Abstract
Head and neck squamous cell carcinoma (HNSCC) is a very heterogeneous cancer with a poor overall response to therapy. One of the reasons for this therapy resistance could be cancer stem cells (CSCs), a small population of cancer cells with self-renewal and tumor-initiating abilities. Tumor cell heterogeneity represents hurdles for therapeutic elimination of CSCs. Different signaling pathway activations, such as Wnt, Notch, and Sonic-Hedgehog (SHh) pathways, lead to the expression of several cancer stem factors that enable the maintenance of CSC features. Identification and isolation of CSCs are based either on markers (CD133, CD44, and aldehyde dehydrogenase (ALDH)), side populations, or their sphere-forming ability. A key challenge in cancer therapy targeting CSCs is overcoming chemotherapy and radiotherapy resistance. However, in novel therapies, various approaches are being employed to address this hurdle such as targeting cell surface markers, other stem cell markers, and different signaling or metabolic pathways, but also, introducing checkpoint inhibitors and natural compounds into the therapy can be beneficial.
1. Introduction
Head and neck cancer is an incredibly heterogeneous cancer and the sixth most common type of cancer in the world. It typically includes tissues of the larynx, pharynx, lips, mouth, nose, and salivary glands. Head and neck squamous cell carcinoma (HNSCC) arises from the epithelia of these tissues and is well known for its ability to metastasize more often than other types of cancers: 50% of cancers have already spread to the lymph nodes by the time of diagnosis. Therapy of HNSCC includes surgery with adjuvant radiotherapy and chemotherapy. However, other therapies such as checkpoint inhibitors are also being developed and tested in clinics with notable results [1]. Nevertheless, even with the novel developing therapies, tumor molecular and cellular heterogeneity and cancer stem cells (CSCs) are two main factors that can contribute to metastatic dissemination, poor response to treatment, and worse outcomes in HNSCC patients. CSCs represent a challenge in therapy as they are a small population of cancer cells with self-renewal capacity and incredible plasticity that allows them to adapt to any environmental change. This results in their ability to contribute to tumor progression and therapy resistance. They are mostly responsible for relapse as they are resistant to most conventional therapies. As tumors can regrow from a single CSC, these cells should be one of the most crucial therapy targets in cancer treatment strategy determination. CSCs may originate from adult tissue-specific stem cells or progenitor cells; however, it is also important to emphasize that they can also initiate from differentiated cells due to genomic instability or hypoxia resulting in their dedifferentiation [2,3]. Moreover, a variety of evidence suggests that CSCs do not only remain in the steady-state but that the plasticity of CSCs is critical and that there is an alteration of phenotypic status through differentiation/dedifferentiation and reprogramming.
2. Cancer Stem Cells
2.1. Evolution Model
There are two models of tumorigenesis, known as the stochastic or clonal evolution model and the hierarchy or cancer stem cell (CSC) model, that currently predominate in describing the initiation, growth, progression, and heterogeneity of tumors [4,5]. The clonal evolution model holds that a tumor consists of a heterogeneous population of cells, each possessing the equal probability to initiate, sustain, and promote tumor growth. In this model, a stochastic acquisition of various genetic and epigenetic changes through time confers a selective advantage to the fittest clones of tumor cells, allowing them to become more aggressive and expand while outcompeting clones with less fitness. However, clonal fitness tends to change spatially and temporally as requirements and conditions vary in different tumor regions over the course of the disease. For instance, hypoxic regions may select for more aggressive and resistant clones, while nutrient-rich regions may boost the rapid-growing clones [6,7]. Furthermore, it was recently shown that mutations in certain genes such as DNA damage response genes may be preferentially selected upon exposure to radiation therapy [8].
Contrary to this, the CSC model proposes that only a minority of cells within a tumor can drive tumor growth by differentiating into phenotypically diverse cells that comprise the bulk of cells in a tumor. This model assumes that tumor growth and progression follow a hierarchical structure reliant on differentiation capacity, where highly tumorigenic CSCs sustain the long-term maintenance of intermediate and terminally differentiated progenitors. Similar to normal stem cells, CSCs exhibit an unlimited self-renewal and regenerative capacity, allowing them to generate more stem cells (symmetric division) as well as give rise to functionally heterogeneous cancer cell lineages with a limited proliferative and tumorigenic capacity (asymmetric division). The hierarchy model was first recognized in hematopoietic malignancies [9,10], and later on, a broad spectrum of specific cell surface markers allowed its identification in solid tumors [11].
However, in the last decade, it became evident that the hierarchy model is more dynamic and complex than originally imagined. Thus, accumulating evidence suggests an alternative model of cellular plasticity which has partly reconciled both the CSC and clonal model by postulating that cancer cells possess a dynamic capacity for bidirectional interconversion between a stem cell and non-self-renewing/non-stem-like states. It is assumed that stemness and CSC plasticity are influenced by diverse microenvironmental stimuli and/or genetic tumor cell alterations that work simultaneously over time, allowing differentiated tumor cells to reacquire stem cell characteristics [12]. Moreover, the newest model of CSC-induced tumorigenesis (monophyletic model of cancer), which has been proposed very recently by Luo and colleagues, suggests that CSCs are stem cells that evade control of differentiation whose primary purpose was to restore damaged tissues [13] (Figure 1). However, as this is a very recent model, it still needs more experimental evidence.
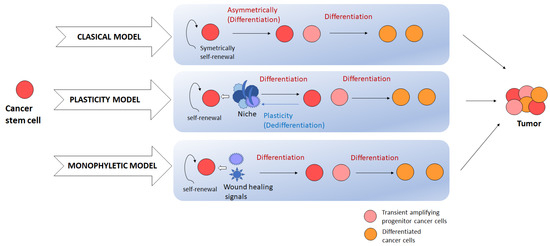
Figure 1.
Current models of CSC-induced tumorigenesis. Classical model presumes the self-renewal of the cancer stem cells by symmetrical division, and heterogeneity is introduced by asymmetrical division. In the plasticity model, CSCs are interchanged from differentiated or transient cancer cells to CSCs in response to niche. Monophyletic model presumes the effect of inflammation and wound healing factors in CSC dynamics and heterogeneity. Nevertheless, all these models postulate the same result: a heterogeneous tumor.
2.2. Stemness-Associated Signaling Pathways
2.2.1. Wnt Signaling Pathway
Numerous signaling pathways are involved in the onset and development of HNSCC, and each one is essential in controlling the proliferation, differentiation, and survival of cells. The Wnt signaling pathway is a highly conserved pathway that plays a key role in embryonic development, cell proliferation, differentiation, and adult tissue homeostasis [14]. β-Catenin is one of the central players in the Wnt signaling pathway, and its stability and nuclear translocations lead to the activation of downstream target genes through T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) transcription factors, thereby promoting cell survival and proliferation [15]. In HNSCC, abnormal activation of the Wnt signaling pathway most probably occurs not like in other cancers due to mutations in Adenomatous polyposis coli (APC) or beta-catenin [16] but probably due to the mutations in other pathways that interact with Wnt pathway (FAT1 and AJUBA) or by EGFR stabilization of β-catenin [17]. According to recent developments, Wnt/β-catenin signaling also has a role in the differentiation, growth, and drug resistance of CSCs in HNSCC [17].
2.2.2. Notch Signaling Pathway
The Wnt signaling pathway crosses paths with other signaling pathways to further enhance its impact on HNSCC progression. Another important element in the pathophysiology of HNSCC is the Notch signaling system. Notch receptors (Notch 1–4) and their ligands (Jagged and Delta-like) have been shown to play crucial roles in the differentiation, proliferation, and determination of cell fate. Targeted gene expression is regulated by the Notch intracellular domain (NICD) which translocates to the nucleus after the cleavage during the activation of Notch signaling [18]. Dysregulation of the Notch signaling pathway is common in HNSCC, and mutations in Notch receptors and ligands are frequently observed. NOTCH1 is one of the most frequently altered genes in squamous cell carcinoma, with inactivating mutations detected in about 10% of all cases, including those involving the oral cavity [19]. Aberrant Notch signaling can promote tumor cell survival, epithelial–mesenchymal transition (EMT), and immune evasion [19]. Furthermore, Notch signaling interacts with other signaling pathways such as Wnt and Sonic-Hedgehog (SHh) to form a complex network of molecular events that drive HNSCC progression.
2.2.3. Sonic-Hedgehog (SHh) Signaling Pathway
Although the SHh signaling system is critical for tissue homeostasis and embryonic development, cancer cells, especially those in HNSCC, have found a way to utilize it. In the absence of Hh ligands, the signaling is inactivated because Smoothened (Smo) is inhibited by Patched (Ptch). In the presence of Shh ligands, Ptch suppression of Smo is abolished, resulting in the nuclear accumulation of Gli1 and activation of target genes [20]. Excessive SHh signaling in HNSCC is typically brought on by mutations in PTCH1, SMO, GLI1, and GLI2 or increased SHh ligand expression. Angiogenesis, EMT, and tumor development are all aided by abnormal SHh signaling, which makes HNSCC more aggressive [21]. Previous research indicates that SHh is essential for the maintenance and function of cancer stem cells. It has also been linked with chemotherapy and radiotherapy resistance [22,23,24]. The Notch, SHh, and Wnt pathway interactions further increase the intricacy of HNSCC [25].
2.2.4. EGFR Signaling Pathway
The epidermal growth factor receptor (EGFR) signaling pathway is another relevant player in HNSCC. EGFR is a receptor tyrosine kinase that supervises cell proliferation, survival, and angiogenesis. Overexpression and overactivation of EGFR are prevalent in the majority of HNSCCs and are related to poor prognosis [26]. Therapies that focus on the inhibition of EGFR, such as cetuximab, have been successfully employed to treat HNSCC, especially in combination with radiotherapy [27]. However, resistance mechanisms, including EGFR mutations, the activation of different signaling pathways, and tumor heterogeneity, often restrain the effectiveness of the therapy [28].
2.2.5. PI3K/AKT/mTOR Signaling Pathway
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of the rapamycin (mTOR) signaling pathway is often dysregulated in HNSCC, where it is active in over 80% of HNSCCs. This happens due to activation of previously mentioned EGFR, but also PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), PI3K (phosphatidylinositol 3-kinase), and PTEN (phosphatase and tensin homolog) mutations [29], as well as the overexpression of mTOR [30]. Dysregulation of the PI3K/AKT/mTOR signaling pathway adds to HNSCC progression and drug resistance [29].
2.2.6. Hippo Pathway
The Hippo pathway plays an important role in tissue homeostasis and is highly conserved. When Hippo-stimulating signals are not present, Hippo kinase cascade is not active, and YAP/TAZ translocate into the nucleus to induce the transcription in association with transcriptional enhanced associate domain (TEAD) family DNA binding proteins. In the presence of Hippo-stimulating signals, after a series of phosphorylation events, YAP/TAZ are also phosphorylated which leads to its cytoplasmic retention or proteolytic degradation [31]. YAP/TAZ are important for cell proliferation and stemness, and therefore, it also has an important role in CSCs maintenance [32]. Genes from the Hippo pathway are often mutated in HNSCC by inactivating mutation and deletion or amplification, depending on the gene/protein function [31]. Additionally, HNSCC is one of the cancers in which YAP and TAZ amplification is most frequently observed [33]. YAP/TAZ activation in HNSCC frequently leads to cancer stemness, progression, poor prognosis, and therapeutic resistance [34,35,36,37].
2.3. Stem Cell Factors
Stemness, embryonic development, and tissue homeostasis are all critically regulated by SRY (Sex-Determining Region Y)-Box (SOX) transcription factors. Different members of the SOX family have been linked to the promotion of CSC characteristics in HNSCC. For example, Sox2 is frequently overexpressed and linked to aggressive behavior in HNSCC tumors [38]. The role of Sox2 in preserving HNSCC CSC pluripotency and self-renewal has also been established [39]. Because Sox2 encourages tumor initiation [40] and therapy resistance [41], it is a desirable target for therapeutic intervention [42]. Moreover, Sox9 has been connected to chemotherapy resistance and CSC characteristics in HNSCC, underlining the significance of Sox family members in these cells [43].
