Innovative Alveolar Socket Preservation Procedure Using Demineralized Tooth Dentin as Graft Biomaterial Covered with Three Reabsorbable Membranes: Human Histological Case Series Evaluation
Abstract
1. Introduction
2. Materials and Methods
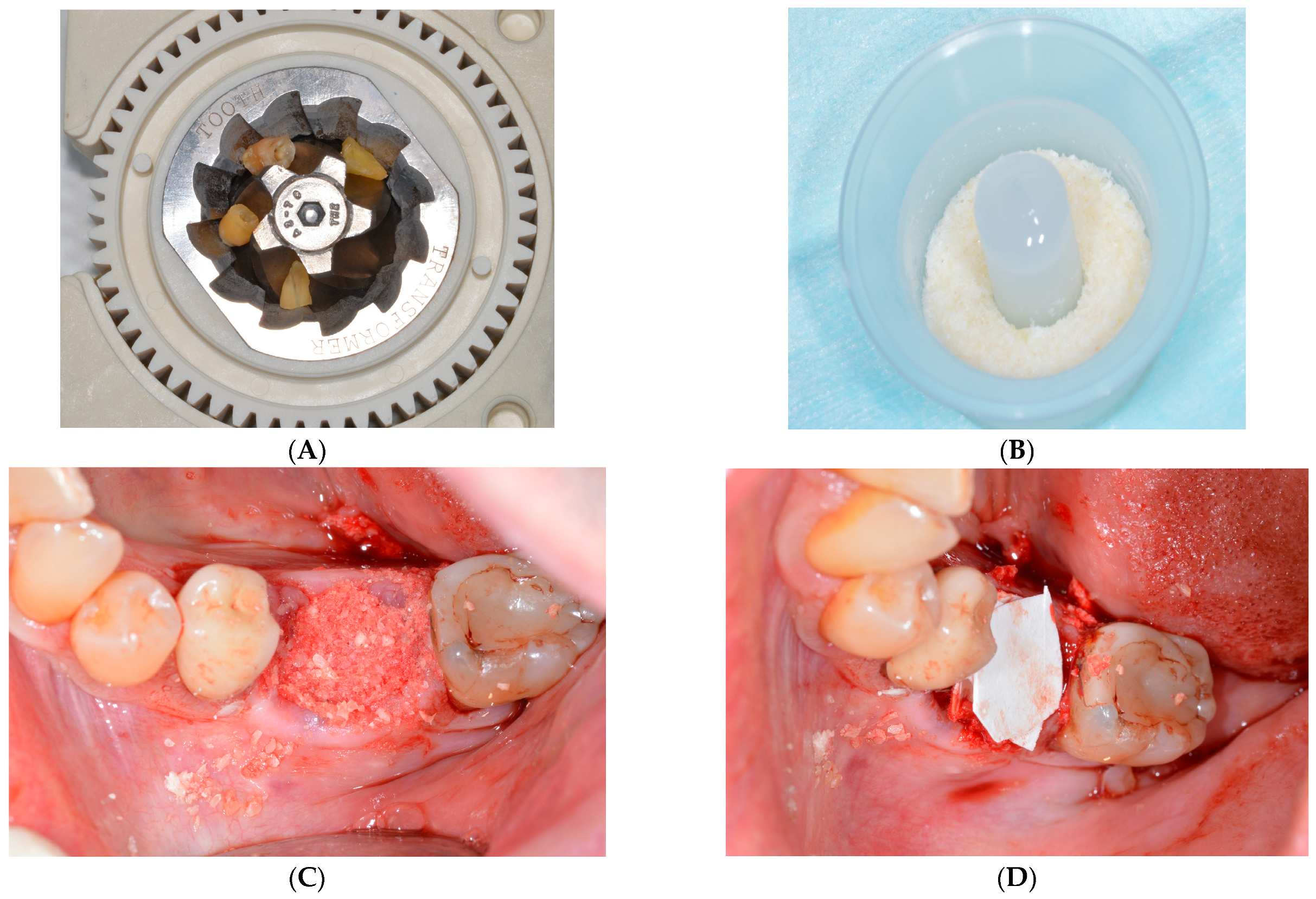
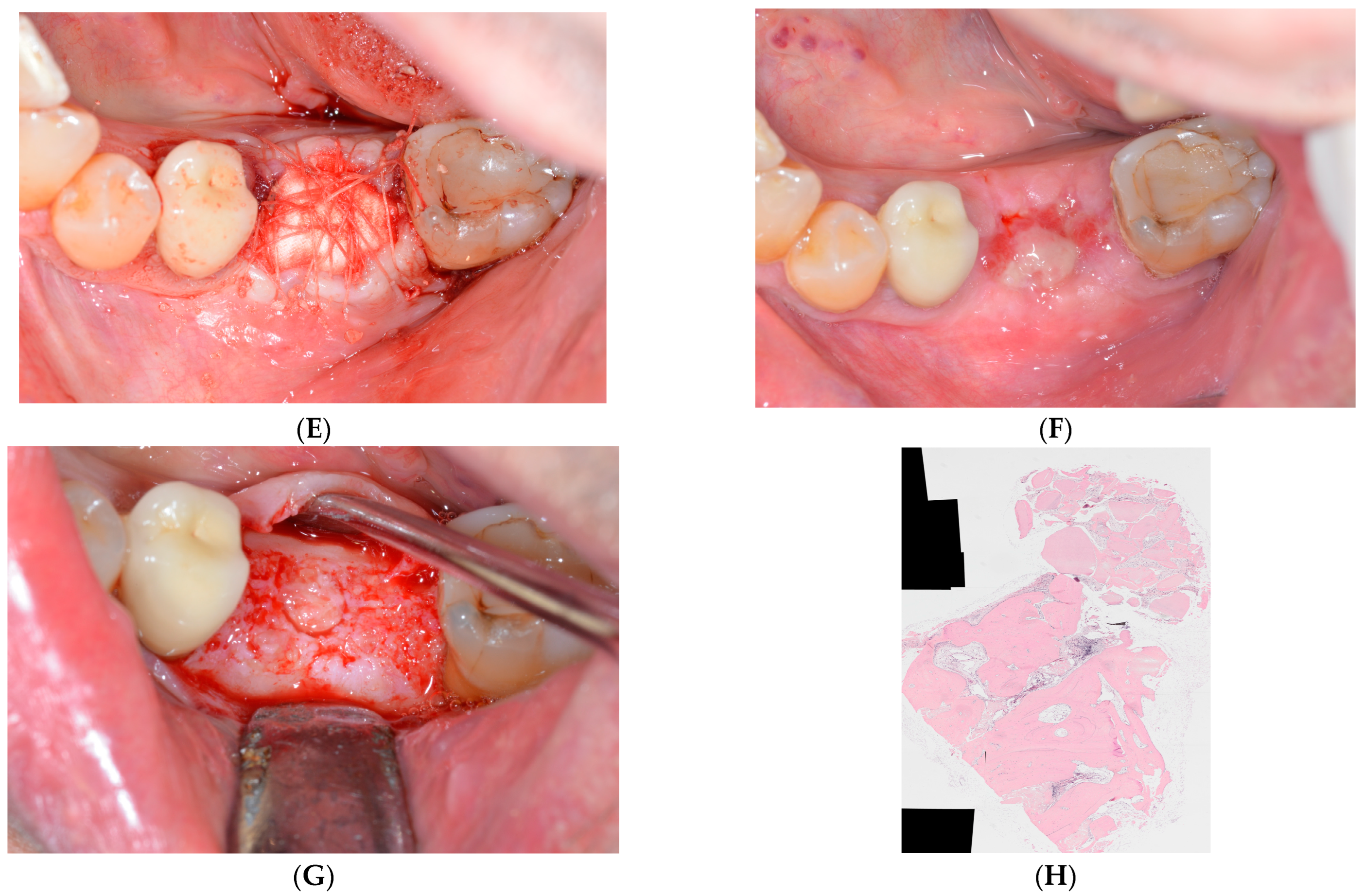
2.1. Surgical Protocol
2.2. Histology
2.3. Statistical Analysis
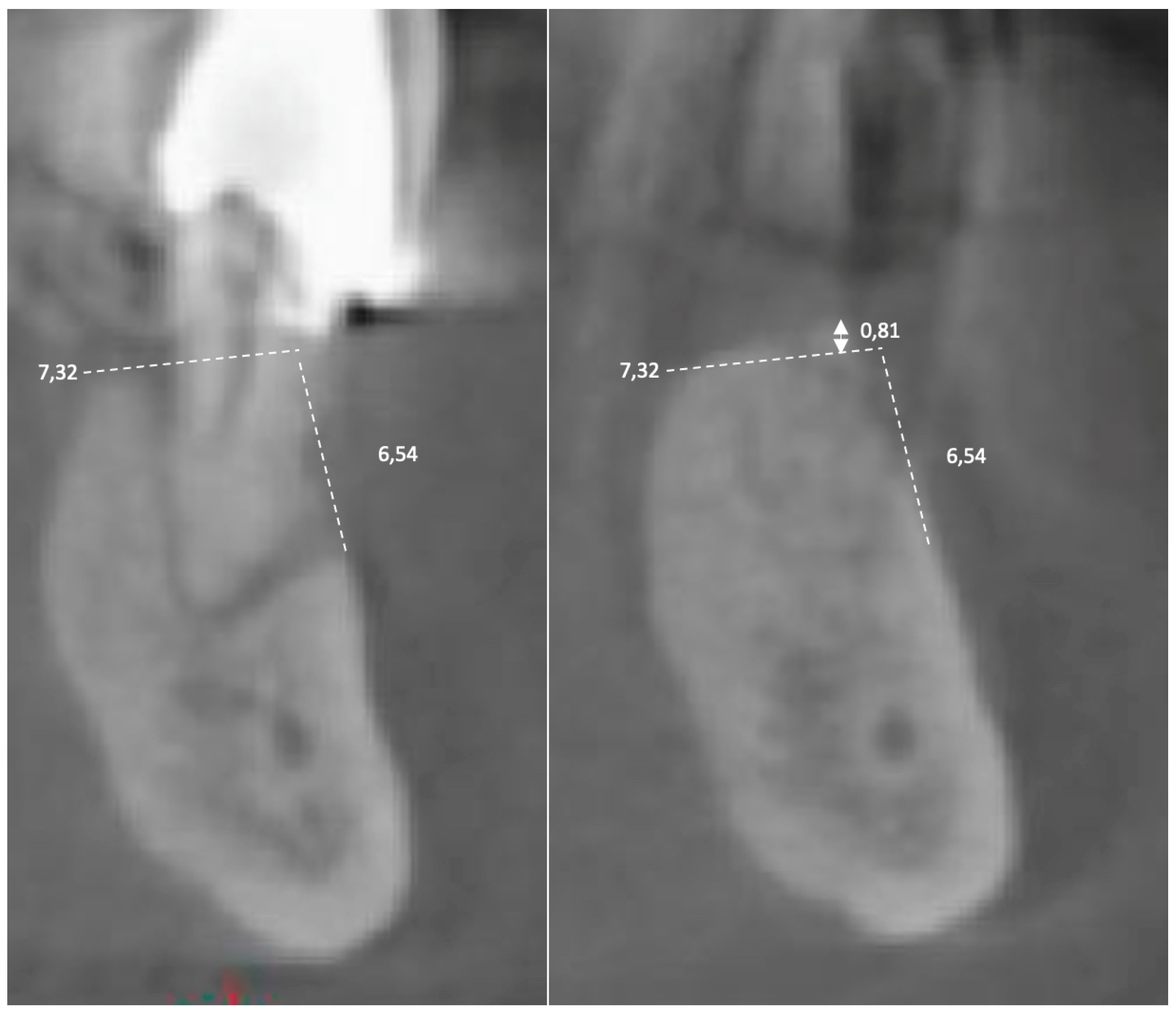
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lekholm, U.; Grondahl, K.; Jemt, T. Outcome of oral implant treatment in partially edentulous jaws followed 20 years in clinical function. Clin. Implant Dent. Relat. Res. 2006, 8, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Healing of extraction socket grafted with deproteinized bovine bone and acellular dermal matrix: Histomorphometric evaluation. Implant. Dent. 2010, 19, 307–313. [Google Scholar] [CrossRef]
- Hammerle, C.H.; Jung, R.E. Bone augmentation by means of membrane membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current membrane membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef]
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of gbr procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 113–123. [Google Scholar] [CrossRef]
- Benic, G.I.; Hammerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Francetti, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric results after postextraction socket healing with different biomaterials: A systematic review of the literature and meta-analysis. Int. J. Oral Maxilofac. Implants 2017, 32, 1001–1017. [Google Scholar] [CrossRef]
