Abstract
Many patients worldwide suffer from constipation, which reduces their quality of life (QOL) over the long term. Carbonated water intake is expected to improve constipation by improving intestinal motility. Conversely, carbonated water intake is believed to alter bowel status via the intestinal contents (gas and liquid) and intestinal distension, and these changes may be reflected in bowel sound (BS) peak-frequency histograms. In this study, to identify changes in intestinal conditions before and after the ingestion of liquid (i.e., water/carbonated water intake), we used a novel evaluation index, namely peak-frequency histogram similarity (PFHS), which measures the changes in the peak-frequency histogram before and after liquid intake. We considered 13 subjects who participated in a liquid intake test, and PFHS values before and after carbonated water intake were found to be significantly lower than those before and after cold water intake (p < 0.01). However, when using conventional frequency-domain features, this difference was not identified. The results obtained in this study suggest that PFHS can identify changes in bowel status (including intestinal gas and distension) that could not be found using conventional BS frequency domain features. Our findings provide a novel method of research for investigators to non-invasively monitor and evaluate intestinal conditions such as the intestinal gas volume and intestinal distention, which are associated with constipation, using a BS-based approach.
1. Introduction
Constipation is a disease that is frequently observed worldwide that causes difficulties in defecation, abdominal pain, and abdominal bloating and reduces quality of life (QOL), thus making treatment difficult. A 2011 meta-analysis estimated the prevalence of constipation to be approximately 14% and reported a higher prevalence in women than in men [1]. Constipation is common in the elderly, and its prevalence increases with age. Constipation is said to be caused by abnormal intestinal motility [2]. However, as there is no simple and noninvasive test to detect intestinal motility disruptions, in many cases in which a patient has not had a bowel movement for several days, he or she is diagnosed with colonic constipation based on an interview and is prescribed laxatives. Given that laxative abuse can lead to watery stools and to anal stenosis [3], it is hoped that constipation can be improved by adopting lifestyle improvements, including improved eating habits. Carbonated water has been shown to contribute to the improvement of constipation by improving intestinal motility [4,5,6].
Carbonated water is an effervescent beverage that introduces gas into the digestive system [7,8]. Carbon dioxide contained in carbonated water stimulates the gastric fundus [5] and gastric distention, which are associated with gastric motility [4]. Furthermore, it has been shown that carbonated water intake improves movements of the entire gastrointestinal apparatus by acting on smooth muscles [5]. Therefore, it is thought that carbonated water intake affects intestinal conditions such as intestinal gas/liquid volume and intestinal distention.
Previously, intestinal motility before and after carbonated water intake was evaluated using bowel sounds (BSs), which are measured noninvasively. In such evaluations, time-domain acoustic features extracted from BS signals (e.g., number of BS occurrences per minute, sound-to-sound interval (SSI), amplitude intensity, and BS length) have been used. Significant changes in these time-domain acoustic features were observed following carbonated water intake compared with their values before carbonated water intake [9,10]. It has also been reported that intestinal motility evaluations can be conducted by using various time-domain BS acoustic features, such as the evaluation of the postoperative intestinal motility recovery state [11,12] and estimation of the intestinal motility phase during interdigestive migrating motor contraction [13]. However, there are few reports on the acoustic features of BSs in the frequency domain compared with those in the time domain. Peak frequency (PF) [10,14,15], first formant (F1) [10,15], spectral flatness (SF) [16], spectral centroid (SC) [10,17], and spectral bandwidth (SBW) [17] have been used in such investigations. These BS features in the frequency domain has been used for the evaluation of intestinal obstruction in patients, as well as the evaluation of food/liquid ingestion. Despite the significant changes in time-domain features following the ingestion of carbonated water (compared with the state before ingestion), there are different opinions concerning the acoustic feature quantity in the frequency domain of BSs, and further investigation is required.
