Wearable Inertial Devices in Duchenne Muscular Dystrophy: A Scoping Review
Abstract
1. Introduction
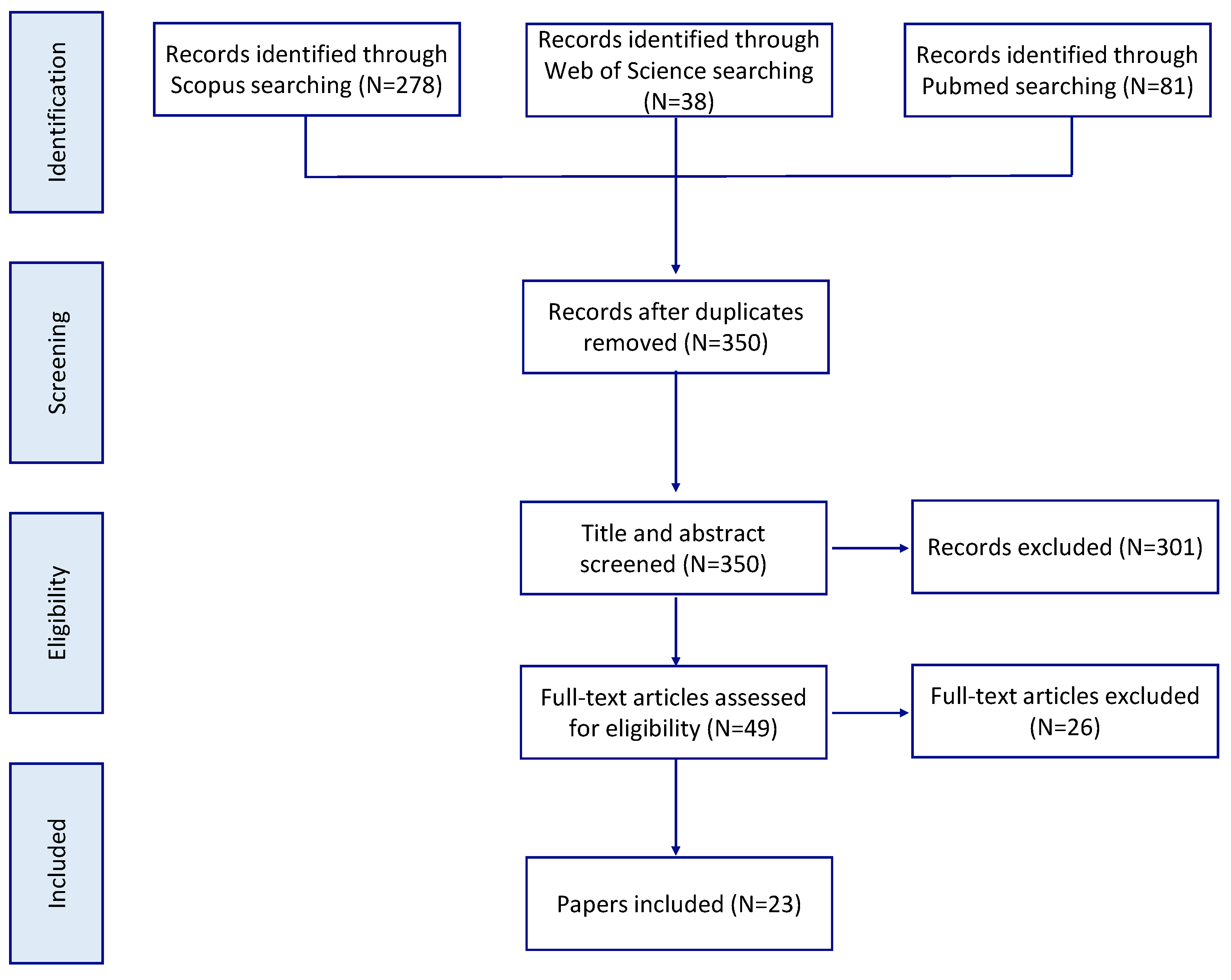
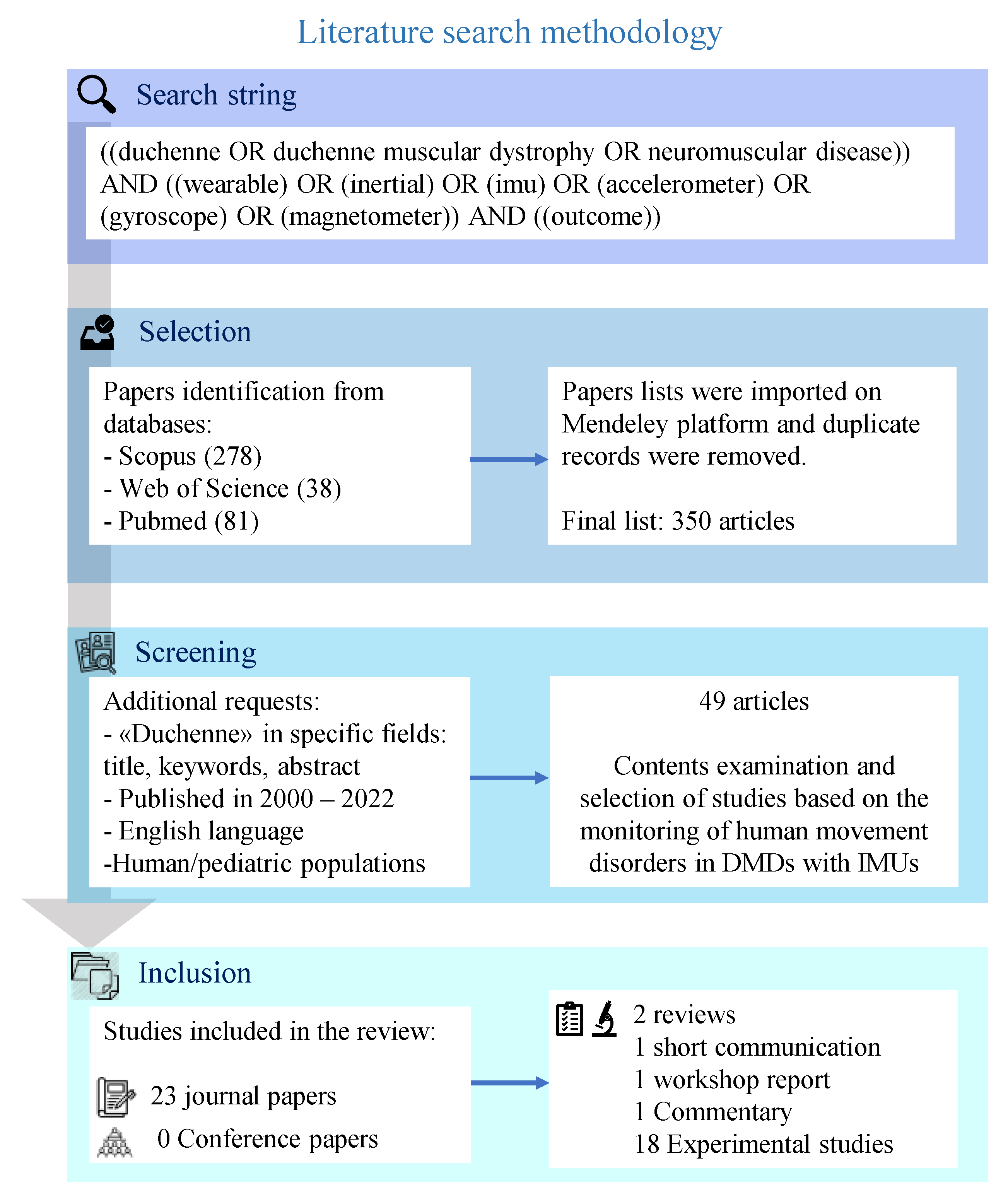
2. Methodology
- Definition of the principal aim of the search and research questions;
- Formulation of relevant keywords;
- Selection of search databases;
- Identification of specific inclusion/exclusion criteria for article selection;
- Elimination of duplicates and unrelated articles;
- In-depth analysis and investigation of selected articles.
2.1. Research Questions
2.2. Search Schemes
- Duchenne muscular dystrophy, neuromuscular disease;
- Wearable, inertial systems, IMU, accelerometer, gyroscope, magnetometer;
- Outcome measures.
2.3. Inclusion Criteria
2.4. Study Characteristics and Classification
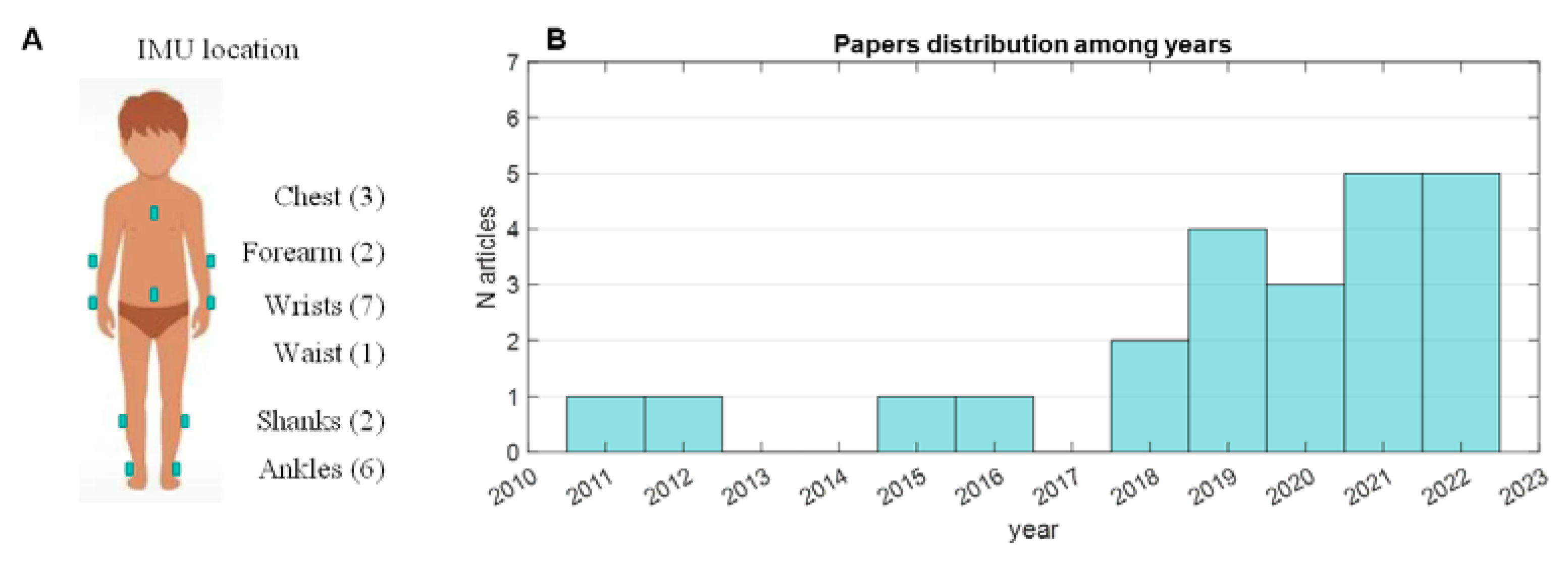
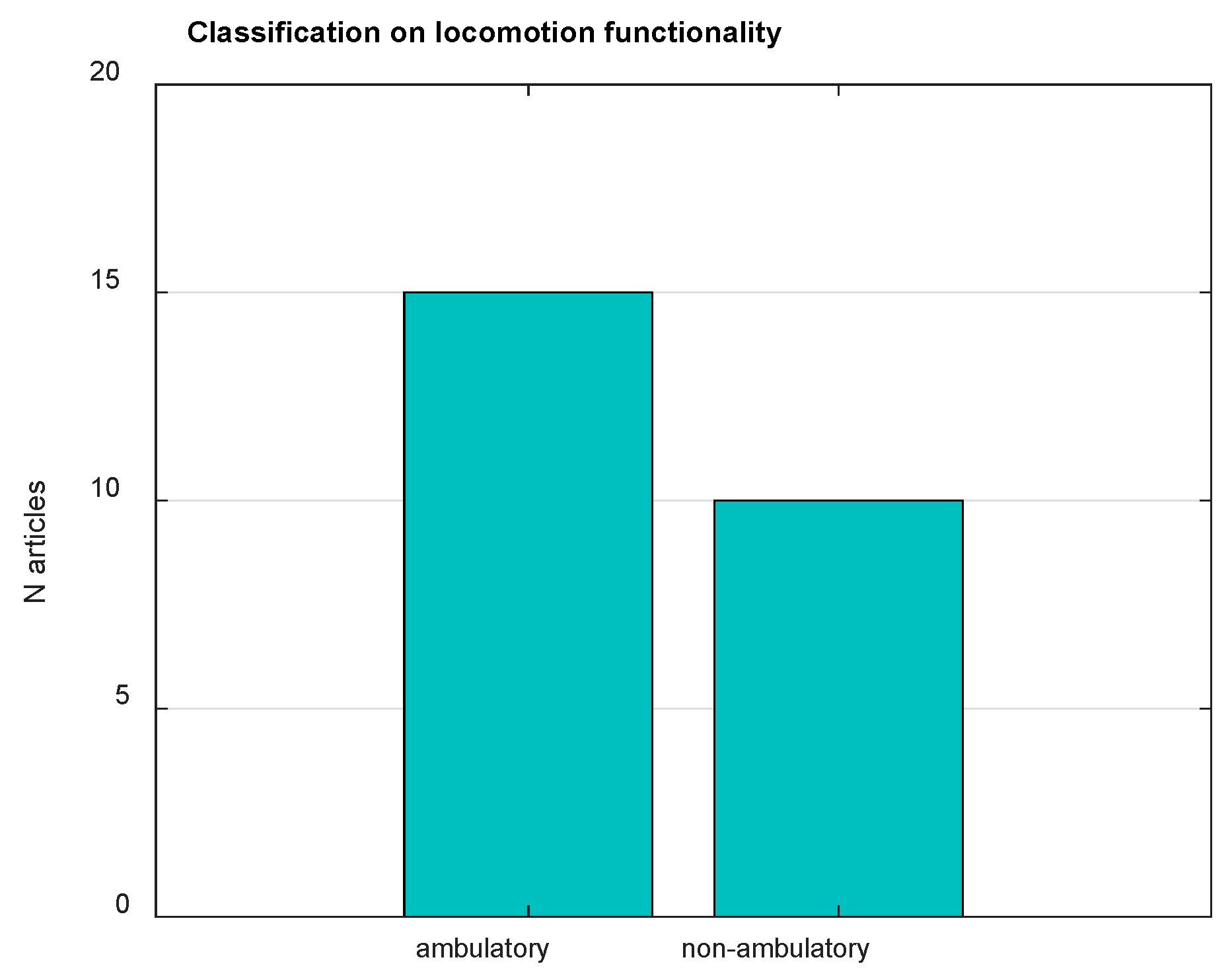
3. Results
4. Discussion
4.1. Technical Perspective
4.2. Biomechanical Perspective
4.3. Clinical Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Duan, D.; Goemans, N.; Takeda, S.I.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Prim. 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Yiu, E.M.; Kornberg, A.J. Duchenne muscular dystrophy. J. Paediatr. Child Health 2015, 51, 759–764. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Catteruccia, M.; Baranello, G.; Politano, L.; Govoni, A.; Previtali, S.C.; Pane, M.; D’Angelo, M.G.; Bruno, C.; Messina, S.; et al. Diagnosis of Duchenne Muscular Dystrophy in Italy in the last decade: Critical issues and areas for improvements. Neuromuscul. Disord. 2017, 27, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, F.; Rossi, R.; Falzarano, M.S.; Ferlini, A. Innovative therapeutic approaches for duchenne muscular dystrophy. J. Clin. Med. 2021, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Bello, L.; Torri, F.; Schirinzi, E.; Pegoraro, E.; Siciliano, G. Therapeutic opportunities and clinical outcome measures in Duchenne muscular dystrophy. Neurol. Sci. 2022, 43, 625–633. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef]
- Vill, K.; Ille, L.; Schroeder, S.A.; Blaschek, A.; Müller-Felber, W. Six-minute walk test versus two-minute walk test in children with Duchenne muscular dystrophy: Is more time more information? Eur. J. Paediatr. Neurol. 2015, 19, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.L.; Iannaccone, S.T. Clinical outcome assessments in Duchenne muscular dystrophy and spinal muscular atrophy: Past, present and future. Neuromuscul. Disord. 2021, 31, 1028–1037. [Google Scholar] [CrossRef]
- Brogna, C.; Coratt, G.; Pane, M.; Ricotti, V.; Messina, S.; D’Amico, A.; Bruno, C.; Vita, G.; Berardinelli, A.; Mazzone, E.; et al. Correction: Long-term natural history data in Duchenne muscular dystrophy ambulant patients with mutations amenable to skip exons 44, 45, 51 and 53. PLoS ONE 2019, 14, e0218683. [Google Scholar] [CrossRef]
- Mercuri, E.; Coratti, G.; Messina, S.; Ricotti, V.; Baranello, G.; D’Amico, A.; Pera, M.C.; Albamonte, E.; Sivo, S.; Mazzone, E.S.; et al. Revised north star ambulatory assessment for young boys with Duchenne muscular dystrophy. PLoS ONE 2016, 11, e0160195. [Google Scholar] [CrossRef]