An important modulator of pluripotency in embryonic stem cells is Nanog, a homeobox transcription factor. Nanog has a critical role in HNSCC in enhancing CSC features. Increased tumor growth, metastasis, and tumor-initiating capacity have all been linked to elevated Nanog expression [44]. Nanog controls genes related to stemness and proliferation, CSC pluripotency maintenance, self-renewal, and therapy resistance [45,46].
Furthermore, Octamer-binding transcription factors (OCTs), such as Oct3/4, Oct1, Oct6, and others, are essential for preserving stemness and controlling cellular differentiation [47,48]. Oct3/4 is critical for HNSCC progression due to its role in tumor growth and CSC maintenance. In HNSCC, Oct3/4 expression is frequently increased, especially in CSC populations [49].
B cell-specific Moloney murine leukemia virus Integration site 1 (Bmi-1) has also been studied extensively in relation to HNSCC. It has been discovered that HNSCC has an aberrant expression of Bmi-1, which may be related to the CSCs’ proliferative behavior within the tumor. For instance, by upregulating Bmi-1, growth factors produced from endothelial cells potently support the survival and self-renewal of cancer stem cells in HNSCC [50]. Additionally, cisplatin treatment of HNSCC can induce Bmi-1 expression and augment CSC populations [51]. Twist1 and Bmi-1 work together in human HNSCC to promote EMT and stemness, suggesting an active function for Bmi-1 in HNSCC metastasis. According to Chen and colleagues, these results led them to speculate that Bmi-1+ tumor cells may be CSCs in HNSCC and could be linked to therapeutic resistance in vivo [52,53].
The transcription factor Klf4 (Kruppel-like factor 4) has recently drawn interest in relation to HNSCC [54]. Klf4 has been shown in several studies to function as a tumor suppressor [55], preventing the growth of cancer cells and promoting differentiation, but it has also been reported that Klf4 acts as an oncogene [56]. The recent study by Tsompana and colleagues corroborates the oncogenic role of Klf4 in HNSCC, demonstrating that Klf4 binds to super-enhancers and directs the transcriptional upregulation of cancer genes promoting cancer progression and global transcriptional changes [54]. Moreover, a worse disease-specific survival was connected with the persistence of Klf4 expression [57]. However, studies analyzing Klf4 expression in HNSCC cases compared to normal tissue showed both decreased and elevated Klf4 expression [57,58].
3. Identification of CSC
Since CSCs may play an important role in the prognosis of cancer and therapeutic strategy, the precise identification of CSC subpopulations is considered essential for a more accurate characterization of patient subtypes and ultimately more personalized and efficient therapeutic approaches. The first proof for the existence of CSCs comes from the study by Bonnet and Dick, who identified a small subpopulation of leukemic cells in the bone marrow samples of AML patients, displaying the immature CD34+ CD38− phenotype and the functional capacity to recapitulate the tumor of origin upon serial transplantation in immunocompromised mice [10]. Since then, the possibility of CSC identification based on the expression of specific stem cell-related markers has paved the way for their detection in multiple types of solid tumors, including HNSCC [11].
In HNSCC, several different markers such as CD44 and CD133, side populations, and aldehyde dehydrogenase (ALDH) activity have been used to identify and isolate highly tumorigenic cells with reduced sensitivity to chemo- and radiotherapy. However, despite significant efforts made to identify a unique marker for CSCs in HNSCC, the specificity of markers as well as their restriction to CSCs still remains the major challenge [59,60].
3.1. Marker-Based Isolation of CSCs
3.1.1. CD44
High expression of the hyaluronic acid receptor CD44 is one of the most common markers for the isolation and enrichment of CSCs in solid cancers, including HNSCC (Figure 2) [61]. The CD44 receptor is a type I transmembrane glycoprotein with a critical role in cell adhesion, migration, proliferation, and angiogenesis [62]. The extracellular segment of CD44 contains a ligand-binding site that primarily binds hyaluronic acid (HA), but it can engage with various other constituents of the extracellular matrix such as osteopontin, integrin, fibronectin, matrix metalloproteinases, and laminin. By working as a signaling platform, CD44 facilitates the activation of multiple cancer-related signaling pathways involved in cell proliferation and survival, such as the epidermal growth factor receptor (EGFR), Src/focal adhesion kinase (FAK), and the hepatocyte growth factor receptor (MET) [62].
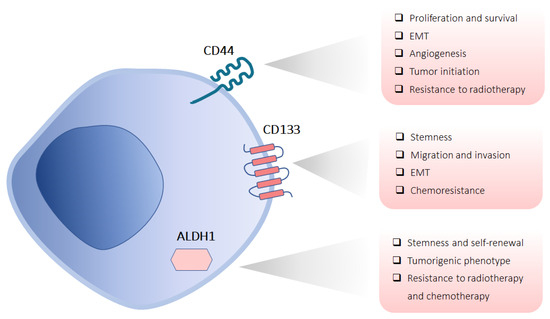
Figure 2.
CSC markers in HNSCC. Schematic representation shows the subcellular localization of the most commonly used CSC markers (CD44, CD133, and ALDH1) with their functional properties in HNSCC.
Increasing evidence suggests that overexpression of CD44 is associated with various aspects of HNSCC tumorigenesis, including proliferation, migration, angiogenesis, epithelial-mesenchymal transition (EMT), and therapy resistance [63,64,65,66]. In addition, several studies demonstrated that CD44 expression is associated with poorly differentiated tumors, regional lymph node metastasis, local recurrence, and a reduced overall survival rate in oral squamous cell carcinoma (OSCC), emphasizing its crucial role in tumor recurrence and metastasis in HNSCC tumors [67].
Accumulating data indicate that CD44 has a key role in the regulation of CSC features such as tumor initiation, self-renewal, and resistance to chemo- and radiotherapy [68]. Biddle and colleagues showed that in OSCC, CSC subpopulations expressing high levels of CD44 exhibit increased phenotypic plasticity that allows a sequential shift between EMT and mesenchymal–epithelial transition (MET) activities and ultimately underlies enhanced therapeutic resistance [69,70]. However, the CD44 gene is regularly subjected to alternative splicing which produces both the standard isoform (CD44s) and several variant isoforms (CD44v), which might play a distinct biological role [71]. For instance, the CD44v3 isoform is highly expressed in CSCs and correlates with HNSCC progression [72,73]. Furthermore, Huang et al. demonstrated that CD44 plays a pivotal role in promoting stemness and the development of HNSCC CSCs. By using HNSCC cell lines, a xenograft model, and patient tumor samples, they showed that the ERK1/2-Nanog signaling pathway is critical for the maintenance of CSC stemness and tumorigenic capacity by upregulating CD44 expression, and therefore, targeting this pathway may prevent or reverse CSC phenotypes that drive tumor progression and metastasis in HNSCC [46].
3.1.2. CD133
CD133 (prominin-1) is a penta-transmembrane glycoprotein that was originally recognized as a hematopoietic stem cell marker (Figure 2). First reported as a CSC marker in CRC [74], CD133 has become the most extensively used cell surface marker for the isolation of putative CSCs from a variety of solid tumors [75]. In HNSCC, a subpopulation of CD133+ CSCs displayed greater cell viability, migratory, and invasive capability as well as chemoresistance when compared with CD133− cells [76,77,78]. Nevertheless, the CD133-mediated molecular processes in regulating stemness properties of HNSCC CSCs are still unclear. Chen et al. demonstrated the importance of CD133/Src signaling in modifying stemness, EMT, and tumorigenicity of HNSCC CSCs by showing that the downregulation of CD133 resulted in a reduced self-renewal ability and downregulated expression of stemness genes while promoting differentiation and apoptosis in CSCs [79].
Nevertheless, although at first, it seemed that CD133 would be a useful marker for HNSCC CSCs, the development of CSC research revealed certain discrepancies even in this type of cancer. For instance, clinical reports demonstrated that the upregulation of CD133 in HNSCC tumor tissue negatively correlates with the survival of HNSCC patients [80]. However, a study by de Moraes et al. reported no CD133 expression in tumor samples from HNSCC patients and no correlation between CD133 expression and prognosis or survival [81].
3.1.3. ALDH1
ALDH1 is a useful putative marker in identifying CSCs of different origins (Figure 2). ALDH1 is a superfamily of cytosolic enzymes that metabolize aldehydes to their corresponding carboxylic acids. Among 19 enzyme isoforms that may be localized to different cellular compartments, such as the cytoplasm, mitochondria, and nucleus, the cytoplasmic isoforms involved in the retinoic acid biosynthesis have proven to be critical in regulating stemness in normal stem cells as well as CSCs [82].
Elevated activity of ALDH1 has also been identified in HNSCC CSCs in several studies, and associated with increased invasiveness, stemness, and self-renewal properties of CSCs [83,84]. While the prognostic relevance of ALDH1 expression in HNSCCs is still debatable [85,86,87], current evidence indicates the potential significance of this CSC marker in the prediction of lymph node metastasis in some types of HNSCCs [88]. For example, it has been demonstrated that ALDH1+ cells are present in both, primary tumors and lymph node metastases from OSCC patients. The association of ALDH1 with tumor progression is further supported by studies showing that ALDH1+ cells from HNSCC patients preferentially co-localize in the tumor-invasive front with other CSC markers including CD44 and CD14, and they endogenously co-express several EMT-related markers such as Snail and MMP-9 [83,85,89].
3.2. Side Population
Another distinguishing characteristic of CSCs is their capability to actively expel chemotherapeutic drugs from within the cell, in a process mediated by drug-efflux proteins of the ATP-binding cassette (ABC) family. The term “side population” (SP) refers to the fraction of cells that eliminate the Hoechst 33342 dye (DNA binding dye) via the ABC transporter and therefore appears as a distinct tail or side branch in flow cytometry analysis [90]. Compared to the main non-SP population, the SP subset is characterized by tumor-initiating capacity, expression of stem cell-related markers, and resistance to chemotherapeutics. For all these reasons, this method is commonly used to identify and isolate CSCs [91]. The side population subset of HNSCC cells was found to be characterized by increased in vitro clonogenic potential, invasiveness, and resistance to chemotherapeutics, as well as enhanced tumorigenicity in vivo [92,93,94].
Among ABC transporters, ABCG2 has been considered a universal marker of CSCs, and its high expression has been observed in different types of malignant tumors, including HNSCC [43,95]. Shen et al. have reported that ABCG2 levels vary among the different HNSCC cell lines as well as the tissues from different types of HNSCC carcinoma, including laryngeal, hypopharyngeal, and nasopharyngeal cancers [95]. Furthermore, ABCG2 expression strongly correlated with the multidrug resistance against various chemotherapeutics in HNSCC cell lines, while a high positivity of ABCG2 was significantly associated with the TNM stage and lymph node metastasis in laryngeal, nasopharyngeal, and hypopharyngeal cancers, suggesting that ABCG2 expression may be a clinically important mechanism of drug resistance [43,95].
3.3. Sphere-Forming Ability
Since epithelial cells typically rely on substrate attachment for their survival, the capacity to grow under anchorage-independent conditions, avoiding anoikis and differentiation, is considered to be a hallmark of stem cells. Therefore, one of the methods for CSC characterization and enrichment relies on their functional property to form non-adherent 3D structures called tumor spheres when grown in ultra-low attachment plates in a serum-free medium or 3D matrices such as soft agar or dishes coated with fibronectin or matrigel. Such sphere-forming cells retain their stem cell characteristics over several generations and the formation of secondary spheres following the dissociation of primary spheres can serve as evidence of their self-renewal ability. Additionally, phenotypic markers are employed for their characterization.
Several studies showed that a sphere-forming assay might be useful in the enrichment of CSCs from HNSCC cell lines or primary tumor samples [96,97,98]. Furthermore, CSCs enriched through the sphere-forming assay from cell lines and HNSCC primary cultures display an increased expression of CSC markers such as CD133, CD44, Sox2, Nanog, and Nestin in comparison to the parental counterparts, as well as enhanced tumorigenic ability in vivo [97,98,99]. However, spheroid-forming capacity does not always reflect tumorigenic ability in vivo [100], which indicates the need for in vitro assays that closely replicate the physiological microenvironment (niche) of CSCs, allowing for the more accurate observation of their functional properties.
