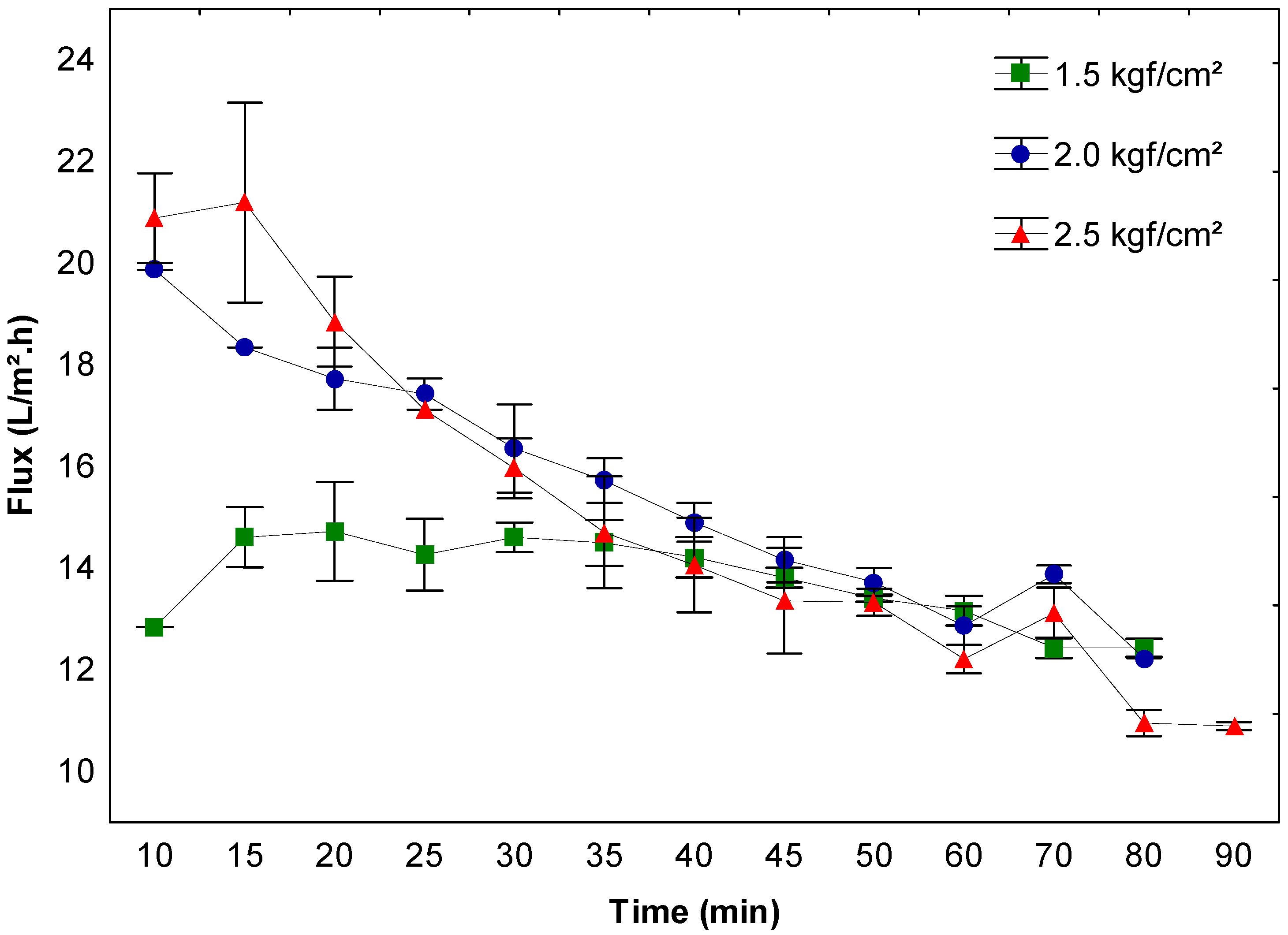
3.1. β-Galactosidase Recovery and Concentration Using Ultrafiltration
3.2. Diafiltration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lemes, A.C.; Machado, J.R.; Brites, M.L.; Di-Luccio, M.; Kalil, S.J. Design Strategies for Integrated β-Galactosidase Purification Processes. Chem. Eng. Technol. 2014, 37, 1–9. [Google Scholar]
- Dekker, P.J.T.; Koenders, D.; Bruins, M.J. Lactose-Free Dairy Products: Market Developments, Production, Nutrition and Health Benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Kocabaş, H.; Ergin, F.; Aktar, T.; Küçükçetin, A. Effect of lactose hydrolysis and salt content on the physicochemical, microbiological, and sensory properties of ayran. Int. Dairy J. 2022, 129, 105360. [Google Scholar] [CrossRef]
- Ugidos-Rodríguez, S.; Matallana-González, M.C.; Sánchez-Mata, M.C. Lactose malabsorption and intolerance: A review. Food Funct. 2018, 9, 4056–4068. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; Manera, A.P.; Ores, J.D.; Sala, L.; Maugeri, F.; Kalil, S.J. Kinetics and Thermal Properties of Crude and Purified β-Galactosidase with Potential for the Production of Galactooligosaccharides. Food Technol. Biotechnol. 2013, 51, 45–52. [Google Scholar]
- Yu, L.; O’Sullivan, D.J. Production of galactooligosaccharides using a hyperthermophilic β-galactosidase in permeabilized whole cells of Lactococcus lactis. J. Dairy Sci. 2014, 97, 694–703. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, S.; Zhao, L.; Chen, L.; Du, M.; Zhang, C.; Yang, S.-T. A novel β-galactosidase from Klebsiella oxytoca ZJUH1705 for efficient production of galacto-oligosaccharides from lactose. Appl. Microbiol. Biotechnol. 2020, 104, 6161–6172. [Google Scholar] [CrossRef]
- Tomal, A.A.B.; Farinazzo, F.S.; Bachega, A.; Bosso, A.; Silva, J.B.; Suguimoto, H. Galacto Oligosaccharides and Human Health Implications. Nutr. Food Sci. Int. J. 2019, 9, 1–4. [Google Scholar]
- Asraf, S.S.; Gunasekaran, P. Current trends of β-galactosidase research and application. Appl Microbiol Biot 2010, 1, 880–890. [Google Scholar]
- Schmidt, C.; Mende, S.; Jaros, D.; Rohm, H. Fermented milk products: Effects of lactose hydrolysis and fermentation conditions on the rheological properties. Dairy Sci. Technol. 2016, 96, 199–211. [Google Scholar] [CrossRef]