According to the mathematical model of BS generation [16], the resonance frequency of the intestinal wall is thought to depend on intestinal conditions such as intestinal distention and contents. Therefore, it is thought that the peak frequency of power spectrum changes due to variations in intestinal distention and the amount of intestinal gas/liquid after carbonated water intake. Previous studies on the frequency domain of BSs used basic statistical estimates of basic features, but it is likely that the shape of the distribution of the feature data, which may be associated with the intestinal conditions, was not accurately represented. Therefore, this study investigates whether changes in the intestinal conditions before and after carbonated water intake can be identified by the PF distribution of BSs. For this purpose, we propose a novel index for estimating changes in intestinal conditions based on PF histograms extracted from multiple BS episodes over a certain time period for the evaluation of intestinal conditions. We evaluated the effectiveness of the proposed method based on the PF histogram similarity that was obtained before and after the ingestion of cold carbonated water and cold water. Furthermore, we verified the effectiveness of the proposed method by comparing it with the conventional frequency-domain features-based method.
2. Materials and Methods
2.1. Subject Database
This study was approved by the Tokushima University Graduate School of Technology, Industrial and Social Sciences Science and Technology Course, and Bioresource Industry Course Research Ethics Committee. All experiments were conducted after the subjects were briefed. Informed consent was obtained from all the subjects.
In this study, 13 subjects participated in the liquid intake test (six men and seven women; age (mean ± standard deviation), 34.23 ± 7.71 years; height, 162.96 ± 7.48 cm; weight, 55.86 ± 8.03 kg; body mass index (BMI), 20.95 ± 1.85). The Rome III diagnostic criteria confirmed that none of the subjects had irritable bowel syndrome (IBS). The liquid intake test consisted of a 5 min rest period before liquid intake and a 10 min rest period after liquid intake. Subjects fasted for approximately 12 h prior to the test onset. The carbonated and cold-water intake experiments were conducted in the morning on different days to avoid post-ingestion and migrating motor complex (MMC) effects. The temperature of the water and carbonated water was ~10 °C or less.
During the liquid intake test, recordings were conducted using an electronic stethoscope (E-scope2, Cardionics Inc., Houston, TX, USA) and a multitrack recorder (R16 Zoom Co., Ltd., Tokyo, Japan). All recordings were made under quiet conditions and in a supine position using an electronic stethoscope that was placed 9 cm to the right of the navel and fixed in a square shape using masking tape. Additionally, although the electronic stethoscope has heart and breath sound modes, recordings were conducted in the breath sound mode, as it was capable of recording within a wider frequency range. Recording data were acquired at a sampling frequency of 44,100 Hz at a digital resolution of 16 bits/sample. The sampling frequency was downsampled to 4000 Hz after considering the frequency characteristics of electronic stethoscopes and BSs.
2.2. BS Detection Method Based on an Artificial Neural Network
We adopted the improved version of the PNCC- and artificial neural network (ANN)-based automatic BS extraction method, published in 2021 [9], to detect BSs in the recorded data. In this automatic detection method, the recorded data were first segmented with a segment length of 64 ms and shift size of 16 ms. The improved PNCC (20 dimensions) that was obtained for each segment can be input to the trained ANN to obtain its binary output. BS segments were detected by applying a threshold to the obtained binary output. Single or consecutive segments were each treated as single BS episodes.
2.3. Peak-Frequency Histogram of BSs
Based on the mathematical model of BS generation [16], BSs can be expressed by the vibration of the intestinal wall. As shown in Equation (1), the resonance frequency of the vibration of the intestinal wall can be expressed using a spring and damper model [18].
Here, M is mass of the intestinal wall including the intestinal contents such as liquid and gas; K is the stiffness that is associated with resistance toward the distention of the intestinal wall; C is a damping coefficient that is associated with fluid resistance.
According to Equation (1), the resonance frequency of the vibration of the intestinal wall is thought to be changed by the variation of the intestinal wall distention and intestinal contents. Therefore, information concerning the intestinal conditions should be reflected in the PF of a BS. As a BS is generated multiple times in various locations in the intestine, the distribution that was created based on the PF of the BSs for a given time period may reflect a representative intestinal condition.
