Abstract
We aimed to investigate the acute effects of blood flow restriction (BFR) combined with neuromuscular electrical stimulation (NMES) on muscle strength, thigh circumference, and knee joint reposition sense in female amateur football players, as well as to determine whether this procedure is safe. Methods: This was a pilot trial. Twenty female amateur football players were randomized into two groups: group 1 (n = 10) received a single NMES session; group 2 (n = 10) received NMES + BFR. The measured variables included maximal voluntary eccentric contraction (MVEC) and maximal voluntary concentric contraction (MVCC), thigh circumference, and knee joint reposition sense test. The type of electrical current used was TENS (symmetrical biphasic rectangular pulse, 350 μs, and 50 Hz), combined simultaneously with active knee extension (75 repetitions in 4 sets, 20% MVCC, 30 s rest between sets), for both groups. Group 2 had BFR added (80% of arterial occlusion pressure). Results: Statistically significant differences (p ≤ 0.05) were obtained for thigh circumference in both groups. The comparison between groups did not show statistically significant differences (p ≤ 0.05) in MVEC, MVCC, thigh circumference, or the knee joint reposition sense test. Conclusions: Both the isolated NMES intervention and its combination with BFR induced immediate changes in thigh circumference without impairing the muscle strength or proprioceptive ability of the football players. However, these results should be interpreted with caution, and future studies including a control group and isolated BFR application are needed.
1. Introduction
Blood flow restriction (BFR) is defined as a novel training method based on the partial occlusion of arterial flow and complete occlusion of venous flow [1]. BFR is performed using pneumatic cuffs applied to the proximal region of the limb. These cuffs apply gradual mechanical compression that favors the occlusion of blood flow, preventing venous return in the targeted muscle [2,3].
From a physiological perspective, pneumatic compression generates hypoxia, increasing capillary blood flow and the possibility of erythema. It can also cause cellular swelling due to the accumulation of metabolites and increased water content in the sarcoplasm, which stimulates protein synthesis and growth hormone release [4]. Previous studies have also reported an increase in arterial distensibility, similar to that seen in high-load training [5,6,7]. This fact explains the muscle hypertrophy observed in young, healthy subjects [8,9] and in subjects with musculoskeletal [3,10,11] or neurological diseases [12].
In addition, the effects of BFR on the training of athletes have been studied, showing significant gains in strength, muscle hypertrophy, and performance enhancement [13,14]. However, the majority of these studies are applied to male athletes, with limited research conducted on female football players [13]. Additionally, BFR training is often combined with low-intensity resistance exercises [15,16], as this is considered a safe technique when performed according to the protocols recommended in the scientific literature [17]. However, there is a knowledge gap regarding its application combined with other techniques.
In this context, there has been a growing interest in analyzing the effects of combining BFR with other muscle strength training methods, such as neuromuscular electrical stimulation (NMES) [8,9]. This electrotherapeutic procedure consists of the ability to recruit motor units through motoneuron depolarization, resulting in the electrical excitation of muscle fibers [18,19]. Isolated NMES has traditionally been used as a reliable and safe technique to improve performance in healthy athletes [13,14] and to prevent muscle atrophy during periods of immobilization in injured football players [20,21]. However, there are no studies that evaluate the acute effects on muscle strength and volume when combined with BFR in women. Most studies aiming to increase strength in healthy subjects apply an occlusion rate of 80% in the BFR [15,16]. Nevertheless, when this percentage is combined with NMES, strength losses attributable to muscle fatigue have been observed [22]. Consequently, the hypothesis of this study suggests that the combined application of these techniques in female amateur footballers could induce immediate changes in strength.
Therefore, the aim of this study was to analyze whether there would be significant changes in the immediate effects on the quadriceps muscle derived from a NMES procedure, either alone or in combination with BFR, in amateur football players in order to determine whether this procedure is safe.
2. Materials and Methods
2.1. Design
A controlled and randomized pilot study was conducted, with a 1:1 allocation ratio, on the study groups. The present study adhered to the ethical guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of the University of Extremadura under reference number 181/2023. It was also registered in ClinicalTrials.gov under the code NCT05924698.
2.2. Participants
A sample of 30 female amateur 11-a-side football players voluntarily participated in the study. The inclusion criteria were: (i) active federation membership in an 11-a-side football team for at least the last two seasons; (ii) age between 16 and 28 years; and (iii) physically active players at the time of the study, regularly practice for at least 5 h per week during the last 2 months. Individuals with medically diagnosed injuries classified according to the Munich Consensus [23] were excluded, as were those with systemic lupus erythematosus, hemophilia, uncontrolled hypertension, a history of pulmonary thromboembolism or stroke, blood clotting disorders, current contraceptive use, nerve root lesion, or previous surgery related to the circulatory system. After applying the inclusion and exclusion criteria, the final sample consisted of 20 participants (Figure 1). All participants maintained a fatigue level of less than 6 points according to the Borg Fatigue Index [24].