Besides being useful in evaluating CSCs’ function, the sphere-forming assay represents an attractive and reliable model for the evaluation of treatment response in CSCs [86]. For example, a study by Lim and colleagues showed that HNSCC tumorspheres, besides the expression of common CSC markers, exhibit increased levels of ABCG2 transporter and were resistant to several chemotherapeutics such as cisplatin, 5-fluorouracil (FU), paclitaxel, and docetaxel [97].
4. Chemotherapy Resistance
Autophagy, epithelial–mesenchymal transition (EMT), drug efflux, CSCs, and metabolic reprogramming are biological processes and phenotypes implicated in cisplatin resistance in HNSCC. In this type of cancer, stemness properties are augmented by the increased expression of stemness-related markers, including Sox2, Bmi-1, CD44, Nanog, CD133, and ALDH, resulting in a cell population that is more likely to be resistant to cisplatin [51,101,102]. Most HNSCC cell lines cultured as spheroids (3D) are more resistant to cetuximab and cisplatin treatment than the same cells growing in 2D monolayers [103]. Elevated expression of a stress-inducible factor Nrf2 mediates multidrug resistance in CD133+ HNSCC CSCs [104], indicating another mechanism of CSC resilience. Also, Fibroblast growth factor receptor (FGFR) signaling regulates the resistance of HNSCC CSCs to cisplatin [105].
Some of the novel mechanisms of cisplatin resistance in CSCs include ABCG2, CD44, and Sox9 as main players since they are upregulated with the acquisition of drug resistance and involved in cancer stem cell features [43]. Another Sox family member, Sox18, facilitates the resistance of Bmi-1-expressing cells to cetuximab in HNSCC via the oxidative phosphorylation pathway [106]. Another novel player involved in cisplatin resistance in oral cancer is the SHh pathway through the regulation of CD10-positive cells [22].
As for the role of the tumor microenvironment in chemotherapy resistance, cancer-associated fibroblasts (CAFs) play an important part. It has been recently shown that CAFs increase the proliferation of HNSCC spheroids originating from the same tumor, and during their co-culture, CAFs increase EGFR expression which affects their treatment response [107]. Additionally, Guan et al. showed that overproduction of Interleukin 4 (IL-4) is also a critical event in cell death resistance for CSCs. Namely, CD133+ cells show increased expression of IL-4 leading to enhanced multidrug resistance [108].
5. Radiotherapy Resistance
CSCs may escape death from radiotherapy exposures through different mechanisms: the control of the cell cycle, elevated free-radical scavenging, more efficient DNA repair, the activation of autophagy, and protection by the microenvironmental niche [109,110,111,112,113]. Different biological factors may also enhance the radioresistance in HNSCC: tumor size, high density of CSCs, negative HPV status, and other parameters such as tumor hypoxia [114].
CD44 is a well-known CSC marker, and it has been suggested that it is responsible for radiotherapy resistance and poor overall survival [115]. It was reported previously that the CD44high/EGFRlow phenotype is associated with therapy resistance [116,117]. Other well-known proteins that have been associated with radioresistance in HNSCC are integrin β1 [118], Oct4 [119], and ALDH [120].
We will focus here on the recent studies reporting novel mechanisms of radiotherapy resistance. Suzuki et al. recently reported that CD98 expression might be a predictive marker of resistance to radiotherapy in HNSCC as the response to chemoradiotherapy or bioradiotherapy was lower in the high CD98 expression group [121]. Also, CD98 heavy chain has been shown to be a regulator of HNSCC radiosensitivity as its high expression levels increase radioresistance in vitro and in vivo [122].
Another interesting study on the radioresistant SQ20B cell line highlighted CSCs characteristics: low EGFR expression, cetuximab-resistant, and highly migratory. However, carbon irradiation in combination with cetuximab strongly inhibited the invasion of SQ20B CSCs signifying that carbon ion irradiation is a better potential choice of therapy for CSCs than photon irradiation [123]. Another study involving the same cell line revealed that the combination of irradiation, cetuximab, and Bcl-2 inhibitor can be a prosperous, novel CSC-targeted therapy. This drug combination inhibits cell proliferation, invasion/migration, and resistance to apoptosis in both 2D and 3D models. Also, this combination therapy effectively delayed tumor growth and improved in vivo lifespan in a nude mouse model with a heterotopic tumor xenograft [124].
Recently, Glycoprotein nonmetastatic melanoma protein B (GPNMB)-positive cells in HNSCC have been shown to enhance sphere formation, invasion, and migration and were more resistant to chemoradiation and bio-radiotherapy, indicating that this protein might be involved in radiation resistance acquisition [125]. Also, a high expression of GPNMB in patients was associated with poor prognosis.
6. Therapeutic Targeting of CSCs
Despite recent advances in HNSCC therapy through the introduction of checkpoint inhibitors, the response to the therapy in HNSCC patients is still limited. Targeting CSCs is a logical option, especially since even one CSC can regrow a tumor, so it is crucial to eradicate all CSCs in order for patients to remain disease-free. There are different ways to aim at CSCs: by targeting their specific markers, signaling pathways, particular receptors important for their maintenance, and PD-1 blockade but also by pursuing different plants or mushrooms as an excellent source of natural compounds that might have CSCs-specific effects (Figure 3).
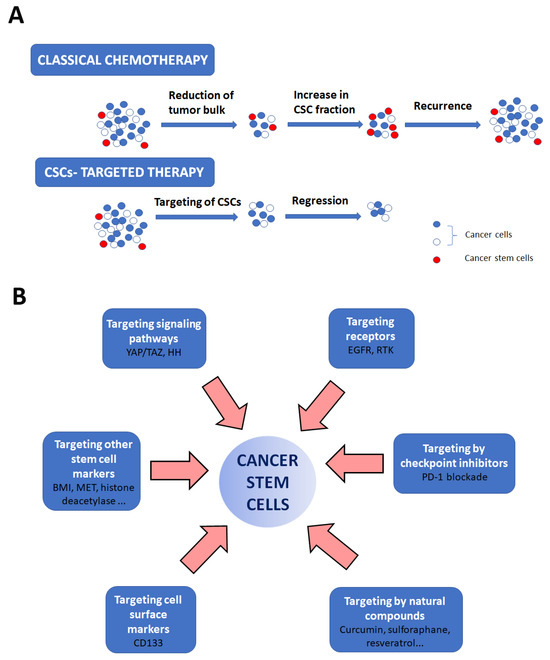
Figure 3.
Strategies for CSC eradication. The importance of CSC-targeted therapy is shown in (A). Classical chemotherapy only reduces tumor bulk but does not destroy CSCs, resulting in cancer relapse. CSC-targeted therapy specifically targets these cells resulting in cancer regression. (B) Aiming specifically at CSCs is feasible by targeting their specific markers, signaling pathways, and particular receptors important for their maintenance, by using PD-1 blockade or by using natural compounds in therapy.
6.1. Targeting Cell Surface Markers
One of the possible strategies for HNSCC stem cell eradication is targeting cell-surface markers. As we already mentioned, several cell-surface markers have been found to be specific for HNSCC stem cells: CD10, CD24, CD29, CD44, CD44/CD27, CD133 [126], CD90, CD98 [127], and CD147 [128]. However, not all of these markers have been demonstrated as good targets for therapy. Nevertheless, CD133 is one of the most studied markers for HNSCC CSCs. CD133+ OSCC cells show enhanced resistance to chemotherapeutic drugs [77], indicating that CD133 is a good target for cancer stem cell suppression. Waldron et al. developed 10 years ago a biological drug targeting EpCam and CD133 which was effective in inhibiting multiple carcinoma lines in vitro and caused regression in HNSCC tumors in vivo [129]. Lai et al. showed that the treatment of HNSCC cells with a newly synthesized flavonoid derivative, 2-(3-hydroxyphenyl)-5-methylnaphthyridin-4-one (CSC-3436), upregulated E-cadherin and downregulated N-cadherin, vimentin, and CD133 while also reducing EMT and stemness [130]. Yu et al. showed that targeting CD133 by shRNA reduces side population (SP) cells and in vivo tumorigenicity [131].
6.2. Targeting Other Stem Cell Markers
Bmi-1 is an important factor in HNSCC CSCs self-renewal and maintenance, and HNSCC progression is associated with this protein [85,132]. Therefore, it has been recognized as a potential therapeutic target for CSC eradication [133,134]. For example, the combination treatment of anti-PD1 and cisplatin, enriched Bmi-1+ CSCs but also inhibited HNSCC growth. Additionally, the inhibition of Bmi-1 in combination with anti-PD1 eliminates CSCs and inhibits HNSCC progression [135]. Also, targeting Bmi-1+ CSCs decreases chemoresistance to cisplatin and eliminates metastases in HNSCC [52].
As ALDH is an important CSC marker, its inhibition has also been investigated in terms of CSC therapy. Disulfiram, an ALDH inhibitor, has been shown to increase radio- and chemo-sensitization in HNSCC CSCs while also inhibiting spheroid formation [136].
Oncofetal antigen 5T4 has been demonstrated as a cancer stem cell marker and two studies have been performed to explore the effect of its inhibition on HNSCC CSCs. The first study explored the strategy of 5T4 inhibition through an innovative antibody–drug conjugate targeted to 5T4 and carrying a DNA-damaging “payload” (pyrrolobenzodiazepine). The authors showed that this inhibition decreases the fraction of HNSCC CSCs in vitro and in vivo and prevents local recurrence in a patient-derived xenograft (PDX) model [137]. Another study aimed at nasopharyngeal carcinoma (NPC) explored chimeric antigen receptor (CAR)-engineered cytokine-induced killer (CIK) cell therapy against NPC. CIK cells are created ex vivo by stimulating peripheral blood mononuclear cells (PBMCs) with IFNγ, anti-human CD3 antibody, and IL-2. This chimeric CAR construct was specific to 5T4, and the co-culture of CIK cells generated using this construct with NPC spheroids successfully eliminated the spheroids [138].
Targeting histone deacetylases has been proposed as a reasonable strategy for CSC suppression. Consequently, pharmacological inhibition of histone deacetylase 6 (HDAC6) decreases cisplatin resistance and inhibits oral CSCs [139]. Another HDAC inhibitor, entinostat, also reduced the number of oral CSCs [140].
Other well-known proteins that have been connected with CSC features and maintenance have also been studied as potential targets for CSC suppression. The importance of integrin β1 in the regulation of stemness and radioresistance has been demonstrated in a recent study [118], suggesting this protein could be the next candidate for CSC therapy. Another recent study by Khedkar et al. identified that higher expressions of MET, STAT3, and AKT were connected with poor overall survival in HNSCC patients, so they synthesized a small molecule HNC018 that targets these molecules. HNC018 decreased sphere formation and also increased the response to cisplatin and suppressed tumor growth in vivo [141]. Another study investigating MET as a CSC therapeutic target showed that MET inhibition induces the radiosensitization of HNSCC [142]. Milan et al. demonstrated recently that β-catenin and histone methyltransferase Enhancer of Zest Homolog 2 (EZH2) were accumulated in cisplatin-resistant and CSC populations and proposed that their inhibition could be a strategy for CSC suppression [143]. The high mobility group AT-hook 2 (HMGA2)-Snai2 axis was shown to be important in tumorigenicity and stemness of HNSCC CSCs, so targeting these two proteins might also be a suitable approach [144]. Moon et al. demonstrated that the inhibition of Slug may represent a new therapeutic target for HNSCC stem-like cells [145].
Dong et al. recently published a very interesting study showing that super-enhancers (SE) are extremely important for CSCs. In order to maintain cell identity and status, master transcription factors assemble SEs at cell-type-determining genes. So, for HNSCC CSCs, these factors are bromodomain-containing protein 4 (BRD4) which employs mediators and NF-κB p65 to create SEs at cancer stemness genes such as TP63, MET, and FOSL1. The disruption of SEs leads to the inhibition of CSC renewal and their elimination [146].
