Abstract
In this study, we investigated the cytoprotective effects of acid-treated sesame leaf extracts against oxidative damage in HepG2 cells. Treatment with 0.1 M HCl of sesame leaves significantly increased their verbascoside content (4.398 g/100 g) compared to non-acid-treated leaves (3.950 g/100 g). Acid-treated sesame leaf extract (ASLE) showed no cytotoxicity in HepG2 cells. ASLE conferred a greater cytoprotective effect against oxidative insult than a methanol extract of sesame leaves (SLE), verbascoside, and a vehicle control group. ASLE treatment also significantly inhibited reactive oxygen species generation in response to oxidative stress. Treatment with tert-butyl hydroperoxide increased malondialdehyde (MDA) levels, and depleted reduced glutathione (GSH). However, ASLE treatment significantly ameliorates this MDA and GSH depletion. Moreover, ASLE increased the activities of antioxidant enzymes including catalase, glutathione reductase, and glutathione peroxidase. Phenolic compounds in ASLE and SLE were characterized using UPLC-Q-TOF/MS analysis. A total of 29 iridoid and phenol compounds were tentatively identified in ASLE, and 27 compounds were observed in SLE. These results suggest that acid treatment of sesame leaves enhances the protective effects of their extract against oxidative stress by modulating antioxidant enzymes in HepG2 cells.
1. Introduction
Excessive reactive oxygen species (ROS) exposure can drive the progression of metabolic disorders including diabetes, obesity, hypertension, and atherosclerosis [1]. Metabolic diseases have become an important global health problem because of their increasing incidence. In response to oxidative stress, antioxidant enzymes, including glutathione reductase (GR), superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), are induced and may play a pivotal role in endogenous defensive metabolism in the liver [2]. Glutathione (GSH) is a well-known potential antioxidant molecule that can neutralize ROS. Oxidative stress depletes cellular GSH [3]. In addition, excessive ROS generation leads to liver damage by inducing pro-inflammatory cytokine production [4]. Based on numerous studies, the endogenous antioxidant defense system is generally considered to be protective against oxidative stress-induced damage [5,6]. Reducing ROS levels could thus serve as an effective approach to managing and preventing liver diseases.
Sesame (Sesamum indicum L.) is an important oilseed crop that is widely cultivated in Asia and Africa [7]. Sesame oil is used in cooking and in folk medicine. Sesame seeds contain polyphenolic compounds, lignans, minerals, and omega-3 fatty acids, which are responsible for their health benefits. In addition, sesame seeds possess hepato-protective properties against oxidative stress in rat hepatocytes [8]. To date, limited information is available on sesame leaf contents and their functions. Previous studies have shown that sesame leaves contain abundant nutrients and phytochemicals [7,9]. Verbascoside, also known as acteoside, is one of the main bioactive compounds in sesame leaves. This compound belongs to the family of phenylethanoid glycosides, which are commonly found throughout the plant kingdom and extracted from medicinal plants [10]. Studies have demonstrated that verbascoside possesses a range of biological functions including antioxidant, anti-inflammatory, hepatoprotective, and neuroprotective effects [11,12,13]. It is widely recognized that aglycones exhibit greater activity within the intestine than their glycoside counterparts, leading to increased bioavailability [14]. Our previous studies have demonstrated that subjecting glycosidic flavonoids to acid treatment enhanced their bioactivity [15,16]. Acid hydrolysis thus has the potential to enhance the bioactivity of phenolic compounds through deglycosylation and the release of bound phenolic compounds. Here, we investigated the cytoprotective effects of acid-treated sesame leaves, including their ability to modulate antioxidant enzyme activity against oxidative stress, using human HepG2 cells.
2. Materials and Methods
2.1. Chemicals
Verbascoside, caffeic acid, tert-butyl hydroperoxide (TBHP), ethyl acetate, hydrogen chloride, 2′,7′-dichlorofluorescein diacetate (DCF-DA), 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hank’s balanced salt solution, Dulbecco’s modified Eagle’s medium, trypsin-ethylenediaminetetraacetic acid, penicillin–streptomycin, and fetal bovine serum (FBS) were purchased from Gibco (Gaithersburg, MD, USA). All other chemicals were of analytical grade.
2.2. Sample Preparation
Sesame leaves were collected from a local farm (Yangsan, Kyungnam, Republic of Korea) in August 2022. The leaves were washed, lyophilized, and ground into a fine powder. To prepare a sesame leaf extract (SLE), 10 g of powder was added to 500 mL of methanol. This mixture was extracted using an ultrasonic bath for 1 h at 24 °C, and then filtered through filter paper (Advantec, No. 2, Toyo Roshi, Tokyo, Japan). Solvent was removed using a vacuum evaporator, and the residue was dissolved in DMSO and stored at −20 °C until use.
To prepare acid-treated sesame leaf extract (ASLE), we used a previously reported method with slight modifications [15]. Sesame leaf powder (10 g) was added to 500 mL of an acidic solvent (100% methanol containing 0.1 M HCl). This mixture was extracted in an ultrasonic bath for 1 h at 24 °C. When the reaction was complete, the reactant was cooled and the pH was adjusted to 7 using sodium hydroxide (1.0 M). The neutralized solution was subjected to vacuum evaporation to remove organic solvents. Subsequently, the residue was dissolved in distilled water and the resulting aqueous solution was washed twice with ethyl acetate. The ethyl acetate layer was concentrated under reduced pressure. The resulting residue was dissolved in DMSO and stored at −20 °C until needed for use.
