Featured Application
The research provides data on practices in imaging employed in radiotherapy services in countries with different levels of income and development. The information will be used in deciding on recommendations to encourage optimization of radiological protection for imaging by radiotherapy facilities and in evaluating what additional dosimetry features might be included on cone beam CT facilities incorporated into radiotherapy equipment. These will be included in a publication on radiological protection aspects of imaging in radiotherapy being prepared by the International Commission on Radiological Protection (ICRP).
Abstract
Dramatic improvements in radiotherapy equipment have allowed radiation fields to be conformed to tumours for more accurate treatment. Successful delivery often requires imaging at every treatment fraction, a method known as image guided radiation therapy (IGRT). But increased X-ray imaging exposes patients to doses that carry risks of inducing second cancers in normal tissues. Therefore, reductions in high-dose treatment margins achieved with IGRT must be balanced against detriments from greater imaging doses. ICRP Task Group 116 has been set up to prepare guidance on radiological protection aspects of IGRT. Factors affecting the optimization of radiological protection are the modalities used, the frequency of imaging, the image acquisition parameters influencing image quality and radiation dose, and the volume of normal tissue included in the images. The Task Group has undertaken two projects: (1) a snapshot survey of radiotherapy imaging practices across six continents, which has shown that use of kV cone beam CT (CBCT) increases with Human Development Index for the country; and (2) a project looking at ways for measuring CBCT doses that could be applied more widely. The results highlight the need for raising awareness of imaging doses, and development of the dose quantities displayed on imaging equipment used in radiotherapy.
1. Introduction
Radiotherapy is an important method for treatment of patients with cancer, with 50–60% of patients undergoing radiotherapy either alone or in combination with surgery or chemotherapy [1,2]. In the last few decades, dramatic improvements have been made in the capability of external beam radiotherapy (EBRT) equipment to conform radiation treatment fields to any shape of tumour. Most radiation treatment plans for EBRT consist of 3D dose distributions calculated from computed tomography (CT) scans, while other medical imaging modalities are often necessary to assist with the identification of the treatment target. The development of tungsten multi-leaf collimators (MLCs) has enabled the radiation field delivered by the radiation source, most frequently a medical linear accelerator (linac), to be conformed to the shape of the tumour target. Conformal treatment fields are then delivered from multiple static directions or via dynamic arc radiotherapy to focus the therapeutic high dose on the tumour and spare the healthy surrounding tissue from the potentially harmful radiation side effects. However, the required high targeting accuracy can only be achieved by ensuring a daily reproducible patient position as close as possible to that of the treatment plan.
The size of the high therapeutic dose region of the treatment field is typically larger than the tumour, as defined by the clinical target volume (CTV) and planning target volume (PTV) concepts [3,4]. The CTV expands directly from the tumour or gross tumour volume (GTV) to account for any cancer cells lying on the periphery of the tumour. The PTV expands from the CTV to account for internal motion and variations in shape of the tumour, and the uncertainties in patient position during radiotherapy treatments. The treatment dose and PTV margin are adjusted to maximize the chance of cancer cure, while minimizing the probability of complications by maintaining doses to nearby organs at risk (OARs) below their respective tolerance doses [5]. When a patient is set up for treatment, images are acquired, and then compared and aligned with the planning images of the patient to reduce positional uncertainty. Pre-treatment imaging allows the size of the high-dose margin to be reduced (Figure 1). Set-up images are frequently recorded at many, if not all, of the fractions in which treatment is delivered. Cases involving motion management or high-dose treatments might also require intra-fraction imaging. This enables changes in patient anatomy to be monitored during treatment, and allows corrections to be made to treatment delivery if required. The process of using images in planning treatments and then taking further images at the time of treatment to guide the delivery is referred to as image guided radiation therapy (IGRT). Additional imaging during treatment planning and delivery can also be used to account for motion, with the recording of multiple images through breathing or other motion cycles.
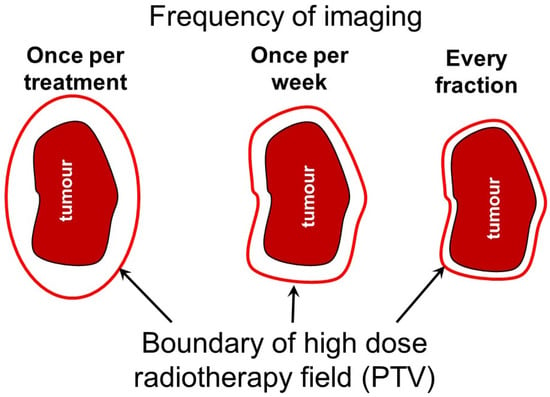
Figure 1.
Schematic diagram illustrating the relationship between the planning target volume (PTV) around a tumour and the frequency of imaging during radiotherapy treatments. High-dose margins around tumour targets are used to account for uncertainty in target location and more frequent imaging allows margins to be reduced to protect normal tissue. There is a balance between reducing the width of these high-dose margins around the target and lowering the dose to surrounding normal tissues from more frequent imaging.
The application of IGRT around the world has increased considerably in the last decade. It should enable more precise treatment delivery and improved safety. IGRT has led to better patient outcomes, and this is still improving steadily [6]. The National Radiotherapy Trials Quality Assurance Group (UK) reviewed the current evidence on the use of IGRT for pelvic tumours and concluded that IGRT was essential for the safe implementation of highly conformal pelvic radiotherapy [7]. IGRT may in some cases decrease overall treatment time, reduce the number of treatment sessions required, and even enable certain patients to receive radiation therapy when that might otherwise not have been possible.