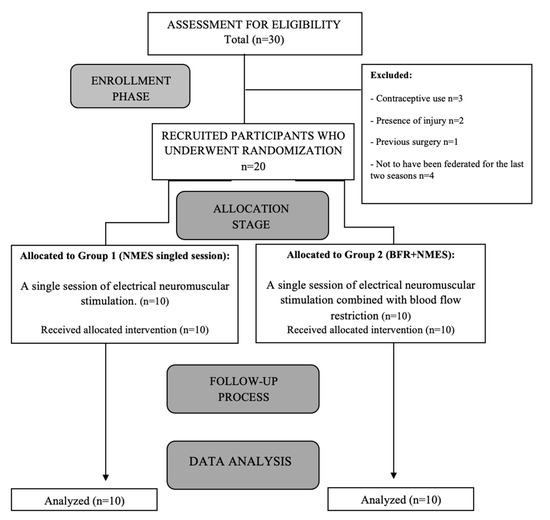
Figure 1.
Flow-chart diagram of the progress of patients through the study phases.
2.3. Randomization
Participants were randomized (1:1) into two groups: Group 1, NMES single session (n = 10 participants), and Group 2, NMES + BFR (n = 10 participants). Randomization was carried out by a simple system in which participants randomly withdrew a number from an opaque envelope. An investigator, who was aware of the study design, was in charge of enrolment and group allocation. Participants in both groups were assessed at the beginning of the session and immediately afterwards. Both the statistician and the physiotherapist performing the assessment were blinded.
2.4. Procedure
Recruitment was conducted at a private sports-medical center between September 2022 and January 2023. Prior to data collection, all participants were informed of the study procedures. All data were collected in a quiet room at a temperature of 23 °C, with participants wearing comfortable clothing (t-shirt and shorts). Height and weight were measured using a standard scale and stadiometer (Seca 285, Seca, Birmingham, UK). Both the assessment tests and the procedure were performed on the dominant lower limb [8], in an identical manner, between assessments.
2.5. Outcome Measures
2.5.1. Muscle Strength
The maximal voluntary eccentric contraction (MVEC) and the maximal voluntary concentric contraction (MVCC) of each dominant lower limb were assessed using the Kineo® isokinetic dynamometer (Globus, Codognè, Italy). Each participant sat in an upright position with their hips flexed at 80°, with the position secured by straps around the pelvis. The medial condyle of the femur was aligned with the machine’s axis of rotation, and a padded lever arm was placed on the tibia just above the anterior part of the ankle joint. To calculate the MVEC, the test started from a 60° knee extension angle. The protocol included five maximal eccentric contractions with 60 s of recovery between trials. After a 2 min rest period, the MVCC was calculated. To accomplish this, participants applied maximal concentric force against the leg pad, starting from a 60° knee flexion position [25]. Participants were verbally instructed to perform the test with maximum force. The recorded data were obtained from the mean value of the five maximal eccentric and concentric contractions.
Isokinetic dynamometry has reported a high intra-class correlation coefficient (ICC) for maximal concentric (0.95–0.96) and eccentric (0.95–0.97) strength measurement in the quadriceps [26]. The standard error of measurement (SEM) has been established as 4% [27].
The intervention programs for both groups were then set at 20% of MVCC, according to previous studies [15,16].
2.5.2. Thigh Circumference
Muscle volume was assessed using thigh circumference, measured with a tape measure. This method has previously been applied in female athletes [28]. Anatomically, the upper pole of the patella was selected as the reference point, and four segments were marked at distances of 5, 10, 15, and 20 cm from this point [29]. Subsequently, by placing the starting point of the measuring tape at the mark of the specific segment, the thigh was encircled while keeping the starting point fixed, coinciding with the measurement as it reached the endpoint. This measurement was taken before the start of the quadriceps exercise, during the knee extension exercise, and after a 15 min rest period at the end of the procedure.
The intra-observer reliability of this measurement tool has been reported to be greater than 0.9 [29]. The SEM was established at 0.46 cm, with a coefficient of variation (CV) of 9.73% for thigh circumference [30].
2.5.3. Knee Joint Position Sense Test
Static proprioception is assessed by evaluating the ability to perceive and reproduce a previously presented and passively explained joint angle.