2.3. Quantitation of Verbascoside and Caffeic Acid Content
Verbascoside and caffeic acid contents were assessed as reported earlier using high-performance liquid chromatography (Hitachi 5000 Chromaster, Hitachi Ltd., Tokyo, Japan) with a UV detector [17]. Verbascoside and caffeic acid standards were obtained from Sigma-Aldrich. Phenomenex ultramex (5 µm, 250 mm × 4.6 mm, Luna., Torrance, CA, USA) was used with a mobile phase of acetonitrile (solvent A) and water (containing 0.2% phosphoric acid, solvent B) with a flow rate of 1.0 mL/min. Column oven temperature was 30 °C.
2.4. LC-Q-TOF MS Analysis
Ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS) was performed as previously described (Agilent Q-TOF 6530 combining Agilent 1290 Infinity II UHPLC, Agilent Technologies, Santa Clara, CA, USA) [18]. Quintuplicate SLE and ASLE samples were prepared. Chromatographic separation was conducted on a Zorbax Eclipse Plus C18 column (2.1 mm × 50 mm, 1.8 µm, Agilent). Column oven temperature was 30 °C, and injection volume was 5 μL. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) with the following gradient elution: 0–5 min, 5% B; 5–8 min, 10% B; 8–20 min, 12% B; 20–23 min, 55% B; 23–28 min, 95% B; 28–35 min, 5% B, at a flow rate of 0.3 mL/min. MS analysis was performed in both positive and negative ion modes using MS scan modes. MS parameters were as follows: mass range, 20–1700 m/z; collision energy, 0 V; gas temperature, 150 °C; drying gas, 10 L/min; nebulizer, 35 psi; sheath gas temperature, 350 °C; sheath gas flow, 11 L/min; capillary, 4000 V; fragmentor, 125 V; Oct 1 RF 750 V).
Data acquisition and processing were performed using Agilent MassHunter Qualitative Analysis software (Version 10.0, Agilent Technologies). Raw MS data files obtained from UPLC-Q-TOF/MS analysis were matched with the METLIN database B 08.00, and an in-house library of data was derived from prior studies for identification [7,19,20].
2.5. Cell Culture and Cytotoxicity
HepG2 cells were purchased from the Korean Collection for Type Cultures (Daejeon, Republic of Korea). SLE and ASLE cytotoxicities were assessed through MTT assay. HepG2 cells were seeded in 96-well plates at a density of 1 × 104 cells/well. The next day, cells were treated with extracts (25 and 50 μg/mL) for 24 h. Then, MTT assays were performed.
To evaluate extracts for cytoprotective effects against TBHP treatment, HepG2 cells were cultured in 96-well plates at a density of 1 × 104 cells/well in FBS-free medium for 18 h, and then incubated in FBS-free medium containing 0.5 mM TBHP and different concentrations of extracts (25 and 50 μg/mL). After 6 h, MTT assays were conducted. Absorbance was measured at 550 nm using a spectrophotometer (BioTek Instruments Inc., Winooski, VT, USA).
2.6. Determination of Intracellular Reactive Oxygen Species Generation
Generation of intracellular ROS was measured using the fluorescent probe 2′,7′–dichlorofluorescin diacetate (DCFH-DA) as previously described [21]. In brief, HepG2 cells were seeded in 96-well black plates at a density of 5 × 104 cells/well, and pretreated with SLE and ASLE. After 1 h, the cells were washed twice with phosphate-buffered saline. Oxidative stress was induced through the addition of 0.5 mM TBHP to the culture medium for 1 h. The cells were washed twice with phosphate-buffered saline and were incubated with 25 μM DCFH-DA in serum-free medium for 1 h at 37 °C. Then, the cells were incubated in Hank’s balanced salt solution. Fluorescence intensity was evaluated using a fluorescence spectrophotometer (Perkin–Elmer, Norwalk, CT, USA) for 90 min at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
2.7. Quantitation of Glutathione and Lipid Peroxidation
HepG2 cells were seeded in 6-well plates at a density of 1 × 106 cells/well. The next day, cells were treated with different concentrations of each extract and TBHP for 6 h, and then harvested to measure malondialdehyde (MDA) and GSH levels. To determine cellular lipid peroxidation, MDA levels were measured using a thiobarbituric-acid-reactive substance assay [22]. Intracellular GSH levels were determined as previously described, with slight modifications [23]. The results are expressed as nmol of MDA/mg of protein using a molar extinction coefficient of 1.56 × 105 M/cm.
2.8. Measurement of Antioxidant Enzyme Activities
HepG2 cells were seeded in 6-well plates at a density of 1 × 106 cells/well to measure antioxidant enzyme activities. The next day, HepG2 cells were treated with each extract (50 μg /mL) and 0.5 mM TBHP for 6 h. Catalase (CAT) [24], glutathione reductase (GR) [25], and glutathione peroxidase (GPx) [26] activities were evaluated using previously reported methods.
2.9. Statistical Analyses
All results are representative of at least three independent experiments and are expressed as means ± standard deviation. Statistical analyses were conducted using one-way analysis of variance (ANOVA) with GraphPad Prism software (GraphPad Prism version 5.0 Software, San Diego, CA, USA), followed by Tukey’s test. The threshold of significance was set at a p value of <0.05.
3. Results and Discussion
3.1. Effects of Acid-Hydrolyzed Sesame Leaves on Verbascoside Content
In this study, acid hydrolysis was employed to increase verbascoside and caffeic acid content in sesame leaves. Verbascoside and caffeic acid contents increased with acid treatment (Table 1).

Table 1.
Sesame leaf verbascoside and caffeic acid content quantitated by acid hydrolysis.