However, increased imaging with X-rays exposes normal tissues surrounding a tumour to additional radiation. This carries a risk of raising doses to OARs close to the PTV boundary above the respective tolerance doses, as imaging doses are not accounted for in treatment planning [5], and thereby increases the risk of deterministic acute or long-term effects. In addition, there will be an increased risk of inducing second cancers in organs and tissues lying within the field being imaged [8,9,10,11,12]. A study of radiotherapy imaging doses in a US hospital reported average cumulative imaging doses to the brain, lungs and red bone marrow of 380, 188, and 491 mGy, respectively, for which the associated additional average lifetime attributable risks of cancer incidence per 100,000 persons were 78, 271 and 510 for brain cancer, lung cancer and leukaemia, respectively [12]. Therefore, the decrease in exposure that can be achieved through use of smaller high-dose treatment margins and reduction of alignment errors need to be balanced against the detriment from doses to surrounding tissues from more frequent imaging. A study of a group of Finnish hospitals showed that cumulative doses to organs surrounding the PTV can vary by factors of ten or more depending on the imaging technique and frequency, indicating that there is considerable scope for optimisation of radiological protection [11]. Making judgements on potential harm from imaging is difficult without information on the doses received, and this information is limited at the present time.
Guidance has been produced about the use of IGRT [13], but this has been largely by high income countries and less information is available on its use in countries at all levels throughout the world and especially on doses delivered by radiologic imaging modalities in radiotherapy. Two projects are described in this paper; the purpose of the first was to gain information about the use of IGRT in countries at different stages of development with varying levels of funding. The type of information sought was how widely IGRT was used, and how much information and knowledge users have about doses from imaging delivered to patients. The second project was to look at methods for recording doses from cone beam CT (CBCT), the imaging method used more widely, and the feasibility of carrying out such measurements in countries with lower levels of funding.
2. Materials and Methods
ICRP set up Task Group 116 to provide recommendations and guidance on radiological protection aspects of IGRT. A report is being prepared to give an overview of imaging use in radiotherapy and provide guidance on optimization of imaging practices. Two aspects of the Task Group work are considerations that relate to the most effective method for recording patient doses and for developing appropriate methods that would allow radiotherapy centres to monitor imaging dose performance at all levels of expertise.
2.1. Imaging Practice Survey
Information available on the use of IGRT is limited largely to more developed nations. Therefore, the Task Group set up a project through the ICRP mentorship programme (https://www.icrp.org/page.asp?id=465, accessed 29 December 2022) to find out more about practices in other parts of the world. This took advantage of the wide range of countries from which mentee applications were received. The survey was conducted online for the countries in which the mentees were resident in 2020. It involved completion of 130 items of information about imaging practices in each radiotherapy centre that agreed to participate, and a total of 97 centres, spread across nine countries in six continents, were included in the analysis [14]. Australia and New Zealand, which work closely together, were combined as one entry for Australasia. In each country, the mentee was responsible for collating the data and investigating any apparently anomalous responses.
The survey of imaging practices in radiotherapy showed that all surveyed countries employed IGRT, but differences were apparent in the extent to which imaging was used in lower- and middle-income countries (LMICs). In order to investigate relationships between practices and the income and development of the countries in the survey, results for individual countries were compared against the Human Development Index (HDI) value, as defined by the United Nations Development Programme [15]. The HDI combines indices of life expectancy and education (literacy rate and receipt of different levels of education) with per capita income, and values increase with the level of development up to a maximum of 1.0. Pearson correlation coefficients (R) were derived to assess relationships of some parameters, with the HDI assuming statistical significance at the p < 0.05 level.
2.2. Imaging Dose Measurement Project
In preparing guidance on radiological protection, it is necessary to consider the balance between doses that patients receive from frequent imaging, often performed multiple times throughout a whole treatment course, and the need to deliver the high therapeutic dose to the PTV with high accuracy (Figure 1). However, the recording of imaging doses from kV cone beam CT (CBCT), the main imaging modality used during treatment procedures, is not well established. Several methods have been proposed in guidance documents for measurement and calibration of doses for CBCT systems, and these are described in Section 4 of this paper. However, these would be impractical to implement for many centres and require time and resources that are unavailable in the majority of centres [16]. Bearing this in mind, a second project was established through the ICRP mentorship programme to consider ways in which quantities relating to patient dose might be measured, and what options might be available for countries with varying resources to carry out surveys of patient doses from imaging.
3. Results
3.1. Survey of IGRT Practices in Different Countries
The countries included in the survey, together with the numbers of radiotherapy centres and linacs, and the HDI are given in Table 1. The proportions of radiotherapy centres from which data were obtained in countries with HDI values of 0.9 and above were 11% or less, but for five countries with HDI values of 0.74–0.90, for which less data are available in the literature, results were obtained for 24% or more of the centres. However, the survey can only be considered as a snapshot showing variations in practices that occur in different regions, rather than being representative of the particular countries, as the centres participating might not be representative of the situation in the whole country.

Table 1.
Data on radiotherapy centres completing the questionnaire for each country [14].
All the centres used CT as the main imaging modality for treatment planning. Other imaging modalities were used in the planning process, with 80–90% of centres in Germany, Australasia, the USA, and Cyprus using magnetic resonance imaging (MRI). Positron emission tomography (PET)/CT was used when appropriate in 65–80% of centres in Germany, Australasia, the USA and Cyprus, and 40–50% of those in the Asian and South American countries. There were significant declines with the country HDI values in the proportions of centres using MRI (R = 0.879, p = 0.002) and PET/CT (R = 0.929, p < 0.0005 (Table 2)) [14]. The use of other modalities (SPECT/CT and ultrasound) showed weaker correlations (R < 0.75) and/or no statistical significance (p > 0.05). Many of the imaging modalities used were sited elsewhere in the hospital complexes, but between 20% and 50% of hospitals in the European, African, and American countries had MRI scanners within the radiotherapy centres.

Table 2.