Before the assessment, four markers were applied to the skin with adhesive tape over the greater trochanter, the iliotibial band, the head of the fibula, and the peroneal malleolus [31]. Each pair of markers represented the axis of the thigh and leg. To record the joint positions, the same camera, mounted on a tripod, was always used. It was placed in the sagittal plane in front of the knee joint, seven meters from the participant.
To begin, the participants were seated on an examination table, wearing shorts and with their legs hanging, but not touching the floor. Their eyes were blindfolded to eliminate any visual feedback. Starting with a knee flexion of 90°, as measured by a goniometer, the examiner slowly moved the leg from the initial position to the test position, with an angle of 120° of flexion. Subsequently, the player maintained this position for five seconds to memorize it. Then, the examiner instructed the player to actively reposition the leg to the initial position and immediately actively extend the knee in an attempt to reproduce the test position with the same limb. The subject then returned to the perceived test position and signaled this to the examiner. This position was held for five seconds, and upon the command “return,” the subject returned to the initial position and repeated the repositioning two more times. In this way, subjects performed three repetitions to reproduce the passively positioned knee angle. This protocol was repeated, starting from an initial position of 135°. Finally, each player was placed in prone position and the process was repeated, obtaining two variables per position (starting from 90° and 135°), defined as the proprioceptive error in angles. The angles were photographed and measured using the SAPO® computer program. The SAPO® software version 0.69 has excellent reliability (ICC = 0.96) when used to assess knee flexion angles [32].
A previous study on football players showed an ICC of 0.91, an SEM of 0.42°, and a minimum detectable change (MDC) of 1.16° for this method of assessing knee joint repositioning ability [31].
2.6. Intervention
The interventions took place in a room adjacent to the measurement site, which was set up and equipped for supervised exercise training for all participants. For both groups, the participants were placed in the supine position, with the knee resting on a wedge with 25° of flexion. The exercise consisted of performing active knee extension simultaneously with the muscle contraction induced by the passage of the electric co-current.
Group 1 (n = 10) received an intervention consisting of NMES (symmetric biphasic pulse, bipolar mode, 50 Hz frequency, 350 μs pulse duration, 1:1 ratio) (S82®; Enraf-Nonius BV, Rotterdam, The Netherlands). A duty cycle of 50% was used according to previous studies [33]. Self-adhesive electrodes (8 × 5 cm2 Pals Platinum©, Axelgaard Manufacturing Co. Ltd., Fallbrook, CA, USA) were placed on the quadriceps muscle. The positive electrodes were placed distally on the rectus femoris and vastus lateralis, and the negative electrodes were placed proximally on the rectus femoris and vastus medialis. The overcurrent program consisted of 8 s (s) of work (ON-phase) and 8 s of rest (OFF-phase). The ON-phase overcurrent program was designed with a rise time of 2 s, a maintenance time of 4 s, and a fall time of 2 s. The intensity of the electric current was increased to the maximum perceived tolerance, achieving a clear contraction without inducing fatigue. The instructions were: “When you begin to feel the electric current with a perception of muscle contraction, it is time to start the exercise. You must maintain the position during the 4 s that the current pulse lasts.” Following the protocol established in previous studies [15,16], work was performed at 20% of MVCC in four sets of 30-15-15-15 repetitions, with 30 s of rest between them (Table 1). The load was applied by strap weights.

Table 1.
Dosage guidelines for both interventions according to the CERT statement.
Group 2 (n = 10) received the same intervention, combined simultaneously with the BFR therapy protocol [34]. A PTS ii portable tourniquet system (Delphi Medical, Vancouver, BC, Canada), along with an Easy-Fit tourniquet cuff of a specific size corresponding to the patient’s thigh, was used. The total limb occlusion pressure (LOP) was achieved using the Delphi PTS system [35]. As is recommended to achieve muscle benefits, LOP was inflated to 80% of the maximum tolerated pressure (50–300 mmHg) [36]. The cuff width was selected based on limb size and was personalized for each player [36] (Figure 2). This individualized approach avoided excessive pressure in participants with lower LOP. Moreover, this system allowed for the precise control of cuff pressure throughout the training, even when natural changes in muscle volume occurred.
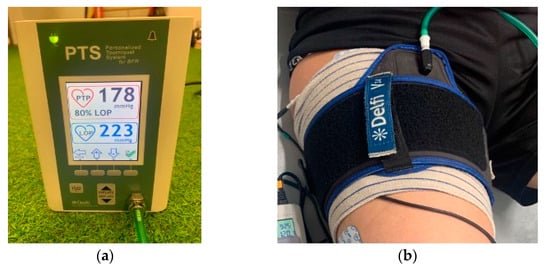
Figure 2.