There are also not-that-well-known targets but with a good potential for the CSCs suppression that have been studied lately. Qin et al. discovered a long noncoding RNA (lncRNA), named PVT1, which was highly expressed in CSCs. This lncRNA was correlated with HNSCC lymph node metastasis. PVT1 inhibition removed CSCs and prevented metastasis, whilst also activating anti-tumor immunity [147]. Garcia-Mayea et al. identified tetraspanin-1 (TSPAN1) as a novel target protein for HNSCC chemoresistance [148]. Tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6) controls tumor spreading through EMT and CSC phenotypes in HNSCC [149]. Furthermore, Heat shock protein 90 (HSP90) inhibitors (KU711 and Ku757) are also efficient in targeting HNSCC CSCs [150].
6.3. Targeting Signaling Pathways
Among signaling pathways that are important for HNSCC stemness, already explored in terms of therapy (Hippo (YAP/TAZ), Wnt, and Notch) [151,152,153,154,155], the SHh signaling pathway has recently been recognized as significant for CSC maintenance and could serve as a possible target for their removal. For example, Gli3 knockdown diminishes stemness, cell proliferation, and invasion in oral squamous cell carcinoma [156]. Furthermore, an SHh pathway inhibitor Vismodegib, a drug currently used for the treatment of basal-cell carcinoma that is also undergoing clinical trials for therapy of different types of cancer, effectively reduced the expression of CSC-related genes in the HNSCC cell line [157].
6.4. Targeting Receptors
A drug approved by the FDA, also used for targeting CSC markers, is cetuximab which targets the EGFR. EGFR enhances the stemness and advancement of oral cancer through the inhibition of autophagic degradation of Sox2 [158].
Several studies are reporting the antitumor activity of cetuximab on HNSCC and other cancers [159,160,161]. Fu et al. recently demonstrated that combined therapy with EGFR/Notch bispecific antibodies and PARP inhibitor decreases the subpopulation of stem-like cells, reduces the frequency of tumor-initiating cells, and delays tumor recurrence after irradiation in a lung cancer model [162]. However, as a recent review article pointed out that Notch signaling pathway is critical in HNSCC, this strategy could be tested in this type of cancer as well [155]. One recent study tested the therapeutic combination of cisplatin, cetuximab, and valproic acid (an antiepileptic drug and a histone deacetylase inhibitor) and showed synergistic antiproliferative and pro-apoptotic effects on four different 3D-self-assembled spheroid models. Also, the combined treatment completely obstructed HNSCC xenograft tumor growth in nude mice [163]. Another study was focused on determining how EGFR inhibition affects different subtypes of cancer stem cells, i.e., cancer stem cells shifting from epithelial to mesenchymal states. They discovered that the inhibition of EGFR reduces cell proliferation but without significant cell death induction. Additionally, cetuximab and erlotinib induced cellular differentiation, which typically means that the cells should be more susceptible to chemotherapy or irradiation therapy [160]. Guy et al. showed that dual monoclonal antibody HER family blockade (cetuximab and pertuzumab) combined with photon irradiation is highly effective in the inhibition of growth of HNSCC CSCs through the blocking of downstream AKT-mTOR and Ras-MAPK signaling [164].
Another receptor tyrosine kinase pan-inhibitor, ponatinib, has been shown to eliminate HNSCC CSCs [165]. Also, an interesting study by Alvarez-Teijeiro et al. showed there is a difference in factors secreted by CAFs and normal fibroblasts, and these factors were connected with EGFR, Insulin-like growth factor (IGFR), and Platelet-derived growth factor receptor (PDGFR). So, when the authors applied different inhibitors of these receptors, they showed reduced sphere formation and anchorage-independent growth [166]. Also, afatinib, a pan-EGFR inhibitor, reduced tumorigenicity and radiosentisized HNSCC cells by specifically targeting CSCs [167].
6.5. Targeting Metabolism
CSC metabolism is often reprogrammed to meet their needs. CSCs preferentially utilize glycolysis for survival, but studies have shown that CSCs may also rely on OXPHOS. Previous research demonstrated that lipid metabolism plays an important role in maintaining the stemness of CSCs and their energy needs [168]. Therefore, targeting either of these pathways should be a good strategy in CSCs therapy. Metformin has been studied frequently as a potential drug for CSC therapy because it may impede glycolysis. There are numbers of studies on the metformin anticancer effect in oral cancer (for a detailed and recent review see [169]), and some of them also show an inhibitory effect in CSCs [170]. In addition, therapy by HIF inhibitors might also be a promising approach for CSC elimination considering the importance of reactive oxygen species (ROS) in CSCs preservation [171].
6.6. Targeting by Checkpoint Inhibitors
In 2016, Anti-PD1 antibodies (Pembrolizumab and Nivolumab) were approved by the FDA for the treatment of HNSCC. However, the response of HNSCC to therapy remained low, probably because of the heterogeneous nature of this cancer. Maybe the combination of treatments comprising checkpoint inhibitors with other inhibitors, such as one from a recent study that combined an inhibitor of Lysine-specific demethylase 1 (LSD1), which is important for CSC maintenance, and a PD-1 blockade for the treatment of HNSCC, will be exploited in future strategies [172]. Or maybe promising therapeutic strategies for CSC therapy will be targeting not PD-L1 directly but indirectly, like in a study by Chen et al. who demonstrated that targeting CKLF Like MARVEL Transmembrane Domain Containing 6 (CMTM6), which is a regulator of PD-L1 expression, represses stem cell-like properties and increases antitumor immunity in HNSCC [173].
6.7. Targeting by Natural Compounds
Curcumin, epigallocatechin-3-gallate (EGCG), sulforaphane, resveratrol, and genistein are the most studied naturally occurring agents with anti-cancer stem cell properties [174,175,176]. For example, resveratrol and oxyresveratrol inhibit the expression of cancer stem-cell markers and could possibly target cancer stem cells in a hypoxia-associated tumor [177]. Even though many compounds that could be exploited in CSC-specific therapy are emerging and being tested every day, there are not many naturally occurring compounds identified and evaluated for HNSCC yet.
Different Chinese herbs have been used in cancer medicine for years, and some of the active ingredients have been shown to be effective in CSC suppression as well. For example, the bioactive macrocyclic diterpenoid compound ovatodiolide, purified from the herb Anisomeles indica (L.) Kuntze, used in Chinese traditional herbal medicine, decreased the tumor-initiating potential of orospheres in a mouse xenograft model [178]. Polysaccharides from Ganoderma lucidum, an oriental fungus that has been used for promoting health and longevity in China, Japan, and other Asian countries, have also shown such an effect in OSCC. These specific polysaccharides decreased colony formation and impaired sphere formation, as well as downregulated EMT and ABC markers of chemoresistance [179]. Butylidenephthalide, a bioactive phthalide compound from Angelica sinensis, which is also an herb used in Chinese medicine, diminishes CSC properties in an oral carcinoma model [180].
Few novel natural compounds have been found in a recent study aimed at finding the inhibitors of the SHh pathway in oral cancer: Butein, Biochanin-A, and Curcumin [181]. It remains to test these compounds in CSC models to see if they are effective, considering the importance of the SHh pathway in CSC. Honokiol, a lignan (low molecular weight polyphenol) isolated from different parts of trees belonging to the genus Magnolia, has shown an inhibitory effect on cell survival and self-renewal of oral carcinoma stem cells. It can suppress CSC markers ALDH1 and CD44, decrease migration and invasion, reduce secretion of IL-6, and decrease phosphorylation of STAT3. This lignan can also synergize with cisplatin in inhibiting cell survival [182]. Interestingly, another compound from Magnolia, magnolol, also suppresses the oral cancer stemness properties [183]. The most abundant diterpene lactone from the leaves and stem of Andrographis paniculata (Burm. f) Ness, an herbal plant generally cultivated in India, Thailand, and China and used as a traditional medicine, is called andrographolide. This substance is effective in therapy against CSCs in oral carcinomas. It can reduce oncogenicity and increase the radiosensitivity of oral CSCs via the increased expression of miR-218 resulting in the downregulation of Bmi-1, a protein important for self-renewal of CSC [184]. Mycelium from the fungus Antrodia cinnamomea and its ethyl acetate extracts have antiproliferative effects against all types of CSCs, including HNSCC CSCs. Additionally, CSC treatment with A. cinnamomea increased the effect of chemo- and radiotherapy [185]. Gallotannin extract from Bouea macrophylla seed, a plant that has a fruit similar to a mango, showed an antiproliferative effect in HNSCC CSCs, while it also enhanced radiosensitivity [186]. Furthermore, propolis has been shown to reduce stemness in HNSCC [187]. Paris saponin II from the plant Paris polyphylla Smith var. yunnanensis, which is used as an anticancer drug in traditional Chinese medicine, causes reduced sphere formation [188]. As for the well-known vegetables, radish (Raphanus sativus L.) seed extracts induce apoptosis and downregulate β-catenin in oral CSCs [189], while broccoli extract (sulforaphane) increases the cytotoxicity of cisplatin and 5-FU and inhibits sphere formation and tumor progression [190].
7. Conclusions and Future Perspectives
CSCs are a small proportion of cells in the tumor tissue; however, as they have the ability to regrow cancer from a single cell, they should represent a major focus in the development of anti-cancer therapies. A combination of drugs might be an appropriate strategy as they usually aim at different targets at the same time which makes them more effective than a single drug. It would be ideal to have a combination of a chemotherapeutic that targets the tumor bulk and reduces its growth and mass and another drug targeting CSCs specifically. However, we are not there yet, and by now, there have only been a few clinical studies conducted on the matter of head and neck cancer stem cell therapy in the last decade (Table 1). More experimental studies directed at the deciphering of CSC regulation and the acquiring of their resistance to chemo- and radiotherapy are required in the future, as well as the consequential clinical studies testing novel drugs or their combination with the already existing ones. Moreover, the emerging number of naturally occurring substances successful at targeting CSCs should also be further tested.

Table 1.
Clinical studies involving the research of head and neck cancer stem cells.