Percentages of radiotherapy centres in each country using different imaging modalities to check patient position during treatment [14].
3.2. Imaging during the Treatment Cycle
The purpose of imaging during the radiotherapy treatment cycle is to align the treatment beam precisely with the targeted tumour and deliver the radiation treatment as accurately as possible. Patient positioning accuracy with IGRT varies, depending on the treatment site, prescription, PTV margin, treatment delivery technique, and immobilization method. A typical set of clinical tolerance for daily alignment ranges from ~10 mm for standard 3D breast treatment (planar MV and kV imaging), down to a few mm for IMRT treatments (3D CBCT imaging), and to sub-mm for intracranial high-dose stereotactic radiosurgery cases [17,18]. The ICRP Task Group 116 report aims to deal with radiological protection aspects of IGRT and—after appropriate patient selection—there are four areas involved in the choices relating to optimization of radiological protection. These are: the imaging modalities used, the frequency of imaging, the image acquisition parameters determining the quality of the image, and the volume of surrounding normal tissue included in the images.
3.2.1. Imaging Modality
kV cone beam CT (CBCT) was the modality used most widely during treatment, being used by all centres in countries with HDIs over 0.8 (Table 2). Two countries with lower HDIs had fewer kV CBCT facilities available. In Colombia, 71% of linacs in surveyed centres had CBCT but most centres had only one linac with kV imaging, and in Egypt only two linacs out of 17 had kV imaging, which resulted in MV imaging being the main modality used in most centres. The soft tissue image contrast with MV X-rays is poor and the absorbed doses delivered to tissues are substantially greater than with kV imaging techniques [11,19,20] and so MV imaging is an option that is used predominantly when kV imaging is not available. Non-ionizing radiation techniques were used widely to monitor patients during treatment with over half of the centres in Germany and the USA using optical surface guidance, and centres in Australasia and Saudi Arabia using more ultrasound. There was a significant decline in the number of centres using optical surface guidance with HDI (R = 0.708, p = 0.033). Between 10% and 25% of centres in countries surveyed with HDI values over 0.85 had access to MRI scanners.
3.2.2. Frequency of Imaging
The frequency of advanced imaging (i.e., CT, kV- and MV-CBCT, and fluoroscopic imaging) has a significant impact on the trade-off between doses to tissues surrounding the target from the therapy beam and those from imaging exposures. When a misalignment is identified by IGRT, the patient is typically repositioned to correct this and, in some cases, imaging may be repeated [17]. Increasing the number of fractions in which imaging is performed will reduce the likelihood of undetected patient alignment errors which could decrease the success of radiotherapy or lead to radiation injury to healthy tissue. The most common option used for all treatments was imaging at every fraction (Figure 2), as this ensures that delivery of treatment is as accurate as possible. A study of lung cancer treatments undertaken with varying frequencies of on-board mega-voltage CT (MVCT) imaging concluded that performing IGRT less than daily increased the rate of geographic misses when small (3 mm) uncertainty margins are chosen and recommended to increase such margin to ≥5 mm when imaging less frequently [21].
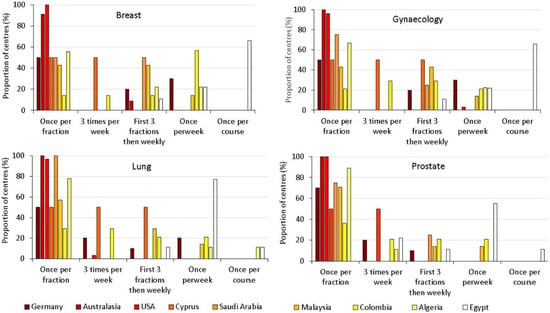
Figure 2.
The frequencies of imaging used most commonly by each radiotherapy centre during treatment of tumours in the trunk, as a proportion of the centres surveyed in the country [14]. The countries are ordered using a temperature scale from dark red, through orange and yellow to white, indicating decreasing HDI.
The frequency was generally higher in countries with higher HDI numbers, although in Germany, the country with the highest HDI, only half of the radiotherapy centres chose imaging at every fraction as the option used most frequently for treatments of the trunk. The frequency of imaging may be chosen to match the standard of care within each individual country and the resources available in specific radiotherapy centres. General trends in frequency of imaging practices within individual countries tended to follow similar patterns for most types of treatment. However, practices did vary with the location of the tumour being treated, and will depend on the treatment protocol and local departmental resources. Centres in Egypt that did not have kV CBCT only imaged either once per week or once during the course of treatment for most tumour types and only carried out imaging once during the course of radiotherapy for breast, gynaecology, and brain treatments.
For some treatments of the head, imaging might be performed twice or even three times during one fraction (Figure 3). This was particularly noticeable for stereotactic radiotherapy in which higher doses of radiation were delivered to the target from many angles, but with fewer fractions. Figure 3a,b shows all the treatment options used in each centre for treatment of the head and Figure 3c,d the ones used most frequently. This indicates that imaging more than once per fraction was only used for a proportion of patients being treated for both brain, and head and neck lesions.
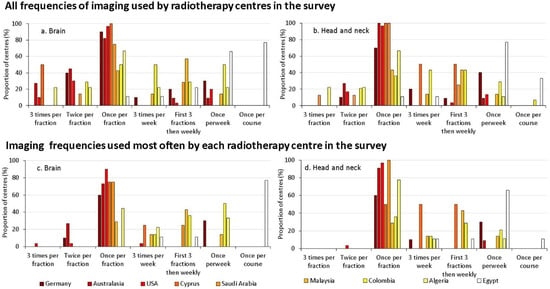
Figure 3.