(a) Detail of the BFR PTS ii system; (b) detail of the occlusion cuff.
The duration of the exercise was 8 s, with a rest period of 8 s after each repetition. The exercise program consisted of one session lasting 10 min. The interventions were conducted by a physiotherapist with 15 years of experience in exercise therapy and NMES. To minimize the risk of bias among participants, the two groups were assessed in separate rooms. None of the study participants had received any theoretical or practical training on the applied procedure prior to the intervention.
The intervention followed the recommendations highlighted by the scientific evidence regarding the use of BFR [1]. Furthermore, the interventions were conducted according to the recommendations of the Consensus on Exercise Reporting Template (CERT) [37] and the Template for Intervention Description and Replication (TIDieR) checklist [38] (Figure 3). No adverse effects were recorded in any of the intervention groups.
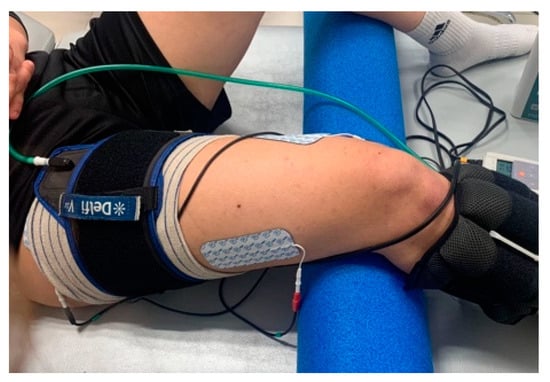
Figure 3.
Detail of the NMES procedure combined with BFR.
2.7. Sample Size
A pilot study was conducted in which two out of the four groups planned in the main trial (control, isolated NMES, isolated BFR, and NMES + BFR) were involved. G*power 3.1 software was used to calculate the sample size required to detect changes in the primary variable (thigh circumference). Calculations were based on the detection of 2.5-point differences (minimum clinically important difference, or MCID, estimated for variance in patients with neck pain of 10 points), assuming a standard deviation (SD) of 2.5 cm.
Assuming an effect size (F-test) of 0.28 for ANOVA, with repeated measures, between-group interaction differences, an alpha level of 0.05, and a power of 80%, a total sample size of four groups with two baseline and two post-intervention measurements was estimated to be 40 participants. This calculation indicated that a sample size of 10 participants per group was required for a 95% confidence interval with a power of 80%, assuming a bilateral significance level of 0.05.
2.8. Statistical Analysis
A descriptive analysis was performed for each of the variables. The normality of the variables was assessed using the Shapiro–Wilk test, which showed a normal distribution for all variables, and, therefore, parametric tests were appropriate. Data are presented as the mean ± SD. The demographic and clinical variables at baseline were compared using the chi-squared test for categorical data and the t-test for independent samples for quantitative data.
A two-way repeated measures analysis of variance (ANOVA) was conducted to analyze the interaction effects of time (at baseline and after treatment) within the two intervention groups (NMES group and NMES + BFR group). Independent and paired sample t-tests were used for between-group and within-group comparisons, respectively. In addition, the effect size was calculated using Cohen’s d coefficient, with values greater than 0.8 considered high, 0.5 considered moderate, and below 0.2 considered low [39].
The significance level was set at p < 0.05, and data analysis was performed with SPSS statistical software, version 26.0 (SPSS Inc., Chicago, IL, USA).
3. Results
Table 2 shows the baseline clinical and demographic characteristics of the participants. There were no significant differences in the analysis between groups at baseline for any of the variables (p > 0.05 for all). No adverse events were recorded.

Table 2.
Baseline characteristics of participants (mean ± standard deviation).
Table 3 shows the baseline and post-intervention scores, as well as the mean differences between and within groups for all of the variables under study. Compared to the baseline values, both the BFR + NMES group and the NMES group showed statistically significant changes in all thigh circumference variables (p < 0.05), as well as a low effect size (d ≤ 0.2). However, no statistically significant differences were found between groups in any variable (all p > 0.05).

Table 3.
Changes in baseline, post-intervention, and mean scores.
4. Discussion
The present study analyzed the effects of an isolated NMES intervention on the quadriceps muscle and its combination with BFR on parameters related to muscle performance in amateur female football players. Rehabilitation strategies for the prevention of muscle injuries in football include programs based on increasing strength as the gold standard [40].
