Abstract
Microalgae, as some of the oldest life forms on Earth, are of significant interest to industry and in terms of environmental policies, due to their ability to perform photosynthesis and consume atmospheric carbon dioxide. Moreover, they contain a wide variety of value-added compounds such as amino acids and proteins, carbohydrates, and fatty acids, which can be exploited in multiple fields like medicine, cosmetics, nutritional supplements, and for the production of biodiesel. In this article, Nannochloropsis gaditana, a type of microalgae that inhabits both fresh and salt water, is studied for fatty acid recovery using deep eutectic solvents (DES). This microalgae species is a natural source of eicosapentaenoic acid (EPA), an omega-3 compound that is commonly used in the nutritional industry. There are numerous extraction techniques and pretreatments to obtain these compounds. In this work, DES are studied as extractive agents due to their advantages as neoteric solvents. Specifically, this work focuses on an assessment of the effect of the composition of DES on the extraction yield of fatty acids from microalgae. Several DES compositions based on choline chloride, ethylene glycol, and fructose are studied to analyze the influence of water content in these phases. The results show that water content significantly influences recovery yields. The DES with higher extractive capacity were those based on choline chloride, ethylene glycol, and water at a molar ratio of 1:2:2. This composition offered 48.7% of the yield obtained with a conventional solvent like methanol for the recovery of EPA (11.2 mg/g microalgae). Furthermore, the choline chloride-fructose-based DES shows the capability of selective extractions of fatty acids with low carbon content—choline chloride:fructose:water (molar ratio 2:1:2) can extract 0.14 mg of decanoic acid/g of microalgae, indicating that this DES composition can recover 35.7% more decanoic acid in comparison to methanol.
1. Introduction
It is becoming increasingly urgent to find new green resources to meet the needs of society. Policies advocating for the reduction of emissions and the use of renewable energies are becoming more stringent in their requirements. In this sense, microalgae can help establish new sustainable pathways for obtaining products for human consumption and use.
Microalgae are rich in valuable compounds, making them highly versatile microorganisms with numerous applications from biofuel production to nutrition and cosmetics [1,2]. Among these compounds, fatty acids are notable, as they can be used in applications like the production of biodiesel and in nutritional supplements [3].
Biodiesel is an excellent alternative to non-renewable sources of energy like fossil fuels. It is formed by fatty acid methyl esters (FAMEs), which can be obtained from vegetal and animal fats [4,5]. Fatty acids are also present in microalgae, and, with an adequate extraction technique, they could be used for the production of biodiesel. Nevertheless, the production of biodiesel that is sourced from microalgae is not currently optimized, as the process consumes so much energy and the production costs are high; hence, this technique is neither economically feasible nor industrially productive at this time [6,7].
On the other hand, the use of microalgae as a source of nutritional supplements is more extended. Human beings are unable to synthesize all the vitamins necessary for their proper growth [8], requiring other sources from which to obtain them. For instance, vitamin B12 is not produced by the human body. This kind of vitamin can be found in several meats, but not in vegetables, which poses a significant challenge for those on vegetarian and vegan diets, as the deficiency of this nutrient can lead to illnesses such as anemia [9]. To solve this problem, individuals following a plant-based diet can incorporate microalgae into their nutritional intake, as microalgae are rich in this vitamin [3,10].
Some of the most consumed fatty acids in nutrition are omega-3 types, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [11,12]. These fatty acids are mainly found in fish, seafood, meat, and dairy products. However, overfishing poses a threat to the sustainability of marine assets. Different species of microalgae (Arthrospira platensis, Crypthecodinium cohnii, Isochrysis galbana, Nannochloropsis sp., Nitzschia sp., Porphyridium sp., and Schizochytrium sp., among others) are good sources of this type of fatty acid, offering an excellent alternative for humans [13,14].
The extraction of fatty acids from microalgae typically involves the use of chemical solvents. Conventional methods are based on organic solvents, such as hexane and methanol [15]. However, the use of these chemicals presents significant environmental and health issues, given their toxic and non-biodegradable nature [16].