The highest verbascoside content (4.398 g/100 g) was observed in 0.1 M HCl-treated leaves. The highest caffeic acid yield (0.534 g/100 g) was also obtained from 0.1 M HCl-treated leaves. However, treatment of 0.5 M HCl into sesame leaves decreased both verbascoside and caffeic acid compared to 0.1 M HCl treatment. The sesame leaf verbascoside content was previously determined to be in the 0.13–4.86 g/100 g range [5]. The observed broad variability in phytochemical content may be due to climatic, seasonal, and geographic conditions; harvest time; and storage conditions [27]. Phytochemicals that exist in plant leaves mainly crosslink with cellulose, lignin, and hemicellulose by glycosidic bonds [28]. Verbascoside is a phenylethanoid glycoside composed of hydroxytyrosol, caffeic acid, and glucose. On the other hand, acid hydrolysis breaks glycosidic bonds. For that reason, we anticipated the increased content of caffeic acid and verbascoside after acid hydrolysis. Our result showed that acid hydrolysis increased the contents of both verbascoside and caffeic acid. Moreover, acid hydrolysis combined with ultrasonic extraction was an efficient method for increasing the bound phenolic contents from leaves of Rubus idaeus L. [29]. On the other hand, verbascoside, the primary bioactive constituent of dietary supplements in Japan, is derived from Sesamum indicum leaves [6]. It also possesses antioxidant, antitumor, antibacterial, and neuroprotective activities [30,31,32,33]. In a previous study, acid-treated rutin was converted to quercetin, which exerted an enhanced cytoprotective activity against oxidative stress and anti-adipogenic and anti-inflammatory activities [15]. These results thus showed that acid treatment effectively increased verbascoside and caffeic acid content, and 0.1 M HCl treatment provided the most efficient conditions for extracting verbascoside and caffeic acid.
3.2. Identification of Phytochemicals in Acid-Hydrolyzed Sesame Leaves
Phytochemical analysis of sesame leaves was conducted using UPLC-Q-TOF/MS. A total of 29 compounds, including iridoid and phenolic compounds, were tentatively identified in SLE and ASLE extracts (Table 2). Three iridoid compounds (sesamoside, lamalbid, and shanzhiside methyl ester) were identified in both SLE and ALSE. Twenty-seven phenolic compounds (3-O-methylquercetin, verbascoside, chlorogenic acid, cistanoside F, diosmetin, eupafolin, ferulic acid, gentisic acid, hydroxytyrosol, luteolin, protocatechuic acid, quinic acid, rhamnetin, caffeic acid, corylifolinin, dioonflavone, epicatechin pentaacetate, ferulic acid, genistein, hesperetin, isorhamnetin, oxyquinoline, P-salicylic acid, rhamnetin, sinapic acid, and tricin) were identified in both SLE and ASLE. Morin and 3-O-methylkaempferol were exclusively detected in ASLE. Verbascoside and its constituent components, caffeic acid and hydroxytyrosol, were detected in both SLE and ASLE. Interestingly, the acid-treated extracts contained more phenolic compounds than the untreated extracts. Phenolic compounds are the major bioactive compounds in sesame leaves and possess strong antioxidant properties [34]. Luteolin is well known for its therapeutic properties, including anticancer, anti-inflammatory, and antihypertensive activities [35]. Additionally, luteolin exhibits antioxidant and neuroprotective properties [36,37]. Morin, a flavonol, is present in free and glycoside forms, including morin-3-O-β-D-glucopyranoside, in plants [38]. Acid hydrolysis cleaves glycosidic linkages in glycosides to produce aglycones from glycosides. Therefore, it appears that morin is only detected in ASLE. Various phytochemicals have been identified in SLE and ASLE, and acid hydrolysis increases the number of phenolic compounds.

Table 2.
Identification of compounds in acid-treated sesame leaves using UPLC-QTOF/MS.
3.3. Effects of Acid-Hydrolyzed Sesame Leaves on Cell Viability and Cytoprotection in HepG2 Cells
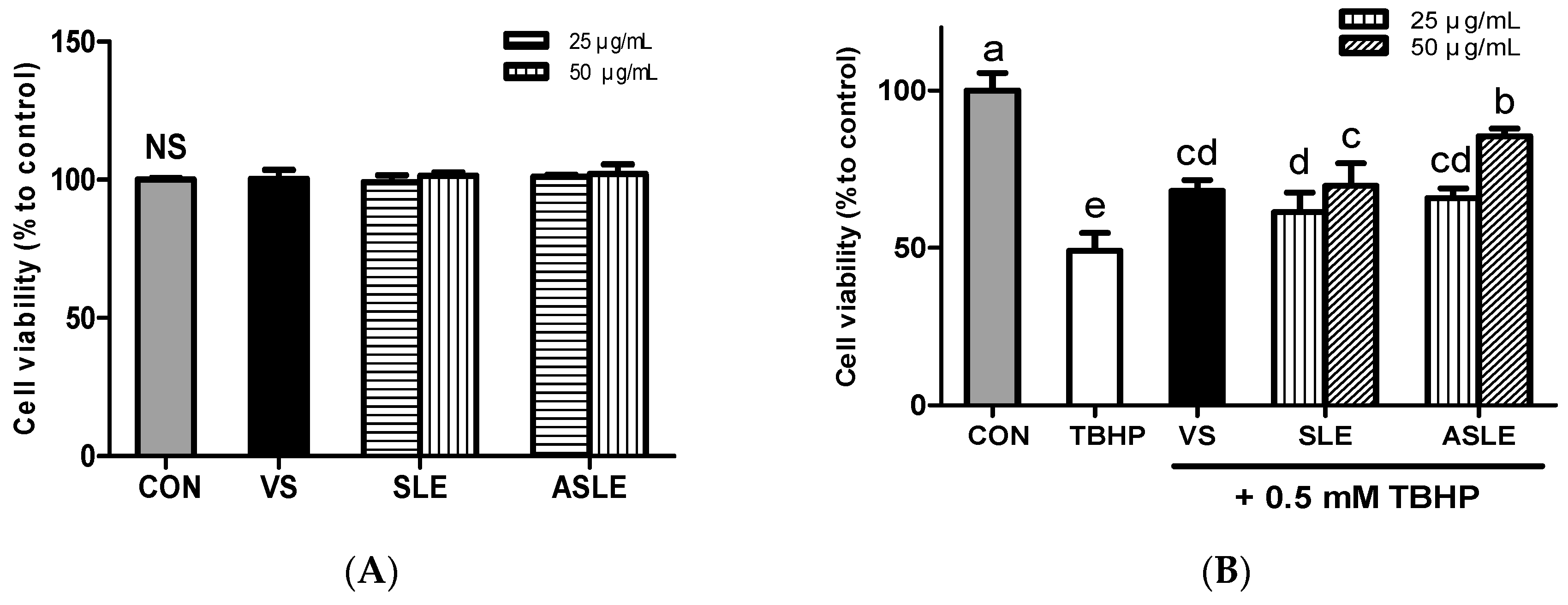
The cytotoxicity of sesame leaves extracts was assessed using the MTT assay. None of the extracts showed cytotoxicity at concentrations up to 50 μg/mL in HepG2 cells after 24 h (Figure 1A).