Therefore, in this study, the PF and histogram of BSs obtained over a certain time length were generated using the following procedure:
- We considered the main frequency components of the BS episodes that were detected by using the BS automatic detection method described in Section 2.2, and applied a third-order Butterworth bandpass filter with cutoff frequencies of 100 and 1000 Hz
- The BS episodes were segmented using a segment length of 64 ms (256 samples) and shift size of 16 ms (64 samples)
- After conducting window processing using a Hamming window (duration: 128 ms, 512 samples) for each BS segment, the amplitude spectral frequency with the maximum amplitude was considered as the peak frequency
- The signal to noise (SN) ratio was considered, and the frequency with the maximum amplitude (among the peak frequencies, which were detected for each segment) was treated as the maximum peak frequency of the BS episode
- The maximum peak frequency of the BS episode that occurred over a certain time period T was used to generate a histogram with the probability of occurrence displayed on the vertical axis and the bin width (BW) presented in Hz. In this study, the generated histogram is called the “peak-frequency histogram” (PFH)
2.4. Peak-Frequency Histogram Similarity for Intestinal Condition Evaluations
Based on the obtained PFH information, we propose a PFH similarity index based on Pearson’s correlation coefficient to identify changes in the intestinal conditions of individual subjects before and after liquid intake. Let A and B be the conditions of the subject at different times. Setting the PFH of the th bin obtained in the two conditions A and as and , respectively, the PFH similarity PFHS can be defined by Equation (2):
where is the number of PFH bins and and are the respective means and standard deviations of the PFH values in conditions A and B, respectively. To calculate PFHS, the preliquid intake PFH must be generated; however, some subjects may have fewer BS episodes in a given time period. Therefore, subjects with at least 150 BS episodes that were confirmed before and after liquid intake were selected in this study to evaluate the effectiveness of the proposed method.
2.5. Frequency Domain Acoustic Features Extracted from BS Episodes
Features in the frequency domain that were used in previous BS analysis studies (i.e., SF [16], SBW [17], SC [10,17], PF [10,14,15]) were extracted. SF is defined as:
where the magnitude represents the amplitude spectrum and M is number of points of the fast Fourier transform. SF is an index representing the flatness of the power spectrum. It approaches a value of one in flat spectra such as that of white noise.
SBW is defined as the frequency range where the signal is higher than half of the maximum amplitude [17]. The value is low in the case of a single spectrum, such as a pure tone. Moreover, SC is defined as Equation (4).
SC is an index that represents the balancing point of the amplitude spectrum. PF is defined as the frequency, which has the maximum amplitude in the amplitude spectrum. PF describes the frequency of the main peak, whereas SC describes the combined power of all the possible peaks and nonpeaks. As BSs have diverse occurrence patterns, the BS episodes were generally not directly calculated; instead, we decomposed them into BS segment units and treated the means of the acoustic features of the BS segments as the representative values of the BS episodes.
2.6. Statistical Analysis
The significance was evaluated using the Wilcoxon signed-rank test. When p < 0.05, it was assumed that the difference between the compared groups was significant.
3. Results
3.1. Peak-Frequency Histogram of BS before and after Liquid Intake
Figure 1 and Figure 2, respectively, show PFH examples before and after carbonated water intake and the PFH before and after water intake in a subject at BW = 23.4. The blue bars represent data obtained before soda intake and the red bars indicate data obtained after soda intake. A comparison of the histogram shapes in Figure 1 and Figure 2 confirms that the change in the PFH before and after carbonated water intake is greater than that in the PFH before and after cold water intake. This trend was also observed in many other subjects. These results suggest that the intestinal condition after liquid intake is affected by the beverage type, thereby suggesting that differences in intestinal condition may be reflected in PFH. PFHS was next used to quantitatively evaluate differences in PFH shapes.