As an alternative, neoteric solvents can be used for the extraction of target compounds from biomass. Among the options for the extraction of microalgal components are supercritical fluids—mainly supercritical carbon dioxide—which are safer while still offering high extraction yields. For example, extraction with supercritical CO2 is a method in which it is possible to achieve high efficiencies by adjusting parameters such as pressure and temperature [17,18]. Other relevant neoteric solvents include ionic liquids (IL) and deep eutectic solvents (DES). IL are low melting-point salts that present a high extractive capacity [19,20], although they still have high costs and their synthesis can be challenging [19,21,22]. Conversely, DES offer simpler preparation and reduced costs, and they are typically more biodegradable [23]. These consist of two or more components that combine to form a structure of hydrogen bonds. One of these components is a hydrogen bond donor (HBD), which is a compound that provides hydrogen bonds, and the other is a hydrogen bond acceptor (HBA), which captures the hydrogen bonds. The combination of these two compounds in the appropriate proportions and at a specific temperature will form the DES [24]. While HBAs are formed, basically, from quaternary ammonium salts, HBDs can include amines, carboxylic acids, or urea [25,26].
Regarding the toxicity of DES, these compounds are commonly regarded as non-toxic substances. However, there is a lack of systematic studies that comprehensively address their potential health effects and environmental toxicity, and these issues are still controversial. The harmless characteristics of the individual components lead to this assumption, especially in the case of natural deep eutectic solvents (NADES) which are a subgroup of DES formed by primary metabolites (e.g., sugar, polyols, and amino acids at a specific molar proportion). Nevertheless, it is essential to consider the potential synergistic effects that might arise from the combination of these DES components, as they could influence the overall hazardous properties and ecological footprint of these eutectic mixtures [27]. It is anticipated that the number of studies on the toxicity of these compounds will increase in the future. However, compared to traditional organic solvents, DES offer clear advantages, such as their negligible vapor pressure and non-flammability. They also exhibit good chemical and thermal stability, ensuring their reliability in various applications. Several studies have shown that, in general, DES can offer less toxicity in comparison to other neoteric solvents such as ILs [27].
One of the key properties of DES is their formation temperature. Unlike many mixtures with a formation temperature that is the average of the melting points of its components, DES have a lower melting point than the respective melting points of their individual constituents. This property is quite relevant in terms of energy cost-savings since less energy is required for DES formation [28,29]. Previously, in our research group, a study was conducted on the efficiency of fatty acid extraction with eutectic solvents from untreated and ultrasound- and osmotic shock-pretreated microalgal biomass [25]. The results showed that the most promising phases were those based on choline chloride. As a novelty in this work, the effect of water content in choline chloride-based eutectic phases on the extraction capacity of the fatty acids present in untreated microalgal biomass is addressed, in order to perform a direct extraction from the starting material. Elsewhere, it has been reported that the use of hydrophilic DES helps disrupt the cell walls of microalgae, eliminating the necessity for pre-treatment procedures [30].
On the one hand, the addition of the right amount of water can reduce the viscosity of DES by weakening the hydrogen bonds in their structure, enhancing the manageability as well as the extractive capacity [28]. On the other hand, an excess of water in DES can result in the disruption of too many hydrogen bonds, causing DES to lose their functionality as extractive agents. Thus, the variation in water amounts in DES, based on choline chloride in combination with ethylene glycol and fructose, respectively, is investigated in this paper.
2. Materials and Methods
2.1. Preparation of DES
The preparation of DES requires the mixing of two components—or three, in the case of using water—at different temperatures, ranging from 60 °C to 90 °C, with magnetic stirring until a completely homogeneous mixture is formed. The synthesized DES were based on choline chloride–ethylene glycol–water with a molar fraction of 1:2:x and choline chloride-fructose-water with a molar fraction of 2:1:x, with x varying from 0 to 3. The water amount in DES was varied to study its effect on the extraction process. In the case of choline chloride–fructose–water, the 2:1:0 composition was dismissed due to its high viscosity and the associated handling difficulties. Table 1 shows the different DES being studied and their compositions.

Table 1.
Molar composition of the DES phases studied.
Table 2 shows the chemical structures of the HBAs and HBDs that form the DES being analyzed.

Table 2.
HBAs and HBDs used in this study.
2.2. Primary Extraction and Sample Collection
Fatty acids were obtained by mixing 0.5 g of the dried microalgae Nannochloropsis gaditana with 45 g of DES in an Erlenmeyer flask under magnetic stirring. The flask was submerged in a water bath for temperature control. Extractions were carried out at 60 °C for 1 h and 30 min.