- Mazzone, E.S.; Messina, S.; Vasco, G.; Main, M.; Eagle, M.; D’Amico, A.; Doglio, L.; Politano, L.; Cavallaro, F.; Frosini, S.; et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul. Disord. 2009, 19, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.M.; Malkus, E.C.; Mendell, J.R.; Flanigan, K.M.; Miller, J.P.; Schierbecker, J.R.; Siener, C.A.; Golumbek, P.T.; Zaidman, C.M.; Mcdonald, C.M. Outcome reliability in non-ambulatory boys/men with Duchenne muscular dystrophy. Muscle Nerve 2015, 51, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Pane, M.; Coratti, G.; Brogna, C.; Mazzone, E.S.; Mayhew, A.; Fanelli, L.; Messina, S.; Amico, A.D.; Catteruccia, M.; Scutifero, M.; et al. Upper limb function in Duchenne muscular dystrophy: 24 month longitudinal data. PLoS ONE 2018, 13, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.G.; Coratti, G.; Mazzone, E.S.; Klingels, K.; James, M.; Pane, M.; Straub, V.; Goemans, N.; Mercuri, E.; Ricotti, V.; et al. Performance of Upper Limb module for Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2020, 62, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.W.; Chang, C.F. Biomechanics of human movement and its clinical applications. Kaohsiung J. Med. Sci. 2012, 28, S13–S25. [Google Scholar] [CrossRef]
- Muro-de-la-Herran, A.; García-Zapirain, B.; Méndez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef]
- Wade, L.; Needham, L.; McGuigan, P.; Bilzon, J. Applications and limitations of current markerless motion capture methods for clinical gait biomechanics. PeerJ 2022, 10, e12995. [Google Scholar] [CrossRef]
- Digo, E.; Agostini, V.; Pastorelli, S.; Gastaldi, L.; Panero, E. Gait Phases Detection in Elderly using Trunk-MIMU System. In Proceedings of the BIODEVICES 2021-14th International Joint Conference on Biomedical Engineering Systems and Technologies, Vienna, Austria, 11–13 February 2021; pp. 58–65. [Google Scholar]
- Panero, E.; Digo, E.; Dimanico, U.; Artusi, C.A.; Zibetti, M.; Gastaldi, L. Effect of deep brain stimulation frequency on gait symmetry, smoothness and variability using IMU. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications, Lausanne, Switzerland, 23–25 June 2021. [Google Scholar] [CrossRef]
- Lopez-Nava, I.H.; Angelica, M.M. Wearable Inertial Sensors for Human Motion Analysis: A review. IEEE Sens. J. 2016, PP, 7821–7834. [Google Scholar] [CrossRef]
- Madej, M.; Ruminski, J. Optimal placement of IMU sensor for the detection of children activity. In Proceedings of the 2022 15th International Conference on Human System Interaction (HSI), Melbourne, Australia, 28–31 July 2022; pp. 8–13. [Google Scholar] [CrossRef]
- Bo, F.; Yerebakan, M.; Dai, Y.; Wang, W.; Li, J.; Hu, B.; Gao, S. IMU-Based Monitoring for Assistive Diagnosis and Management of IoHT: A Review. Healthcare 2022, 10, 1210. [Google Scholar] [CrossRef]
- Bortolani, S.; Brusa, C.; Rolle, E.; Monforte, M.; De Arcangelis, V.; Ricci, E.; Mongini, T.E.; Tasca, G. Technology outcome measures in neuromuscular disorders: A systematic review. Eur. J. Neurol. 2022, 29, 1266–1278. [Google Scholar] [CrossRef]