Regarding muscle strength, there was an increasing trend in both MVEC and MVCC in the NMES group and a decreasing trend in the NMES + BFR group for both variables, although these differences were not statistically significant either within or between groups. A loss of muscle strength after intense physical exercise can affect performance, reduce the quality of training, and increase the risk of injury [41]. Our findings differ from previous studies that found improvements in quadriceps muscle strength after the simultaneous application of NMES and BFR [8,9]. These differences could be explained by the timing of the measurement. Our results correspond to immediate post-intervention effects. According to previous studies performed with BFR [14,15], at least two weeks are required to induce significant architectural changes and achieve muscular hypertrophy. Likewise, these immediate effects may be influenced by fatigue. The fact that the NMES + BFR group showed strength loss trends (Table 3) could be explained by the combination of a 50% duty cycle with vascular occlusion by BFR. This parameter has been associated with the incipient development of muscle fatigue in healthy subjects [33].
For fatigue control, the intensity of the stimulation was increased during each exercise included in the procedure in accordance with participant tolerance [42], and the load was 20% of the MVCC [33]. De Almeida-Paz et al. observed differences in fatigue induced in conditions which used different types of current (low frequency vs. medium frequency). In healthy athletes, when the aim is to generate more muscle work with less fatigue during training, the preferred option is NMES using a symmetric biphasic pulse [33], as applied in the present study.
Regarding muscle volume, thigh circumference showed statistically significant increases in all measurements in both groups, showing a small effect size. All changes were above the SEM. However, no significant changes were observed between groups, which could indicate that the increase was not due to an adverse reaction of muscle damage after the application of BFR [43,44]. Natsume et al. [8] observed an increase in muscle thickness after combining NMES and BFR at a low load for 2 weeks, which was lost after the same period without training. According to Yasuda et al. [15], metabolic fatigue can trigger an increase in acute cellular inflammation. These phenomena can increase the rate of muscle protein synthesis to trigger muscle hypertrophy [8]. Egner et al. [45] highlighted post-exercise muscle inflammation as a factor contributing to hypertrophy. Future studies should analyze the architectural parameters of the muscle to understand immediate changes, their relationship with muscle growth, and how long adaptations can prevail.
On the other hand, knee repositioning capacity did not show statistically significant differences either within or between groups. Salgado et al. [31] observed how the sensory acuity of the knee joint position decreases after the onset of fatigue during exercise in amateur football players. This proprioceptive reduction has been attributed to increased concentrations of metabolites and inflammatory substances in the fatigued muscles [46]. A deficit in proprioceptive acuity or joint stability could affect motor control accuracy and efficiency of movement during a game, increasing the risk of injuries [47]. The absence of immediate changes in this parameter could be beneficial for avoiding proprioceptive impairment in players.
4.1. Practical Implications
The increase in thigh circumference could indicate muscular adaptations [8]. However, the tendency for strength loss after simultaneous application of NMES + BFR leads us to recommend athletes or coaches not to use this procedure prior to or integrated into training because of the possible adverse effects on performance. Future studies should evaluate the immediate effects of combining NMES with different degrees of vascular occlusion on strength in female football players.
4.2. Study Limitations
This is an initial pilot study, in which two experimental groups were included. Future studies should include a control group and a group with isolated BFR intervention to determine its immediate effects. Also, further research with a greater number of sessions and longer follow-up periods should investigate the immediate effects of the combination of NMES and BFR.
Likewise, future studies should assess the level of fatigue derived from the procedures in this study.
5. Conclusions
Although the addition of BFR to an NMES procedure on the quadriceps muscle achieved an increase in thigh circumference, with no adverse effects on proprioception, the trend of strength loss does not lead us to recommend this combination before training or exercise.
