Multi-Electron Ionization and Coulomb Explosion of the IBr Molecule in the Near-Infrared Femtosecond Laser Field
Abstract
1. Introduction
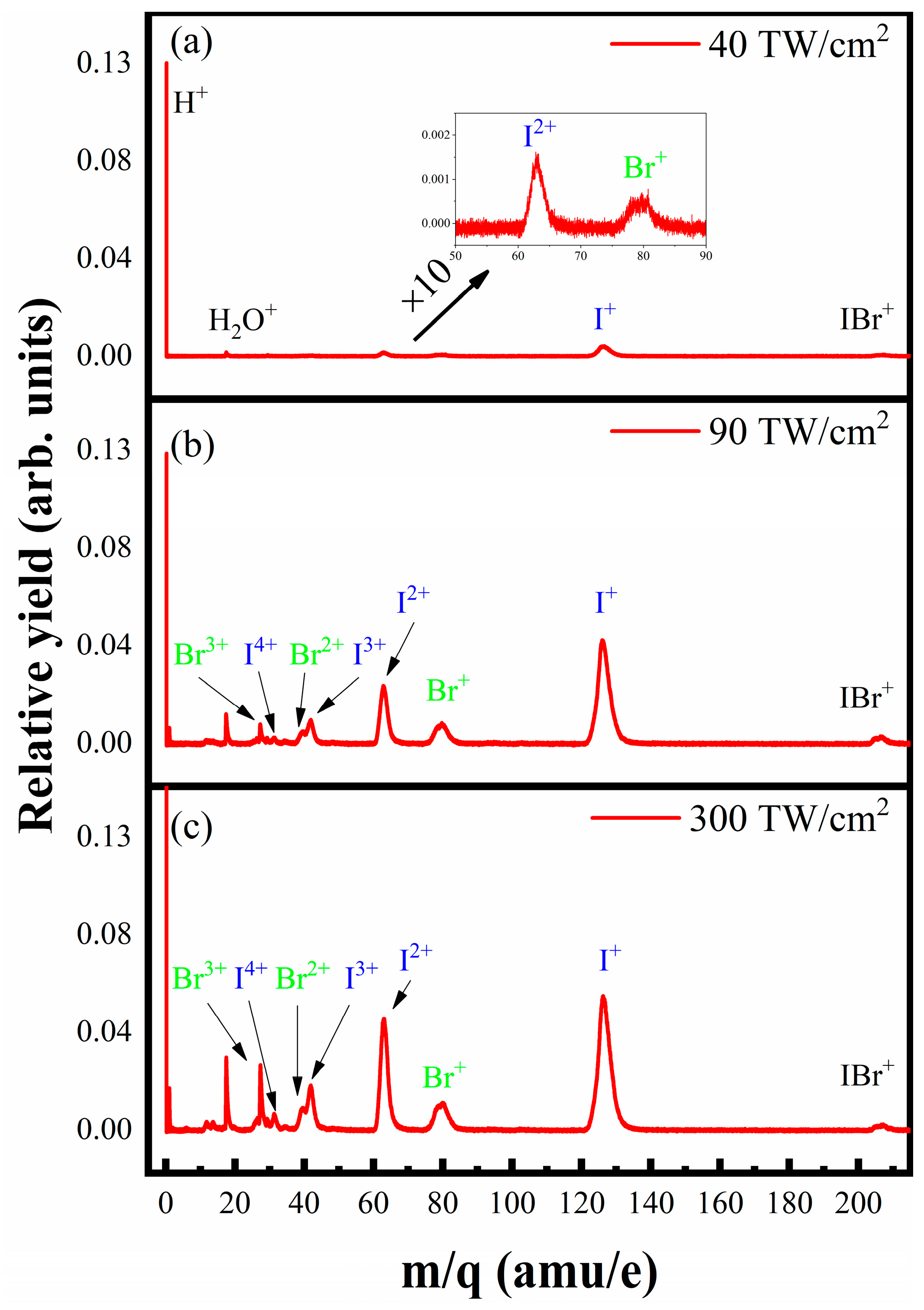
2. Experiment and Theory Calculation
3. Results
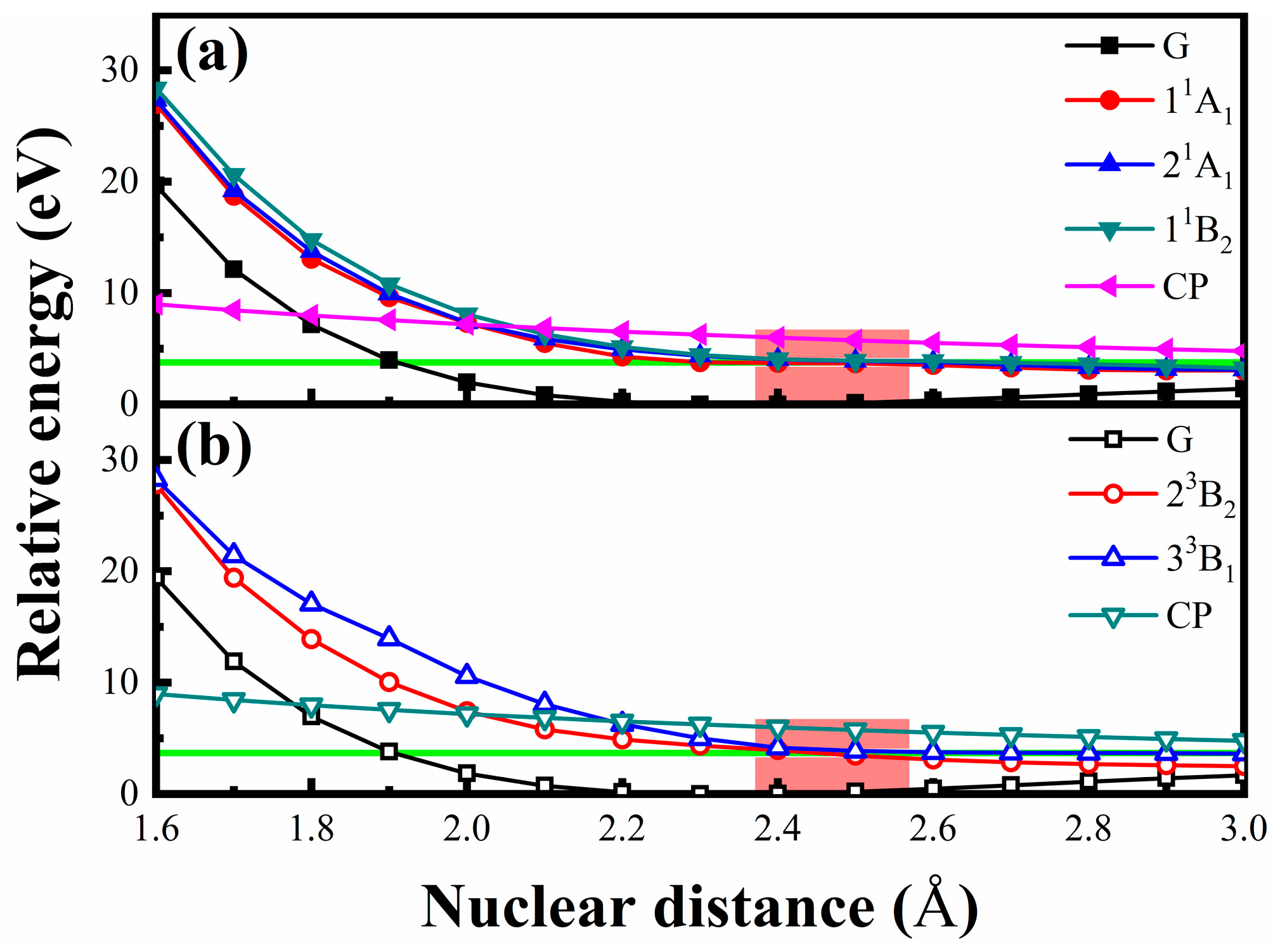
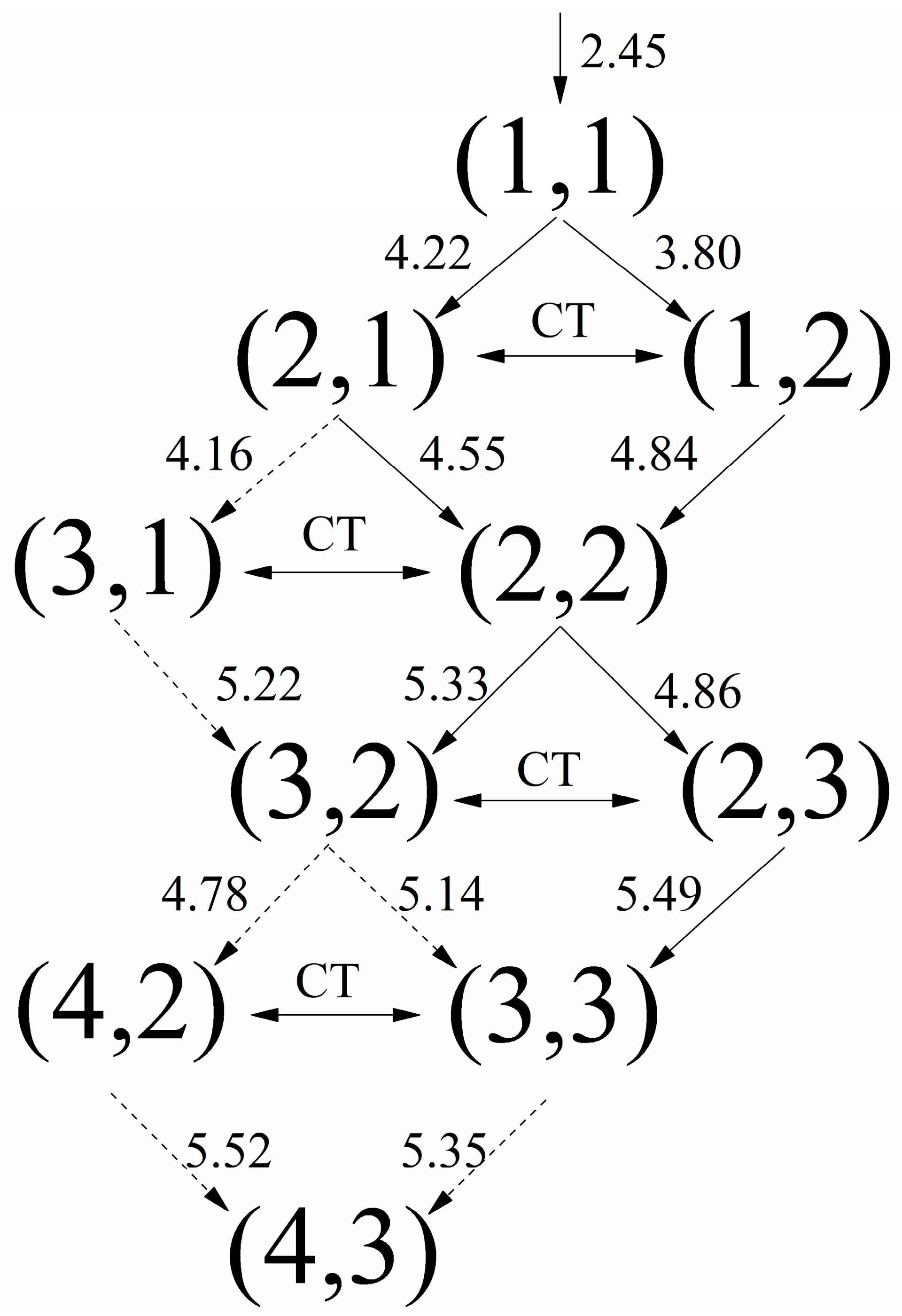
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Q.-B.; Shi, L.-K.; Zhang, K.; Kang, S.-J.; Li, Z.-F.; Wu, Y.-M.; Qin, L.-L.; Zhai, C.-Y.; Liu, A.-H.; Li, Y.-B. Electron dynamics of molecular frustrated double ionization driven by strong laser fields. Commun. Theor. Phys. 2023, 75, 035502. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Tong, A. Controlling multiple returnings in non-sequential double ionization with orthogonal two-color laser pulses. Front. Phys. 2023, 11, 1177359. [Google Scholar] [CrossRef]
- Kang, H.; Chen, S.; Chen, J.; Paulus, G.G. Frustrated double ionization of atoms in circularly polarized laser fields. New J. Phys. 2021, 23, 033041. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Yu, S.; Sun, R.; Liu, X.; Dorner-Kirchner, M.; Erattupuzha, S.; Larimian, S.; Koch, M.; Hanus, V.; et al. Laser-Induced Electron Transfer in the Dissociative Multiple Ionization of Argon Dimers. Phys. Rev. Lett. 2020, 125, 063202. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-Y.; Ben, S.; Sun, Y.; Xu, H.-F.; Liu, X.-S.; Lv, H.; Guo, J. Non-sequential double ionization of triatomic molecules OCS in intense laser fields. Chem. Phys. Lett. 2020, 747, 137326. [Google Scholar] [CrossRef]
- Li, S.; Sierra-Costa, D.; Michie, M.J.; Ben-Itzhak, I.; Dantus, M. Control of electron recollision and molecular nonsequential double ionization. Commun. Phys. 2020, 3, 35. [Google Scholar] [CrossRef]