- Vandekerckhove, I.; Hauwe, M.V.D.; De Beukelaer, N.; Stoop, E.; Goudriaan, M.; Delporte, M.; Molenberghs, G.; Van Campenhout, A.; De Waele, L.; Goemans, N.; et al. Longitudinal Alterations in Gait Features in Growing Children with Duchenne Muscular Dystrophy. Front. Hum. Neurosci. 2022, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Minosse, S.; Favetta, M.; Romano, A.; Pisano, A.; Summa, S.; Schirinzi, T.; Vasco, G.; Castelli, E.; Petrarca, M. Comparison of the Gait Biomechanical Constraints in Three Different Type of Neuromotor Damages. Front. Hum. Neurosci. 2022, 16, 822205. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, M.; Van den Hauwe, M.; Dekeerle, J.; Verhelst, L.; Molenaers, G.; Goemans, N.; Desloovere, K. Gait deviations in Duchenne muscular dystrophy—Part 1. A systematic review. Gait Posture 2018, 62, 247–261. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.A.; Cezarani, A.; Lizzi, E.A.D.S.; Davoli, G.B.d.Q.; Mattiello, S.M.; Jones, R.; Mattiello-Sverzut, A.C. The use of the gait profile score and gait variable score in individuals with Duchenne Muscular Dystrophy. J. Biomech. 2020, 98, 109485. [Google Scholar] [CrossRef]
- Rinaldi, M.; Petrarca, M.; Romano, A.; Vasco, G.; D’Anna, C.; Schmid, M.; Castelli, E.; Conforto, S. EMG-based Indicators of Muscular Co-Activation during Gait in Children with Duchenne Muscular Dystrophy. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3845–3848. [Google Scholar] [CrossRef]
- Romano, A.; Favetta, M.; Schirinzi, T.; Summa, S.; Minosse, S.; D’Amico, A.; Catteruccia, M.; Petrarca, M.; Castelli, E.; Bertini, E.; et al. Evaluation of gait in Duchenne Muscular Dystrophy: Relation of 3D gait analysis to clinical assessment. Neuromuscul. Disord. 2019, 29, 920–929. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 31. [Google Scholar] [CrossRef]
- Jeannet, P.-Y.; Aminian, K.; Bloetzer, C.; Najafi, B.; Paraschiv-Ionescu, A. Continuous monitoring and quantification of multiple parameters of daily physical activity in ambulatory Duchenne muscular dystrophy patients. Eur. J. Paediatr. Neurol. 2011, 15, 40–47. [Google Scholar] [CrossRef]
- Ganea, R.; Jeannet, P.-Y.; Paraschiv-Ionescu, A.; Goemans, N.M.; Piot, C.; Van Den Hauwe, M.; Aminian, K. Gait assessment in children with duchenne muscular dystrophy during long-distance walking. J. Child Neurol. 2012, 27, 30–38. [Google Scholar] [CrossRef]
- Davidson, Z.E.; Ryan, M.M.; Kornberg, A.J.; Walker, K.Z.; Truby, H. Strong correlation between the 6-minute walk test and accelerometry functional outcomes in boys with duchenne muscular dystrophy. J. Child Neurol. 2015, 30, 357–363. [Google Scholar] [CrossRef]
- Le Moing, A.-G.; Seferian, A.M.; Moraux, A.; Annoussamy, M.; Dorveaux, E.; Gasnier, E.; Hogrel, J.-Y.; Voit, T.; Vissière, D.; Servais, L. A movement monitor based on magneto-inertial sensors for non-ambulant patients with Duchenne muscular dystrophy: A pilot study in controlled environment. PLoS ONE 2016, 11, e0156696. [Google Scholar] [CrossRef]
- Jacques, M.F.; Onambele-Pearson, G.L.; Reeves, N.D.; Stebbings, G.K.; Smith, J.; Morse, C.I. Relationships between muscle size, strength, and physical activity in adults with muscular dystrophy. J. Cachexia. Sarcopenia Muscle 2018, 9, 1042–1052. [Google Scholar] [CrossRef]
- Straub, V.; Mercuri, E. Report on the workshop: Meaningful outcome measures for Duchenne muscular dystrophy, London, UK, 30–31 January 2017. Neuromuscul. Disord. 2018, 28, 690–701. [Google Scholar] [CrossRef]
- Fujii, T.; Takeshita, E.; Iwata, Y.; Yajima, H.; Nozaki, F.; Mori, M.; Kumada, T. Cumulative jerk as an outcome measure in nonambulatory Duchenne muscular dystrophy. Brain Dev. 2019, 41, 796–802. [Google Scholar] [CrossRef]