The frequencies of imaging used more commonly by each radiotherapy centre during treatment of the brain (a,c) and head and neck tumours (b,d), as a proportion of the centres surveyed in the country; (a,b) representing all the frequency options used, and (c,d) the ones used most frequently [14]. The countries are ordered using a temperature scale from white to dark red indicating increasing HDI.
Imaging at every fraction is the choice favoured by most radiotherapy centres for most types of treatment providing close monitoring of variations over time and enabling the avoidance of random errors. A substantial number of centres in countries with HDIs between 0.75 and 0.9 also image for the first three fractions and then cut back to weekly, a strategy that helps to identify systematic differences between treatment planning and delivery.
3.2.3. The Balance between Image Quality and Imaging Dose
Less effort has been devoted to optimization of radiological protection for imaging in radiotherapy than in diagnostic radiology, as staff are accustomed to dealing with the much higher doses from therapeutic radiation. However, since exposures are repeated and normal tissues surrounding the tumour target are irradiated, imaging protocols should be optimized with radiological protection considerations, especially since the cumulative dose from daily kV CBCT over a whole treatment course can reach the level of a radiotherapy treatment fraction dose [13,19,22]. The level of image quality required for delineation of organs and target alignment in radiotherapy involves a trade-off with radiation exposure. Ideally the dose level for imaging should be the minimum necessary for providing the level of detail needed to verify alignments for accurate treatment delivery. This should not in general be as detailed as that required for diagnosis of disease. But, before optimization of radiological protection can begin, measures of the image quality linked to clinical requirements and a knowledge of the dose levels delivered during imaging are required. The survey showed that the majority of centres in most countries carried out objective evaluations of image quality, but only about half made dose measurements in Australasia, the USA, Colombia, Algeria and Egypt (Figure 4) as part of their quality control (QC) program. The proportion of centres carrying out QC on a monthly basis for imaging equipment declined with HDI (R = 0.784, p = 0.012).
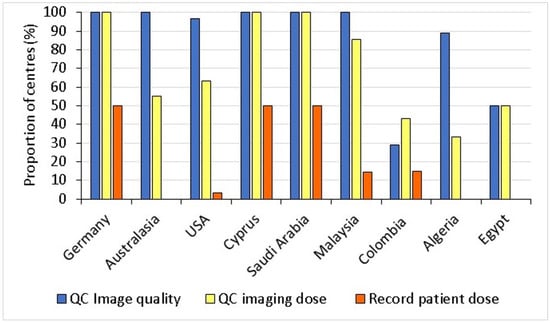
Figure 4.
Proportion of radiotherapy centres in each country carrying out QC tests on image quality and dose, and those recording patient dose quantities [14].
The survey further revealed that although patient doses from kV CBCT and kV planar imaging were recorded by 50% of the radiotherapy centres that took part in the survey in Cyprus, Germany, and Saudi Arabia, there was little recording of dose information at other centres. The recording of patient doses from imaging requires equipment, funding, and experience, but these were not necessarily the barriers, since countries with higher HDIs, such as Australia/New Zealand and the USA also did not record this information.
3.2.4. Imaging Field of View
The volume of normal tissue surrounding a tumour target that is irradiated during imaging is determined by the size of the X-ray field used. This should be restricted to the minimum to achieve the required accuracy of target positioning with the need to ensure that appropriate markers are visible on the images. All centres in Cyprus, Malaysia and Egypt used standard adult protocols provided by the vendors, with limited adjustment of field size for individual patients (Table 3). Between 38% and 60% of the radiotherapy centres surveyed in other countries adjusted the field size for individual adult patients [14]. A slightly greater proportion of the centres performing paediatric treatments adjusted field sizes for individual patients.

Table 3.
Percentages of radiotherapy centres in each country adapting protocols for individual patients and involvement of diagnostic medical physicists in optimization [14].
4. Dosimetry Methods for Cone Beam CT Systems
Optimization of radiological protection requires a knowledge of patient doses from imaging, but the survey has shown that only 50% of radiotherapy centres in European countries recorded patient doses and less than 10% in other parts of the world record patient doses (Figure 4). There is a need for raising awareness of imaging doses, and adaptation and development of the dose quantities displayed on imaging equipment used in radiotherapy to allow calibration and assessment to be performed more readily. The imaging technique used most widely during radiotherapy is kV CBCT [14,16], so this study is focused on that technique. The challenge is then to find measures of dose that can be made by centres in a variety of countries, many with limited resources.
Few centres have a methodology available that could be used to measure CBCT dose currently. Bearing this in mind, another mentee project has been initiated to investigate ways for measuring CBCT doses that could be applied more widely. The aim of the project is to trial ways in which dosimetry measurements could be carried out on CBCT systems on radiotherapy linacs. The dosimetry equipment and phantoms available in different countries vary widely and methods are required that could be used in centres with varying facilities, and ways developed in which comparisons of results could be made between centres.
The dose metric displayed on most CBCT systems is a version of the CT dose index (CTDI), which gives a measurement of dose with a 100 mm long ionization chamber. Measurements made at the centre and periphery of head or body phantoms made of polymethyl methacrylate (PMMA) and having diameters of 160 or 320 mm, respectively, and lengths of 150 mm, are combined to give a weighted result (CTDIw) that is suitable for dose surveys and optimization of practices. CTDIw values are not accurate reflections of doses to individual patients of varying size, but nevertheless provide a starting point through which comparisons can be made with doses from CT scanning more generally. The CTDI values displayed on CBCT scanners will depend on the model, and may be the standard CTDI for narrow beam CT or a measure for a wide beam, as this is an area that is evolving, so care is required in choice of any method for calibration. All vendors are encouraged to include displays of assessed imaging dose quantities that can be used for patient dose surveys. The calibration of the displayed quantity should be verified to allow future comparison against national or international standards.