- Kang, H.P.; Chen, S.; Chu, W.; Yao, J.P.; Chen, J.; Liu, X.J.; Cheng, Y.; Xu, Z.Z. Nonsequential double ionization of alkaline-earth metal atoms by intense mid-infrared femtosecond pulses. Opt. Express 2020, 28, 19325–19333. [Google Scholar] [CrossRef]
- Kang, H.; Chen, S.; Wang, Y.; Chu, W.; Yao, J.; Chen, J.; Liu, X.; Cheng, Y.; Xu, Z. Wavelength-dependent nonsequential double ionization of magnesium by intense femtosecond laser pulses. Phys. Rev. A 2019, 100, 033403. [Google Scholar] [CrossRef]
- Cheng, C.; Vindel-Zandbergen, P.; Matsika, S.; Weinacht, T. Electron correlation in channel-resolved strong-field molecular double ionization. Phys. Rev. A 2019, 100, 053405. [Google Scholar] [CrossRef]
- Henrichs, K.; Waitz, M.; Trinter, F.; Kim, H.; Menssen, A.; Gassert, H.; Sann, H.; Jahnke, T.; Wu, J.; Pitzer, M.; et al. Observation of Electron Energy Discretization in Strong Field Double Ionization. Phys. Rev. Lett. 2013, 111, 113003. [Google Scholar] [CrossRef]
- Beylerian, C.; Saugout, S.E.; Cornaggia, C. Non-sequential double ionization of H2 using ultrashort 10 fs laser pulses. J. Phys. B At. Mol. Opt. Phys. 2006, 39, L105–L112. [Google Scholar] [CrossRef]
- Baldit, E.; Saugout, S.; Cornaggia, C. Coulomb explosion of N2 using intense 10- and 40-fs laser pulses. Phys. Rev. A 2005, 71, 021403. [Google Scholar] [CrossRef]
- Urbain, X.; Fabre, B.; Staicu-Casagrande, E.M.; de Ruette, N.; Andrianarijaona, V.M.; Jureta, J.; Posthumus, J.H.; Saenz, A.; Baldit, E.; Cornaggia, C. Intense-Laser-Field Ionization of Molecular Hydrogen in the Tunneling Regime and Its Effect on the Vibrational Excitation of H2+. Phys. Rev. Lett. 2004, 92, 163004. [Google Scholar] [CrossRef] [PubMed]