- van der Geest, A.; Essers, J.M.N.; Bergsma, A.; Jansen, M.; de Groot, I.J.M. Monitoring daily physical activity of upper extremity in young and adolescent boys with Duchenne muscular dystrophy: A pilot study. Muscle Nerve 2020, 61, 293–300. [Google Scholar] [CrossRef]
- Haberkamp, M.; Moseley, J.; Athanasiou, D.; de Andres-Trelles, F.; Elferink, A.; Rosa, M.M.; Magrelli, A. European regulators’ views on a wearable-derived performance measurement of ambulation for Duchenne muscular dystrophy regulatory trials. Neuromuscul. Disord. 2019, 29, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Siegel, B.I.; Cakmak, A.; Reinertsen, E.; Benoit, M.; Figueroa, J.; Clifford, G.D.; Phan, H.C. Use of a wearable device to assess sleep and motor function in Duchenne muscular dystrophy. Muscle Nerve 2020, 61, 198–204. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Xie, Z.; Jia, F.; Wang, Z.; Yuan, Y.; Zhang, J.; Fang, J. Quantitative coordination evaluation for screening children with Duchenne muscular dystrophy. Chaos 2020, 30, 023116. [Google Scholar] [CrossRef]
- Arteaga, D.; Donnelly, T.; Crum, K.; Markham, L.; Killian, M.; Burnette, W.B.; Soslow, J.; Buchowski, M.S. Assessing Physical Activity Using Accelerometers in Youth with Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2020, 7, 331–342. [Google Scholar] [CrossRef]
- Killian, M.; Buchowski, M.S.; Donnelly, T.; Burnette, W.B.; Markham, L.W.; Slaughter, J.C.; Xu, M.; Crum, K.; Damon, B.M.; Soslow, J.H. Beyond ambulation: Measuring physical activity in youth with Duchenne muscular dystrophy. Neuromuscul. Disord. 2020, 30, 277–282. [Google Scholar] [CrossRef]
- Lott, D.J.; Taivassalo, T.; Senesac, C.R.; Willcocks, R.J.; Harrington, A.M.; Zilke, K.; Cunkle, H.; Powers, C.; Finanger, E.L.; Rooney, W.D.; et al. Walking activity in a large cohort of boys with Duchenne muscular dystrophy. Muscle Nerve 2021, 63, 192–198. [Google Scholar] [CrossRef] [PubMed]
- McErlane, F.; Davies, E.H.; Ollivier, C.; Mayhew, A.; Anyanwu, O.; Harbottle, V.; Donald, A. Wearable Technologies for Children with Chronic Illnesses: An Exploratory Approach. Ther. Innov. Regul. Sci. 2021, 55, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Poleur, M.; Ulinici, A.; Daron, A.; Schneider, O.; Farra, F.D.; Demonceau, M.; Annoussamy, M.; Vissière, D.; Eggenspieler, D.; Servais, L. Normative data on spontaneous stride velocity, stride length, and walking activity in a non-controlled environment. Orphanet J. Rare Dis. 2021, 16, 318. [Google Scholar] [CrossRef] [PubMed]
- Servais, L.; Camino, E.; Clement, A.; McDonald, C.M.; Lukawy, J.; Lowes, L.P.; Eggenspieler, D.; Cerreta, F.; Strijbos, P. First Regulatory Qualification of a Novel Digital Endpoint in Duchenne Muscular Dystrophy: A Multi-Stakeholder Perspective on the Impact for Patients and for Drug Development in Neuromuscular Diseases. Digit. Biomark. 2021, 5, 183–190. [Google Scholar] [CrossRef]
- Youn, B.-Y.; Ko, Y.; Moon, S.; Lee, J.; Ko, S.-G.; Kim, J.-Y. Digital biomarkers for neuromuscular disorders: A systematic scoping review. Diagnostics 2021, 11, 1275. [Google Scholar] [CrossRef]
- Jacques, M.F.; Onambele-Pearson, G.L.; Reeves, N.D.; Stebbings, G.K.; Dawson, E.A.; Stockley, R.C.; Edwards, B.; Morse, C.I. 12-Month changes of muscle strength, body composition and physical activity in adults with dystrophinopathies. Disabil. Rehabil. 2022, 44, 1847–1854. [Google Scholar] [CrossRef]