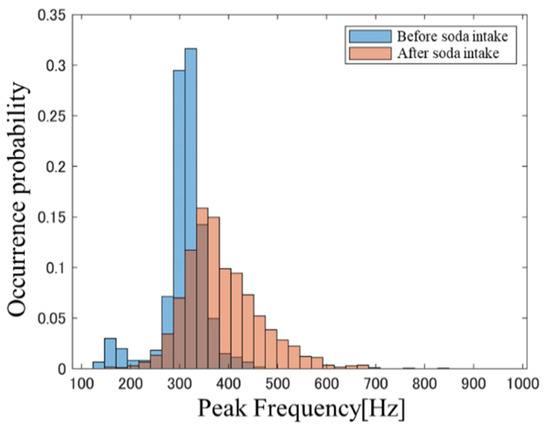
Figure 1.
PFH before and after carbonated water intake.
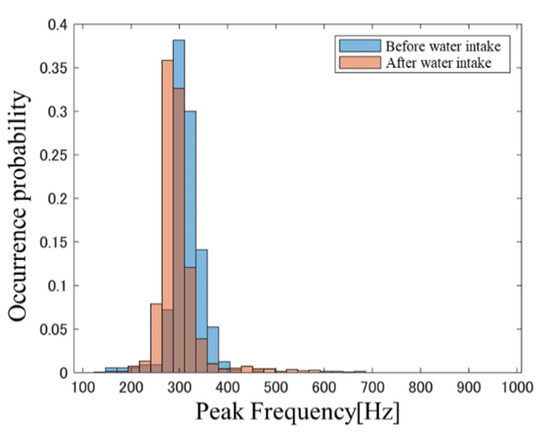
Figure 2.
PFH before and after cold water intake.
3.2. Bin Width and Selection of the Number of BS Episodes for PFHS of BS
PFHS depends on the BW and number of BS episodes used when generating the PFH. Therefore, we first investigated how these parameters affect PFHS when evaluating intestinal conditions before and after liquid intake.
The recorded data were divided into the following intervals: before carbonated water intake (BSI), after carbonated water intake (ASI), before water intake (BWI), and after water intake (AWI). The N BS episodes detected in each recording interval were sampled without replacement to create the PFH within the BW. We calculated the PFHS between BSI-BWI, BSI-ASI, and BWI-AWI based on this PFH. The PFHS was obtained through sampling without replacement in each subject. This approach was repeated 30 times, and the mean PFHS was then computed. Figure 3 shows the relationship between the mean PFHS values obtained as functions of N and BW.
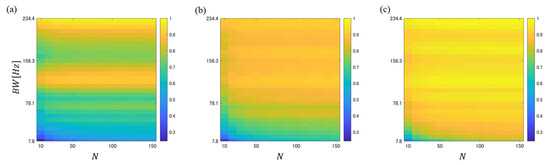
Figure 3.
PFHS plots between (a) BSI-BWI, (b) BSI-ASI, and (c) BWI-AWI when the number of BS episodes and BW were varied during the creation of the PFH.
It is confirmed from Figure 3a that the PFHS between BSI-BWI tended to be relatively low, regardless of the liquid intake. This suggests that PFHS was affected by the difference in intestinal conditions on different experimental days. Figure 3b,c show that the PFHS between BSI-ASI was generally smaller than that between BWI-AWI, but it can be confirmed that it tended to be low regardless of N, especially in the range where BW was relatively small. To evaluate the difference between these two systems, a significance test was conducted for the PFHSs between BSI-ASI and BWI-ASI, and the significance level was calculated. The -value was obtained through sampling without replacement in each subject. This approach was repeated 30 times, and the mean p-value was then computed. Figure 4 shows the relationships between the mean -value and BW as a function of N.
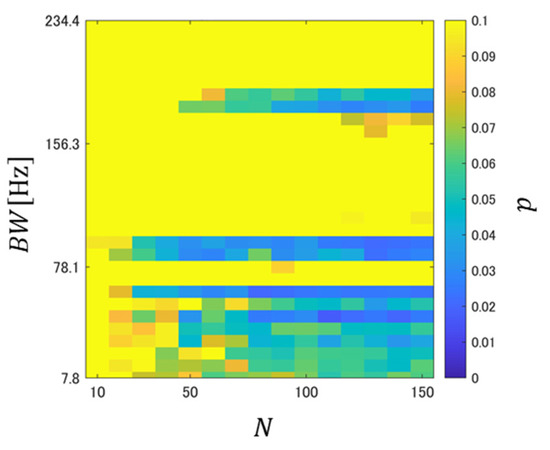
Figure 4.