- Cornaggia, C.; Hering, P. Nonsequential double ionization of small molecules induced by a femtosecond laser field. Phys. Rev. A 2000, 62, 023403. [Google Scholar] [CrossRef]
- Cornaggia, C. Large-amplitude nuclear motions in the laser-induced Coulomb explosion of carbon dioxide molecules. Phys. Rev. A 1996, 54, R2555–R2558. [Google Scholar] [CrossRef] [PubMed]
- Cornaggia, C.; Schmidt, M.; Normand, D. Laser-induced nuclear motions in the Coulomb explosion of C2H2+ ions. Phys. Rev. A 1995, 51, 1431–1437. [Google Scholar] [CrossRef]
- Cornaggia, C.; Lavancier, J.; Normand, D.; Morellec, J.; Liu, H.X. Intensity dependence of the multielectron dissociative ionization of N2 at 305 and 610 nm. Phys. Rev. A 1990, 42, 5464–5472. [Google Scholar] [CrossRef]
- Cornaggia, C.; Lavancier, J.; Normand, D.; Morellec, J.; Agostini, P.; Chambaret, J.P.; Antonetti, A. Multielectron dissociative ionization of diatomic molecules in an intense femtosecond laser field. Phys. Rev. A 1991, 44, 4499–4505. [Google Scholar] [CrossRef]
- Keldysh, L. Ionization in the field of a strong electromagnetic wave. Sov. Phys. JETP 1965, 20, 1307–1314. [Google Scholar]
- DeWitt, M.J.; Levis, R.J. Observing the transition from a multiphoton-dominated to a field-mediated ionization process for polyatomic molecules in intense laser fields. Phys. Rev. Lett. 1998, 81, 5101–5104. [Google Scholar] [CrossRef]
- Hatherly, P.A.; Stankiewicz, M.; Codling, K.; Frasinski, L.J.; Cross, G.M. The multielectron dissociative ionization of molecular iodine in intense laser fields. J. Phys. B At. Mol. Opt. Phys. 1994, 27, 2993. [Google Scholar] [CrossRef]
- Schmidt, M.; Normand, D.; Cornaggia, C. Laser-induced trapping of chlorine molecules with pico- and femtosecond pulses. Phys. Rev. A 1994, 50, 5037–5045. [Google Scholar] [CrossRef]