- Kaslow, J.A.; Sokolow, A.G.; Donnelly, T.; Buchowski, M.S.; Damon, B.M.; Markham, L.W.; Burnette, W.B.; Soslow, J.H. Leveraging cardiac magnetic resonance imaging to assess skeletal muscle progression in Duchenne muscular dystrophy. Neuromuscul. Disord. 2022, 32, 390–398. [Google Scholar] [CrossRef]
- Servais, L.; Yen, K.; Guridi, M.; Lukawy, J.; Vissiere, D.; Strijbos, P.; Vissière, D.; Strijbos, P. Stride Velocity 95th Gentile: Insights into Gaining Regulatory Qualification of the First Wearable-Derived Digital Endpoint for use in Duchenne Muscular Dystrophy Trials. J. Neuromuscul. Dis. 2022, 9, 335–346. [Google Scholar] [CrossRef]
- Morse, C.I.; Onambele-Pearson, G.; Edwards, B.; Wong, S.C.; Jacques, M.F. Objective and subjective measures of sleep in men with Muscular Dystrophy. PLoS ONE 2022, 17, e0274970. [Google Scholar] [CrossRef]
- Nair, K.S.; Lott, D.J.; Forbes, S.C.; Barnard, A.M.; Willcocks, R.J.; Senesac, C.R.; Daniels, M.J.; Harrington, A.T.; Tennekoon, G.I.; Zilke, K.; et al. Step Activity Monitoring in Boys with Duchenne Muscular Dystrophy and its Correlation with Magnetic Resonance Measures and Functional Performance. J. Neuromuscul. Dis. 2022, 9, 423–436. [Google Scholar] [CrossRef] [PubMed]
| Paper | Subjects | Instruments | Variables |
|---|---|---|---|
| Jeannet et al. [32], 2011 | 5 DMD (age: 4–6 years) | Non-commercialized ASUR monitor with 3D accelerometer and gyroscope positioned on the chest | Posture parameters, no. of walking episodes, cadence, maximum duration of walking, total steps |
| Ganea et al. [33], 2012 | 25 DMD (age: 5–12 years) 20 healthy children as control group | 2 ASUR units with a 1-axis gyroscope fixed on the shank, 1 BioAGM unit with 3-axis accelerometer fixed on the trunk | Stride length, shank peak angular velocity, stride velocity, cadence, double support, power spectral entropy |
| Davidson et al. [34], 2015 | 16 DMD (age: 5–13 years) 13 healthy children as control group | StepWatch accelerometer worn at the ankle joint | Time inactivity, time in low activity, time in high activity, total steps |
| Le Moing et al. [35], 2016 | 7 non-ambulatory DMD (around 18 years) | ActiMyo (3 axis-MIMU) worn on the wrist | Rotation rate, ratio of the vertical component in the overall acceleration, hand elevation rate, power |
| Jacques et al. [36], 2018 | 15 DMD, 16 healthy, 46 other dystrophies (mean age 24) | GENEActiv with a 3-axis accelerometer worn on the wrist | Daily average minutes being physically active, % sedentary behavior |
| Straub et al. [37], 2018 | / | / | Stride length, cadence, knee extension strength, heart rate, PUL, 6MWT, NSAA |
| Fujii et al. [38], 2019 | 7 non-ambulatory DMD (age: 12–24 years) | Silmee Bar-type Lite 3-axis accelerometer worn on the dominant wrist | Cumulative sum of jerk, Brooke Upper Extremity Scale, muscle strength |
| Van der Geest et al. [39], 2019 | 16 DMD (age: 7–17 years) | 3-axis accelerometer MOX worn on upper arm and lower arm | Intensity (activity counts), level of arm elevation, elevation rate, Brooke Upper Extremity Scale, PUL |
| Haberkamp et al. [40], 2019 | / | / | Stride Velocity 95th Centile (SV95C) defined as a new endpoint in therapeutic DMD trials |