Relationship of of PFHS in BWI-AWI and BSI-ASI when the number of BS episodes and BW were varied during the creation of PFH. The colors represent significance levels; a yellow color indicates a significance level of ≥0.1.
It is confirmed from Figure 4 that there are regions with significance levels < 0.05 and regions with significance levels <0.1. These results showed that the PFH changes were larger when carbonated water was ingested than those obtained when cold water was ingested. Therefore, the effect of carbon dioxide on intestinal conditions was reflected in the PFH.
In this study, we identified a trend toward lower PFHS values when comparing PFH before liquid intake on different experimental days. Therefore, in the next section, we will investigate whether the calculation of PFHS before and after liquid intake was affected by the PFH generated before liquid intake on the day of experiment.
3.3. PFHS of BSs for the Evaluations of the before- and after-Liquid-Intake States
To investigate whether the PFHS calculation was affected by the PFH generated before liquid intake on the day of experiment, the recorded data were first divided into 2.5 min analysis intervals (before liquid intake: B1 and B2; after liquid intake: A1 to A4), as shown in Figure 5. For each subject, a PFH was generated from the BS episode obtained in each analysis interval. In this study, = 85.9 and = 50 were applied to generate PFHs because, in a specific subject, only approximately 55 BS episodes could be confirmed within the analysis interval.
Figure 5.
Analysis interval based on a 2.5-min time interval; before liquid intake: B1, B2; after liquid intake: A1, A2, A3, A4.
PFHS was calculated for each analysis interval based on the first analysis interval from the onset of the recording (i.e., B1). Figure 6 shows a plot of the mean values of the results. In the significant difference tests, the comparison results with the PFHS of B1-A1, B1-A2, B1-A3, and B1-A4 are shown based on the PFHS of B1-B2, which is the subject of analysis before liquid intake.
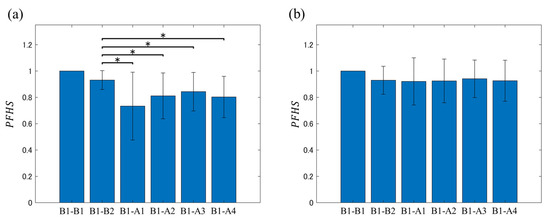
Figure 6.
Comparison of results obtained with the of B1-A1, B1-A2, B1-A3, and B1-A4 from (a) carbonated water intake and (b) cold water intake ( of B1-B2 was set as a reference). * Significant difference at p < 0.05.
It can be confirmed from Figure 6 that the mean PFHS value was almost unchanged before and after water intake, and no significant change from B1 to B2 was confirmed. Meanwhile, in the before and after carbonated water intake cases, there was variation in the mean PFHS value, but these values tended to be lower after intake compared with those before the intake. Additionally, a significant decrease in PFHS was observed in all analysis intervals in the second half compared with the PFHS of the analysis targets B1–B2.
The presented results indicate that carbonated water intake caused sustained changes in intestinal conditions. Additionally, the difference in mean value was minor, the PFHS of B1–B2 before carbonated water intake was 0.909 ± 0.077, that of B1-B2 before water intake was 0.912 ± 0.079, and no significant differences were identified ( = 0.644). These results suggest that the PFH before liquid intake did not change significantly on the experiment day, and the PFHS before and after liquid intake could be calculated.
3.4. Comparison of the Conventional and Proposed Methods
To compare the effectiveness of the conventional and proposed methods, we calculated the PFHS between BWI-AWI and BSI-ASI using = 85.9 and = 50 (as used in Section 3.3) for the proposed method (Case 1). Furthermore, as the results of Figure 4 confirmed areas below the significance level, we calculated the mean PFHS between BWI-AWI and BSI-ASI in the range of = 7.8–93.8 and = 10–150 (Case 2). In both cases, PFHS was obtained through sampling without replacement in each subject, and after this approach was repeated 30 times, the mean PFHS was used as the representative value. In the case of the conventional method, the rate of change for each subject was calculated by dividing the mean feature value obtained from the second-half by the mean feature value obtained from the first half of the liquid intake process.