Author Contributions
Conceptualization, L.E.-A.; methodology, L.E.-A., C.F.-M. and F.L.-M.; software, C.F.-M. and M.A.-C.; validation, I.A.-A.; formal analysis, M.A.-C.; investigation, L.E.-A., C.F.-M. and I.A.-A.; resources, L.E.-A.; data curation, I.A.-A., M.d.l.Á.C.-D. and M.A.-C.; writing—original draft preparation, L.E.-A. and C.F.-M.; writing—review and editing, C.F.-M. and M.d.l.Á.C.-D.; visualization, F.L.-M.; supervision, L.E.-A. and M.d.l.Á.C.-D.; project administration, M.A.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of the University of Extremadura (protocol code 181/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with Blood Flow Restriction: An Updated Evidence-Based Approach for Enhanced Muscular Development. Sports Med. 2015, 45, 313–325. [Google Scholar] [CrossRef]
- Wernbom, M.; Augustsson, J.; Raastad, T. Ischemic Strength Training: A Low-Load Alternative to Heavy Resistance Exercise? Scand. J. Med. Sci. Sports 2008, 18, 401–416. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Wilson, J.M.; Marín, P.J.; Zourdos, M.C.; Bemben, M.G. Low Intensity Blood Flow Restriction Training: A Meta-Analysis. Eur. J. Appl. Physiol. 2012, 112, 1849–1859. [Google Scholar] [CrossRef]
- Farup, J.; De Paoli, F.; Bjerg, K.; Riis, S.; Ringgard, S.; Vissing, K. Blood Flow Restricted and Traditional Resistance Training Performed to Fatigue Produce Equal Muscle Hypertrophy. Scand. J. Med. Sci. Sports 2015, 25, 754–763. [Google Scholar] [CrossRef]
- Saltin, B.; Rådegran, G.; Koskolou, M.D.; Roach, R.C. Skeletal Muscle Blood Flow in Humans and Its Regulation during Exercise. Acta. Physiol. Scand. 1998, 162, 421–436. [Google Scholar] [CrossRef]
- Grant Mouser, J.; Dankel, S.J.; Jessee, M.B.; Mattocks, K.T.; Buckner, S.L.; Counts, B.R.; Loenneke, J.P. A Tale of Three Cuffs: The Hemodynamics of Blood Flow Restriction. Eur. J. Appl. Physiol. 2017, 117, 1493–1499. [Google Scholar] [CrossRef]
- Credeur, D.P.; Hollis, B.C.; Welsch, M.A. Effects of Handgrip Training with Venous Restriction on Brachial Artery Vasodilation. Med. Sci. Sports Exerc. 2010, 42, 1296–1302. [Google Scholar] [CrossRef]
- Natsume, T.; Ozaki, H.; Saito, A.I.; Abe, T.; Naito, H. Effects of Electrostimulation with Blood Flow Restriction on Muscle Size and Strength. Med. Sci. Sports Exerc. 2015, 47, 2621–2627. [Google Scholar] [CrossRef]
- Slysz, J.T.; Burr, J.F. The Effects of Blood Flow Restricted Electrostimulation on Strength and Hypertrophy. J. Sport Rehabil. 2018, 27, 257–262. [Google Scholar] [CrossRef]
- Cancio, J.M.; Sgromolo, N.M.; Rhee, P.C. Blood Flow Restriction Therapy after Closed Treatment of Distal Radius Fractures. J. Wrist Surg. 2019, 8, 288–294. [Google Scholar] [CrossRef]
- McEwen, J.A.; Owens, J.G.; Jeyasurya, J. Why Is It Crucial to Use Personalized Occlusion Pressures in Blood Flow Restriction (BFR) Rehabilitation? J. Med. Biol. Eng. 2019, 39, 173–177. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Timmons, M.K.; Dolbow, D.R.; Bengel, J.; Fugate-Laus, K.C.; Michener, L.A.; Gater, D.R. Electrical Stimulation and Blood Flow Restriction Increase Wrist Extensor Cross-Sectional Area and Flow Mediated Dilation Following Spinal Cord Injury. Eur. J. Appl. Physiol. 2016, 116, 1231–1244. [Google Scholar] [CrossRef]
- Wortman, R.; Brown, S.; Savage-Elliott, I.; Finley, Z.; Mulcahey, M. Blood Flow Restriction Training for Athletes: A Systematic Review. Am. J. Sports Med. 2020, 49, 1938–1944. [Google Scholar] [CrossRef]
- Luebbers, P.E.; Fry, A.C.; Kriley, L.M.; Butler, M.S. The Effects of a 7-Week Practical Blood Flow Restriction Program on Well-Trained Collegiate Athletes. J. Strength Cond. Res. 2014, 28, 2270–2280. [Google Scholar] [CrossRef]
- Yasuda, T.; Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Effects of Blood Flow Restricted Low-Intensity Concentric or Eccentric Training on Muscle Size and Strength. PLoS ONE 2012, 7, E52843. [Google Scholar] [CrossRef]