- Lima, P.C.; Gazoni, I.; de Carvalho, A.M.G.; Bresolin, D.; Cavalheiro, D.; de Oliveira, D.; Rigo, E. β-galactosidase from Kluyveromyces lactis in genipin-activated chitosan: An investigation on immobilization, stability, and application in diluted UHT milk. Food Chem. 2021, 349, 129050. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C.; Braga, A.R.C.; Gautério, G.V.; Fernandes, K.F.; Egea, M.B. Application of Membrane Technology for Production of Bioactive Peptides. In Bioactive Peptides: Production, Bioavailability, Health Potential and Regulatory Issues; Onuh, J.O., Selvamuthukumaran, M., Pathak, Y.V., Eds.; CRC Press: Boca Raton, FL, USA, 2021; Volume 1, pp. 253–279. [Google Scholar]
- Cui, Z. Protein separation using ultrafiltration: An example of multi-scale complex systems. China Particuology 2005, 3, 343–348. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Cui, Z.F. Protein purification by ultrafiltration with pre-treated membrane. J. Membr. Sci. 2000, 167, 47–53. [Google Scholar] [CrossRef]
- Lu, R.R.; Xu, S.Y.; Wang, Z.; Yang, R.J. Isolation of lactoferrin from bovine colostrum by ultrafiltration coupled with strong cation exchange chromatography on a production scale. J. Membr. Sci. 2007, 297, 152–161. [Google Scholar] [CrossRef]
- Van Audenhaege, M.; Garnier-Lambrouin, F.; Piot, M.; Gésan-Guiziou, G. Unexpected displacement of the equilibrium between the apo and the holo form during ultrafiltration of the metalloprotein α-lactalbumin. J. Membr. Sci. 2012, 401–402, 195–203. [Google Scholar] [CrossRef]
- Iltchenco, S.; Preci, D.; Bonifacino, C.; Fraguas, E.F.; Steffens, C.; Panizzolo, L.A.; Colet, R.; Fernandes, I.A.; Abirached, C.; Valduga, E.; et al. Whey protein concentration by ultrafiltration and study of functional properties. Ciência Rural 2018, 48, 778–780. [Google Scholar] [CrossRef]
- Patsioura, A.; Galanakis, C.M.; Gekas, V. Ultrafiltration optimization for the recovery of β-glucan from oat mill waste. J. Membr. Sci. 2011, 373, 53–63. [Google Scholar] [CrossRef]
- Xie, J.H.; Shen, M.Y.; Nie, S.P.; Zhao, Q.; Li, C.; Xie, M.Y. Separation of water-soluble polysaccharides from Cyclocarya paliurus by ultrafiltration process. Carbohydr. Polym. 2014, 101, 479–483. [Google Scholar] [CrossRef]
- Sala, L.; Gautério, G.V.; Younan, F.F.; Brandelli, A.; Moraes, C.C.; Kalil, S.J. Integration of ultrafiltration into an aqueous two-phase system in the keratinase purification. Process Biochem. 2014, 49, 2016–2024. [Google Scholar] [CrossRef]
- Long, X.; Meng, Q.; Sha, R.; Huang, Q.; Zhang, G. Two-step ultrafiltration of rhamnolipids using PSU-g-PEG membrane. J. Membr. Sci. 2012, 409–410, 105–112. [Google Scholar] [CrossRef]
- Tres, M.V.; Racoski, J.C.; Di Luccio, M.; Oliveira, J.V.; Treichel, H.; de Oliveira, D.; Mazutti, M.A. Separation of soybean oil/n-hexane and soybean oil/n-butane mixtures using ceramic membranes. Food Res Int 2014, 63 Part A, 33–41. [Google Scholar] [CrossRef]