- Ten- Heggeler, J.M.; De Van Der Weijden, G.A. Effect of socket preservation therapies following tooth extraction in non-molar regions in humens. A systematic review. Clin. Oral Implants Res. 2011, 22, 779–788. [Google Scholar] [CrossRef]
- Lindhe, J.; Cecchinato, D.; Tomasi, C.; Donati, M.; Liljenberg, B. Ridge preservation with the use of deproteinized bovine bone mineral. Clin. Oral Implants Res. 2014, 25, 786–790. [Google Scholar] [CrossRef]
- Yeomans, J.D.; Urist, M.R. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch. Oral Biol. 1967, 12, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Bang, G.; Urist, M.R. Bone induction in excavation chambers in matrix of decalcified dentin. Arch. Surg. 1967, 94, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S. Orban’s Oral Histology & Embryology, 14th ed.; Elsevier: New Delhi, India, 2015; pp. 40–71. [Google Scholar]
- Manavella, V.; Romani, F.; Corano, L.; Bignardi, C.; Aimetti, M. Three dimensional volumetric changes in severely resorbed alveolar sockets after ridge augmentation with bovine derived xenograft and resorbable membrane: A preliminary study on CBCT imaging. Int. J. Oral Maxillofaccial Implants 2018, 33, 373–382. [Google Scholar] [CrossRef]
- Grassi, A.; Bernardello, F.; Cavani, F.; Palumbo, C.; Spinato, S. Three-punch alveolar ridge reconstruction technique: A novel flapless approach in eight consecutive cases. Int. J. Periodontics Restor. Dent. 2021, 41, 875–884. [Google Scholar] [CrossRef]
- Minetti, E.; Giacometti, E.; Gambardella, U.; Contessi, M.; Ballini, A.; Marenzi, G.; Celko, M.; Mastrangelo, F. Alveolar Socket Preservation with Different Autologous Graft Materials: Preliminary Results of a Multicenter Pilot Study in Human. Materials 2020, 13, 1153. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Celko, M.; Contessi, M.; Carini, F.; Gambardella, U.; Giacometti, E.; Santillana, J.; Beca Campoy, T.; Schmitz, J.H.; Libertucci, M.; et al. Implants Survival Rate in Regenerated Sites with Innovative Graft Biomaterials: 1 Year Follow-Up. Materials 2021, 14, 5292. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Corbella, S.; Taschieri, S.; Canullo, L. Tooth as graft material: Histologic study. Clin. Implant Dent. Relat. Res. 2022, 24, 488–496. [Google Scholar] [CrossRef]
- Hazballa, D.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; Minetti, E.; Di Venere, D.; Limongelli, L.; Bordea, I.R.; Scarano, A.; et al. The effectiveness of autologous demineralized tooth graft for the bone ridge preservation: A systematic review of the literature. J. Biol. Regul. Homeost. Agents 2020, 35 (Suppl. 1), 283–294. [Google Scholar]
- Spinato, S.; Galindo-Moreno, P.; Zaffe, D.; Bernardello, F.; Soardi, C.M. Is pocket healing conditioned by buccal pate thickness?a clinical and histologic study 4-months after mineralized human allografting. Clin. Oral Implants Res. 2014, 25, e120–e126. [Google Scholar] [CrossRef]
- Nevins, M.; Camelo, M.; De Paoli, S. A study of the fate of the buccal wall of extraction sockets of the teeth with prominent roots. Int. J. Periodontics Restor. Dent. 2006, 26, 19–29. [Google Scholar] [CrossRef]
- Ferrus, J.; Cecchinato, D.; Pietursson, E.; Iang, N.P.; Sanz, M.; Lindhe, J. Factors influencing ridge alterations following immediate implant placement into extraction socket. Clin. Oral Implants Res. 2012, 21, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 195–223. [Google Scholar] [CrossRef] [PubMed]