After extraction, fatty acids were recovered from the mixture of microalgae–DES for analytical purposes. Then, 25 mL of hexane was added to the flask and mixed under magnetic stirring for 5 min. Two phases were formed, comprising the supernatant containing the extracted fatty acids and the DES phase. The hexane phase was separated, and the DES was centrifuged for 3 min at 1000 rpm to recover the maximum hexane amount since some of it could be retained within the DES phase.
2.3. Transesterification
The fatty acids extracted from the microalgae biomass underwent a transesterification process to convert them into fatty acid methyl esters (FAMEs), which were subsequently quantified using gas chromatography. The transesterification reaction was catalyzed using a 10% v/v solution of hydrochloric acid (HCl) in methanol. To facilitate the reaction, hexane samples were mixed with the HCl solution in methanol and stirred using a magnetic stirrer. The reaction mixture was maintained under reflux conditions at a temperature of 55 ºC for a duration of 1 h and 30 min. After completion of the reaction, the hexane phases were efficiently separated from the methanol phases using a separating funnel. The hexane phase containing the FAMEs was then stored at a freezing temperature of −20 °C until further analysis was conducted. This experimental procedure was conducted to assess and quantify the FAME content in the microalgae-derived fatty acids.
2.4. Gas Chromatography
Hexane samples containing FAMEs were filtered through a 0.22 μm nylon membrane. Subsequently, 2 mL were transferred to a clean vial for analysis. The analysis was performed with an Agilent Technologies 6890 N gas chromatograph, equipped with a single quadrupole mass detector (Agilent Technologies 5975, Agilent, Santa Clara, CA, USA) and a DB-23 column (250 µm × 60 m × 0.25 µm). The injector temperature was 240 °C, while the source and quadrupole temperatures of the mass spectrometer (MS detector) were set to 230 °C and 150 °C, respectively. Helium gas served as the carrier gas, and in each run, 1 µL of the sample was injected. FAMEs were identified and quantified by comparing their retention times and mass fragments with those of a certified standard solution containing the fatty acids as reference compounds. At the same time, several standard samples were prepared to create calibration curves that correlated the area obtained through gas chromatography with the concentration of fatty acids in each sample. These calibration samples were prepared using a commercial standard (Supelco® 37 Component FAME Mix, Sigma-Aldrich, St. Louis, MO, United States). The fatty acid standard samples were prepared at concentrations of 4, 12, 16, 20, 24, and 40 ppm, respectively, for the construction of the calibration curves.
2.5. Infrared Spectroscopy
Fourier-transform infrared spectrophotometry (FTIR) was used to study water composition in the synthesized DES phases. An FTIR Thermo Nicolet 5700 (Thermo Electron Scientific Instruments Corp., Madison, WI, USA) equipped with a DTGS detector and a KBr window with a spectral interval between 350 and 12,500 cm−1, was employed for the analysis. The specific range used in this study was 500–4000 cm−1.
3. Results and Discussion
3.1. Characterization of DES with FTIR
The FTIR spectra of the synthesized DES, based on choline chloride and ethylene glycol with the named compositions of CE0, CE1, CE2, and CE3, are shown in Figure 1.
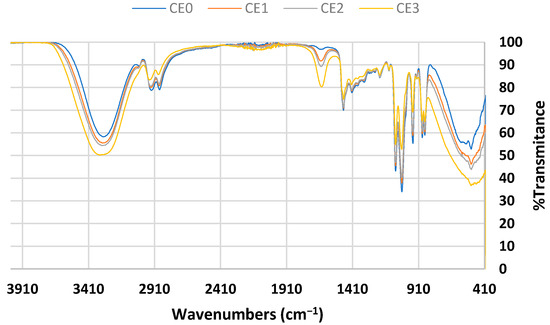
Figure 1.
FTIR spectra of choline chloride–ethylene glycol-based DES.
In all cases, after mixing the starting materials (HBAs and HBDs), homogenous and transparent phases were obtained, indicating the formation of the eutectic phases. The formation of the DES was also confirmed with the IR spectrum of the DES without water (CE0), as previously reported in [31]. The O-H vibration of the DES appears at 3300 cm−1, increasing in the presence of water. Additionally, in all choline chloride–ethylene glycol-based DES spectra, there is a peak at 1086 cm−1, which is indicative of the C-N vibration [31]. As seen here, the differences between the spectrum of the original DES and the spectra of the compositions with water, CE1, CE2, and CE3, are only related to the amount of water. This suggests that the DES retains its functionality for the potential extraction of fatty acids, even when water is added. The rise in the amplitude of some peaks is linked to the presence of water. This increase can be observed for the peak at 3300 cm−1, corresponding to the stretching of the bond O-H, and it is produced by an increase in the amount of water from CE1 to CE3. Conversely, there is also an increase in the peak at 1650 cm−1 when comparing the spectra of CE1, CE2, and CE3, which corresponds to the bending of the O-H bond due to the presence of water [32].