- Biron, D.d.S.; Zeni, M.; Bergmann, C.P.; Santos, V.d. Analysis of Composite Membranes in the Separation of Emulsions Sunflower oil/water. Mater. Res. 2017, 20, 843–852. [Google Scholar] [CrossRef]
- Firdaous, L.; Dhulster, P.; Amiot, J.; Gaudreau, A.; Lecouturier, D.; Kapel, R.; Lutin, F.; Vézina, L.-P.; Bazinet, L. Concentration and selective separation of bioactive peptides from an alfalfa white protein hydrolysate by electrodialysis with ultrafiltration membranes. J. Membr. Sci. 2009, 329, 60–67. [Google Scholar] [CrossRef]
- Lemes, A.C.; Sala, L.; Ores, J.C.; Braga, A.R.; Egea, M.B.; Fernandes, K.F. A Review of the Latest Advances in Encrypted Bioactive Peptides from Protein-Rich Waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [PubMed]
- Hatzinikolaou, D.G.; Katsifas, E.; Mamma, D.; Karagouni, A.D.; Christakopoulos, P.; Kekos, D. Modeling of the simultaneous hydrolysis–ultrafiltration of whey permeate by a thermostable β-galactosidase from Aspergillus niger. Biochem. Eng. J. 2005, 24, 161–172. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Athanasopoulos, V.I.; Niranjan, K.; Rastall, R.A. Synthesis of galacto-oligosaccharide from lactose using beta-galactosidase from Kluyveromyces lactis: Studies on batch and continuous UF membrane-fitted bioreactors. Biotechnol. Bioeng. 2005, 89, 434–443. [Google Scholar] [CrossRef]
- Palai, T.; Mitra, S.; Bhattacharya, P.K. Kinetics and design relation for enzymatic conversion of lactose into galacto-oligosaccharides using commercial grade beta-galactosidase. J. Biosci. Bioeng. 2012, 114, 418–423. [Google Scholar] [CrossRef]
- Hemavathi, A.B.; Raghavarao, K.S.M.S. Differential partitioning of β-galactosidase and β-glucosidase using aqueous two phase extraction. Process Biochem. 2011, 46, 649–655. [Google Scholar] [CrossRef]
- Veide, A.; Lindbäck, T.; Enfors, S.-O. Recovery of β-galactosidase from a poly (ethylene glycol) solution by diafiltration. Enzym. Microb Tech. 1989, 11, 744–751. [Google Scholar] [CrossRef]
- Lemes, A.C.; Pavón, Y.; Lazzaroni, S.; Rozycki, S.; Brandelli, A.; Kalil, S.J. A new milk-clotting enzyme produced by Bacillus sp. P45 applied in cream cheese development. LWT Food Sci. Technol. 2016, 66, 217–224. [Google Scholar] [CrossRef]
- Manera, A.P.; Ores, J.C.; Ribeiro, V.A.; Burkert, C.A.V.; Kalil, S.J. Optimization of the culture medium for the production of beta-galactosidase from Kluyveromyces marxianus CCT 7082. Food Technol. Biotech. 2008, 46, 66–72. [Google Scholar]
- Pinheiro, R.; Belo, I.; Mota, M. Growth and beta-galactosidase activity in cultures of Kluyveromyces marxianus under increased air pressure. Lett. Appl. Microbiol. 2003, 37, 438–442. [Google Scholar] [CrossRef]