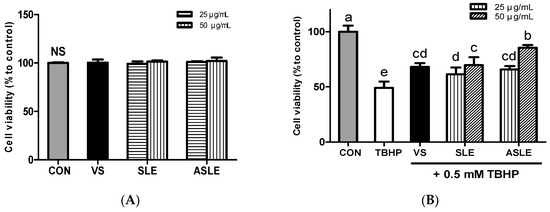
Figure 1.
Cytotoxic effect of sesame leaves (A) and cytoprotective effect of various concentrations of sesame leaf methanolic and acid-treated extracts (25 and 50 µg/mL) against 0.5 mM tert-butyl-hydroperoxide-induced cytotoxicity (B). a~e Means with different letters are significantly different according to Duncan’s multiple range test with significance threshold at p < 0.05. NS, not significant; CON, control; TBHP, tert-butyl hydroperoxide; VS, verbascoside (50 µM); SLE, sesame leaf extract; ASLE, acid-treated sesame leaf extract.
To explore the extracts’ protective efficacy on TBHP-treated HepG2 cells, cells were exposed to TBHP and each extract for 6 h. TBHP treatment decreased cell viability by approximately 50% compared to control cells. Treatment with verbascoside, SLE, and ASLE significantly increased HepG2 cell viability after oxidative stress challenge (Figure 1B). An amount of 50 μg/mL of ASLE resulted in the highest cell viability. Oxidative stress increases hepatocyte cell death. Dietary interventions using natural antioxidants have thus been utilized to reduce hepatocyte oxidative stress and protect against cellular damage. Several flavonoids, including luteolin, quercetin, and kaempferol, exert well-known protective effects against oxidative insults [39]. An earlier study showed that verbascoside effectively mitigated ROS-induced cytotoxicity in HT-29 