Various methods have been developed for dose measurement and calibration of CBCT systems. The CTDIw concept has shortcomings in that it does not capture all the scattered radiation that would contribute to patient dose [23,24,25] and it is designed for CT scanners in which the fan beam is narrower, so that X-rays are incident at close to a perpendicular angle to the scanner axis. Various modifications have therefore been made to adapt the concept for CBCT. The International Electrotechnical Commission (IEC) has proposed making measurements with a narrower reference beam and adjusting these based on a ratio of CTDI100 measurements in air [26,27,28] to provide a measurement that is relatively independent of beam width [29]. An alternative approach taken by the American Association of Physicists in Medicine (AAPM) task group is based on measuring the cumulative dose with a small ion chamber in the centre of a phantom that is sufficiently long to include most of the contribution from scattered radiation [30,31]. However, neither of these techniques is straightforward to perform. The IEC method requires a series of CTDI measurements at different positions to replicate a longer ionization chamber, while the AAPM method requires the availability of a larger phantom.
Since imaging patient dose surveys are new to radiotherapy treatment imaging exposures, any survey that plans to obtain results from a large number of centres will require an approach that is easy to follow. A dose survey needs comparisons of performance across as many systems as possible in order to be successful. Use of a measurement of cumulative dose at the centre of a standard 150 mm long CT phantom with either a 0.6 cc Farmer ionization chamber or a 100 mm CTDI chamber with a wide beam are considered to be the easiest practical solutions for deriving a typical cumulative dose for a CBCT scan in mGy mAs−1 (Figure 5). The reason for using different chambers at this stage is that access to 100 mm chambers is limited in many radiotherapy centres, and use of a 0.6 cc chamber would be a viable alternative. The final choice of which chamber to use will depend on the availability in different countries, but at this stage the use of either is proposed. Conversion factors between the two would then be established from Monte Carlo calculations and experimental measurements. Monte Carlo calculations employing 109–1010 photon histories can reduce uncertainty to the order of 2% or lower [32]. It is proposed that measurements are made for the maximum field size used in clinical practice for the kV/filter combinations used according to a set protocol provided in a spreadsheet. Measurements would be combined with the accepted 2:1 weighting for CTDI measurements [28], to derive a weighted value of the cumulative dose per mAs for any given kV/filter combination.
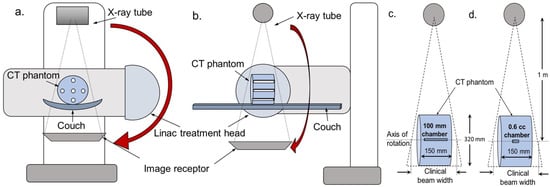
Figure 5.
Arrangement being used for measurement of cumulative dose at the centre of a CT body phantom. The X-ray source and image receptor are typically mounted at 90° from the treatment head of a medical linear accelerator. The figure shows schema of the measurement set-up in (a) the axial plane and (b) the sagittal plane. Measurements would be made with in a CT standard phantom with (c) a 100 mm CTDI ionization chamber and/or (d) a 0.6 cc Farmer type ionization chamber.
Because the beam will be larger than the phantom, the efficiency in terms of making a measure of dose to tissues in the body will only be 40–50% [32]. But the fact that this is not an absolute measure of dose to a patient does not matter at this stage, as these measurements are designed primarily for comparing the performance of CBCT protocols. They capture a simple measure related to the level of dose to the patient, and the effects of kV and filtration. Placing more constraints on measurement of the dose quantity to make a measure more meaningful in terms of dose to the patient would increase the difficulty of the measurement and inevitably reduce the number of centres able to take part.
Mentees have been asked first to take measurements at their own centres to establish the technique and then to ask colleagues to carry out similar measurements at their centres. The next stage will be to collect exposure factor data on standard treatment protocols for several specified treatments at each centre. The measurements can then be combined with exposure data in clinical protocols to obtain some information on patient dose levels. The measurements proposed should give dose values suitable for benchmarking and comparing the performance of equivalent CBCT protocols for the same clinical indication. The project is still at an early stage, but measurements have been made at a few centres and reasons for differences in results are being investigated. The project has already identified that one centre used similar exposure factors for imaging the thorax and the pelvis, whereas the exposure of the thorax at other centres was between a half and a quarter of the pelvic value, and this is now being investigated. A number of centres are unable to obtain access to equipment required. It is hoped that all centres will have at least a 0.6 cc Farmer chamber for which they can obtain a calibration for 120 kV X-rays, and if the project is successful a recommendation may be made that all centres arrange access to standard CT phantoms for the measurements.
5. Discussion
This study is not a comprehensive survey, but it has shown that kV CBCT is the imaging modality of choice for imaging immediately prior to treatment. The appropriate use of non-ionizing techniques may help to reduce numbers of X-ray exposures, where facilities are available. However, countries with lower HDI levels have fewer of these options available. Once the imaging modality has been decided, there remains a question as to whether the current use of advanced imaging techniques for IGRT is always appropriate. Beyond focusing on the PTV margin and geometric accuracy, the justification for daily imaging should also be on a patient-specific basis and take account of patient age and disposition during treatment, disease site and stage, radiotherapy dose and treatment delivery technique, as well as the availability of the imaging modality. In principle, the high targeting accuracy required for small PTV margins can only be conserved with a high imaging frequency (Figure 1). However, the clinical impact of imaging frequency on the potential targeting accuracy and cancer cure can only be determined on a patient-specific basis, as it depends on the tumour site and on the type of localization and positioning errors to be expected. Centres that only image once per week or once per course of treatment in this study were predominantly those without kV imaging facilities. The potential widening of the application of optical surface guidance has been suggested recently by an AAPM Task Group [33]. It is an approach that could help in reducing the frequency for X-ray imaging, but although optical surface guidance was employed in more high-income countries, only 11% of centres on average used it in countries with values of HDI less than 0.88. Since this is a less expensive alternative, it could assist in the optimisation of imaging in these countries. Globally, the frequency of imaging during treatment will be affected by the availability of appropriate equipment, resources and personnel, but more studies evaluating how imaging affects the accuracy of treatment delivery and treatment outcome are required to determine and support the optimal approaches.