- Medeiros, F.O.; Veiga Burkert, C.A.; Juliano Kalil, S. Purification of β-Galactosidase by Ion Exchange Chromatography: Elution Optimization Using an Experimental Design. Chem. Eng. Technol. 2012, 35, 911–918. [Google Scholar] [CrossRef]
- Medeiros, F.O.; Alves, F.G.; Lisboa, C.R.; Martins, D.S.; Burkert, C.A.V.; Kalil, S.J. Ondas ultrassônicas e pérolas de vidro: Um novo método de extração de β-galactosidase para uso em laboratório. Química Nova 2008, 31, 336–339. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Lemes, A.C.; Kalil, S.J. Single Chromatographic Step for β-Galactosidase Purification: Influence of Salt and Elution Parameters. Sep. Sci. Technol. 2014, 49, 1817–1824. [Google Scholar] [CrossRef]
- Heidtmann, R.B.; Duarte, S.H.; Pereira, L.P.; Braga, A.R.C.; Kalil, S.J. Kinetics and thermodynamic characterization of β-galactosidase from Kluyveromyces marxianus CCT 7082 fractionated with ammonium sulphate. Braz. J. Food Technol. 2012, 15, 41–49. [Google Scholar] [CrossRef]
- Inchaurrondo, V.A.; Flores, M.V.; Voget, C.E. Growth and β-galactosidase synthesis in aerobic chemostat cultures of Kluyveromyces lactis. J. Ind. Microbiol. Biotech. 1998, 20, 291–298. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ghosh, R.; Cui, Z.F. Purification of lysozyme using ultrafiltration. Biotechnol. Bioeng. 2000, 68, 191–203. [Google Scholar] [CrossRef]
- Ni, L.; Shi, Q.; Wu, M.; Ma, J.; Wang, Y. Fouling behavior and mechanism of hydrophilic modified membrane in anammox membrane bioreactor: Role of gel layer. J. Membr. Sci. 2021, 620, 118988. [Google Scholar] [CrossRef]
- Cortés, G.; Trujillo-Roldán, M.A.; Ramírez, O.T.; Galindo, E. Production of β-galactosidase by Kluyveromyces marxianus under oscillating dissolved oxygen tension. Process Biochem. 2005, 40, 773–778. [Google Scholar] [CrossRef]
- Pan, I.H.; Yao, H.J.; Li, Y.K. Effective extraction and purification of β-xylosidase from Trichoderma koningii fermentation culture by aqueous two-phase partitioning. Enzym. Microb. Tech. 2001, 28, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Medeiros e Silva, G.M.; Porto, C.S.; Cavalcanti, M.T.H.; Neto, B.B.; Lima-Filho, J.L.; Converti, A.; Porto, A.L.F.; Pessoa, A., Jr. Liquid–liquid extraction of proteases from fermented broth by PEG/citrate aqueous two-phase system. Chem. Eng. Process. Process Intensif. 2008, 47, 716–721. [Google Scholar] [CrossRef]
- Saha, N.K.; Balakrishnan, M.; Ulbricht, M. Polymeric membrane fouling in sugarcane juice ultrafiltration: Role of juice polysaccharides. Desalination 2006, 189, 59–70. [Google Scholar] [CrossRef]