| Siegel et al. [41], 2020 | 54 DMD (age: 5–17 years) | Actigraphy Actiwatch 2 worn on the wrist | Rest activity, sleep quality, and 6-minute walk test (6MWT) |
| Ann et al. [42], 2020 | 100 DMD and 100 healthy controls (age: 2–13 years) | 5 APDM OPAL accelerometers applied on forearms, shanks, chest | Relative coupling coefficient (RCC) |
| Arteaga et al. [43], 2020 | 49 DMD (mean age 13 years) | Accelerometer Actigraph GT3X worn on wrist and ankle | Total vector magnitude (VM), awake vector magnitude |
| Killian et al. [44], 2020 | 48 DMD (mean age 13 years) | Accelerometer Actigraph GT3X worn on wrist and ankle | Total vector magnitude, awake vector magnitude |
| Lott et al. [45], 2021 | 70 DMD (age: 8 years) and 10 controls | Accelerometer Actigraph GT3X worn on waist | Daily steps count |
| McErlane et al. [46], 2021 | 8 DMD (age: 6–16 years) | Wrist-worn accelerometer | Average daily maximum, average daily steps, average steps per epochs |
| Poleur et al. [47], 2021 | 91 healthy subjects (mean age: 16 years) | ActiMyo (3 axis-MIMU) worn on the wrist and ankle | Stride length, stride velocity, meters walked per hour |
| Servais et al. [48], 2021 | / | ActiMyo (3 axis-MIMU) worn on the wrist and ankle | Stride Velocity 95th Centile (SV95C) defined as a new endpoint in therapeutic DMD trials |
| Youn et al. [49], 2021 | / | / | Several activity biomarkers based on previous studies |
| Jacques et al. [50], 2022 | 15 DMD (mean age: 25 years) | GENEActiv with a 3-axis accelerometer worn on the wrist | Percentage of time spent sedentary (SB%), total time spent physically active |
| Kaslow et al. [51], 2022 | 49 DMD (mean age: 13 years) | Accelerometer Actigraph GT3X worn on wrist and ankle | Minutes per day of wearing, minutes per day of wearing and awake, VMs generated while wearing, VMs generated per minute while wearing, VMs generated per minute while wearing and awake |
| Servais et al. [52], 2022 | - | ActiMyo (3 axis-MIMU) worn on the ankle | Stride length, stride velocity, no. of meters walked per hour |
| Morse et al. [53], 2022 | 53 MD men (mean age: 40 years) | GENEActiv with a 3-axis accelerometer worn on the wrist | Sleep time, sleep efficiency, activity periods, activity times |
| Nair et al. [54], 2022 | 114 DMD (age:5–15 years) and 24 healthy controls | Accelerometer Actigraph GT3X worn on waist | Step activity, quality of muscle health |
| Sensor | Component Description | Technical Data |
|---|---|---|
| ASUR-Autonomous Sensing Unit Recorder | 3-axis accelerometer3-axis gyroscope | Sample rate = 25 HzNon-commercialized sensor |
| Physiolog BioAGM | 3-axis accelerometer3-axis gyroscope 3-axis magnetometer | Sample rate = 1–500 HzAcc range = ±2 g/±10 g |
| StepWatch | 3-axis accelerometer | Sample rate = 200 Hz |
| ActiMyo | 3-axis accelerometer3-axis gyroscope3-axis magnetometer | Sample rate = 100 Hz |
| GENEActiv | 3-axis accelerometer | Sample rate = 10–100 HzAcc range = ±8 g |
| Silmee Bar-type Lite | 3-axis accelerometer | Sample rate = 15–125 HzAcc range = ±2 g |
| MOX | 3-axis accelerometer | Sample rate = 25–100 HzAcc range = ±8 g |
| Actiwatch 2 | 3-axis accelerometer | Sample rate = 32 Hz |
| OPAL | 3-axis accelerometer3-axis gyroscope 3-axis magnetometer | Sample rate = 20–128 HzAcc range = ±16 g Gyr range = ± 2000 deg/sMagn range = ±8 Gauss |
| Actigraph GT3X/GT3X+ | 3-axis accelerometer | Sample rate = 30–100 HzAcc range = ±6 g |