Methods for the checking of dose performance of imaging equipment and recording of patient doses from imaging need to be introduced generally in radiotherapy centres and awareness among radiotherapy staff raised through education and training. The recording of patient dose is the first step that needs to be taken before optimization of radiological protection can begin. The lack of information on imaging dose apparent in this study is likely to result in less attention being paid to optimization of radiological protection, as the impact of any changes cannot be evaluated. It is also probably a factor behind the use of standard adult imaging protocols supplied by the vendor for all patients in most countries, often with limited optimization for individual patients (Table 3).
The majority of medical physicists working in radiotherapy do not have expertise in diagnostic imaging and will not be familiar with the dose quantities used. Therefore, introducing imaging dose measurements and surveys of patient imaging doses will require both involvement of diagnostic radiology medical physicists and training of radiotherapy imaging physicists. The survey showed that some centres did use diagnostic physicists, but the proportion in the different countries was variable (Table 3). More training courses in diagnostic imaging are required for both radiotherapy physicists and diagnostic imaging specialists who can also be involved in imaging for radiotherapy. The attention to dose levels should be associated with assessments of image quality, not only as a constancy check on imaging performance, but also to determining the level of image quality required for making the alignments necessary to ensure accurate treatment delivery.
If more assessment of imaging doses is carried out, there may be other simple steps that can be taken to reduce doses in the early stages. For example, similar protocols are generally used throughout the treatment of a patient, and since imaging of the same region around the tumour is often repeated at each treatment fraction, further adaptation of exposure factors to optimize radiological protection should be possible based on knowledge derived from the initial exposures.
Another aspect of optimization is the size of the radiation field used and the adjustment to suit the size of the patient. If a standard field size is used for patients of varying height, this will include a greater proportion of organs and tissues on the periphery in smaller patients. The size-specific effective dose that will be received by a person who is 5 cm shorter than the standard height will be between 3% and 10% greater [34], but use of standard field sizes for patients of all heights treated for a particular type of malignancy could result in much higher increases in dose than this. For example, upgrades in treatment planning software or radiation oncology information systems may include changes in imaging protocols, and associated increases in field sizes or exposure factors that could lead to gradual increases in imaging dose [35]. Such changes should be monitored as part of the evaluation process to check effects on imaging doses and ensure that any changes are reasonable in terms of potential improvements in treatment delivery.
Conventional CT scanners enable the fields to be included in a scan to be determined from scan projection radiographs and include automatic tube current modulation to reduce dose levels both for smaller patients and in parts of the scan where attenuation is lower. Facilities are included in some CBCT systems to enable the exposure arcs to be limited to protect radiosensitive organs, but the field size is not based on recorded images and is determined either by simple manual adjustment of collimation or selected from small, medium, and large options and there is no automatic exposure control. Tools to allow field sizes to be set based on an initial radiographic image from a single projection and radiation exposure levels to be adapted automatically to sizes of patients being imaged might be included by CBCT vendors to function in a similar manner to devices used in CT scanners. This type of development could be used together with appropriate selection of beam direction and bow-tie filters [20] to enable further optimization not only of dose but also image quality, and vendors are encouraged to consider potential use of an initial image for both adjustment of field size and exposure factors.
One aim of the Task Group is to provide guidance on the recording of imaging doses linked to programmes for dose optimization that will be applicable in all countries. However, the resources for CBCT CTDI dose measurement may not be readily available in all institutions in low- and middle-income countries, in terms of the phantom, measurement tools, and expertise. It should not be an issue for large teaching hospitals in most countries to perform such measurements, particularly for hospitals where diagnostic imaging medical physicists are available. While the radiation oncology medical physicists can be trained to perform the CTDI measurement, it can be a challenge for these institutions to justify the procurement of the CTDI phantom required for such measurements. Inclusion of the equipment in the procurement budgets may help to improve availability. Development of expertise within large teaching hospitals, resource sharing between institutions, and roll-out of techniques through training to other hospitals may be ways in which imaging dose measurement can be promoted in low- and middle-income countries.
6. Conclusions
Data from the projects described are feeding into development of the Task Group report with recommendations for users, managers, and equipment vendors to facilitate improvements in the application and optimization of radiological protection aspects in the use of imaging in radiotherapy. The main imaging modality employed during treatment is CBCT, and this is frequently used at every treatment fraction. But there are significant differences in what available imaging techniques can offer, both in terms of the amount of information provided and the dose level, so decisions are required about optimum choices for different types of treatment, and particular treatment sites. More information on development of guidance on the frequency of imaging for different types of treatment would be useful, but the approaches are going to depend on the facilities in each country. More research is needed to provide information on image quality as well as dose levels appropriate for imaging during radiotherapy treatment that can contribute to the overall optimization of radiological protection and clinical outcome for radiotherapy treatments.
The survey of imaging practice has made clear that radiotherapy centres throughout the world, even in some high-income countries, have little information on patient doses from imaging linked to radiation therapy treatments. A knowledge of patient dose is fundamental to starting the process of optimization of radiological protection, as without it there is no indication of what dose levels are used, and so it is not possible to evaluate the impact of any changes. Improvement in the situation requires efforts from radiotherapy departments, in terms of surveys of patient imaging doses. The methods described in the second project in this paper could be used by other radiotherapy centres to carry out initial evaluations of imaging doses that could be compared between centres in individual countries or more widely.