Figure 2 shows the spectra of the DES based on choline chloride and fructose. Specifically, the spectra of the compositions CF2 and CF3 are presented. As in the case of choline chloride–ethylene glycol-based DES, CF2 and CF3 exhibit increasing peaks that correspond to the stretching and bending of the O-H bond in water, with no other significant differences between them.
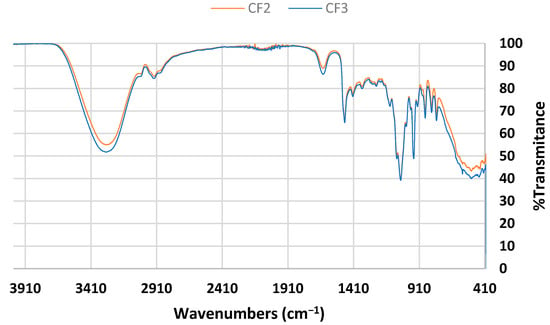
Figure 2.
FTIR spectra of choline chloride–fructose-based DES.
3.2. Formation Temperature of DES
Table 3 displays the different formation temperatures for each DES composition analyzed. In general, the addition of water leads to a decrease in the formation temperature of the DES compared to the formation temperature of the base components. This could potentially translate into energy savings if this technique were to be implemented on a larger scale. This effect can be explained by water acting as an agent that partially disrupts the hydrogen bonds formed by the HBAs and the HBDs. This, in turn, decreases the viscosity of DES and lowers its melting temperature.

Table 3.
Formation temperature of DES phases.
Conversely, the data in Table 3 indicate that choline chloride–ethylene glycol-based DES are formed at lower temperatures than the rest of the DES, from 45 °C to 63 °C. This suggests that these DES phases form more readily at relatively mild temperatures. In contrast, choline chloride–fructose (CF)-based DES require higher formation temperatures. The minimum temperature observed for CF-based DES is 73 °C when the system contains a higher proportion of water. Conversely, choline chloride–fructose-based DES need an initial addition of water during their preparation. Without the inclusion of water, the melting point of this DES rises significantly, resulting in impractically high viscosities. Consequently, the composition of 2:1:0 could not be studied in terms of extraction capacity.
3.3. Extractive Capacity of DES
The total amounts of fatty acids in the form of methyl esters that were obtained with each DES are shown in Figure 3. According to these results, the DES based on choline chloride and ethylene glycol outperformed the phases based on choline chloride and fructose. Specifically, the compositions CE1 and CE2 offered the highest extraction yields, with values of over 11.2 and 10.06 mg/g of microalgae. These extraction capacities were higher than those obtained in the absence of water (CE0). The compositions based on choline chloride and fructose (from CF1 to CF2) did not offer significant extraction capacities regarding total fatty acids, with values below 0.35 mg/g. The extraction of fatty acids from microalgae was also performed with methanol as a conventional organic solvent. The traditional solvent was able to recover a higher quantity of total fatty acids in comparison with DES, with a final value of 23 mg/g of microalgae. Although DES can only recover approximately half of the total amount of fatty acids that can be obtained with a traditional solvent, they offer additional advantages over these types of solvents, as indicated in Section 1.
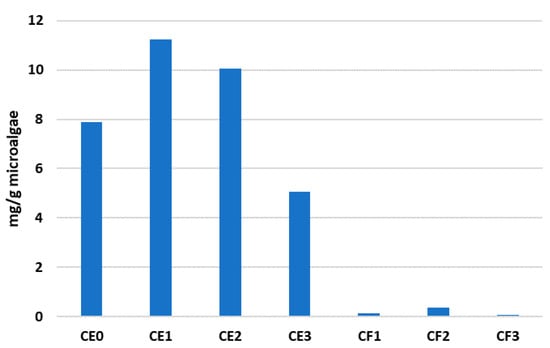
Figure 3.
Total fatty acids extracted with each eutectic.