cells through ROS scavenging [12]. Hydroxytyrosol can neutralize free radicals and inhibit LDL oxidation in vitro [40,41,42]. ASLE contained various phytochemicals including ferulic acid, luteolin, and morin, as well as verbascoside (Table 2). It seems that these phytochemicals were attributed to the increased cell viability compared to treatment with verbascoside alone. These results show that all treatments, including verbascoside, SLE, and ASLE, provided cytoprotective effects against oxidative stress, and the extract of acid-hydrolyzed sesame leaves provided the most effective cytoprotection.
3.4. Intracellular Antioxidative Activities of Acid-Hydrolyzed Sesame Leaves
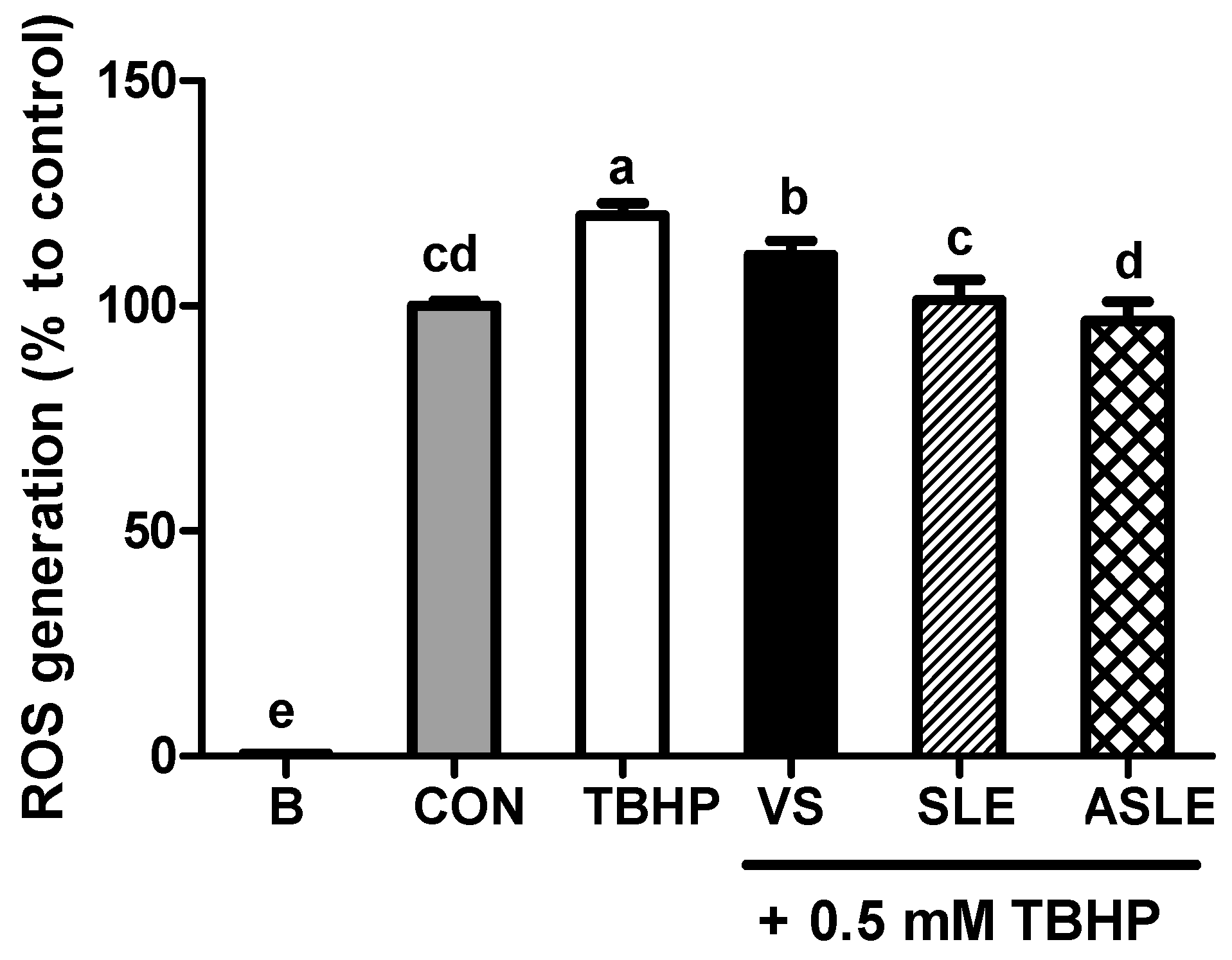
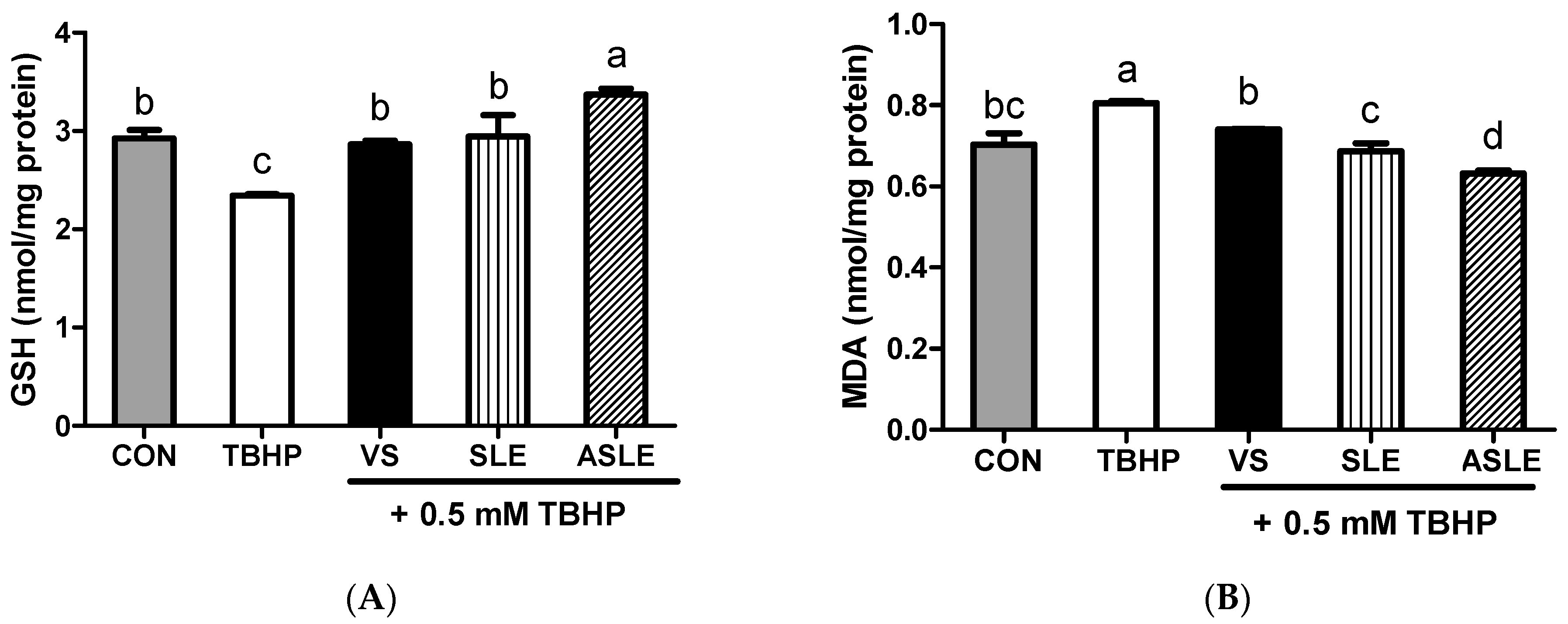
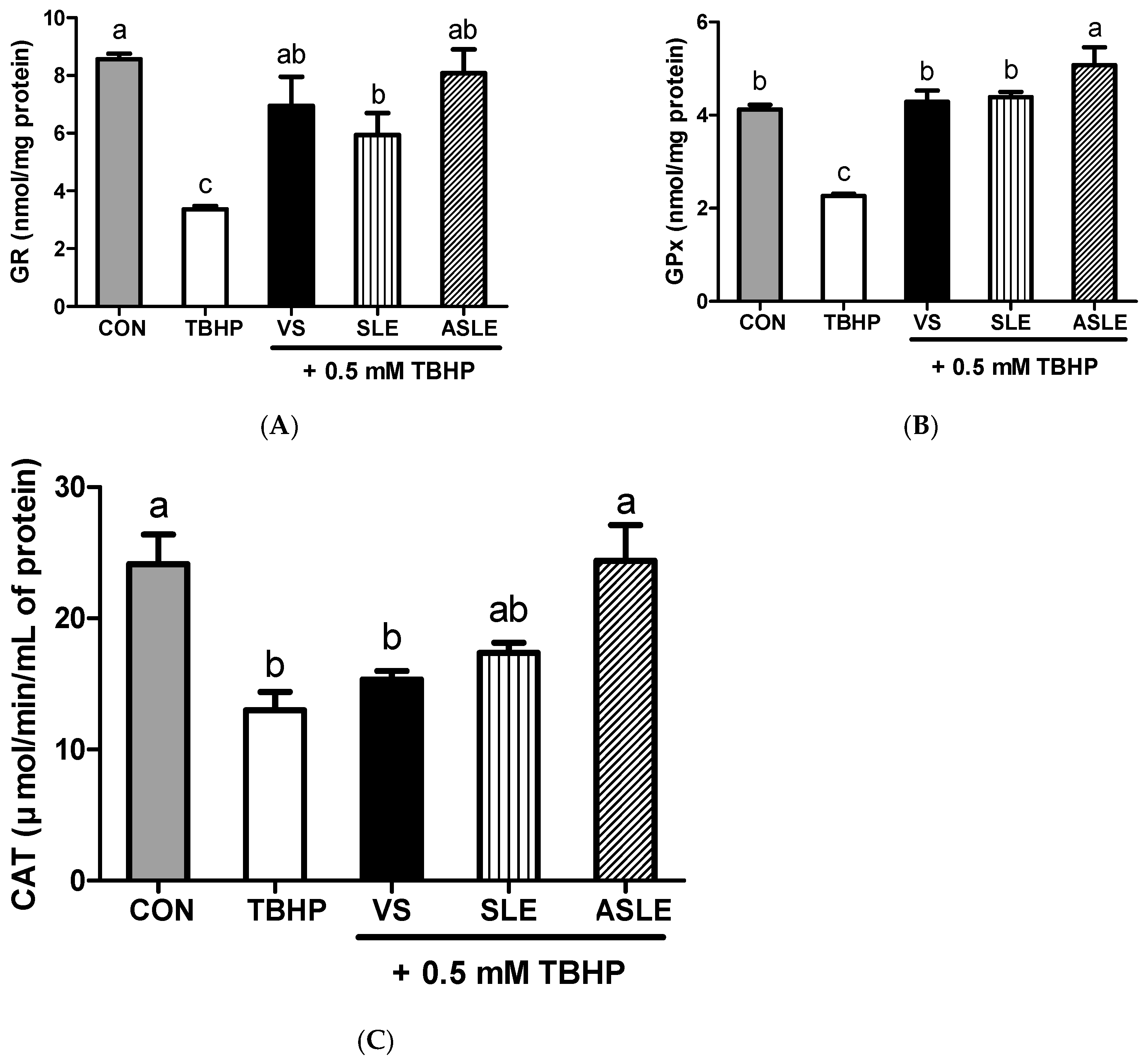
To evaluate the effect of acid hydrolysis in sesame leaves on redox effectors including ROS, MDA, and GSH, and antioxidant enzymes, including GPx, CAT, and GR, sample-treated HepG2 cells were collected after 24 h. As shown in Figure 2, TBHP increased intracellular ROS generation. All extract-treated samples showed markedly decreased ROS generation compared to that in the TBHP-alone group. ASLE treatment decreased ROS generation to almost basal levels. We then measured GSH and MDA levels to gauge the HepG2 cell response to oxidative stress. TBHP significantly decreased GSH levels compared to control cells (Figure 