| Study | Results | Limitations |
|---|---|---|
| Jeannet et al. [32] | A wide range of detailed parameters of daily activity can be reliably measured and quantified in DMD patients using a single monitoring device worn on the patient’s chest | Small number of patients, no statistical analysis, no information about activity organization throughout the day, possible extrinsic factors that may have influenced the measure |
| Ganea et al. [33] | Significant differences in stride velocity, stride length, and variability of stride velocity. Moderate correlation between spatio-temporal parameters and clinical scale. Possibility to recognize and classify DMD patients with different levels of motor dysfunction | Small numbers of investigated gait parameters, only one clinical scale and only two groups characterizing the functional status |
| Davidson et al. [34] | Strong correlation between clinical 6MWT and accelerometry data | Small sample size, no investigation into the sensitivity of accelerometry data on the natural history of change in DMD |
| Le Moing et al. [35] | No difference between dominant and non-dominant hands, strong correlation of instrumental outcomes with clinical scores | Small sample of patients and large heterogeneity among them, difficult to establish reliability of results |
| Jacques et al. [36] | Significant relationship between muscle weakness and sedentary behavior in MD patients | No different level of physical activities |
| Straub et al. [37] | Inertial sensors were confirmed as suitable and reliable instruments for the monitoring of motion activity both in ambulatory and non-ambulatory DMD patients | Previous studies suffered from the lack of natural history data available at the time the trails were scheduled, no ideal outcome that can be used for all the studies |
| Fujii et al. [38] | Strong and significant correlation between the cumulative jerk of the acceleration norm and the clinical score | Small sample size, only 8 h of monitoring, small sample rate (15 Hz) of data acquisition |
| Van der Geest et al. [39] | Strong and significant correlation between objective outcomes and the clinical score | Small sample size, not all data available for all patients, no specific inclusion criteria for the selection of patients |
| Haberkamp et al. [40] | Stride Velocity 95th Centile continuously monitored in home environment was recognized as a new endpoint in DMD patients by European regulators | - |
| Siegel et al. [41] | Non-ambulatory participants had significantly lower sleep efficiencies, less wake time after sleep onset, and less daytime activity than those in the ambulatory group. There were no significant correlations between rest-activity data and SDSC and PedsQL questionnaires | Small sample size and limited statistical power to detect significant association with clinical data |
| Ann et al. [42] | The proposed RCC is a sensitive index to distinguish children with DMD and controls at the same age in terms of motor coordination | The complex methodology for the formulation of the coordination index |
| Arteaga et al. [43] | DMD patients spent most of their time in sedentary and low-intensity activities. Age and locomotion ability affected the monitoring of acceleration results | Small sample size and unequal distribution of participants among ambulatory, non-ambulatory, and control. No inclusion of anthropometric and clinical data |