Regarding the individual fatty acids obtained from microalgae with DES, in the case of the choline chloride–ethylene glycol-based CE1, the yields in the form of methyl esters were: hexadecenoic acid (30.13%), eicosapentaenoic acid or EPA (29.62%), octadecenoic acid (9.26%), hexadecenoic acid (8.54%), eicosatetraenoic acid (7.56%), octadecadienoic acid (7.47%), octadecanoic acid (3.47%), myristic acid (2.81%), pentadecanoic acid (0.47%), dodecanoic acid (0.35%), decanoic acid (0.17%), and octanoic acid (0.08%). As seen, EPA is the second most abundant fatty acid recovered from microalgae.
For the choline chloride–fructose mixture CF2, the individual yields were: decanoic acid (40.1%), hexadecenoic acid (29.54%), hexadecanoic acid (10.20%), octadecanoic acid (6.10%), octadecenoic acid (4.67%), octadecadienoic acid (3.17%), myristic acid (2.49%), octanoic acid (2.15%), and dodecanoic acid (1.58%).
The analysis of the capacity extraction of individual fatty acids also offers interesting results. Hexadecenoic acid was among the most abundant fatty acids recovered from microalgae. Figure 4 shows the capacity extractions of each DES for this specific fatty acid (in the form of methyl ester), obtaining the same patterns as those shown in Figure 3 for the total amount of fatty acids. This means that the choline chloride–ethylene glycol-based DES with a composition of 1:2:1 and 1:2:2 (CE1 and CE2) were able to recover the highest quantities of hexadecenoic acid, with values of 3.38 and 3.09 mg/g.
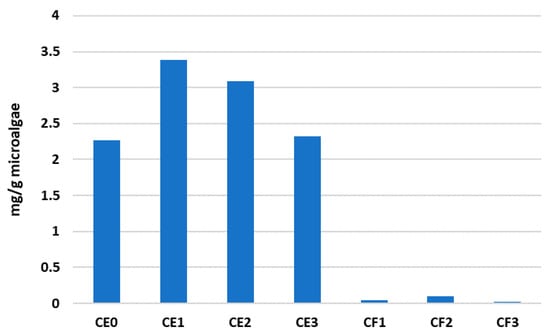
Figure 4.
Recovery performance of hexadecenoic acid methyl ester with each DES mixture.
On the other hand, although the DES phase based on choline chloride and fructose yielded low recovery amounts of total fatty acids, such phases could be useful for the selective extraction of short-chain fatty acids. As can be seen in Figure 5, the DES mixture based on choline chloride and fructose, especially the composition CF2, could recover higher amounts of decanoic acid, with a total amount of 0.14 mg/g of microalgae. These yields were higher in comparison to the DES based on choline chloride and ethylene glycol for this specific fatty acid.
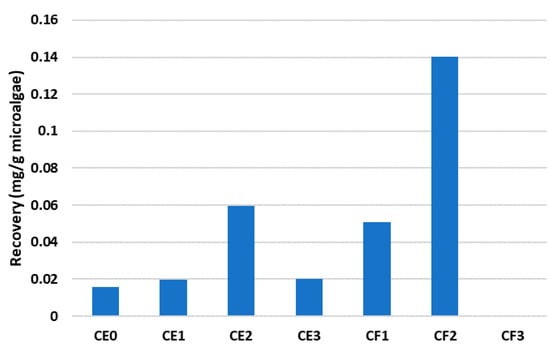
Figure 5.
Recovery performance of decanoic acid with each DES mixture.
The results shown in Figure 3 and Figure 4 highlight the effect of water content on the extractive capacity of DES. The extraction capacity of the DES based on choline chloride and ethylene glycol is higher in the presence of water. The composition CE1 yielded over 11 mg of total fatty acids per g of microalgae, while the composition CE0 (without water) only offered 7.9 mg/g microalgae. The addition of water produces a decrease in the viscosity of DES, facilitating fatty acid extraction. However, if water is added in excess, fatty acid capacity can be affected. For instance, the composition CE3, corresponding to a 1:2:3 molar composition with the highest amount of water, yielded 5 mg/g of microalgae in terms of total fatty acid recovery, which is less than half of the total amount of fatty acids obtained with CE1. This excess of water in the DES phases can produce excessive rupture of the hydrogen bonds, reducing the extraction capacity. Thus, in light of these results, it is necessary to find a balance in terms of water content for each DES composition. Moreover, fatty acids, especially those of higher polar character, are bound to other biomolecules (such as proteins and carbohydrates) through electrostatic forces, posing a challenge regarding their extraction. However, it has been reported that water can intervene in the solvent–cellulose interaction, disrupting the bonds between the fatty acids and the biomolecules. This presence of water facilitates ion dissociation and solvation, potentially aiding in the release of fatty acids for more effective extraction [30].