- Taschieri, S.; Morandi, B.; Alberti, A.; Tarasenko, S.; Diachkova, E.; Francetti, L.; Corbella, S. Immediate implant positioning using tooth-derived bone substitute material for alveolar ridge preservation: Preliminary results at 6 months. Clin. Exp. Dent. Res 2022, 1–8, online ahead of print. [Google Scholar] [CrossRef]
- Murata, M.; Kabir, A.M.D.; Hirose, Y.; Ochi, M.; Okubo, N.; Akazawa, T.; Kashiwazaki, H. Histological Evidences of Autograft of Dentin/Cementum Granules into Unhealed Socket at 5 Months after Tooth Extraction for Implant Placement. J. Funct. Biomater. 2022, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ku, J.-K.; Um, I.W.; Seok, H.; Leem, D.H. Impact of Autogenous Demineralized Dentin Matrix on Mandibular Second Molar after Third Molar Extraction: Retrospective Study. J. Funct. Biomater. 2023, 14, 4. [Google Scholar] [CrossRef]
- Cenicante, J.; Botelho, J.; Machado, F.; Mendes, J.J.; Mascarenhas, P.; Alcoforado, G.; Santos, A. The Use of Autogenous Teeth for Alveolar Ridge Preservation: A Literature Review. Appl. Sci. 2021, 11, 1853. [Google Scholar] [CrossRef]

| Group A | Patients | % Vertical Bone Variation | % Horizontal Bone Variation | % New Bone | % Vital Bone | % Residual Graft |
|---|---|---|---|---|---|---|
| A | 65 | 85 | 54.14 | 51.76 | 02.38 | |
| B | 97 | 99 | 61.69 | 61.52 | 00.17 | |
| C | 99 | 98 | 57.15 | 42.72 | 14.43 | |
| D | 90 | 86 | 66.67 | 59.06 | 07.60 | |
| E | 92 | 92 | 74.25 | 72.63 | 01.62 | |
| Mean Value | 89 | 92 | 62.78 | 57.53 | 05.24 | |
| Group B | Patients | % Vertical Bone Variation | % Horizontal Bone Variation | % Bone Volume | % Vital Bone | % Residual Graft |