- Bullón, J.; Belleville, M.P.; Rios, G.M. Preparation of gelatin formed-in-place membranes: Effect of working conditions and substrates. J. Membr. Sci. 2000, 168, 159–165. [Google Scholar] [CrossRef]
- Palecek, S.P.; Mochizuki, S.; Zydney, A.L. Effect of ionic environment on BSA filtration and the properties of BSA deposits. Desalination 1993, 90, 147–159. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 1–20. [Google Scholar] [CrossRef]
- Cassini, A.S.; Tessaro, I.C.; Marczak, L.D.F.; Pertile, C. Ultrafiltration of wastewater from isolated soy protein production: A comparison of three UF membranes. J. Clean. Prod. 2010, 18, 260–265. [Google Scholar] [CrossRef]
- Chollangi, A.; Hossain, M.M. Separation of proteins and lactose from dairy wastewater. Chem. Eng. Process. Process Intensif. 2007, 46, 398–404. [Google Scholar] [CrossRef]
- Shahid, M.; Faure, C.; Ottoboni, S.; Lue, L.; Price, C. Employing Constant Rate Filtration To Assess Active Pharmaceutical Ingredient Washing Efficiency. Org. Process Res. Dev. 2022, 26, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.S.; Bilad, M.R.; Shamsuddin, N.; Suhaimi, H.; Ismail, N.M.; Jaafar, J.; Ismail, A.F. Confounding Effect of Wetting, Compaction, and Fouling in an Ultra-Low-Pressure Membrane Filtration: A Review. Polymers 2022, 14, 2073. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Leow, H.F. Microfiltration of activated sludge wastewater—The effect of system operation parameters. Sep. Purif. Technol. 2002, 29, 189–198. [Google Scholar] [CrossRef]
- Aghajani, M.; Maruf, S.H.; Wang, M.; Yoshimura, J.; Pichorim, G.; Greenberg, A.; Ding, Y. Relationship between permeation and deformation for porous membranes. J. Membr. Sci. 2017, 526, 293–300. [Google Scholar] [CrossRef]
- Van de Ven, W.J.C.; Sant, K.v.t.; Pünt, I.G.M.; Zwijnenburg, A.; Kemperman, A.J.B.; van der Meer, W.G.J.; Wessling, M. Hollow fiber dead-end ultrafiltration: Influence of ionic environment on filtration of alginates. J. Membr. Sci. 2008, 308, 218–229. [Google Scholar] [CrossRef]
- Leberknight, J.; Wielenga, B.; Lee-Jewett, A.; Menkhaus, T.J. Recovery of high value protein from a corn ethanol process by ultrafiltration and an exploration of the associated membrane fouling. J. Membr. Sci. 2011, 366, 405–412. [Google Scholar] [CrossRef]
- Becht, N.O.; Malik, D.J.; Tarleton, E.S. Evaluation and comparison of protein ultrafiltration test results: Dead-end stirred cell compared with a cross-flow system. Sep. Purif. Technol. 2008, 62, 228–239. [Google Scholar] [CrossRef]