The effect of water is not only seen for the choline chloride–ethylene glycol-based DES but also for the choline chloride–fructose-based phase. As is shown, the recovery of decanoic acid increased from CF1 (0.05 mg/g) to CF2 (0.14 mg/g), but the CF3 mixture was not able to obtain decanoic acid.
Due to its advantages as a food supplement, the recovery yield of EPA is of special relevance in the analysis. As seen before, EPA was the second most abundant fatty acid recovered from microalgae with the DES based on ethylene glycol. Figure 6 displays the recovery of EPA with each DES. As can be seen, choline chloride–ethylene glycol-based DES was the only phase with an extraction capacity regarding EPA. The maximum amount that was recovered was obtained with the CE1 composition, with around 3.3 mg/g microalgae, which is in line with the results reported for total fatty acids. This was followed by the composition CE2, achieving 2.89 mg/g of microalgae. However, EPA was not found in the samples of the choline chloride–fructose phases. The extractive capacity of the traditional solvent (methanol) was still higher in comparison to the DES phases. It could extract 8.41 mg/g microalgae, which was almost three times the quantity of EPA extracted by CE1. While the extraction results are not yet comparable to those obtained with the traditional organic solvent, they do need to be optimized, and DES could potentially provide a new sustainable pathway for the extraction of fatty acids from microalgae biomass, especially if the recyclability of these solvents is proven.
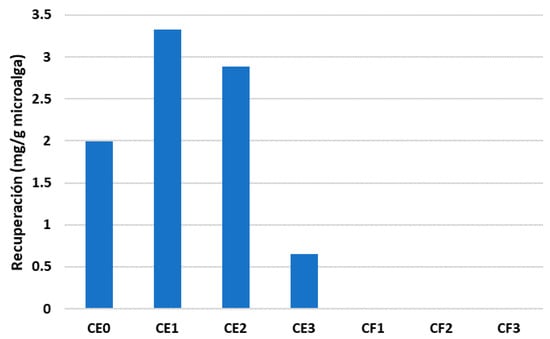
Figure 6.
Recovery of EPA with each DES.
In this work, the extraction of fatty acids from DES phases was not addressed as a research objective. To achieve a fully sustainable process, the development of an optimized re-extraction method represents a critical avenue for future research. It is worth mentioning that the addition of water to the DES phase may be a viable strategy to facilitate these re-extraction methods, as water can weaken the interactions between individual components of the DES. Furthermore, different strategies are being studied to enhance the recyclability of DES. For instance, the utilization of supercritical fluids presents a promising option for the recovery and purification of fatty acids of special interest, such as EPA, as demonstrated in the literature [33], enabling the reuse of the DES phase. This research sets the stage for future investigations into the fine-tuning, recyclability, and optimization of re-extraction methodologies, all of which are essential for advancing the sustainability of DES-based processes. Furthermore, future studies should systematically investigate the influence of DES polarity, aiming to elucidate the specific extraction mechanisms of these materials. This may involve employing quantum chemical calculations with modeling techniques.
4. Conclusions
Deep eutectic solvents (DES) have emerged as a promising and environmentally friendly option for extractive processes. Their extractive capacity in sourcing fatty acids from microalgae has been shown to be closely linked to their water content. The addition of water reduces DES viscosity, enhancing their extractive efficiency and ease of handling. Nevertheless, an excessive water content can disrupt the hydrogen bonds within DES, potentially compromising their functionality. This underscores the significance of identifying an optimal water-to-DES ratio to maximize the extractive capacity. Choline chloride–ethylene glycol-based DES were shown to be the most efficient phase at a molar composition of 1:2:1 (choline chloride:ethylene glycol:water), yielding 11.2 mg/g of microalgae. Beyond this point, further water addition resulted in a decreased DES extractive capacity. Also, this composition offered the highest extraction capacity for EPA, an omega-3 fatty acid with health benefits for humans. Furthermore, when comparing the two types of DES studied, choline chloride–ethylene glycol-based DES demonstrated the capacity to extract a broader range of fatty acids, while choline chloride–fructose-based DES exhibited the capacity for selective extraction, particularly targeting fatty acids with lower carbon content. The addition of water was also advantageous for reducing the formation temperature of DES phases. The results reaffirm the potential of DES as a versatile solution for the extraction of fatty acids from microalgae, fostering opportunities for further optimization. Finally, ensuring the recyclability of eutectic phases should be a crucial aspect to be addressed in future research, to guarantee the development of fully sustainable extraction processes.