| Killian et al. [44] | Moderate–strong correlation between QMT and acceleration measures | No analysis of correlation between the accelerometric measures and locomotion clinical tests (6MWT) |
| Lott et al. [45] | 2 to 5 days of activity monitoring predicted weekly step activity | Waist-worn device, large natural history of participants |
| McErlane et al. [46] | Utility of remote and continuous monitoring of physical activity in different pediatric diseases | Small sample size |
| Poleur et al. [47] | Significant positive correlations of the stride length with age and height of participants, significant increase of the median stride length. 95th centile stride velocity stable after one year | No upper limb movement analysis |
| Servais et al. [48] | Reliability, sensitivity, and efficacy of objective endpoints for DMD patients evaluated with wearable inertial devices | - |
| Youn et al. [49] | Potential use of digital biomarkers for several neuromuscular disorders | - |
| Jacques et al. [50] | No significant differences after 12 months from baseline in physical activity monitored with the accelerometer | Sample size, short time monitoring |
| Kaslow et al. [51] | Imaging of the upper extremity musculature (triceps and biceps) demonstrated the most robust correlations with accelerometry | No distinction between ambulatory and non-ambulatory patients, limitations in the CMR protocol |
| Servais et al. [52] | Significant decrease of stride length 95th percentile, median stride velocity, and SV95C after 6 months. All variables have moderate–strong correlations with clinical scores | - |
| Morse et al. [53] | Possibility to differentiate sleep and activity phases through measuring the accelerometer data; no significant differences among different groups of muscular disease | Small sample size for each pathological groups, patients from the same clinical center |
| Nair et al. [54] | Significant correlation between accelerometry, magnetic resonance, and functional measures. Significant decrease of step activity in older patients | No covariation with external factors, different genetic mutations among DMD patients, no examination of intensity of physical activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panero, E.; D’Alessandro, R.; Cavallina, I.; Davico, C.; Mongini, T.; Gastaldi, L.; Ricci, F. Wearable Inertial Devices in Duchenne Muscular Dystrophy: A Scoping Review. Appl. Sci. 2023, 13, 1268. https://doi.org/10.3390/app13031268
Panero E, D’Alessandro R, Cavallina I, Davico C, Mongini T, Gastaldi L, Ricci F. Wearable Inertial Devices in Duchenne Muscular Dystrophy: A Scoping Review. Applied Sciences. 2023; 13(3):1268. https://doi.org/10.3390/app13031268
Chicago/Turabian StylePanero, Elisa, Rossella D’Alessandro, Ilaria Cavallina, Chiara Davico, Tiziana Mongini, Laura Gastaldi, and Federica Ricci. 2023. "Wearable Inertial Devices in Duchenne Muscular Dystrophy: A Scoping Review" Applied Sciences 13, no. 3: 1268. https://doi.org/10.3390/app13031268
APA StylePanero, E., D’Alessandro, R., Cavallina, I., Davico, C., Mongini, T., Gastaldi, L., & Ricci, F. (2023). Wearable Inertial Devices in Duchenne Muscular Dystrophy: A Scoping Review. Applied Sciences, 13(3), 1268. https://doi.org/10.3390/app13031268