- Yasuda, T.; Brechue, W.F.; Fujita, T.; Shirakawa, J.; Sato, Y.; Abe, T. Muscle Activation during Low-Intensity Muscle Contractions with Restricted Blood Flow. J. Sports Sci. 2009, 27, 479–489. [Google Scholar] [CrossRef]
- Queiros, V.; Dantas, M.; Neto, G.; Silva, L.; Assis, M.; Almeida-Neto, P.; Dantas, P.; Cabral, B. Application and Side Effects of Blood Flow Restriction Technique. Medicine 2021, 100, e25794. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Black, C.D.; Elder, C.P.; Dudley, G.A. Effects of Electrical Stimulation Parameters on Fatigue in Skeletal Muscle. J. Orthop. Sports Phys. Ther. 2009, 39, 684–692. [Google Scholar] [CrossRef]
- Takahashi, M.; Takeda, K.; Otaka, Y.; Osu, R.; Hanakawa, T.; Gouko, M.; Ito, K. Event-Related Desynchronization-Modulated Functional Electrical Stimulation System for Stroke Rehabilitation: A Feasibility Study. J. Neuroeng. Rehabil. 2012, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schardong, J.; Dipp, T.; Bozzeto, C.B.; da Silva, M.G.; Baldissera, G.L.; Ribeiro, R.D.C.; Valdemarca, B.P.; do Pinho, A.S.; Sbruzzi, G.; Plentz, R.D.M. Effects of Intradialytic Neuromuscular Electrical Stimulation on Strength and Muscle Architecture in Patients with Chronic Kidney Failure: Randomized Clinical Trial. Artif. Organs 2017, 41, 1049–1058. [Google Scholar] [CrossRef]
- Erickson, L.N.; Hickey Lucas, K.C.; Davis, K.A.; Jacobs, C.A.; Thompson, K.L.; Hardy, P.A.; Andersen, A.H.; Fry, C.S.; Noehren, B.W. Effect of Blood Flow Restriction Training on Quadriceps Muscle Strength, Morphology, Physiology, and Knee Biomechanics Before and After Anterior Cruciate Ligament Reconstruction: Protocol for a Randomized Clinical Trial. Phys. Ther. 2019, 99, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Head, P.; Waldron, M.; Theis, N.; Patterson, S.D. Acute Neuromuscular Electrical Stimulation (NMES) With Blood Flow Restriction: The Effect of Restriction Pressures. J. Sport Rehabil. 2020, 30, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Wohlfahrt, H.W.; Haensel, L.; Mithoefer, K.; Ekstrand, J.; English, B.; McNally, S. Terminology and Classification of Muscle Injuries in Sport: The Munich Consensus Statement. Br. J. Sports Med. 2013, 47, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; Aitchison, T.; Henderson, E.; Christie, J.; Zare, S.; McMurray, J.; Dargie, H. A Comparison of the Reproducibility and the Sensitivity to Change of Visual Analogue Scales, Borg Scales, and Likert Scales in Normal Subjects during Submaximal Exercise. Chest 1999, 116, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.; Cahalane, E.; Conroy, R.; Fitzgerald, D.; Hardiman, O. Maximum Voluntary Isometric Contraction: Reference Values and Clinical Application. Amyotroph. Lateral. Scler. 2007, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.C.P.; Caserotti, P.; Carvalho, C.M.P.; Abade, E.A.; Sampaio, A.J.E. Reliability of Concentric, Eccentric and Isometric Knee Extension and Flexion When Using the REV9000 Isokinetic Dynamometer. J. Hum. Kinet. 2013, 37, 47–53. [Google Scholar] [CrossRef]
- Chamorro, C.; Armijo-Olivo, S.; De La Fuente, C.; Fuentes, J.; Chirosa, L.J. Absolute Reliability and Concurrent Validity of Hand Held Dynamometry and Isokinetic Dynamometry in the Hip, Knee and Ankle Joint: Systematic Review and Meta-Analysis. Open Med. Wars 2017, 12, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, E. Asymmetry of Limb Circumferences in Female Athletes. In The Female Athlete; Karger Publishers: Basel, Switzerland, 1981; Volume 15, pp. 142–149. [Google Scholar]
- Santonja Medina, F.; Alberto Peña Ramírez, W.; Medina Leal, D.; Ferrer-López, V. Metodología y Fiabilidad de La Medición Del Perímetro de Muslo. Rev. Act. Fis Des. Hum. 2012, 4, 150–154. [Google Scholar]
- Bragança, S.; Arezes, P.; Carvalho, M.; Ashdown, S.P.; Castellucci, I.; Leão, C. A Comparison of Manual Anthropometric Measurements with Kinect-Based Scanned Measurements in Terms of Precision and Reliability. Work 2018, 59, 325–339. [Google Scholar] [CrossRef]