Author Contributions
Investigation, P.A.G.-S.; conceptualization, V.M.O.-M. and S.S.-S.; methodology, P.A.G.-S. and M.I.S.d.S.; validation, M.J.S.-G.; formal analysis, M.I.S.d.S. and M.J.S.-G.; data curation, P.A.G.-S.; writing—original draft preparation, P.A.G.-S. and V.M.O.-M.; writing—review and editing, M.J.S.-G. and M.I.S.d.S.; supervision, S.S.-S. and V.M.O.-M. All authors have read and agreed to the published version of the manuscript.
Funding
Sergio Sánchez-Segado and this research were supported by the Spanish Ministry of Education and Vocational Training, grant number “Beatriz Galindo” BEAGAL18/00079.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request to the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Nikolaos, K.; Karapanagiotidis, I.T.; Papapolymerou, G. Current and Potential Applications of Microalgae: A Mini Review. Oceanogr. Fish. Open Access J. 2019, 11, 555811. [Google Scholar] [CrossRef]
- Monika; Banga, S.; Pathak, V.V. Biodiesel Production from Waste Cooking Oil: A Comprehensive Review on the Application of Heterogenous Catalysts. Energy Nexus 2023, 10, 100209. [Google Scholar] [CrossRef]
- Al-Zuhair, S. Production of Biodiesel: Possibilities and Challenges. Biofuels Bioprod. Biorefining 2007, 1, 57–66. [Google Scholar] [CrossRef]
- Nazloo, E.K.; Moheimani, N.R.; Ennaceri, H. Biodiesel Production from Wet Microalgae: Progress and Challenges. Algal Res. 2022, 68, 102902. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.W. Methods of Downstream Processing for the Production of Biodiesel from Microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef]
- Smith, A.D.; Warren, M.J.; Refsum, H. Vitamin B12. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 83, pp. 215–279. [Google Scholar]
- Oh, R.C.; Brown, D.L. Vitamin B 12 Deficiency Clinical Manifestations of Vitamin B 12 Deficiency. Am. Fam. Physician 2003, 67, 979–986. [Google Scholar]
- Grossman, A. Nutrient Acquisition: The Generation of Bioactive Vitamin B12 by Microalgae. Curr. Biol. 2016, 26, R319–R321. [Google Scholar] [CrossRef]
- Byelashov, O.A.; Sinclair, A.J.; Kaur, G. Dietary Sources, Current Intakes, and Nutritional Role of Omega-3 Docosapentaenoic Acid. Lipid Technol. 2015, 27, 79–82. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Jakhwal, P.; Kumar Biswas, J.; Tiwari, A.; Kwon, E.E.; Bhatnagar, A. Genetic and Non-Genetic Tailoring of Microalgae for the Enhanced Production of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA)—A Review. Bioresour. Technol. 2022, 344, 126250. [Google Scholar] [CrossRef] [PubMed]
- Sharntell, M.; Water, S.U.; Rajagopaul, R. Evaluation and Selection of an Appropriate Automatic Coagulant Dose Control System for Water Treatment Plants; Water Research Commission: Petroria, South Africa, 2016. [Google Scholar]
- Shin, H.Y.; Shim, S.H.; Ryu, Y.J.; Yang, J.H.; Lim, S.M.; Lee, C.G. Lipid Extraction from Tetraselmis Sp. Microalgae for Biodiesel Production Using Hexane-Based Solvent Mixtures. Biotechnol. Bioprocess Eng. 2018, 23, 16–22. [Google Scholar] [CrossRef]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green Extraction of Value-Added Compounds Form Microalgae: A Short Review on Natural Deep Eutectic Solvents (NaDES) and Related Pre-Treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Moghadam, A.J.; Aghababai Beni, A. Comparison of Biodiesel Production from Dunaliella Salina Teodor and Chlorella Vulgaris Microalgae Using Supercritical Fluid Technique. S. Afr. J. Chem. Eng. 2022, 41, 150–160. [Google Scholar] [CrossRef]