Developments from equipment manufacturers and vendors could aid in assessment of imaging doses through improvement in displayed dose quantities that can readily be measured and calibrated. Manufacturers are also encouraged to consider the introduction of techniques through which more control can be exercised over imaging exposure levels in CBCT. This might be achieved through exposure assessments based on an initial radiographic image that could be used both for a form of automatic exposure control in adjustment of X-ray tube current and as an image from which field sizes could be adjusted, as in standard CT, providing a next step in taking optimization of radiological protection for imaging forward.
Author Contributions
C.J.M. conceptualized the projects and methodologies described in the paper that were administered through the Task Group, and supervised development of the questionnaires and spreadsheets. He has coordinated the investigation and supervised several of the contributors to the project, overseeing data collation and curation, and formal analysis of the results. He wrote the initial draft of the paper and signed off the final version. S.G. has assisted in formal analysis of survey data and in trialing methods for the dosimetry measurements being undertaken. He has written sections of the paper and edited and reviewed the rest. T.K. has mentored participants in the project, assisted in formal analysis of data for the survey of practices, and reviewed and edited the draft paper. T.J.W. has been involved in conceptualizing and designing the survey questionnaire, trialing methods for dosimetry measurements. He prepared spreadsheets for recording the dosimetry results, led discussions of dosimetry methods, and reviewed the draft paper. J.V. has been involved in preparation of the survey questionnaire, supervised participants in the dosimetry project, assisted in formal analysis of data for the survey of practices, and reviewed and edited the paper. W.S.J. has mentored participants in the project and reviewed and edited the draft paper. U.N.M. has contributed to the formal analysis of survey data and reviewed sections of the paper relating to dosimetry measurements that might be undertaken in low- and middle-income countries. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from this study is only available in this paper and in reference [14].
Acknowledgments
The authors wish to acknowledge contributions of participants in the ICRP mentorship programme linked to Task Group 116 to collection of survey data, Y. Roussakis, M.C. Plazas, A.-H. Benali, M. Djukelic, H. Ragab, and A. Abuhaimed, and other members of the Task Group A. Isambert, D. Berger, S. Korreman, C. Lee, and T. Merchant for discussion of requirements for optimization of imaging.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Delaney, G.P.; Barton, M.B. Evidence-based estimates of the demand for radiotherapy. Clin. Oncol. 2015, 27, 70–76. [Google Scholar] [CrossRef]
- Abshire, D.; Lang, M.K. The evolution of radiation therapy in treating cancer Semin. Oncol. Nurs. 2018, 34, 151–157. [Google Scholar] [CrossRef]
- International Commission on Radiation Units and Measurements. Prescribing, Recording and Reporting photon Beam Therapy, ICRU Report 50, ICRU, 1993. Available online: https://journals.sagepub.com/toc/crub/os-26/1 (accessed on 7 October 2021).
- International Commission on Radiation Units and Measurements. Prescribing, Recording, and Reporting Intensity-Modulated Photon-Beam Therapy (IMRT), ICRU Report 83, ICRU, 2010, Bethesda, USA. Available online: https://journals.sagepub.com/toc/crua/10/1 (accessed on 9 August 2021).
- Joiner, M.C.; van der Kogel, A.J. Basic Clinical Radiobiology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Tiong, A.; Lao, L.; MacKean, J.; Goonetilleke, M.; Kron, T. Faculty of Radiation Oncology Position Paper on the use of Image-Guided Radiation Therapy. J. Med. Imaging Radiat. Oncol. 2016, 60, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.; Appelt, A.L.; Eminowicz, G. Image-guided radiotherapy for pelvic cancers: A review of current evidence and clinical utilization. Clin. Oncol. 2020, 32, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Dzierma, Y.; Minko, P.; Ziegenhain, F.; Bell, K.; Buecker, A.; Rübe, C.; Jagoda, P. Abdominal imaging dose in radiology and radiotherapy—Phantom point dose measurements, effective dose and secondary cancer risk. Phys. Med. 2017, 43, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Dzierma, Y.; Mikulla, K.; Richter, P.; Bell, K.; Melchior, P.; Nuesken, F.; Rübe, C. Imaging dose and secondary cancer risk in image-guided radiotherapy of pediatric patients. Radiat. Oncol. 2018, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Chung, W.K.; Yoon, M. Imaging doses and secondary cancer risk from kilovoltage cone-beam CT in radiation therapy. Health Phys. 2013, 104, 499–503. [Google Scholar] [CrossRef]
- Siiskonen, T.; Kaijaluoto, S.; Florea, T. Imaging practices and radiation doses from imaging in radiotherapy. Phys. Med. 2017, 42, 247–252. [Google Scholar] [CrossRef]
- Zhou, L.; Bai, S.; Zhang, Y.; Ming, X.; Zhang, Y.; Deng, J. Imaging Dose, Cancer Risk and Cost Analysis in Image-guided Radiotherapy of Cancers. Sci. Rep. 2018, 8, 10076. [Google Scholar] [CrossRef]
- Ding, G.X.; Alaei, P.; Curran, B.; Flynn, R.; Gossman, M.; Mackie, T.R.; Miften, M.; Morin, R.; Xu, X.G.; Zhu, T.C. Image guidance doses delivered during radiotherapy: Quantification, management, and reduction: Report of the AAPM Therapy Physics Committee Task Group 180. Med. Phys. 2018, 45, e84–e99. [Google Scholar] [CrossRef]
- Martin, C.J.; Kron, T.; Vassileva, J.; Wood, T.J.; Joyce, C.; Ung, N.M.; Small, W.; Gros, S.; Roussakis, Y.; Plazas, M.C.; et al. An international survey of imaging practices in radiotherapy. Phys. Med. 2021, 90, 53–65. [Google Scholar] [CrossRef] [PubMed]
- United Nations, United Nations Development Programme. Human Development Index (HDI). Available online: http://hdr.undp.org/en/content/human-development-index-hdi (accessed on 29 December 2022).