Author Contributions
Conceptualization, T.M.G.; writing—original draft preparation, T.M.G., K.V.Đ. and T.V.; writing—review and editing, K.V.Đ. and T.M.G.; visualization, T.M.G.; supervision, T.M.G.; funding acquisition, T.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Croatian Science Foundation (project number: IP-2020-02-4225 Toll-like receptor 3 in the development and treatment of human head and neck cancer: the role of endogenous ligands) and the Young Researchers’ career development project—training of doctoral students (Croatian Science foundation).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dang, S.; Zhang, S.; Zhao, J.; Li, X.; Li, W. Efficacy and safety of immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma: A systematic review and meta-analysis of randomized clinical trials. Cancer Med. 2023, 12, 20277–20286. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wan, W.W.; Xiong, S.L.; Feng, H.; Wu, N. Cancer stem-like cells can be induced through dedifferentiation under hypoxic conditions in glioma, hepatoma and lung cancer. Cell Death Discov. 2017, 3, 16105. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhong, Z.; Huang, Y.; Deng, W.; Cao, J.; Tsao, G.; Liu, Q.; Pei, D.; Kang, T.; Zeng, Y.X. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J. Biol. Chem. 2010, 285, 4931–4940. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in cancer: Cancer stem cells versus clonal evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Altea-Manzano, P.; Cuadros, A.M.; Broadfield, L.A.; Fendt, S.M. Nutrient metabolism and cancer in the in vivo context: A metabolic game of give and take. EMBO Rep. 2020, 21, e50635. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Evolutionary determinants of cancer. Cancer Discov. 2015, 5, 806–820. [Google Scholar] [CrossRef]
- Bolton, K.L.; Ptashkin, R.N.; Gao, T.; Braunstein, L.; Devlin, S.M.; Kelly, D.; Patel, M.; Berthon, A.; Syed, A.; Yabe, M.; et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet 2020, 52, 1219–1226. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Nairuz, T.; Mahmud, Z.; Manik, R.K.; Kabir, Y. Cancer stem cells: An insight into the development of metastatic tumors and therapy resistance. Stem Cell Rev. Rep. 2023, 19, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. How does multistep tumorigenesis really proceed? Cancer Discov. 2015, 5, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Liu, P.; Yu, P.; Qin, T. Cancer Stem Cells are Actually Stem Cells with Disordered Differentiation: The Monophyletic Origin of Cancer. Stem Cell Rev. Rep. 2023, 19, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Willert, K.; Jones, K.A. Wnt signaling: Is the party in the nucleus? Genes Dev. 2006, 20, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal. Transduct. Target Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Iwai, S.; Katagiri, W.; Kong, C.; Amekawa, S.; Nakazawa, M.; Yura, Y. Mutations of the APC, beta-catenin, and axin 1 genes and cytoplasmic accumulation of beta-catenin in oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2005, 131, 773–782. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Lu, Y.G.; Zheng, D.L. Roles of the Wnt Signaling Pathway in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2020, 7, 590912. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal. Transduct. Target Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Sakamoto, K. Notch signaling in oral squamous neoplasia. Pathol. Int. 2016, 66, 609–617. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; Spohr, T. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef]
- Cierpikowski, P.; Leszczyszyn, A.; Bar, J. The Role of Hedgehog Signaling Pathway in Head and Neck Squamous Cell Carcinoma. Cells 2023, 12, 2083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Xu, L.; Chen, J.; Pu, Y.; Wang, L.; Sun, H.; Guo, Y.; Guo, C. Cancer stemness of CD10-positive cells regulated by Hedgehog pathway promotes the resistance to cisplatin in oral squamous cell carcinoma. Oral Dis. 2021, 27, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Z.; Huang, H.; Wang, H. Hedgehog signaling promotes multidrug resistance by regulation of ABC transporters in oral squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Gan, G.N.; Eagles, J.; Keysar, S.B.; Wang, G.; Glogowska, M.J.; Altunbas, C.; Anderson, R.T.; Le, P.N.; Morton, J.J.; Frederick, B.; et al. Hedgehog signaling drives radioresistance and stroma-driven tumor repopulation in head and neck squamous cancers. Cancer Res. 2014, 74, 7024–7036. [Google Scholar] [CrossRef] [PubMed]
- Patni, A.P.; Harishankar, M.K.; Joseph, J.P.; Sreeshma, B.; Jayaraj, R.; Devi, A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma—Clinical implications. Cell Oncol. (Dordr.) 2021, 44, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tie, Y.; Alu, A.; Ma, X.; Shi, H. Targeted therapy for head and neck cancer: Signaling pathways and clinical studies. Signal. Transduct. Target Ther. 2023, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Bonner, J.A.; Bredel, M. EGFR Mutations in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3818. [Google Scholar] [CrossRef]
- Marquard, F.E.; Jucker, M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharmacol. 2020, 172, 113729. [Google Scholar] [CrossRef]
- Tan, F.H.; Bai, Y.; Saintigny, P.; Darido, C. mTOR Signalling in Head and Neck Cancer: Heads Up. Cells 2019, 8, 333. [Google Scholar] [CrossRef]
- Faraji, F.; Ramirez, S.I.; Anguiano Quiroz, P.Y.; Mendez-Molina, A.N.; Gutkind, J.S. Genomic Hippo Pathway Alterations and Persistent YAP/TAZ Activation: New Hallmarks in Head and Neck Cancer. Cells 2022, 11, 1370. [Google Scholar] [CrossRef] [PubMed]
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.; Abujarour, R.; et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010, 24, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Maglic, D.; Dill, M.T.; Mojumdar, K.; Ng, P.K.; Jeong, K.J.; Tsang, Y.H.; Moreno, D.; Bhavana, V.H.; et al. Comprehensive Molecular Characterization of the Hippo Signaling Pathway in Cancer. Cell Rep. 2018, 25, 1304–1317.e5. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Noguchi, K.; Nakano, Y.; Yamamura, M.; Takaoka, K.; Hashimoto-Tamaoki, T.; Kishimoto, H. The Hippo pathway transcriptional co-activator, YAP, confers resistance to cisplatin in human oral squamous cell carcinoma. Int. J. Oncol. 2015, 46, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Zhu, Y.; Yuan, C.; Wang, D.; Zhang, W.; Qi, B.; Qiu, J.; Song, X.; Ye, J.; et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 2015, 9, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Hiemer, S.E.; Zhang, L.; Kartha, V.K.; Packer, T.S.; Almershed, M.; Noonan, V.; Kukuruzinska, M.; Bais, M.V.; Monti, S.; Varelas, X. A YAP/TAZ-Regulated Molecular Signature Is Associated with Oral Squamous Cell Carcinoma. Mol. Cancer Res. 2015, 13, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Tsinias, G.; Nikou, S.; Mastronikolis, N.; Bravou, V.; Papadaki, H. Expression and prognostic significance of YAP, TAZ, TEAD4 and p73 in human laryngeal cancer. Histol. Histopathol. 2020, 35, 983–995. [Google Scholar]
- Liu, X.; Qiao, B.; Zhao, T.; Hu, F.; Lam, A.K.; Tao, Q. Sox2 promotes tumor aggressiveness and epithelial-mesenchymal transition in tongue squamous cell carcinoma. Int. J. Mol. Med. 2018, 42, 1418–1426. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, S.Y.; Do, S.I.; Lee, H.J.; Kang, H.J.; Rho, Y.S.; Bae, W.J.; Lim, Y.C. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br. J. Cancer 2014, 111, 2122–2130. [Google Scholar] [CrossRef]
- Boumahdi, S.; Driessens, G.; Lapouge, G.; Rorive, S.; Nassar, D.; Le Mercier, M.; Delatte, B.; Caauwe, A.; Lenglez, S.; Nkusi, E.; et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 2014, 511, 246–250. [Google Scholar] [CrossRef]
- Keysar, S.B.; Le, P.N.; Miller, B.; Jackson, B.C.; Eagles, J.R.; Nieto, C.; Kim, J.; Tang, B.; Glogowska, M.J.; Morton, J.J.; et al. Regulation of Head and Neck Squamous Cancer Stem Cells by PI3K and SOX2. J. Natl. Cancer Inst. 2017, 109, djw189. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.; Mannoor, K.; Cao, J.; Qadri, F.; Song, X. SOX2 in cancer stemness: Tumor malignancy and therapeutic potentials. J. Mol. Cell. Biol. 2020, 12, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Umemura, N.; Adachi, M.; Motoki, M.; Ohkoshi, E. ABCG2, CD44 and SOX9 are increased with the acquisition of drug resistance and involved in cancer stem cell activities in head and neck squamous cell carcinoma cells. Exp. Ther. Med. 2022, 24, 722. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ohnishi, Y.; Inoue, H.; Wato, M.; Tanaka, A.; Kakudo, K.; Nozaki, M. NANOG expression correlates with differentiation, metastasis and resistance to preoperative adjuvant therapy in oral squamous cell carcinoma. Oncol. Lett. 2014, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, T.; Nath, N.; Mishra, P.; Jha, A.; Nagini, S.; Mishra, R. Pluripotency transcription factor Nanog and its association with overall oral squamous cell carcinoma progression, cisplatin-resistance, invasion and stemness acquisition. Head Neck 2020, 42, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yoon, C.; Zhou, X.H.; Zhou, Y.C.; Zhou, W.W.; Liu, H.; Yang, X.; Lu, J.; Lee, S.Y.; Huang, K. ERK1/2-Nanog signaling pathway enhances CD44(+) cancer stem-like cell phenotypes and epithelial-to-mesenchymal transition in head and neck squamous cell carcinomas. Cell Death Dis. 2020, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Major, A.G.; Pitty, L.P.; Farah, C.S. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013, 2013, 319489. [Google Scholar] [CrossRef]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Scholer, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Habu, N.; Imanishi, Y.; Kameyama, K.; Shimoda, M.; Tokumaru, Y.; Sakamoto, K.; Fujii, R.; Shigetomi, S.; Otsuka, K.; Sato, Y.; et al. Expression of Oct3/4 and Nanog in the head and neck squamous carcinoma cells and its clinical implications for delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma. BMC Cancer 2015, 15, 730. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Dong, Z.; Vodopyanov, D.; Imai, A.; Helman, J.I.; Prince, M.E.; Wicha, M.S.; Nor, J.E. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010, 70, 9969–9978. [Google Scholar] [CrossRef]
- Nor, C.; Zhang, Z.; Warner, K.A.; Bernardi, L.; Visioli, F.; Helman, J.I.; Roesler, R.; Nor, J.E. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 2014, 16, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, M.; Li, Y.; Chang, I.; Yuan, Q.; Ekimyan-Salvo, M.; Deng, P.; Yu, B.; Yu, Y.; Dong, J.; et al. Targeting BMI1(+) Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma. Cell Stem Cell 2017, 20, 621–634.e6. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.E.; Somayaji, R.; Nor, J.E. Bmi-1: A master regulator of head and neck cancer stemness. Front. Oral Health 2023, 4, 1080255. [Google Scholar] [CrossRef] [PubMed]