Table 1 lists the mean values across all subjects for the rate of change of acoustic features (SC, SF, SBW, PF), as well as the PFHS (Case 1) and PFHS (Case 2) of BWI-AWI and BSI-ASI.

Table 1.
Characteristics of the frequency domain features of BSs.
Table 1 shows that a comparison of the rate of change of the conventional feature quantities between BWI-AWI and BSI-ASI did not confirm any significant differences in any feature quantities. Meanwhile, significant differences could be confirmed in PFHS for Case 1 and Case 2. This suggests that the proposed method reflects information on changes in intestinal conditions due to carbonated water intake that were not observed when using the conventional method.
4. Discussion
This study confirmed, based on the cold water/carbonated water intake test, that PFHS before and after carbonated water intake is significantly lower than before and after cold water intake. Further, PFH, which expresses the peak-frequency distribution of BSs, changed to a larger extent after carbonated water intake. It has been reported that it takes approximately 15 min for carbon dioxide supplied to the intestine to disappear [19,20]. In the present experiment, recordings were conducted for 10 min after ingestion. Accordingly, it was thus considered that carbon dioxide gas remained in the intestinal tract of the subject during the recording period after the ingestion of carbonated water. Therefore, it was suggested that the proportion of gas in the intestinal contents remained at an elevated level compared with its level before carbonated water intake. When tablets are ingested in a fasting state, carbonated water promotes gastrointestinal motility, resulting in faster drug absorption from the intestinal tract than achieved with tap water [8]. Considering the mathematical model of BS generation [16], the resonance frequency of the intestinal wall is thought to be related to intestinal distention and intestomal contents. Additionally, it is confirmed that intraluminal gas is bubble-shaped and a smaller intraluminal gas bubble radius results in a higher BS dominant frequency [21]. Therefore, compared with water intake, carbonated water intake is thought to exhibit more changes in intestinal motility and intestinal conditions wherein the intestinal distention and amount of intraluminal gas may result in additional PFH changes. It was confirmed in the present experiment that the PFHS tended to stabilize as the number of BS episodes increased when the PFH was obtained, and we were unexpectedly able to confirm this tendency from a relatively small number of BS episodes. This suggests that changes in intestinal conditions can be identified based on relatively short data recordings upon the creation of PFHs.
Regarding the conventional BS frequency-domain feature quantities, reports have indicating that there are no differences in the dominant and peak frequencies of BSs between patients with and without intestinal obstructions [15,22]. However, there are also reports that have indicated that BSs with high-frequency components could be confirmed when intestinal obstruction occurs [14]. Additionally, it has been reported that SC and SBW changed depending on dietary intake [17]. Meanwhile, another report showed that SC, F1, and PF did not change, even after the intake of carbonated water [10]. These results provide evidence for the ambivalent opinions on the feature quantities of this system in the frequency domain. In this study, we calculated the rate of change of the frequency-domain feature values of conventional BSs before and after liquid intake. Then, we compared the results between water and carbonated water intake experiments; however, no significant differences were found. A previous report also indicated the possibility of capturing changes in postprandial intestinal motility using frequency-domain features by setting the fasting time to a sufficiently long period [17]. Therefore, long-term recordings may be required when the basic statistics of these frequency-domain features are used to estimate changes in intestinal conditions. It was confirmed that PFH changed significantly when the measurement days differed, thereby suggesting that lifestyle changes, such as diet, sleep, and exercise, may influence intestinal conditions. In this study, PFHS is thought to reflect information regarding intestinal gas and distention based on the results of the liquid intake tests conducted on the same day.
It is known that patients with IBS exhibit gas retention, which is accompanied by distension [23], and patients with ileus bowel obstruction exhibit increased intestinal gas [24]. Our findings may be used for the evaluation of such patients.