| F | 117 | 91 | 46.56 | 42.11 | 04.44 | |
| G | 97 | 85 | 38.42 | 29.97 | 08.45 | |
| H | 110 | 138 | 63.59 | 63.43 | 00.16 | |
| I | 100 | 98 | 44.99 | 33.17 | 11.82 | |
| L | 102 | 82 | 46.64 | 43.37 | 01.59 | |
| Mean Value | 102 | 100 | 48.08 | 42.41 | 05.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minetti, E.; Grassi, A.; Beca Campoy, T.; Palermo, A.; Mastrangelo, F. Innovative Alveolar Socket Preservation Procedure Using Demineralized Tooth Dentin as Graft Biomaterial Covered with Three Reabsorbable Membranes: Human Histological Case Series Evaluation. Appl. Sci. 2023, 13, 1411. https://doi.org/10.3390/app13031411
Minetti E, Grassi A, Beca Campoy T, Palermo A, Mastrangelo F. Innovative Alveolar Socket Preservation Procedure Using Demineralized Tooth Dentin as Graft Biomaterial Covered with Three Reabsorbable Membranes: Human Histological Case Series Evaluation. Applied Sciences. 2023; 13(3):1411. https://doi.org/10.3390/app13031411
Chicago/Turabian StyleMinetti, Elio, Andrea Grassi, Tomas Beca Campoy, Andrea Palermo, and Filiberto Mastrangelo. 2023. "Innovative Alveolar Socket Preservation Procedure Using Demineralized Tooth Dentin as Graft Biomaterial Covered with Three Reabsorbable Membranes: Human Histological Case Series Evaluation" Applied Sciences 13, no. 3: 1411. https://doi.org/10.3390/app13031411
APA StyleMinetti, E., Grassi, A., Beca Campoy, T., Palermo, A., & Mastrangelo, F. (2023). Innovative Alveolar Socket Preservation Procedure Using Demineralized Tooth Dentin as Graft Biomaterial Covered with Three Reabsorbable Membranes: Human Histological Case Series Evaluation. Applied Sciences, 13(3), 1411. https://doi.org/10.3390/app13031411