- International Commission on Radiological Protection. Radiological Protection in Cone Beam Computed Tomography (CBCT). ICRP Publication 129. Ann. ICRP 2016, 44, 9–127. [Google Scholar] [CrossRef]
- Luh, J.Y.; Albuquerque, K.V.; Cheng, C.; Ermoian, R.P.; Nabavizadeh, N.; Parsai, H.; Roeske, J.C.; Weiss, S.; Wynn, R.B.; Yu, Y.; et al. ACR–ASTRO Practice Parameter for Image-guided Radiation Therapy (IGRT). Am. J. Clin. Oncol. 2020, 43, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Chinsky, B.; Patel, R.; Panfil, J.; Surucu, M.; Roeske, J.C. A survey on table tolerances and couch overrides in radiotherapy. J. Appl. Clin. Med. Phys. 2016, 17, 405–420. [Google Scholar] [CrossRef]
- Stock, M.; Palm, A.; Altendorfer, A.; Steiner, E.; Georg, D. IGRT induced dose burden for a variety of imaging protocols at two different anatomical sites. Radiother. Oncol. 2012, 102, 355–363. [Google Scholar] [CrossRef]
- Ding, G.X.; Munro, P. Radiation exposure to patients from image guidance procedures and techniques to reduce the imaging dose. Radiother. Oncol. 2013, 108, 91–98. [Google Scholar] [CrossRef]
- Yu, Y.; Michaud, A.L.; Sreeraman, R.; Liu, T.; Purdy, J.A.; Chen, A.M. Comparison of daily versus nondaily image-guided radiotherapy protocols for patients treated with intensity-modulated radiotherapy for head and neck cancer. Head Neck 2014, 36, 992–997. [Google Scholar] [CrossRef]
- Alaei, P.; Spezi, E. Imaging dose from cone beam computed tomography in radiation therapy. Phys. Med. 2015, 31, 647–658. [Google Scholar] [CrossRef]
- Boone, J.M. The trouble with CTD100. Med. Phys. 2007, 34, 1364–1371. [Google Scholar] [CrossRef]
- Kyriako, Y.; Deak, P.; Langner, O.; Kalender, W.A. Concepts for dose determination in flat-detector CT. Phys. Med. Biol. 2008, 53, 3551–3566. [Google Scholar] [CrossRef]
- Geleijns, J.; Salvado Artells, M.; De Bruin, P.W.; Matter, R.; Muramatsu, Y.; Mcnitt-Gray, M.F. Computed tomography dose assessment for a 160 mm wide, 320 detector row, cone beam CT scanner. Phys. Med. Biol. 2009, 54, 3141–3159. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Status of Computed Tomography Dosimetry for Wide Cone Beam Scanners; IAEA Human Health Reports 5; IAEA: Vienna, Austria, 2011. [Google Scholar]
- Platten, D.J.; Castellano, I.A.; Chapple, C.L.; Edyvean, S.; Jansen, J.T.; Johnson, B.; Lewis, M.A. Radiation dosimetry for wide-beam CT scanners: Recommendations of a working party of the Institute of Physics and Engineering in Medicine. Br. J. Radiol. 2013, 86, 20130089. [Google Scholar] [CrossRef] [PubMed]
- International Electrotechnical Commission. Medical Electrical Equipment—Part 2–44: Particular Requirements for the Basic Safety and Essential Performance of X-ray Equipment for Computed Tomography; IEC, International Electrotechnical Commission: Geneva, Switzerland, 2009. [Google Scholar]
- Abuhaimed, A.; Martin, C.J.; Sankaralingam, M.; Gentle, D.J.; McJury, M. An assessment of the efficiency of methods for measurement of the computed tomography dose index (CTDI) for cone beam (CBCT) dosimetry by Monte Carlo simulation. Phys. Med. Biol. 2014, 59, 6307–6326. [Google Scholar] [CrossRef] [PubMed]
- American Association of Physicists in Medicine. Comprehensive Methodology for the Evaluation of Radiation Dose in X-ray Computed Tomography. Report of AAPM Task Group 111, 2010. Available online: www.aapm.org/pubs/reports/RPT_111.pdf (accessed on 29 December 2022).
- American Association of Physicists in Medicine. The Design and Use of the ICRU/AAPM CT Radiation Dosimetry Phantom: An Implementation of AAPM Report 111. The Report of AAPM Task Group 200, 2014. Available online: www.aapm.org/pubs/reports/RPT_200.pdf (accessed on 29 December 2022).
- Abuhaimed, A.; Martin, C.J.; Sankaralingam, M.; Gentle, D.J. A Monte Carlo investigation of cumulative dose measurements for cone beam computed tomography (CBCT) dosimetry. Phys. Med. Biol. 2015, 60, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Al-Hallaq, H.A.; Cerviño, L.; Gutierrez, A.N.; Havnen-Smith, A.; Higgins, S.A.; Kügele, M.; Padilla, L.; Pawlicki, T.; Remmes, N.; Smith, K.; et al. AAPM task group report 302: Surface-guided radiotherapy. Med. Phys. 2022, 49, e82–e112. [Google Scholar] [CrossRef]
- Martin, C.J.; Abuhaimed, A. Variations in size-specific effective dose with patient stature and beam width for kV cone beam CT imaging in radiotherapy. J. Radiol. Prot. 2022, 42, 031512. [Google Scholar] [CrossRef] [PubMed]
- Abuhaimed, A.; Martin, C.J.; Sankaralingam, M.A. A Monte Carlo study of organ and effective doses of cone beam computed tomography (CBCT) scans in radiotherapy. J. Radiol. Prot. 2018, 38, 61–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).