3A). However, all extract-treated samples showed increased GSH levels compared to those treated with TBHP alone. ASLE treatment most strongly preserved GSH levels in HepG2 cells facing oxidative stress. The MDA level was also significantly elevated in TBHP-treated cells relative to control cells (Figure 3B), and verbascoside, SLE, and ASLE significantly abated the TBHP-mediated elevation of MDA levels. Remarkably, treatment with SLE and ASLE reduced MDA levels to near or below control values. To evaluate the effect of sesame leaf acid hydrolysates on antioxidant enzyme activity, GPx, CAT, and GR levels in HepG2 cells were evaluated. TBHP treatment decreased antioxidant enzyme activities, and verbascoside, SLE, and ASLE markedly restored GR and GPx activities in TBHP-treated cells (Figure 4A,B). CAT activity was increased only by ASLE (verbascoside and SLE did not increase this activity) (Figure 4C).
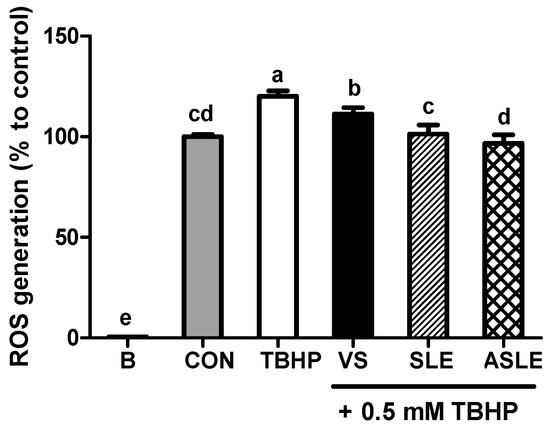
Figure 2.
Mitigating effects of sesame leaf extracts on 0.5 mM tert-butyl-hydroperoxide-mediated intracellular generation of reactive oxygen species (ROS) by HepG2 cells. a~e Means with different letters are significantly different through Duncan’s multiple range test with significance threshold at p < 0.05. B, blank; CON, control; TBHP, tert-butyl hydroperoxide; VS, verbascoside (50 µM); SLE, sesame leaf extract (50 µg/mL); ASLE, acid-treated sesame leaf extract (50 µg/mL).