5. Limitations
One of the limitations in this study is the relatively small subject sample size (n = 13). However, to avoid confounding factors included in the investigation, we used a subject database that does not include IBS. Furthermore, it cannot be stated that there is a one-to-one relationship between the BS peak-frequency and the proportion of gas or distention of the intestinal wall. Finally, the relationship between the proposed method and amount of carbon dioxide was not investigated. These aspects need to be investigated to improve the proposed method in future studies.
6. Conclusions
While invasive approaches such as radiography are needed to evaluate intestinal conditions, BS-based approaches can be easily and non-invasively be performed using low-cost equipment. In this study, the use of PFHS in a liquid intake experiment enabled us to confirm changes in intestinal conditions before and after carbonated water intake that could not be confirmed using conventional frequency-domain features. Based on the results obtained in this study, we can infer that the proposed method can serve as a non-invasive monitoring tool to evaluate changes in intestinal conditions.
Author Contributions
Conceptualization, T.E.; methodology, T.E., T.H. (Takeyuki Haraguchi), T.H. (Takahiro Hirayama), M.K., Y.I. and T.H. (Tomoya Hirano); validation, T.E., T.H. (Takeyuki Haraguchi), T.H. (Takahiro Hirayama), M.K., Y.I. and T.H. (Tomoya Hirano); formal analysis, T.H. (Takeyuki Haraguchi); investigation, T.E., T.H. (Takeyuki Haraguchi), T.H. (Takahiro Hirayama), M.K., Y.I. and T.H. (Tomoya Hirano); data curation, T.H. (Takeyuki Haraguchi); writing—original draft preparation, T.H. (Takeyuki Haraguchi); writing—review and editing, T.E.; project administration, T.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by DAIKIN INDUSTRIES, Ltd., Japan, and was partly supported by Grants-in-Aid for Scientific Research, Japan Society for the Promotion of Science (JSPS) (Scientific Research (C) 20K12755).
Institutional Review Board Statement
This study was conducted after obtaining approval from the ethics review boards of the Division of Science and Technology, Graduate School of Technology, Industrial and Social Sciences (No. 14011).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suares, N.C.; Ford, A.C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. ACG 2011, 106, 1582–1591. [Google Scholar] [CrossRef]
- Chapman, M.J.; Nguyen, N.Q.; Deane, A.M. Gastrointestinal dysmotility: Evidence and clinical management. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 209–216. [Google Scholar] [CrossRef]
- Brisinda, G.; Vanella, S.; Cadeddu, F.; Marniga, G.; Mazzeo, P.; Brandara, F.; Maria, G. Surgical treatment of anal stenosis. World J. Gastroenterol. 2009, 15, 1921. [Google Scholar] [CrossRef]
- Wakisaka, S.; Nagai, H.; Mura, E.; Matsumoto, T.; Moritani, T.; Nagai, N. The effects of carbonated water upon gastric and cardiac activities and fullness in healthy young women. J. Nutr. Sci. Vitaminol. 2012, 58, 333–338. [Google Scholar] [CrossRef]
- Cuomo, R.; Grasso, R.; Sarnelli, G.; Capuano, G.; Nicolai, E.; Nardone, G.; Pomponi, D.; Budillon, G.; Ierardi, E. Effects of carbonated water on functional dyspepsia and constipation. Eur. J. Gastroenterol. Hepatol. 2002, 14, 991–999. [Google Scholar] [CrossRef]
- Mun, J.-H.; Jun, S.S. Effects of carbonated water intake on constipation in elderly patients following a cerebrovascular accident. J. Korean Acad. Nurs. 2011, 41, 269–275. [Google Scholar] [CrossRef]
- Krinsky, D.L. Intestinal gas. Pharm. Today 2021, 27, 14. [Google Scholar] [CrossRef]
- Van Den Abeele, J.; Brouwers, J.; Deloose, E.; Tack, J.; Augustijns, P. The effect of sparkling water on intraluminal formulation behavior and systemic drug performance. J. Pharm. Sci. 2017, 106, 2472–2482. [Google Scholar] [CrossRef]