- Athès, V.; Combes, D. Influence of Additives on High Pressure Stability of β-Galactosidase from Kluyveromyces Lactis and Invertase from Saccharomyces Cerevisiae. Enzym. Microb. Tech. 1998, 22, 532–537. [Google Scholar] [CrossRef]
- Lin, S.H.; Hung, C.L.; Juang, R.S. Effect of operating parameters on the separation of proteins in aqueous solutions by dead-end ultrafiltration. Desalination 2008, 234, 116–125. [Google Scholar] [CrossRef]
- Teng, M.-Y.; Lin, S.-H.; Wu, C.-Y.; Juang, R.-S. Factors affecting selective rejection of proteins within a binary mixture during cross-flow ultrafiltration. J. Membr. Sci. 2006, 281, 103–110. [Google Scholar] [CrossRef]
- Kelly, S.T.; Zydney, A.L. Protein fouling during microfiltration: Comparative behavior of different model proteins. Biotechnol. Bioeng. 1997, 55, 91–100. [Google Scholar] [CrossRef]
- Lebendiker, M.; Danieli, T. Production of prone-to-aggregate proteins. FEBS Lett. 2014, 588, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Arakawa, T.; Shiraki, K. Effect of additives on protein aggregation. Curr. Pharm. Biotechnol. 2009, 10, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C. Purification of β-galactosidase: Process design. In School of Chemistry and Food; Federal University of Rio Grande: Rio Grande, Brazil, 2015; 95p, Available online: https://repositorio.furg.br (accessed on 26 December 2022).
- Duarte, R.M.B.O.; Santos, E.B.H.; Duarte, A.C. Comparison between diafiltration and concentration operation modes for the determination of permeation coefficients of humic substances through ultrafiltration membranes. Anal. Chim. Acta 2001, 442, 155–164. [Google Scholar] [CrossRef]
| pH | MWCO (kDa) | %R A | PF B | RP(%) C | Fc (V) D | Fc (A) E | Fc(P) F |
|---|---|---|---|---|---|---|---|
| 6.5 | 30 | 93.0 ± 0.01 b | 0.93 ± 0.03 cd | 98.5 ± 1.16 ab | 6.7 ± 0.0 ab | 5.79 ± 0.68 ab | 6.31 ± 0.84 a |
| 50 | 79.5 ± 4.8 b | 0.89 ± 0.06 d | 99.4 ± 0.66 ab | 6.4 ± 0.26 b | 5.0 ± 0.3 b | 5.67 ± 0.05 ab | |
| 60 | 89.3 ± 2.9 ab | 0.95 ± 0.09 cd | 99.0 ± 0.96 ab | 6.4 ± 0.5 b | 5.7 ± 0.30 ab | 5.93 ± 0.58 ab | |
| 100 | 96.2 ± 2.2 ab | 1.08 ± 0.08 abcd | 99.2 ± 0.96 ab | 7.2 ± 0.5 ab | 6.9 ± 0.66 a | 6.16 ± 0.53 ab | |
| 7.0 | 30 | 98.1 ± 1.4 ab | 1.09 ± 0.02 abcd | 99.9 ± 0.16 a | 7.6 ± 0.64 ab | 7.03 ± 0.64 a | 6.44 ± 0.59 a |
| 50 | 96.4 ± 2.7 ab | 1.2 ± 0.08 ab | 99.7 ± 0.12 ab | 6.8 ± 0.67 ab | 6.54 ± 0.73 a | 5.42 ± 0.54 ab | |
| 60 | 96.3 ± 2.7 ab | 1.03 ± 0.11 bcd | 99.6 ± 0.13 ab | 6.3 ± 0.36 b | 6.03 ± 0.36 ab | 5.83 ± 0.7 ab | |
| 100 | 99.5 ± 0.3 ab | 1.19 ± 0.06 ab | 99.0 ± 0.42 ab | 6.4 ± 0.36 b | 6.42 ± 0.46 ab | 5.38 ± 0.24 ab | |
| 7.5 | 30 | 86.0 ± 6.2 ab | 1.14 ± 0.03 abc | 99.1 ± 0.64 b | 8.1 ± 0.25 a | 6.97 ± 0.36 a | 6.12 ± 0.46 ab |