- Chelkowski, S.; Bandrauk, A.D. Two-step Coulomb explosions of diatoms in intense laser fields. J. Phys. B At. Mol. Opt. Phys. 1995, 28, L723. [Google Scholar] [CrossRef]
- Zuo, T.; Bandrauk, A.D. Charge-resonance-enhanced ionization of diatomic molecular ions by intense lasers. Phys. Rev. A 1995, 52, R2511–R2514. [Google Scholar] [CrossRef]
- Nibarger, J.P.; Menon, S.V.; Gibson, G.N. Comprehensive analysis of strong-field ionization and dissociation of diatomic nitrogen. Phys. Rev. A 2001, 63, 053406. [Google Scholar] [CrossRef]
- Boyer, K.; Luk, T.S.; Solem, J.C.; Rhodes, C.K. Kinetic energy distributions of ionic fragments produced by subpicosecond multiphoton ionization of N2. Phys. Rev. A 1989, 39, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.N.; Li, M.; Guo, C.; Nibarger, J.P. Direct evidence of the generality of charge-asymmetric dissociation of molecular iodine ionized by strong laser fields. Phys. Rev. A 1998, 58, 4723–4727. [Google Scholar] [CrossRef]
- Pavičić, D.; Kiess, A.; Hänsch, T.W.; Figger, H. Intense-Laser-Field Ionization of the Hydrogen Molecular Ions H2+ and D2+ at Critical Internuclear Distances. Phys. Rev. Lett. 2005, 94, 163002. [Google Scholar] [CrossRef] [PubMed]
- Cornaggia, C. Electronic dynamics of charge resonance enhanced ionization probed by laser-induced alignment in C2H2. J. Phys. B At. Mol. Opt. Phys. 2016, 49, 19LT01. [Google Scholar] [CrossRef]
- Cornaggia, C. Statistical analysis of fragmentation channels of small multicharged molecular ions. J. Phys. B At. Mol. Opt. Phys. 2012, 45, 085602. [Google Scholar] [CrossRef]
- Eremina, E.; Liu, X.; Rottke, H.; Sandner, W.; Schatzel, M.G.; Dreischuh, A.; Paulus, G.G.; Walther, H.; Moshammer, R.; Ullrich, J. Influence of molecular structure on double ionization of N2 and O2 by high intensity ultrashort laser pulses. Phys. Rev. Lett. 2004, 92, 173001. [Google Scholar] [CrossRef]