- Horiyama, K.; Emoto, T.; Haraguchi, T.; Uebanso, T.; Naito, Y.; Gyobu, T.; Kanemoto, K.; Inobe, J.; Sano, A.; Akutagawa, M.; et al. Bowel sound-based features to investigate the effect of coffee and soda on gastrointestinal motility. Biomed. Signal. Process. Control. 2021, 66, 102425. [Google Scholar] [CrossRef]
- Emoto, T.; Abeyratne, U.R.; Gojima, Y.; Nanba, K.; Sogabe, M.; Okahisa, T.; Akutagawa, M.; Konaka, S.; Kinouchi, Y. Evaluation of human bowel motility using non-contact microphones. Biomed. Phys. Eng. Express. 2016, 2, 045012. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Lin, B.-S.; Luo, Y.-W.; Lin, C.-Y. Recovery evaluation system of bowel functions following orthopedic surgery and gastrointestinal endoscopy. IEEE Access 2021, 9, 67829–67837. [Google Scholar] [CrossRef]
- Wang, G.; Wang, M.; Liu, H.; Zhao, S.; Liu, L.; Wang, W. Changes in bowel sounds of inpatients undergoing general anesthesia. BioMed. Eng. Online 2020, 1, 13. [Google Scholar] [CrossRef]
- Tomomasa, T.; Morikawa, A.; Sandler, R.H.; Mansy, H.A.; Koneko, H.; Masahiko, T.; Hyman, P.E.; Itoh, Z. Gastrointestinal sounds and migrating motor complex in fasted humans. Am. J. Gastroenterol. 1999, 94, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Abe, Y.; Yoshino, T.; Ohsato, K. Clinical application of spectral analysis of bowel sounds in intestinal obstruction. Dis. Colon Rectum 1990, 33, 753–757. [Google Scholar] [CrossRef]
- Ching, S.S.; Yih, K.T. Spectral analysis of bowel sounds in intestinal obstruction using an electronic stethoscope. World J. Gastroenterol. 2012, 18, 4585. [Google Scholar] [CrossRef]
- Du, X.; Allwood, G.; Webberley, K.M.; Osseiran, A.; Wan, W.; Volikova, A.; Marshall, B.J. A mathematical model of bowel sound generation. J. Acoust. Soc. Am. 2018, 144, EL485–EL491. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Testa, A.; Marshall, B.J. Development of a bowel sound detector adapted to demonstrate the effect of food intake. BioMed. Eng. Online 2022, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morse, P.M. Vibration and Sound; American Institute of Physics: New York, NY, USA, 1936. [Google Scholar]
- Sumanac, K.; Zealley, I.; Fox, B.M.; Rawlinson, J.; Salena, B.; Marshall, J.K.; Stevenson, G.W.; Hunt, R.H. Minimizing postcolonoscopy abdominal pain by using CO2 insufflation: A prospective, randomized, double blind, controlled trial evaluating a new commercially available CO2 delivery system. Gastrointest. Endosc. 2002, 56, 190–194. [Google Scholar] [CrossRef]
- Bretthauer, M.; Thiis-Evensen, E.; Huppertz-Hauss, G.; Gisselsson, L.; Grotmol, T.; Skovlund, E.; Hoff, G. NORCCAP (Norwegian colorectal cancer prevention): A randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut 2002, 50, 604–607. [Google Scholar] [CrossRef]
- Liu, C.J.; Huang, S.C.; Chen, H.I. Oscillating gas bubbles as the origin of bowel sounds: A combined acoustic and imaging study. Chin. J. Physiol. 2010, 53, 245–253. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, L.; Jiang, J.; Hu, B.; Tang, C.; Li, J. Opinions on computer audition for bowel sounds analysis in intestinal obstruction: Opportunities and challenges from a clinical point of view. Front. Med. 2021, 8, 655298. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Whorwell, P.J. Towards a better understanding of abdominal bloating and distension in functional gastrointestinal disorders. Neurogastroenterol. Motil. 2005, 17, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Hale, J.J.; Hall, D.P.; Dunn, M.J. Physiology of patient transfer by land and air. Anaesth. Intensive Care Med. 2019, 20, 595–599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).