| 50 | 108.9 ± 4.2 a | 1.22 ± 0.06 ab | 96.9 ± 2.7 ab | 6.6 ± 0.18 ab | 7.15 ± 0.41 a | 5.88 ± 0.06 ab | |
| 60 | 96.4 ± 2.3 ab | 1.28 ± 0.14 ab | 98.9 ± 0.3 ab | 6.8 ± 0.78 ab | 6.17 ± 0.39 ab | 4.82 ± 0.38 b | |
| 100 | 97.9 ± 1.76 ab | 1.05 ± 0.04 bcd | 98.9 ± 0.45 ab | 7.1 ± 1.0 ab | 6.14 ± 0.39 ab | 5.75 ± 0.27 ab |
| Salt | Concentration (mol.L) | %R | FC(A) | PF |
|---|---|---|---|---|
| Control | - | 108.9 ± 4.2 a | 7.15 ± 0.4 a | 1.22 ± 0.06 a |
| KCl | 0.01 | 92.4 ± 4.1 b | 4.6 ± 0.7 b | 0.9 ± 0.05 b |
| 0.05 | 88.3 ± 7.3 bc | 5.9 ± 0.1 abc | 0.8 ± 0.1 b | |
| 0.1 | 73.9 ± 4.7 cd | 3.2 ± 0.6 c | 0.7 ± 0.09 b | |
| NaCl | 0.01 | 85.9 ± 3.9 bc | 4.3 ± 0.4 c | 0.8 ± 0.08 b |
| 0.05 | 63.7 ± 6.1 d | 3.1 ± 0.7 c | 0.7 ± 0.1 b | |
| 0.1 | 60.2 ± 5.7 d | 3.4 ± 0.3 c | 0.7 ± 0.06 b |
| Purification Steps | Salts | Diafiltration Cicles * | % R | PF |
|---|---|---|---|---|
| Aqueous two-phase system | Potassium phosphate | 6 | 93.4 ± 2.7 a | 1.1 ± 0.1 a |
| Precipitation | Ammonium sulfate | 97.2 ± 3.4 a | 1.0 ± 0.1 a | |
| Ion exchange chromatography | Potassium phosphate | 93.4 ± 2.1 a | 1.0 ± 0.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemes, A.C.; Molon, F.d.O.; Fagundes, A.d.S.; Egea, M.B.; Di Luccio, M.; Kalil, S.J. Membrane Technology as a Strategy for Improving β-Galactosidase Concentration Processes: The Influence of the pH, Membrane Molecular Weight, Pressure, and Ionic Strength in the Process. Appl. Sci. 2023, 13, 1626. https://doi.org/10.3390/app13031626
Lemes AC, Molon FdO, Fagundes AdS, Egea MB, Di Luccio M, Kalil SJ. Membrane Technology as a Strategy for Improving β-Galactosidase Concentration Processes: The Influence of the pH, Membrane Molecular Weight, Pressure, and Ionic Strength in the Process. Applied Sciences. 2023; 13(3):1626. https://doi.org/10.3390/app13031626
Chicago/Turabian StyleLemes, Ailton Cesar, Fabrício de Oliveira Molon, Alexandre da Silva Fagundes, Mariana Buranelo Egea, Marco Di Luccio, and Susana Juliano Kalil. 2023. "Membrane Technology as a Strategy for Improving β-Galactosidase Concentration Processes: The Influence of the pH, Membrane Molecular Weight, Pressure, and Ionic Strength in the Process" Applied Sciences 13, no. 3: 1626. https://doi.org/10.3390/app13031626
APA StyleLemes, A. C., Molon, F. d. O., Fagundes, A. d. S., Egea, M. B., Di Luccio, M., & Kalil, S. J. (2023). Membrane Technology as a Strategy for Improving β-Galactosidase Concentration Processes: The Influence of the pH, Membrane Molecular Weight, Pressure, and Ionic Strength in the Process. Applied Sciences, 13(3), 1626. https://doi.org/10.3390/app13031626