- Hussain, A.N.; Roberts, G. Wave packet dynamics of IBr predissociation. J. Chem. Phys. 1999, 110, 2474–2488. [Google Scholar] [CrossRef]
- Ohmura, H.; Nakanaga, T.; Arakawa, H.; Tachiya, M. The interference effects induced by two-color excitation in the photodissociation of IBr. Chem. Phys. Lett. 2002, 363, 559–566. [Google Scholar] [CrossRef]
- Lu, J.; Shao, F.-W.; Fan, K.-N. Coherent control of the photodissociation of CH3I and IBr. Chem. Phys. Lett. 2000, 329, 461–468. [Google Scholar] [CrossRef]
- Ohmura, H.; Nakanaga, T.; Tachiya, M. Coherent control of photofragment separation in the dissociative ionization of IBr. Phys. Rev. Lett. 2004, 92, 113002. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Yang, Y. Multi-ionization of the Cl2 molecule in the near-infrared femtosecond laser field. RSC Adv. 2020, 10, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Li, Z.; Sun, H.; Zhang, S.; Sun, Z. Channel-resolved multiorbital double ionization of molecular Cl2 in an intense femtosecond laser field. Phys. Rev. A 2018, 98, 043402. [Google Scholar] [CrossRef]
- Guo, C.L.; Li, M.; Gibson, G.N. Charge asymmetric dissociation induced by sequential and nonsequential strong field ionization. Phys. Rev. Lett. 1999, 82, 2492–2495. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D. 01; Gaussian Inc.: Wallingford, UK, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Stanton, J.F.; Bartlett, R.J. The equation of motion coupled-cluster method. A systematic biorthogonal approach to molecular excitation energies, transition probabilities, and excited state properties. J. Chem. Phys. 1993, 98, 7029–7039. [Google Scholar] [CrossRef]