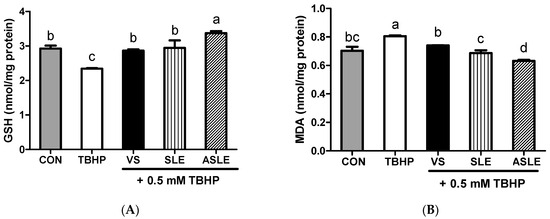
Figure 3.
Effects of sesame leaves on 0.5 mM tert-butyl-hydroperoxide-stimulated GSH (A) and MDA (B) levels in HepG2 cells. a~d Means with different letters are significantly different through Duncan’s multiple range test with significance threshold at p < 0.05. GSH, glutathione; MDA, malondialdehyde; CON, control; TBHP, tert-butyl hydroperoxide; VS, verbascoside (50 µM); SLE, sesame leaf extract (50 µg/mL); ASLE, acid-treated sesame leaf extract (50 µg/mL).
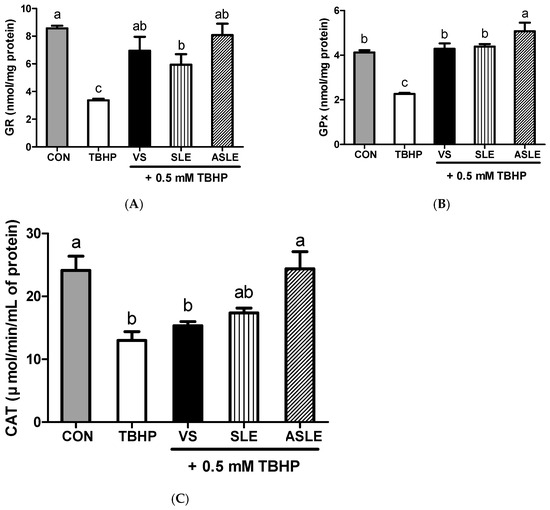
Figure 4.
Effects of sesame leaves on 0.5 mM tert-butyl-hydroperoxide-stimulated GR (A), GPx (B), and CAT (C) activities in HepG2 cells. a~c Means with different letters are significantly different through Duncan’s multiple range test with significance threshold at p < 0.05. GR, glutathione reductase; GPx, glutathione peroxidase; CAT, catalase; CON, control; TBHP, tert-butyl hydroperoxide; VS, verbascoside (50 µM); SLE, sesame leaf extract (50 µg/mL); ASLE, acid-treated sesame leaf extract (50 µg/mL).
Excessive intracellular ROS levels indicate an oxidative insult in living cells, and predict damage to lipids, proteins, and DNA [39]. Cellular MDA is a product of lipid peroxidation in cell membranes resulting from reactions between unsaturated fatty acids and radical species [43]. Oxidative stress-induced liver damage leads to reduced levels of glutathione (GSH), which plays a central role in defending cells against oxidative stress and detoxifying their environment [44]. Numerous dietary phytochemicals have been studied and have shown significant effects on antioxidant enzymes under oxidative stress.
For example, flavonoids and phenolic-acid-rich jujube extracts increase cell viability against oxidative stress by decreasing ROS production and MDA and GSH depletion via nuclear factor erythroid 2–related factor 2 activation [45]. Liao et al. previously reported that diosmetin exerts a strong antioxidant activity by inhibiting ROS generation and restoring intracellular antioxidant enzyme activity in response to oxidative stress [46]. In addition, verbascoside significantly suppressed ROS and MDA levels and increased GPx and SOD activities to counter oxidative stress [47]. The alterations in GR, CAT, and GPx activities in response to TBHP + sample treatment, compared with TBHP treatment alone, clearly indicate the capacity of the cell defense system to react to oxidative stress. Collectively, these results suggest that phytochemicals in sesame leaves exert cytoprotective effects. In addition, acid hydrolysis of sesame leaves enhances antioxidant capacity by modulating antioxidant enzymes and/or scavenging free radicals.
4. Conclusions
In conclusion, acid hydrolysis increased the verbascoside and caffeic acid content extractable from sesame leaves. Among the tested treatments, ASLE exposure most strongly preserved cell viability against oxidative stress. ASLE also effectively reduced ROS generation, GSH depletion, and the elevation in malondialdehyde (MDA) levels in HepG2 cells. ASLE significantly increased antioxidant enzyme activity in response to oxidative damage in HepG2 cells. Twenty-nine and twenty-seven bioactive compounds were tentatively identified in ASLE and SLE, respectively. These findings illustrate that acid hydrolysis of sesame leaves enhances their antioxidant potential, offering a new modality to enhance the functional attributes of sesame leaves.
Author Contributions
Investigation and formal analysis, H.S.; formal analysis, writing—original draft preparation, Y.K. (Yoonjeong Kim); conceptualization, writing—review and editing, Y.K. (Younghwa Kim). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Basic Science Institute (National Research Facilities and Equipment Center), a grant funded by the Ministry of Education (2019R1A6C1010044), and the BB21plus (2023) funded by Busan metropolitan City and Busan Techno Park.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The research data that support this study will be shared upon reasonable request to the corresponding author. The data are not publicly available due to the funding conditions.