- Tsompana, M.; Gluck, C.; Sethi, I.; Joshi, I.; Bard, J.; Nowak, N.J.; Sinha, S.; Buck, M.J. Reactivation of super-enhancers by KLF4 in human Head and Neck Squamous Cell Carcinoma. Oncogene 2020, 39, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Paparella, M.L.; Abrigo, M.; Bal de Kier Joffe, E.; Raimondi, A.R. Oral-specific ablation of Klf4 disrupts epithelial terminal differentiation and increases premalignant lesions and carcinomas upon chemical carcinogenesis. J. Oral Pathol. Med. 2015, 44, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Rowland, B.D.; Bernards, R.; Peeper, D.S. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat. Cell Biol. 2005, 7, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.K.; Yang, M.H.; Chang, S.Y.; Chang, Y.C.; Li, W.Y.; Tsai, T.L.; Wang, Y.F.; Chu, P.Y.; Hsieh, S.L. Persistent Kruppel-like factor 4 expression predicts progression and poor prognosis of head and neck squamous cell carcinoma. Cancer Sci. 2011, 102, 895–902. [Google Scholar] [CrossRef]
- Ingruber, J.; Savic, D.; Steinbichler, T.B.; Sprung, S.; Fleischer, F.; Glueckert, R.; Schweigl, G.; Skvortsova, I.I.; Riechelmann, H.; Dudas, J. KLF4, Slug and EMT in Head and Neck Squamous Cell Carcinoma. Cells 2021, 10, 539. [Google Scholar] [CrossRef]
- Lan, L.; Behrens, A. Are There Specific Cancer Stem Cell Markers? Cancer Res. 2023, 83, 170–172. [Google Scholar] [CrossRef]
- Kim, W.T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Rajarajan, A.; Stokes, A.; Bloor, B.K.; Ceder, R.; Desai, H.; Grafstrom, R.C.; Odell, E.W. CD44 expression in oro-pharyngeal carcinoma tissues and cell lines. PLoS ONE 2012, 7, e28776. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Szczepanski, M.J.; Gluszko, A.; Szafarowski, T.; Azambuja, J.H.; Dolg, L.; Gellrich, N.C.; Kampmann, A.; Whiteside, T.L.; Zimmerer, R.M. CD44(+) tumor cells promote early angiogenesis in head and neck squamous cell carcinoma. Cancer Lett. 2019, 467, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.I.; Ozato, K.; Liu, K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Investig. 2018, 128, 5549–5560. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, D.; Kuku, G.; Selvaraju, R.; Nestor, M. Characterization of CD44 variant expression in head and neck squamous cell carcinomas. Tumour Biol. 2014, 35, 2053–2062. [Google Scholar] [CrossRef]
- Mishra, A.; Sriram, H.; Chandarana, P.; Tanavde, V.; Kumar, R.V.; Gopinath, A.; Govindarajan, R.; Ramaswamy, S.; Sadasivam, S. Decreased expression of cell adhesion genes in cancer stem-like cells isolated from primary oral squamous cell carcinomas. Tumour Biol. 2018, 40, 1010428318780859. [Google Scholar] [CrossRef]
- Skandalis, S.S.; Karalis, T.T.; Chatzopoulos, A.; Karamanos, N.K. Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell Signal. 2019, 63, 109377. [Google Scholar] [CrossRef]
- Biddle, A.; Liang, X.; Gammon, L.; Fazil, B.; Harper, L.J.; Emich, H.; Costea, D.E.; Mackenzie, I.C. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011, 71, 5317–5326. [Google Scholar] [CrossRef]
- Biddle, A.; Gammon, L.; Liang, X.; Costea, D.E.; Mackenzie, I.C. Phenotypic Plasticity Determines Cancer Stem Cell Therapeutic Resistance in Oral Squamous Cell Carcinoma. eBioMedicine 2016, 4, 138–145. [Google Scholar] [CrossRef]
- Tolg, C.; Hofmann, M.; Herrlich, P.; Ponta, H. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res. 1993, 21, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Earle, C.; Shiina, M. Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1849. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, E.J.; Weed, D.T.; Civantos, F.J.; Goodwin, W.J.; Bourguignon, L.Y. A novel CD44 v3 isoform is involved in head and neck squamous cell carcinoma progression. Otolaryngol. Head Neck Surg. 2001, 124, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Sheng, W.Q.; Du, X. CD133: A cancer stem cells marker, is used in colorectal cancers. World J. Gastroenterol. 2013, 19, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. CD133: A stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Gao, W.; Li, F.; Bo, Y.; Zhu, M.; Fu, R.; Liu, Q.; Wen, S.; Wang, B. Identification and characterization of CD133(+)CD44(+) cancer stem cells from human laryngeal squamous cell carcinoma cell lines. J. Cancer 2017, 8, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, S.; Yen, Y.; Brown, J.; Ta, J.Q.; Le, A.D. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010, 289, 151–160. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, C.; Liu, X.; Fang, F.; Liu, S.; Liao, X.; Tao, S.; Mai, H. Characterisation of a subpopulation of CD133(+) cancer stem cells from Chinese patients with oral squamous cell carcinoma. Sci. Rep. 2020, 10, 8875. [Google Scholar] [CrossRef]
- Chen, Y.S.; Wu, M.J.; Huang, C.Y.; Lin, S.C.; Chuang, T.H.; Yu, C.C.; Lo, J.F. CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS ONE 2011, 6, e28053. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, L.; Wu, S.; Gong, X.; Feng, Z.; Ma, L.; Zhu, B.; Yao, N.; Wang, D.; Dong, H. Clinicopathological significance of cancer stem cells marked by CD133 and KAI1/CD82 expression in laryngeal squamous cell carcinoma. World J. Surg. Oncol. 2014, 12, 118. [Google Scholar] [CrossRef]
- de Moraes, F.P.; Lourenco, S.V.; Ianez, R.C.; de Sousa, E.A.; Silva, M.M.; Damascena, A.S.; Kowalski, L.P.; Soares, F.A.; Coutinho-Camillo, C.M. Expression of stem cell markers in oral cavity and oropharynx squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, Y.W.; Hsu, H.S.; Tseng, L.M.; Huang, P.I.; Lu, K.H.; Chen, D.T.; Tai, L.K.; Yung, M.C.; Chang, S.C.; et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun. 2009, 385, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Xu, X.R.; Wu, T.F.; Sun, Z.J.; Zhang, W.F. Correlation of ALDH1, CD44, OCT4 and SOX2 in tongue squamous cell carcinoma and their association with disease progression and prognosis. J. Oral Pathol. Med. 2014, 43, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Lo, W.L.; Chen, Y.W.; Huang, P.I.; Hsu, H.S.; Tseng, L.M.; Hung, S.C.; Kao, S.Y.; Chang, C.J.; Chiou, S.H. Bmi-1 Regulates Snail Expression and Promotes Metastasis Ability in Head and Neck Squamous Cancer-Derived ALDH1 Positive Cells. J. Oncol. 2011, 2011, 609259. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Tsakmaki, V.; Danielidis, V.; Sivridis, E. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br. J. Cancer 2012, 106, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, Y.; Yu, X.; Qian, F.; Bian, B.S.; Xiao, H.L.; Wang, W.G.; Xu, S.L.; Yang, J.; Cui, W.; et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod. Pathol. 2014, 27, 775–783. [Google Scholar] [CrossRef]
- Qian, X.; Wagner, S.; Ma, C.; Coordes, A.; Gekeler, J.; Klussmann, J.P.; Hummel, M.; Kaufmann, A.M.; Albers, A.E. Prognostic significance of ALDH1A1-positive cancer stem cells in patients with locally advanced, metastasized head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 1151–1158. [Google Scholar] [CrossRef]
- Sterz, C.M.; Kulle, C.; Dakic, B.; Makarova, G.; Bottcher, M.C.; Bette, M.; Werner, J.A.; Mandic, R. A basal-cell-like compartment in head and neck squamous cell carcinomas represents the invasive front of the tumor and is expressing MMP-9. Oral Oncol. 2010, 46, 116–122. [Google Scholar] [CrossRef]
- Hirschmann-Jax, C.; Foster, A.E.; Wulf, G.G.; Nuchtern, J.G.; Jax, T.W.; Gobel, U.; Goodell, M.A.; Brenner, M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14228–14233. [Google Scholar] [CrossRef]
- Wu, C.; Alman, B.A. Side population cells in human cancers. Cancer Lett. 2008, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chang, I.; Chen, Z.; Kang, M.; Wang, C.Y. Characterization of side populations in HNSCC: Highly invasive, chemoresistant and abnormal Wnt signaling. PLoS ONE 2010, 5, e11456. [Google Scholar] [CrossRef] [PubMed]
- Loebinger, M.R.; Giangreco, A.; Groot, K.R.; Prichard, L.; Allen, K.; Simpson, C.; Bazley, L.; Navani, N.; Tibrewal, S.; Davies, D.; et al. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br. J. Cancer 2008, 98, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Tabor, M.H.; Clay, M.R.; Owen, J.H.; Bradford, C.R.; Carey, T.E.; Wolf, G.T.; Prince, M.E. Head and neck cancer stem cells: The side population. Laryngoscope 2011, 121, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Dong, P.; Li, D.; Gao, S. Expression and function of ABCG2 in head and neck squamous cell carcinoma and cell lines. Exp. Ther. Med. 2011, 2, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Nor, J.E. Orosphere assay: A method for propagation of head and neck cancer stem cells. Head Neck 2013, 35, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Oh, S.Y.; Cha, Y.Y.; Kim, S.H.; Jin, X.; Kim, H. Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol. 2011, 47, 83–91. [Google Scholar] [CrossRef]
- Pozzi, V.; Sartini, D.; Rocchetti, R.; Santarelli, A.; Rubini, C.; Morganti, S.; Giuliante, R.; Calabrese, S.; Di Ruscio, G.; Orlando, F.; et al. Identification and characterization of cancer stem cells from head and neck squamous cell carcinoma cell lines. Cell Physiol. Biochem. 2015, 36, 784–798. [Google Scholar] [CrossRef]
- Kaseb, H.O.; Fohrer-Ting, H.; Lewis, D.W.; Lagasse, E.; Gollin, S.M. Identification, expansion and characterization of cancer cells with stem cell properties from head and neck squamous cell carcinomas. Exp. Cell Res. 2016, 348, 75–86. [Google Scholar] [CrossRef]
- Kuch, V.; Schreiber, C.; Thiele, W.; Umansky, V.; Sleeman, J.P. Tumor-initiating properties of breast cancer and melanoma cells in vivo are not invariably reflected by spheroid formation in vitro, but can be increased by long-term culturing as adherent monolayers. Int. J. Cancer 2013, 132, E94–E105. [Google Scholar] [CrossRef]
- Lee, J.; Park, M.; Ko, Y.; Kim, B.; Kim, O.; Hyun, H.; Kim, D.; Sohn, H.; Moon, Y.L.; Lim, W. Ectopic overexpression of CD133 in HNSCC makes it resistant to commonly used chemotherapeutics. Tumour Biol. 2017, 39, 1010428317695534. [Google Scholar] [CrossRef] [PubMed]
- Kulsum, S.; Sudheendra, H.V.; Pandian, R.; Ravindra, D.R.; Siddappa, G.; Nisheena, R.; Chevour, P.; Ramachandran, B.; Sagar, M.; Jayaprakash, A.; et al. Cancer stem cell mediated acquired chemoresistance in head and neck cancer can be abrogated by aldehyde dehydrogenase 1 A1 inhibition. Mol. Carcinog. 2017, 56, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019, 19, 16. [Google Scholar] [CrossRef]
- Lu, B.C.; Li, J.; Yu, W.F.; Zhang, G.Z.; Wang, H.M.; Ma, H.M. Elevated expression of Nrf2 mediates multidrug resistance in CD133(+) head and neck squamous cell carcinoma stem cells. Oncol. Lett. 2016, 12, 4333–4338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDermott, S.C.; Rodriguez-Ramirez, C.; McDermott, S.P.; Wicha, M.S.; Nor, J.E. FGFR signaling regulates resistance of head and neck cancer stem cells to cisplatin. Oncotarget 2018, 9, 25148–25165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Z.; Gao, N.; Xiong, G.; Chen, P.; Li, H.; Chen, D.; He, Q.; Peng, L. SOX18 meditates the resistance of Bmi1-expressing cells to cetuximab in HNSCC. Oral Dis. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Magan, M.; Wiechec, E.; Roberg, K. CAFs affect the proliferation and treatment response of head and neck cancer spheroids during co-culturing in a unique in vitro model. Cancer Cell Int. 2020, 20, 599. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.F.; Tang, X.X.; Zhang, D.J.; Zheng, Y.; Yu, D.J.; Zhao, Y.; Lu, Y.Q.; Zhu, L. Constitutive secretion of Interleukin-4 dictates CD133+ side population cells to resist drug treatment and cell death. J. BUON 2015, 20, 1350–1359. [Google Scholar]