- Kállay, M.; Gauss, J. Calculation of excited-state properties using general coupled-cluster and configuration-interaction models. J. Chem. Phys. 2004, 121, 9257–9269. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, L.; Sun, S.; Zhang, J.; Chen, Y.; Zhang, S.; Jia, T.; Sun, Z. Dissociative double ionization of 1-bromo-2-chloroethane irradiated by an intense femtosecond laser field. J. Chem. Phys. 2011, 135, 064303. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, Y.; Wu, Z.; Chen, B.; Dong, H.; Liu, X.; Deng, Y.; Liu, H.; Liu, Y.; Gong, Q. Coulomb explosion of nitrogen and oxygen molecules through non-Coulombic states. Phys. Chem. Chem. Phys. 2011, 13, 18398. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Sheehy, B.; DiMauro, L.F.; Agostini, P.; Schafer, K.J.; Kulander, K.C. Precision Measurement of Strong Field Double Ionization of Helium. Phys. Rev. Lett. 1994, 73, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Nibarger, J.P.; Gibson, G.N. A framework for understanding molecular ionization in strong laser fields. J. Phys. B At. Mol. Opt. Phys. 2002, 35, 2961–2974. [Google Scholar] [CrossRef][Green Version]
- Kawata, I.; Kono, H.; Fujimura, Y.; Bandrauk, A.D. Intense-laser-field-enhanced ionization of two-electron molecules: Role of ionic states as doorway states. Phys. Rev. A 2000, 62, 031401. [Google Scholar] [CrossRef]
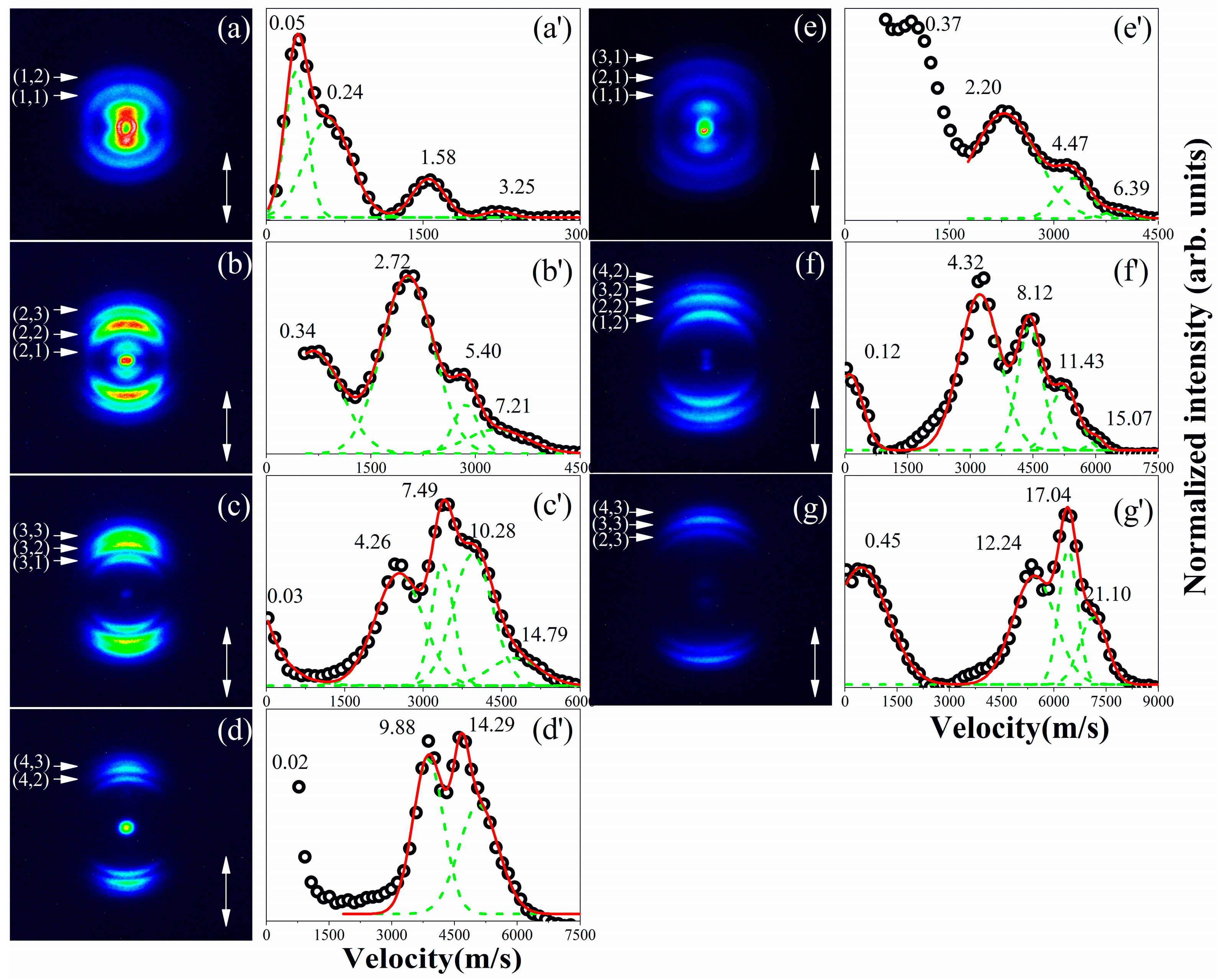
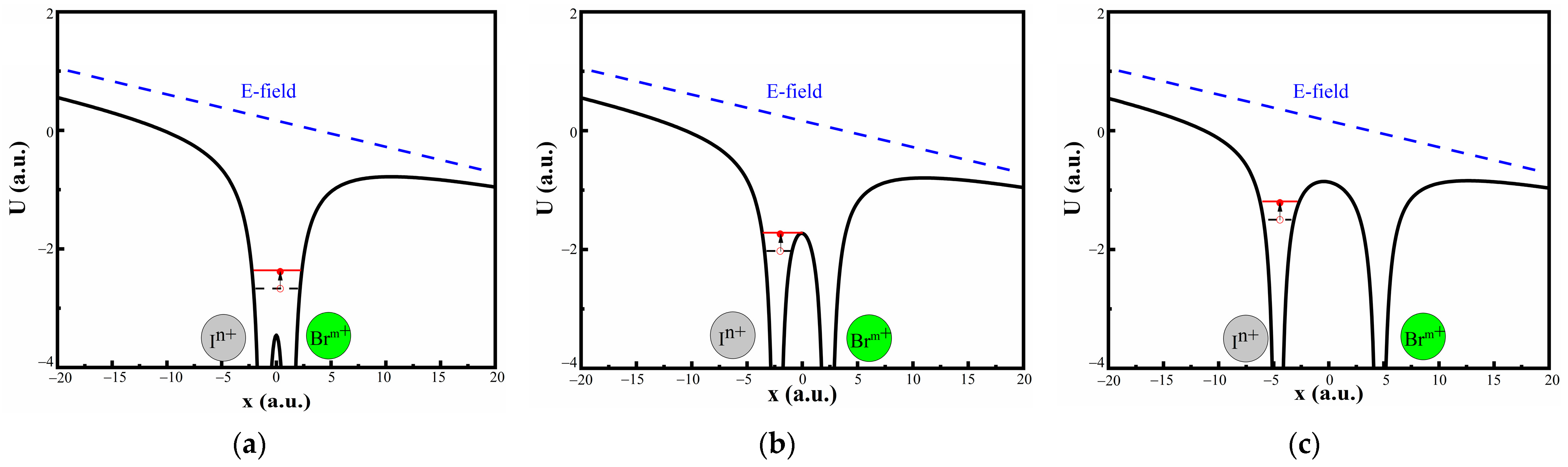
| (n, m) | CE Channels | KER_In+ (eV) | KER_Bm+ (eV) | KER_total (eV) | Rc (Å) |
|---|---|---|---|---|---|
| (1, 1) | IBr2+→I+ + Br+ | 1.58 | 2.20 | 3.78 | 3.81 |
| (1, 2) | IBr3+→I+ + Br2+ | 3.25 | 4.32 | 7.57 | 3.80 |
| (2, 1) | IBr3+→I2+ + Br+ | 2.72 | 4.47 | 7.19 | 4.01 |