- Canela, A.P.R.F.; Rosa, P.T.V.; Marques, M.O.M.; Meireles, M.A.A. Supercritical Fluid Extraction of Fatty Acids and Carotenoids from the Microalgae Spirulina Maxima. Ind. Eng. Chem. Res. 2002, 41, 3012–3018. [Google Scholar] [CrossRef]
- Eppink, M.H.M.; Ventura, S.P.M.; Coutinho, J.A.P.; Wijffels, R.H. Multiproduct Microalgae Biorefineries Mediated by Ionic Liquids. Trends Biotechnol. 2021, 39, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Chiappe, C.; Mezzetta, A.; Pomelli, C.S.; Iaquaniello, G.; Gentile, A.; Masciocchi, B. Development of Cost-Effective Biodiesel from Microalgae Using Protic Ionic Liquids. Green Chem. 2016, 18, 4982–4989. [Google Scholar] [CrossRef]
- Piedade, P.J.; Kochańska, E.; Lukasik, R.M. Biodegradable Ionic Liquids in Service of Biomass Upgrade. Curr. Opin. Green Sustain. Chem. 2022, 35, 100609. [Google Scholar] [CrossRef]
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D.; Mjalli, F.S. Formation of Type III Deep Eutectic Solvents and Effect of Water on Their Intermolecular Interactions. Fluid Phase Equilibria 2017, 441, 43–48. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Q.; Jing, W.; Tian, H.; Yan, H.; Bi, W.; Jiang, Y.; Chen, D.D.Y. Insight into the Deep Eutectic Solvent Extraction Mechanism of Flavonoids from Natural Plant. ACS Sustain. Chem. Eng. 2020, 8, 19169–19177. [Google Scholar] [CrossRef]
- Zaib, Q.; Masoumi, Z.; Aich, N.; Kyung, D. Review of the Synthesis and Applications of Deep Eutectic Solvent-Functionalized Adsorbents for Water Treatment. J. Environ. Chem. Eng. 2023, 11, 110214. [Google Scholar] [CrossRef]
- Moreno Martínez, P.; Ortiz-Martínez, V.M.; Sánchez Segado, S.; Salar-García, M.J.; de los Ríos, A.P.; Hernández Fernández, F.J.; Lozano-Blanco, L.J.; Godínez, C. Deep Eutectic Solvents for the Extraction of Fatty Acids from Microalgae Biomass: Recovery of Omega-3 Eicosapentaenoic Acid. Sep. Purif. Technol. 2022, 300, 121842. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Martínez, G.M.; Townley, G.G.; Martínez-Espinosa, R.M. Controversy on the Toxic Nature of Deep Eutectic Solvents and Their Potential Contribution to Environmental Pollution. Heliyon 2022, 8, e12567. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of Water Addition on Choline Chloride/Glycol Deep Eutectic Solvents: Characterization of Their Structural and Physicochemical Properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Wijffels, R.H.; Eppink, M.H.M. Lipid Extraction from Fresh Nannochloropsis Oceanica Using Semi-Hydrophobic Eutectic Solvents. Algal Res. 2023, 72, 103117. [Google Scholar] [CrossRef]
- Sivrikaya, S. A Novel Vortex-Assisted Liquid Phase Microextraction Method for Parabens in Cosmetic Oil Products Using Deep Eutectic Solvent. Int. J. Environ. Anal. Chem. 2019, 99, 1575–1585. [Google Scholar] [CrossRef]
- Vrancken, N.; Sergeant, S.; Vereecke, G.; Doumen, G.; Holsteyns, F.; Terryn, H.; De Gendt, S.; Xu, X.M. Superhydrophobic Breakdown of Nanostructured Surfaces Characterized in Situ Using ATR-FTIR. Langmuir 2017, 33, 3601–3609. [Google Scholar] [CrossRef]
- Molino, A.; Martino, M.; Larocca, V.; Di Sanzo, G.; Spagnoletta, A.; Marino, T.; Karatza, D.; Iovine, A.; Mehariya, S.; Musmarra, D. Eicosapentaenoic Acid Extraction from Nannochloropsis Gaditana Using Carbon Dioxide at Supercritical Conditions. Mar. Drugs 2019, 17, 132. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).