- Reid, P.A.; Wilson, P.; Li, Y.; Marcu, L.G.; Bezak, E. Current understanding of cancer stem cells: Review of their radiobiology and role in head and neck cancers. Head Neck 2017, 39, 1920–1932. [Google Scholar] [CrossRef]
- Zhang, M.; Atkinson, R.L.; Rosen, J.M. Selective targeting of radiation-resistant tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2010, 107, 3522–3527. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, S.L.; Finniss, S.; Xiang, C.; Decarvalho, A.; Umansky, F.; Kalkanis, S.N.; Mikkelsen, T.; Brodie, C. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int. J. Cancer 2009, 125, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Rovida, E.; Peppicelli, S.; Bono, S.; Bianchini, F.; Tusa, I.; Cheloni, G.; Marzi, I.; Cipolleschi, M.G.; Calorini, L.; Sbarba, P.D. The metabolically-modulated stem cell niche: A dynamic scenario regulating cancer cell phenotype and resistance to therapy. Cell Cycle 2014, 13, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Dubrovska, A.; Linge, A.; Baumann, M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv. Drug Deliv. Rev. 2017, 109, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Gupta, R.; Mishra, A.; Kumar, V.; Bhadauria, S.; Bhatt, M.L.B. Evaluation of correlation between CD44, radiotherapy response, and survival rate in patients with advanced stage of head and neck squamous cell carcinoma (HNSCC). Cancer Med. 2022, 11, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.C.; La Fleur, L.; Melissaridou, S.; Roberg, K. The relationship between EMT, CD44(high)/EGFR(low) phenotype, and treatment response in head and neck cancer cell lines. J. Oral Pathol. Med. 2016, 45, 640–646. [Google Scholar] [CrossRef] [PubMed]
- La Fleur, L.; Johansson, A.C.; Roberg, K. A CD44high/EGFRlow subpopulation within head and neck cancer cell lines shows an epithelial-mesenchymal transition phenotype and resistance to treatment. PLoS ONE 2012, 7, e44071. [Google Scholar] [CrossRef]
- Park, S.J.; Min, H.J.; Yoon, C.; Kim, S.H.; Kim, J.H.; Lee, S.Y. Integrin beta1 regulates the perineural invasion and radioresistance of oral squamous carcinoma cells by modulating cancer cell stemness. Cell Signal. 2023, 110, 110808. [Google Scholar] [CrossRef]
- Nathansen, J.; Lukiyanchuk, V.; Hein, L.; Stolte, M.I.; Borgmann, K.; Lock, S.; Kurth, I.; Baumann, M.; Krause, M.; Linge, A.; et al. Oct4 confers stemness and radioresistance to head and neck squamous cell carcinoma by regulating the homologous recombination factors PSMC3IP and RAD54L. Oncogene 2021, 40, 4214–4228. [Google Scholar] [CrossRef]
- Kurth, I.; Hein, L.; Mabert, K.; Peitzsch, C.; Koi, L.; Cojoc, M.; Kunz-Schughart, L.; Baumann, M.; Dubrovska, A. Cancer stem cell related markers of radioresistance in head and neck squamous cell carcinoma. Oncotarget 2015, 6, 34494–34509. [Google Scholar] [CrossRef]
- Suzuki, H.; Kawasaki, Y.; Suzuki, S.; Yamada, T.; Ito, A.; Suzuki, M.; Miura, M.; Hatakeyama, H.; Omori, Y. CD98 expression can be a predictive factor of resistance to radiotherapy in head and neck squamous cell carcinoma. Pol. J. Pathol. 2023, 74, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Digomann, D.; Kurth, I.; Tyutyunnykova, A.; Chen, O.; Lock, S.; Gorodetska, I.; Peitzsch, C.; Skvortsova, I.I.; Negro, G.; Aschenbrenner, B.; et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity. Clin. Cancer Res. 2019, 25, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Moncharmont, C.; Guy, J.B.; Wozny, A.S.; Gilormini, M.; Battiston-Montagne, P.; Ardail, D.; Beuve, M.; Alphonse, G.; Simoens, X.; Rancoule, C.; et al. Carbon ion irradiation withstands cancer stem cells’ migration/invasion process in Head and Neck Squamous Cell Carcinoma (HNSCC). Oncotarget 2016, 7, 47738–47749. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.B.; Espenel, S.; Louati, S.; Gauthier, A.; Garcia, M.A.; Vial, N.; Malesys, C.; Ardail, D.; Alphonse, G.; Wozny, A.S.; et al. Combining radiation to EGFR and Bcl-2 blockade: A new approach to target cancer stem cells in head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Suzuki, H.; Suzuki, S.; Yamada, T.; Suzuki, M.; Ito, A.; Hatakeyama, H.; Miura, M.; Omori, Y. GPNMB-Positive Cells in Head and Neck Squamous Cell Carcinoma-Their Roles in Cancer Stemness, Therapy Resistance, and Metastasis. Pathol. Oncol. Res. 2022, 28, 1610450. [Google Scholar] [CrossRef] [PubMed]
- Elkashty, O.A.; Abu Elghanam, G.; Su, X.; Liu, Y.; Chauvin, P.J.; Tran, S.D. Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas. Carcinogenesis 2020, 41, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, C.Y. Targeting cancer stem cells in squamous cell carcinoma. Precis. Clin. Med. 2019, 2, 152–165. [Google Scholar] [CrossRef]
- Mai, Y.; Su, J.; Yang, C.; Xia, C.; Fu, L. The strategies to cure cancer patients by eradicating cancer stem-like cells. Mol. Cancer 2023, 22, 171. [Google Scholar] [CrossRef]
- Waldron, N.N.; Barsky, S.H.; Dougherty, P.R.; Vallera, D.A. A bispecific EpCAM/CD133-targeted toxin is effective against carcinoma. Target Oncol. 2014, 9, 239–249. [Google Scholar] [CrossRef]
- Lai, Y.J.; Yu, W.N.; Kuo, S.C.; Ho, C.T.; Hung, C.M.; Way, T.D.; Chen, C.T. CSC-3436 inhibits TWIST-induced epithelial-mesenchymal transition via the suppression of Twist/Bmi1/Akt pathway in head and neck squamous cell carcinoma. J. Cell Physiol. 2019, 234, 9118–9129. [Google Scholar] [CrossRef]
- Yu, C.C.; Hu, F.W.; Yu, C.H.; Chou, M.Y. Targeting CD133 in the enhancement of chemosensitivity in oral squamous cell carcinoma-derived side population cancer stem cells. Head Neck 2016, 38 (Suppl. S1), E231–E238. [Google Scholar] [CrossRef]
- Kalish, J.M.; Tang, X.H.; Scognamiglio, T.; Zhang, T.; Gudas, L.J. Doxycycline-induced exogenous Bmi-1 expression enhances tumor formation in a murine model of oral squamous cell carcinoma. Cancer Biol. Ther. 2020, 21, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mirshahidi, S.; Simental, A.; Lee, S.C.; De Andrade Filho, P.A.; Peterson, N.R.; Duerksen-Hughes, P.; Yuan, X. Cancer stem cell self-renewal as a therapeutic target in human oral cancer. Oncogene 2019, 38, 5440–5456. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Schober, M. Joining Forces: Bmi1 Inhibition and Cisplatin Curb Squamous Carcinogenesis. Cell Stem Cell 2017, 20, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, W.; Wang, C.Y. BMI1 Inhibition Eliminates Residual Cancer Stem Cells after PD1 Blockade and Activates Antitumor Immunity to Prevent Metastasis and Relapse. Cell Stem Cell 2020, 27, 238–253.e6. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Qian, X.; Ochsenreither, S.; Soldano, F.; DeLeo, A.B.; Sudhoff, H.; Oppel, F.; Kuppig, A.; Klinghammer, K.; Kaufmann, A.M.; et al. Disulfiram Acts as a Potent Radio-Chemo Sensitizer in Head and Neck Squamous Cell Carcinoma Cell Lines and Transplanted Xenografts. Cells 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Kerk, S.A.; Finkel, K.A.; Pearson, A.T.; Warner, K.A.; Zhang, Z.; Nor, F.; Wagner, V.P.; Vargas, P.A.; Wicha, M.S.; Hurt, E.M.; et al. 5T4-Targeted Therapy Ablates Cancer Stem Cells and Prevents Recurrence of Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2017, 23, 2516–2527. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, H.; Luo, W.; Zhang, Q.; Liu, J.; Yao, K. 5T4-specific chimeric antigen receptor modification promotes the immune efficacy of cytokine-induced killer cells against nasopharyngeal carcinoma stem cell-like cells. Sci. Rep. 2017, 7, 4859. [Google Scholar] [CrossRef]
- Tavares, M.O.; Milan, T.M.; Bighetti-Trevisan, R.L.; Leopoldino, A.M.; de Almeida, L.O. Pharmacological inhibition of HDAC6 overcomes cisplatin chemoresistance by targeting cancer stem cells in oral squamous cell carcinoma. J. Oral Pathol. Med. 2022, 51, 529–537. [Google Scholar] [CrossRef]
- Marques, A.E.M.; do Nascimento Filho, C.H.V.; Marinho Bezerra, T.M.; Guerra, E.N.S.; Castilho, R.M.; Squarize, C.H. Entinostat is a novel therapeutic agent to treat oral squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 771–779. [Google Scholar] [CrossRef]
- Khedkar, H.N.; Chen, L.C.; Kuo, Y.C.; Wu, A.T.H.; Huang, H.S. Multi-Omics Identification of Genetic Alterations in Head and Neck Squamous Cell Carcinoma and Therapeutic Efficacy of HNC018 as a Novel Multi-Target Agent for c-MET/STAT3/AKT Signaling Axis. Int. J. Mol. Sci. 2023, 24, 10247. [Google Scholar] [CrossRef] [PubMed]
- Luttich, L.; Besso, M.J.; Heiden, S.; Koi, L.; Baumann, M.; Krause, M.; Dubrovska, A.; Linge, A.; Kurth, I.; Peitzsch, C. Tyrosine Kinase c-MET as Therapeutic Target for Radiosensitization of Head and Neck Squamous Cell Carcinomas. Cancers 2021, 13, 1865. [Google Scholar] [CrossRef] [PubMed]
- Milan, T.M.; Eskenazi, A.P.E.; Oliveira, L.D.; Silva, G.D.; Bighetti-Trevisan, R.L.; Freitas, G.P.; Almeida, L.O. Interplay between EZH2/beta-catenin in stemness of cisplatin-resistant HNSCC and their role as therapeutic targets. Cell Signal. 2023, 109, 110773. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, X.; Li, J.; Yu, S.; Ke, X.; Yan, T.; Zhu, Y.; Cheng, J.; Yang, J. HMGA2-Snai2 axis regulates tumorigenicity and stemness of head and neck squamous cell carcinoma. Exp. Cell Res. 2022, 418, 113271. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Lee, S.H.; Koo, B.S.; Kim, J.M.; Huang, S.; Cho, J.H.; Eun, Y.G.; Shin, H.A.; Lim, Y.C. Slug is a novel molecular target for head and neck squamous cell carcinoma stem-like cells. Oral Oncol. 2020, 111, 104948. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, J.; Li, Y.; Ma, Z.; Yu, Y.; Wang, C.Y. Transcriptional super-enhancers control cancer stemness and metastasis genes in squamous cell carcinoma. Nat. Commun. 2021, 12, 3974. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, W.; Liu, S.; Wang, Y.; Peng, X.; Jia, L. PVT1 inhibition stimulates anti-tumor immunity, prevents metastasis, and depletes cancer stem cells in squamous cell carcinoma. Cell Death Dis. 2023, 14, 187. [Google Scholar] [CrossRef]
- Garcia-Mayea, Y.; Mir, C.; Carballo, L.; Castellvi, J.; Temprana-Salvador, J.; Lorente, J.; Benavente, S.; Garcia-Pedrero, J.M.; Allonca, E.; Rodrigo, J.P.; et al. TSPAN1: A Novel Protein Involved in Head and Neck Squamous Cell Carcinoma Chemoresistance. Cancers 2020, 12, 3269. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.C.; Wu, L.; Yu, G.T.; Zhang, W.F.; Huang, C.F.; Sun, Z.J. TRAF6 regulates tumour metastasis through EMT and CSC phenotypes in head and neck squamous cell carcinoma. J. Cell Mol. Med. 2018, 22, 1337–1349. [Google Scholar] [CrossRef]
- Subramanian, C.; Kovatch, K.J.; Sim, M.W.; Wang, G.; Prince, M.E.; Carey, T.E.; Davis, R.; Blagg, B.S.J.; Cohen, M.S. Novel C-Terminal Heat Shock Protein 90 Inhibitors (KU711 and Ku757) Are Effective in Targeting Head and Neck Squamous Cell Carcinoma Cancer Stem cells. Neoplasia 2017, 19, 1003–1011. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wu, Y.; Wang, Y.; Wang, D.; Zhang, W.; Yuan, H.; Ye, J.; Song, X.; Yang, J.; et al. The Hippo effector TAZ promotes cancer stemness by transcriptional activation of SOX2 in head neck squamous cell carcinoma. Cell Death Dis. 2019, 10, 603. [Google Scholar] [CrossRef]