| (2, 2) | IBr4+→I2+ + Br2+ | 5.40 | 8.12 | 13.52 | 4.26 |
| (2, 3) | IBr5+→I2+ + Br3+ | 7.21 | 12.24 | 19.45 | 4.44 |
| (3, 1) | IBr4+→I3+ + Br+ | 4.26 | 6.39 | 10.65 | 4.06 |
| (3, 2) | IBr5+→I3+ + Br2+ | 7.49 | 11.43 | 18.92 | 4.57 |
| (3, 3) | IBr6+→I3+ + Br3+ | 10.28 | 17.04 | 27.32 | 4.74 |
| (4, 2) | IBr6+→I4+ + Br2+ | 9.88 | 15.07 | 24.95 | 4.62 |
| (4, 3) | IBr7+→I4+ + Br3+ | 14.29 | 21.10 | 35.39 | 4.88 |
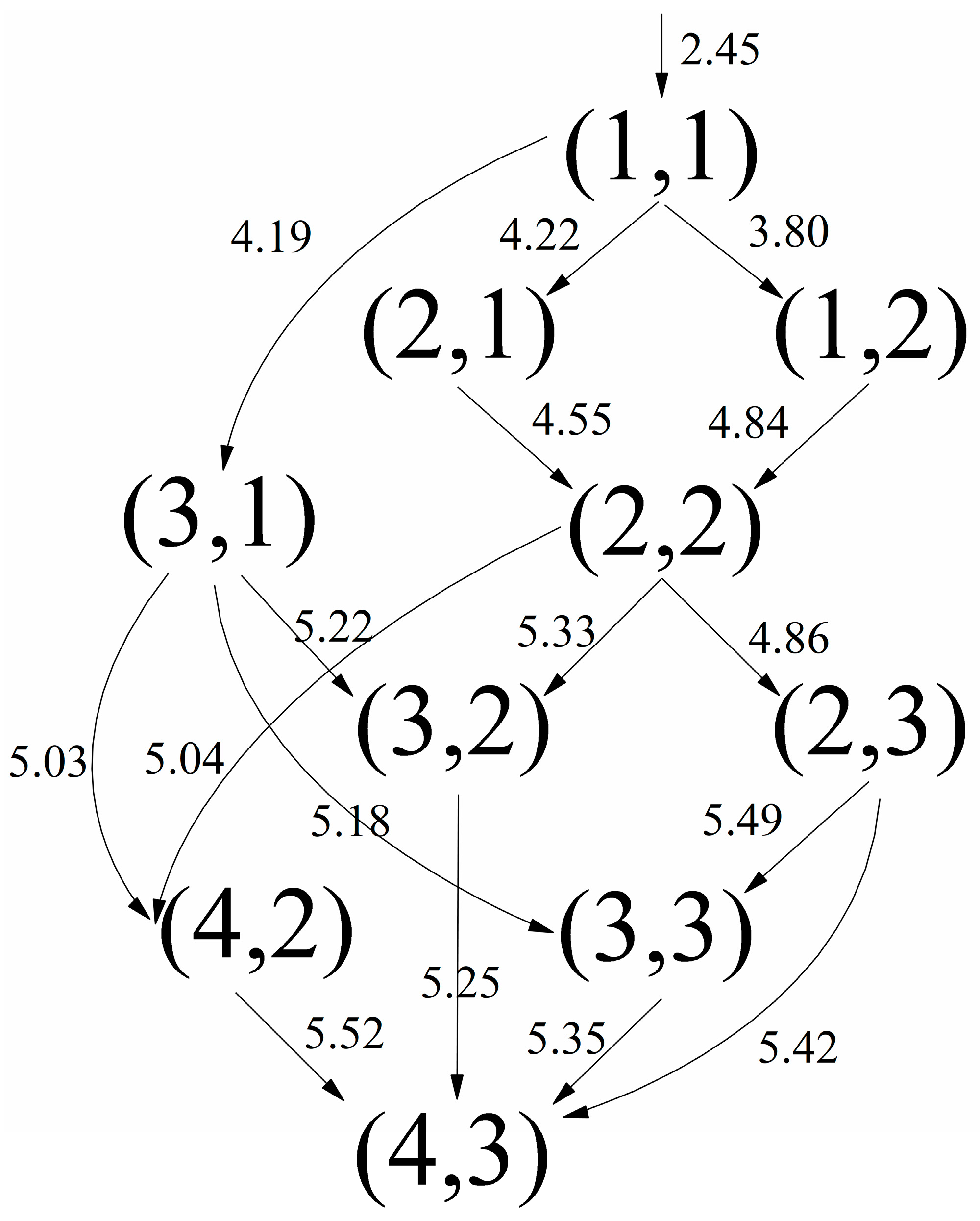
| Process | Channel | Ri,j (Å) | Tij→nm (fs) |
|---|---|---|---|
| (a) | (1, 1)→(1, 2) | 3.80 | 17.55 |
| (1, 2)→(2, 2) | 4.84 | 16.25 | |
| (2, 2)→(2, 3) | 4.86 | 7.28 | |
| (2, 3)→(3, 3) | 5.49 | 9.13 | |
| (3, 3)→(4, 3) | 5.35 | / | |
| (b) | (1, 1)→(1, 2) | 3.80 | 17.55 |
| (1, 2)→(2, 2) | 4.84 | 16.25 | |
| (2, 2)→(3, 2) | 5.33 | 11.73 | |
| (3, 2)→(3, 3) | 5.14 | / | |
| (c) | (1, 1)→(2, 1) | 4.22 | 20.55 |
| (2, 1)→(2, 2) | 4.55 | 9.86 | |
| (2, 2)→(2, 3) | 4.86 | 7.28 | |
| (2, 3)→(3, 3) | 5.49 | 9.13 | |
| (3, 3)→(4, 3) | 5.35 | / | |
| (d) | (1, 1)→(2, 1) | 4.22 | 20.55 |
| (2, 1)→(2, 2) | 4.55 | 9.86 | |
| (2, 2)→(3, 2) | 5.33 | 11.73 | |
| (3, 2)→(3, 3) | 5.14 | / | |
| (e) | (1, 1)→(2, 1) | 4.22 | 20.55 |
| (2, 1)→(3, 1) | 4.16 | / |
| Channels | Ri,j (Å) | 50 TW/cm2 | 100 TW/cm2 | 150 TW/cm2 | 200 TW/cm2 | 250 TW/cm2 | 300 TW/cm2 |
|---|---|---|---|---|---|---|---|
| (1, 1)→(1, 2)↔(2, 1) | 3.80 | 3.43 | 3.19 | 2.94 | 2.82 | 2.82 | 2.70 |
| (1, 1)→(2, 1)↔(1, 2) | 4.22 | 3.68 | 3.31 | 3.19 | 3.06 | 2.94 | 2.82 |
| (1, 2)→(2, 2)↔(3, 1) * | 4.84 | 3.68 | 3.68 | 3.68 | 3.68 | 3.68 | 3.68 |
| (2, 1)→(2, 2)↔(3, 1) * | 4.55 | 3.68 | 3.68 | 3.68 | 3.68 | 3.68 | 3.68 |
| (2, 2)→(3, 2)↔(2, 3) | 5.33 | 4.17 | 3.92 | 3.67 | 3.55 | 3.43 | 3.43 |
| (2, 2)→(2, 3)↔(3, 2) | 4.86 | 3.80 | 3.55 | 3.43 | 3.31 | 3.31 | 3.18 |