- Salgado, E.; Ribeiro, F.; Oliveira, J. Joint-Position Sense Is Altered by Football Pre-Participation Warm-up Exercise and Match Induced Fatigue. J. Knee 2005, 22, 243–248. [Google Scholar] [CrossRef]
- Ferreira, A.G.; Duarte, M.; Maldonado, E.P.; Burke, T.N.; Marques, A.P. Postural Assessment Software (PAS/SAPO): Vadilidation and Reliability. Clinics 2010, 65, 675–681. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Paz, I.; Rigo, G.T.; Sgarioni, A.; Baroni, B.M.; Frasson, V.B.F.; Vaz, M.A. Alternating Current Is More Fatigable Than Pulsed Current in People Who Are Healthy: A Double-Blind, Randomized Crossover Trial. Phys. Ther. 2021, 101, Pzab056. [Google Scholar] [CrossRef]
- Tennent, D.J.; Hylden, C.M.; Johnson, A.E.; Burns, T.C.; Wilken, J.M.; Owens, J.G. Blood Flow Restriction Training After Knee Arthroscopy: A Randomized Controlled Pilot Study. Clin. J. Sport Med. 2017, 27, 245–252. [Google Scholar] [CrossRef]
- Callanan, M.C.; Plummer, H.A.; Chapman, G.L.; Opitz, T.J.; Rendos, N.K.; Anz, A.W. Blood Flow Restriction Training Using the Delfi System Is Associated with a Cellular Systemic Response. Arthrosc. Sports Med. Rehabil. 2020, 27, E189–E198. [Google Scholar] [CrossRef]
- Madarame, H.; Kurano, M.; Takano, H.; Iida, H.; Sato, Y.; Ohshima, H.; Abe, T.; Ishii, N.; Morita, T.; Nakajima, T. Effects of Low-Intensity Resistance Exercise with Blood Flow Restriction on Coagulation System in Healthy Subjects. Clin. Physiol. Funct. Imaging 2010, 30, 210–213. [Google Scholar] [CrossRef]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R.; Beck, B.; Bennell, K.; Brosseau, L.; Costa, L.; Cramp, F.; Cup, E. Consensus on Exercise Reporting Template (CERT): Modified Delphi Study. Phys. Ther. 2016, 96, 1514–1524. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M. Better Reporting of Interventions: Template for Intervention Description and Replication (TIDieR) Checklist and Guide. BMJ 2014, 348. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences Lawrence Earlbaum Associates; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Beato, M.; Maroto-Izquierdo, S.; Turner, A.N.; Bishop, C. Implementing Strength Training Strategies for Injury Prevention in Soccer: Scientific Rationale and Methodological Recommendations. Int. J. Sports Physiol. Perform. 2021, 16, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Kasahara, K.; Yoshida, R.; Yahata, K.; Sato, S.; Murakami, Y.; Aizawa, K.; Nakamura, M. Relationship between Eccentric-Exercise-Induced Loss in Muscle Function to Muscle Soreness and Tissue Hardness. Healthcare 2022, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A. Physiological and Methodological Considerations for the Use of Neuromuscular Electrical Stimulation. Eur. J. Appl. Physiol. 2010, 110, 223–239. [Google Scholar] [CrossRef]
- Umbel, J.D.; Hoffman, R.L.; Dearth, D.J.; Chleboun, G.S.; Manini, T.M.; Clark, B.C. Delayed-Onset Muscle Soreness Induced by Low-Load Blood Flow-Restricted Exercise. Eur. J. Appl. Physiol. 2009, 107, 687–695. [Google Scholar] [CrossRef]
- Wernbom, M.; Paulsen, G.; Nilsen, T.S.; Hisdal, J.; Raastad, T. Contractile Function and Sarcolemmal Permeability after Acute Low-Load Resistance Exercise with Blood Flow Restriction. Eur. J. Appl. Physiol. 2012, 112, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Egner, I.M.; Bruusgaard, J.C.; Eftestøl, E.; Gundersen, K. A Cellular Memory Mechanism Aids Overload Hypertrophy in Muscle Long after an Episodic Exposure to Anabolic Steroids. J. Physiol. 2013, 591, 6221–6230. [Google Scholar] [CrossRef]
- Pedersen, J.; Lonn, J.; Hellstrom, F.; Djupsjobacka, M.; Johansson, H. Localized Muscle Fatigue Decreases the Acuity of the Movement Sense in the Human Shoulder. Med. Sci. Sports Exerc. 1999, 31, 1047–1052. [Google Scholar] [CrossRef]
- Miura, K.; Ishibashi, Y.; Tsuda, E.; Okamura, Y.; Otsuka, H.; Toh, S. The Effect of Local and General Fatigue on Knee Proprioception. Arthroscopy 2004, 20, 414–418. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).