- Omori, H.; Sato, K.; Nakano, T.; Wakasaki, T.; Toh, S.; Taguchi, K.; Nakagawa, T.; Masuda, M. Stress-triggered YAP1/SOX2 activation transcriptionally reprograms head and neck squamous cell carcinoma for the acquisition of stemness. J. Cancer Res. Clin. Oncol. 2019, 145, 2433–2444. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Zhang, L.; Huang, C.F.; Ma, S.R.; Bu, L.L.; Liu, J.F.; Yu, G.T.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; et al. NOTCH1 inhibition enhances the efficacy of conventional chemotherapeutic agents by targeting head neck cancer stem cell. Sci. Rep. 2016, 6, 24704. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Do, S.I.; Lee, H.J.; Kang, H.J.; Koo, B.S.; Lim, Y.C. Notch1 signaling contributes to stemness in head and neck squamous cell carcinoma. Lab. Investig. 2016, 96, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Kalafut, J.; Czerwonka, A.; Anameric, A.; Przybyszewska-Podstawka, A.; Misiorek, J.O.; Rivero-Muller, A.; Nees, M. Shooting at Moving and Hidden Targets-Tumour Cell Plasticity and the Notch Signalling Pathway in Head and Neck Squamous Cell Carcinomas. Cancers 2021, 13, 6219. [Google Scholar] [CrossRef]
- Rodrigues, M.; Miguita, L.; De Andrade, N.P.; Heguedusch, D.; Rodini, C.O.; Moyses, R.A.; Toporcov, T.N.; Gama, R.R.; Tajara, E.E.; Nunes, F.D. GLI3 knockdown decreases stemness, cell proliferation and invasion in oral squamous cell carcinoma. Int. J. Oncol. 2018, 53, 2458–2472. [Google Scholar] [CrossRef] [PubMed]
- Kleszcz, R.; Frackowiak, M.; Dorna, D.; Paluszczak, J. Combinations of PRI-724 Wnt/beta-Catenin Pathway Inhibitor with Vismodegib, Erlotinib, or HS-173 Synergistically Inhibit Head and Neck Squamous Cancer Cells. Int. J. Mol. Sci. 2023, 24, 10448. [Google Scholar] [CrossRef]
- Lv, X.X.; Zheng, X.Y.; Yu, J.J.; Ma, H.R.; Hua, C.; Gao, R.T. EGFR enhances the stemness and progression of oral cancer through inhibiting autophagic degradation of SOX2. Cancer Med. 2020, 9, 1131–1140. [Google Scholar] [CrossRef]
- Sato, F.; Kubota, Y.; Natsuizaka, M.; Maehara, O.; Hatanaka, Y.; Marukawa, K.; Terashita, K.; Suda, G.; Ohnishi, S.; Shimizu, Y.; et al. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biol. Ther. 2015, 16, 933–940. [Google Scholar] [CrossRef]
- Setubal Destro Rodrigues, M.F.; Gammon, L.; Rahman, M.M.; Biddle, A.; Nunes, F.D.; Mackenzie, I.C. Effects of Cetuximab and Erlotinib on the behaviour of cancer stem cells in head and neck squamous cell carcinoma. Oncotarget 2018, 9, 13488–13500. [Google Scholar] [CrossRef]
- Tanei, T.; Choi, D.S.; Rodriguez, A.A.; Liang, D.H.; Dobrolecki, L.; Ghosh, M.; Landis, M.D.; Chang, J.C. Antitumor activity of Cetuximab in combination with Ixabepilone on triple negative breast cancer stem cells. Breast Cancer Res. 2016, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Li, G.; Lei, C.; Qian, K.; Zhang, S.; Zhao, J.; Hu, S. Bispecific antibodies targeting EGFR/Notch enhance the response to talazoparib by decreasing tumour-initiating cell frequency. Theranostics 2023, 13, 3641–3654. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Zotti, A.I.; Roca, M.S.; Grumetti, L.; Lombardi, R.; Moccia, T.; Vitagliano, C.; Milone, M.R.; Ciardiello, C.; Bruzzese, F.; et al. Valproic Acid Synergizes With Cisplatin and Cetuximab in vitro and in vivo in Head and Neck Cancer by Targeting the Mechanisms of Resistance. Front. Cell Dev. Biol. 2020, 8, 732. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.B.; Mery, B.; Ollier, E.; Espenel, S.; Vallard, A.; Wozny, A.S.; Simonet, S.; Lauret, A.; Battiston-Montagne, P.; Ardail, D.; et al. Dual “mAb” HER family blockade in head and neck cancer human cell lines combined with photon therapy. Sci. Rep. 2017, 7, 12207. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, Y.; Sedhom, W.; Lu, L.; Liu, Y.; Wang, H.; Oka, M.; Bornstein, S.; Said, S.; Song, J.; et al. Distinct roles of PIK3CA in the enrichment and maintenance of cancer stem cells in head and neck squamous cell carcinoma. Mol. Oncol. 2020, 14, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Teijeiro, S.; Garcia-Inclan, C.; Villaronga, M.A.; Casado, P.; Hermida-Prado, F.; Granda-Diaz, R.; Rodrigo, J.P.; Calvo, F.; Del-Rio-Ibisate, N.; Gandarillas, A.; et al. Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets. Cancers 2018, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Macha, M.A.; Rachagani, S.; Qazi, A.K.; Jahan, R.; Gupta, S.; Patel, A.; Seshacharyulu, P.; Lin, C.; Li, S.; Wang, S.; et al. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget 2017, 8, 20961–20973. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pages, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 113. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, J.; Qiao, X. Research Progress of Metformin in the Treatment of Oral Squamous Cell Carcinoma. Endocrinology 2023, 164, bqad139. [Google Scholar] [CrossRef]
- Patil, S. Metformin treatment decreases the expression of cancer stem cell marker CD44 and stemness related gene expression in primary oral cancer cells. Arch. Oral Biol. 2020, 113, 104710. [Google Scholar] [CrossRef]
- Marles, H.; Biddle, A. Cancer stem cell plasticity and its implications in the development of new clinical approaches for oral squamous cell carcinoma. Biochem. Pharmacol. 2022, 204, 115212. [Google Scholar] [CrossRef]
- Han, Y.; Xu, S.; Ye, W.; Wang, Y.; Zhang, X.; Deng, J.; Zhang, Z.; Liu, L.; Liu, S. Targeting LSD1 suppresses stem cell-like properties and sensitizes head and neck squamous cell carcinoma to PD-1 blockade. Cell Death Dis. 2021, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Q.C.; Li, Y.C.; Yang, L.L.; Liu, J.F.; Li, H.; Xiao, Y.; Bu, L.L.; Zhang, W.F.; Sun, Z.J. Targeting CMTM6 Suppresses Stem Cell-Like Properties and Enhances Antitumor Immunity in Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.L.; Junior, T.C.T.; Rangel, M.C. Sulforaphane: An emergent anti-cancer stem cell agent. Front. Oncol. 2023, 13, 1089115. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Chatterjee, S.; Hembram, K.C.; Sethy, C.; Mandal, M.; Kundu, C.N. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J. Nutr. Biochem. 2021, 92, 108624. [Google Scholar] [CrossRef] [PubMed]
- Amorntaveechai, A.; Osathanon, T.; Pavasant, P.; Sooampon, S. Effect of resveratrol and oxyresveratrol on deferoxamine-induced cancer stem cell marker expression in human head and neck squamous cell carcinoma. J. Oral Biol. Craniofac. Res. 2022, 12, 253–257. [Google Scholar] [CrossRef]
- Lin, C.S.; Bamodu, O.A.; Kuo, K.T.; Huang, C.M.; Liu, S.C.; Wang, C.H.; Tzeng, Y.M.; Chao, T.Y.; Yeh, C.T. Investigation of ovatodiolide, a macrocyclic diterpenoid, as a potential inhibitor of oral cancer stem-like cells properties via the inhibition of the JAK2/STAT3/JARID1B signal circuit. Phytomedicine 2018, 46, 93–103. [Google Scholar] [CrossRef]
- de Camargo, M.R.; Frazon, T.F.; Inacio, K.K.; Smiderle, F.R.; Amor, N.G.; Dionisio, T.J.; Santos, C.F.; Rodini, C.O.; Lara, V.S. Ganoderma lucidum polysaccharides inhibit in vitro tumorigenesis, cancer stem cell properties and epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Ethnopharmacol. 2022, 286, 114891. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chao, S.C.; Hsieh, P.L.; Liao, Y.W.; Chu, P.M.; Harn, H.J.; Yu, C.C. Butylidenephthalide Abrogates the Snail-Induced Cancer Stemness in Oral Carcinomas. Int. J. Mol. Sci. 2022, 23, 6157. [Google Scholar] [CrossRef]
- Patel, H.; Joshi, J.; Raval, A.; Shah, F. Identification of Natural Compounds to Inhibit Sonic Hedgehog Pathway in Oral Cancer. Anticancer Agents Med. Chem. 2022, 22, 905–913. [Google Scholar] [PubMed]
- Chang, M.T.; Lee, S.P.; Fang, C.Y.; Hsieh, P.L.; Liao, Y.W.; Lu, M.Y.; Tsai, L.L.; Yu, C.C.; Liu, C.M. Chemosensitizing effect of honokiol in oral carcinoma stem cells via regulation of IL-6/Stat3 signaling. Environ. Toxicol. 2018, 33, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.; Yu, C.C.; Huang, C.C.; Liao, Y.W.; Hsieh, P.L.; Chu, P.M.; Yu, C.H.; Lin, S.S. Magnolol inhibits cancer stemness and IL-6/Stat3 signaling in oral carcinomas. J. Formos. Med. Assoc. 2022, 121 Pt 1, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Y.; Hsieh, P.L.; Wang, T.H.; Yu, C.C.; Lu, M.Y.; Liao, Y.W.; Lee, T.H.; Peng, C.Y. Andrographolide impedes cancer stemness and enhances radio-sensitivity in oral carcinomas via miR-218 activation. Oncotarget 2017, 8, 4196–4207. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.K.; Shih, P.H.; Lee, W.H.; Bamodu, O.A.; Wu, A.T.H.; Huang, C.C.; Tzeng, Y.M.; Hsiao, M.; Yeh, C.T.; Lin, C.M. Antrodia cinnamomea sensitizes radio-/chemo-therapy of cancer stem-like cells by modulating microRNA expression. J. Ethnopharmacol. 2017, 207, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kantapan, J.; Dechsupa, N.; Tippanya, D.; Nobnop, W.; Chitapanarux, I. Gallotannin from Bouea macrophylla Seed Extract Suppresses Cancer Stem-like Cells and Radiosensitizes Head and Neck Cancer. Int. J. Mol. Sci. 2021, 22, 9253. [Google Scholar] [CrossRef] [PubMed]
- Nor, F.; Nor, C.; Bento, L.W.; Zhang, Z.; Bretz, W.A.; Nor, J.E. Propolis reduces the stemness of head and neck squamous cell carcinoma. Arch. Oral Biol. 2021, 125, 105087. [Google Scholar] [CrossRef]
- Qi, W.; Zhu, F.; Wang, M.; Teng, Z.; Xu, R.; Xi, Y.; Meng, Q.; Wu, X.; Zhao, H.; Ma, M.; et al. The Antitumoral Effect of Paris Saponin II on Head and Neck Squamous Cell Carcinomas Mediated via the Nitric Oxide Metabolic Pathway. Front. Cell Dev. Biol. 2021, 9, 803981. [Google Scholar] [CrossRef]
- Ahn, K.; Ji, H.; Kim, H.E.; Cho, H.; Sun, Q.; Shi, S.; He, Y.; Kim, B.G.; Kim, O. Raphanus sativus L. seed extracts induce apoptosis and reduce migration of oral squamous cell carcinoma KB and KB(CD133+)cells by downregulation of beta-catenin. Nutr. Cancer 2020, 72, 1378–1389. [Google Scholar] [CrossRef]
- Elkashty, O.A.; Tran, S.D. Broccoli extract increases drug-mediated cytotoxicity towards cancer stem cells of head and neck squamous cell carcinoma. Br. J. Cancer 2020, 123, 1395–1403. [Google Scholar] [CrossRef]
- Farag, A.F.; Hassabou, N.F. CD24-gold nanocomposite as promising and sensitive biomarker for cancer stem cells in salivary gland tumors. Nanomedicine 2022, 46, 102598. [Google Scholar] [CrossRef] [PubMed]
- Ning, N.; Pan, Q.; Zheng, F.; Teitz-Tennenbaum, S.; Egenti, M.; Yet, J.; Li, M.; Ginestier, C.; Wicha, M.S.; Moyer, J.S.; et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012, 72, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Bowles, D.W.; Keysar, S.B.; Eagles, J.R.; Wang, G.; Glogowska, M.J.; McDermott, J.D.; Le, P.N.; Gao, D.; Ray, C.E.; Rochon, P.J.; et al. A pilot study of cetuximab and the hedgehog inhibitor IPI-926 in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016, 53, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, H.; Sauter, A.; Golze, W.; Hanft, G.; Schroen, C.; Hoermann, K.; Erhardt, T.; Gronau, S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008, 44, 823–829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).