| (2, 3)→(3, 3)↔(4, 2) * | 5.49 | 3.80 | 3.80 | 3.80 | 3.80 | 3.80 | 3.80 |
| Process | Channel | Ri,j (Å) | Tij→nm (fs) |
|---|---|---|---|
| (f) | (1, 1)→(1, 2) | 3.80 | 17.55 |
| (1, 2)→(2, 2) | 4.84 | 16.25 | |
| (2, 2)→(2, 3) | 4.86 | 7.28 | |
| (2, 3)→(4, 3) | 5.42 | 8.59 | |
| (g) | (1, 1)→(1, 2) | 3.80 | 17.55 |
| (1, 2)→(2, 2) | 4.84 | 16.25 | |
| (2, 2)→(4, 2) | 5.04 | 9.21 | |
| (4, 2)→(4, 3) | 5.52 | 8.47 | |
| (h) | (1, 1)→(3, 1) | 4.19 | 20.34 |
| (3, 1)→(3, 2) | 5.22 | 14.62 | |
| (3, 2)→(4, 3) | 5.25 | 2.10 | |
| (i) | (1, 1)→(3, 1) | 4.19 | 20.34 |
| (3, 1)→(3, 3) | 5.18 | 14.32 | |
| (3, 3)→(4, 3) | 5.35 | 4.49 | |
| (j) | (1, 1)→(3, 1) | 4.19 | 20.34 |
| (3, 1)→(4, 2) | 5.03 | 13.16 | |
| (4, 2)→(4, 3) | 5.52 | 8.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Li, Z.; Sun, Z.; Yang, Y. Multi-Electron Ionization and Coulomb Explosion of the IBr Molecule in the Near-Infrared Femtosecond Laser Field. Appl. Sci. 2023, 13, 13185. https://doi.org/10.3390/app132413185
Liu B, Li Z, Sun Z, Yang Y. Multi-Electron Ionization and Coulomb Explosion of the IBr Molecule in the Near-Infrared Femtosecond Laser Field. Applied Sciences. 2023; 13(24):13185. https://doi.org/10.3390/app132413185
Chicago/Turabian StyleLiu, Botong, Zhipeng Li, Zhenrong Sun, and Yan Yang. 2023. "Multi-Electron Ionization and Coulomb Explosion of the IBr Molecule in the Near-Infrared Femtosecond Laser Field" Applied Sciences 13, no. 24: 13185. https://doi.org/10.3390/app132413185
APA StyleLiu, B., Li, Z., Sun, Z., & Yang, Y. (2023). Multi-Electron Ionization and Coulomb Explosion of the IBr Molecule in the Near-Infrared Femtosecond Laser Field. Applied Sciences, 13(24), 13185. https://doi.org/10.3390/app132413185