Acknowledgments
We are grateful to Sunghyun Jin, Department of Food Science and Biotechnology, Kyungsung University, for support in the collection of leaves of Sesame indicum from a local farm.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ando, K.; Fujita, T. Metabolic syndrome and oxidative stress. Free Radic. Biol. Med. 2009, 47, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Tausz, M.; Sircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, S.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Fuji, Y.; Uchida, A.; Fukahori, K.; Chino, M.; Ohtsuki, T.; Matsufuji, H. Chemical characterization and biological activity in young sesame leaves (Sesamum indicum L.) and changes in iridoid and polyphenol content at different growth stages. PLoS ONE 2018, 13, e0194449. [Google Scholar] [CrossRef]
- Hosseini, M.J.; Shahraki, J.; Tafreshian, S.; Salimi, A.; Kamalinejad, M.; Pourahmad, J. Protective effects of Sesamum indicum extract against oxidative stress induced by vanadium on isolated rat hepatocytes. Environ. Toxicol. 2016, 31, 979–985. [Google Scholar] [CrossRef]
- Fuji, Y.; Ohtsuki, T.; Matsufuji, H. Accumulation and Subcellular Localization of Acteoside in Sesame Plants (Sesamum indicum L.). ACS Omega 2018, 3, 17287–17294. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Chou, Y.S.; Ho, Y.L.; Ding, C.W.; Chang, Y.S. New antioxidant phenylethanol glycosides from Torenia concolor. J. Asian Nat. Prod. Res. 2009, 11, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Nigro, O.; Tuzi, A.; Tartaro, T.; Giaquinto, A.; Vallini, I.; Pinotti, G. Biological effects of verbascoside and its anti-inflammatory activity on oral mucositis: A review of the literature. Anticancer Drugs 2020, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Conversion of Rutin to Quercetin by Acid Treatment in Relation to Biological Activities. Prev. Nutr. Food Sci. 2019, 24, 313–320. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.; Kim, Y. Effect of Deglycosylated Rutin by Acid Hydrolysis on Obesity and Hyperlipidemia in High-Fat Diet-Induced Obese Mice. Nutrients 2020, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Feng, C.; Hu, L.; Zhao, X.; Tang, X.; Huang, Y.; Luo, J.; Xu, M.; Xie, W. Exploration of a ternary deep eutectic solvent for the efficient extraction of plantamajoside, acteoside, quercetin and kaempferol from Plantago asiatica L. Phytochem. Anal. 2022, 33, 94–104. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, Y. Effect of Purple Sweet Potato Using Different Cooking Methods on Cytoprotection against Ethanol-Induced Oxidative Damage through Nrf2 Activation in HepG2 Cells. Antioxidants 2023, 12, 1650. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Microwave-assisted enzymatic extraction of plant protein with antioxidant compounds from the food waste sesame bran: Comparative optimization study and identification of metabolomics using LC/Q-TOF/MS. J. Food Process. Preserv. 2019, 44, 14304. [Google Scholar] [CrossRef]
- Olalere, O.A.; Abdurahman, H.N.; Gan, C.Y. Microwave-enhanced extraction and mass spectrometry fingerprints of polyphenolic constituents in Sesamum indicum leaves. Ind. Crops Prod. 2019, 131, 151–159. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Ham, H.; Jeong, H.S.; Lee, J. Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. Food Chem. 2013, 137, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Flamini, G.; Cioni, P.L.; Morelli, I. Composition and antimicrobial properties of essential oils of four Mediterranean Lamiaceae. J. Ethnopharmacol. 1993, 39, 167–170. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.; Tsao, R. Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and α-Glucosidase and Pancreatic Lipase Inhibitory Effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, A.; Li, W.; Li, C.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crops Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Aly, S.M.; Ali, H.; Babiker, A.Y.; Srikar, S.; Khan, A.A. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int. J. Clin. Exp. Med. 2014, 7, 483–491. [Google Scholar] [PubMed]
- Tanaka, T.; Kojima, T.; Kawamori, T.; Wang, A.; Suzui, M.; Okamoto, K.; Mori, H. Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogenesis 1993, 14, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Sul, D.; Kim, H.S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Wanasundara, P.K. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Tian, X.H.; Yi, Y.S.; Jiang, W.S.; Zhou, Y.J.; Cheng, W.J. Luteolin-induced protection of H(2)O(2)-induced apoptosis in PC12 cells and the associated pathway. Mol. Med. Rep. 2015, 12, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.H.; Yao, X.L.; Zhang, Y.; Zhang, S.F.; Hu, J.C. Luteolin Could Improve Cognitive Dysfunction by Inhibiting Neuroinflammation. Neurochem. Res. 2018, 43, 806–820. [Google Scholar] [CrossRef]
- Hussain, J.; Ali, L.; Khan, A.L.; Rehman, N.U.; Jabeen, F.; Kim, J.S.; Al-Harrasi, A. Isolation and bioactivities of the flavonoids morin and morin-3-O-beta-D-glucopyranoside from Acridocarpus orientalis-A wild Arabian medicinal plant. Molecules 2014, 19, 17763–17772. [Google Scholar] [CrossRef]
- Chagas, M.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Goncalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Montedoro, G.; Galli, C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis 1995, 117, 25–32. [Google Scholar] [CrossRef]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Jove, M.; Mota-Martorell, N.; Pradas, I.; Martin-Gari, M.; Ayala, V.; Pamplona, R. The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Dysregulation of glutathione synthesis in liver disease. Liver Res. 2020, 4, 64–73. [Google Scholar] [CrossRef]
- Hong, S.; Kim, Y.; Sung, J.; Lee, H.; Heo, H.; Jeong, H.S.; Lee, J. Jujube (Ziziphus jujuba Mill.) Protects Hepatocytes against Alcohol-Induced Damage through Nrf2 Activation. Evid. Based Complement. Alternat. Med. 2020, 2020, 6684331. [Google Scholar] [CrossRef]
- Liao, W.; Ning, Z.; Chen, L.; Wei, Q.; Yuan, E.; Yang, J.; Ren, J. Intracellular antioxidant detoxifying effects of diosmetin on 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. J. Agric. Food Chem. 2014, 62, 8648–8654. [Google Scholar] [CrossRef]
- Ji, S.L.; Cao, K.K.; Zhao, X.X.; Kang, N.X.; Zhang, Y.; Xu, Q.M.; Yang, S.L.; Liu, Y.L.; Wang, C. Antioxidant activity of phenylethanoid glycosides on glutamate-induced neurotoxicity. Biosci. Biotechnol. Biochem. 2019, 83, 2016–2026. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).