Abstract
Background: Isolated gastrocnemius tightness (IGT) is a prevalent condition linked to various foot pathologies. In a previous quantitative gait analysis study, we identified an increase in knee flexion during the midstance phase in IGT patients compared with controls. Although stretching and eccentric exercises (the Stanish protocol) are commonly used for IGT management, their impact on gait parameters remains poorly understood. This study aimed to assess the influence of a Stanish protocol on gait parameters in bilateral IGT subjects. Methods: We enrolled 10 asymptomatic bilateral IGT subjects and 10 controls. Quantitative gait analysis and dynamic baropodometry were carried out on each subject. A Stanish protocol was applied for 4 weeks (five sessions/week) by the IGT group, followed by a similar gait analysis. The ankle and knee range of motion and foot pressure distribution were assessed during the midstance phase of the gait. Results: An increase in knee flexion was initially present in the IGT group compared with controls (8.9 +/− 4.6 vs. 3.4 +/− 2.3 degrees, p < 0.001). There was no difference in the ankle range of motion and foot pressures between the groups at that time. Significant reductions in knee flexion during gait were observed in the IGT subjects after the Stanish protocol (8.9 +/− 4.6 to 3.7 +/− 2.3 degrees, p < 0.001) with a normalization of this parameter (3.4 +/− 2.3 in controls vs. 3.7 +/− 2.3 degrees in IGT, p = 0.72). There was no change in ankle range of motion and foot pressure after the Stanish protocol. Conclusions: Our findings support the effectiveness of the Stanish protocol in reducing knee flexion and normalizing gait in IGT subjects. This protocol not only offers a noninvasive approach for IGT-related issues management but could also enable prophylactic care in asymptomatic cases.
1. Introduction
Isolated gastrocnemius tightness (IGT) is a widely encountered condition associated with various foot pathologies, including metatarsalgia and hallux valgus [1,2,3]. The prevalence of IGT is challenging to determine accurately due to the lack of a consensus definition and validated measuring device [4]. However, IGT is believed to be a common condition [4], with some studies reporting a prevalence of up to 50% in the general population [5]. Furthermore, in cases involving foot pathology, the prevalence of IGT can reach as high as 96.5% [6]. In the surgical community, it is generally assumed that IGT induces ankle dorsiflexion restriction during the midstance phase of the gait, which would induce an increase in forefoot plantar pressure [7]. This hypothesis is supported by studies involving 3D models or cadaveric specimens [8,9]. However, it is important to note that these studies were not conducted on living subjects and remain speculative.
Laboratory gait analyses conducted on living subjects have yielded inconsistent results regarding the restriction of ankle dorsiflexion in individuals with IGT. Contrary to the hypothesis, studies have not consistently found evidence supporting restricted ankle dorsiflexion during the midstance phase except in specific populations with neuropathic involvement [10,11,12]. Moreover, gait analysis studies have reported increased knee flexion during the gait without restricted ankle dorsiflexion [13,14]. However, these studies focused on unilateral IGT patients with associated forefoot pathologies and utilized force plates, which do not provide detailed information on localized forefoot pressure [13,14].
In an attempt to address these limitations, we conducted a study comparing asymptomatic bilateral IGT subjects and controls, using quantitative gait analysis and dynamic baropodometry [15]. Similarly, our findings contradicted our initial hypothesis, as we did not observe restricted ankle dorsiflexion or increased forefoot pressure during the midstance phase in the individuals with bilateral IGT. Instead, we observed knee flexion during this phase, as was previously reported [13,14]. The discrepancy between the clinical and laboratory results can be attributed to compensatory mechanisms such as increased knee flexion, which allows gastrocnemius relaxation, thus releasing the ankle dorsiflexion restriction [15]. However, the exact pathophysiological link between IGT and foot pathologies remains unexplained, highlighting the incomplete understanding of gastrocnemius tightness and the potential for management errors such as inappropriate lengthening procedures [16]. Knowing the gait pattern of IGT subjects is still valuable, and we can assume that correcting gait disorders in these subjects could treat or prevent IGT-related pathologies. Assuming that knee flexion is a compensatory mechanism in IGT subjects to allow ankle dorsiflexion during the midstance phase, we can hypothesize that IGT treatment could result in the disappearance of this gait compensation. Although stretching and eccentric exercises are commonly used for IGT management, their impact on gait parameters remains poorly understood.
Isolated gastrocnemius tightness can be treated in a variety of ways, from simple stretching to surgical lengthening [16,17,18,19]. There is currently no consensus on the type of stretching or physical therapy to use to treat IGT. However, the Stanish protocol, although originally described for Achilles tendinopathy, includes calf stretching and eccentric exercises, both of which are recognized as effective for IGT [17,20,21,22]. In our center, this is the protocol we routinely use, as it is well known and easy to implement, whether with a physiotherapist or as self-rehabilitation. From a research point of view, the use of this predefined, well-known protocol will contribute to improved external consistency and clinical applicability of study outcomes.
Therefore, our study aimed to assess the influence of the Stanish protocol on gait parameters in bilateral IGT subjects. We hypothesized that the Stanish protocol would normalize the gait pattern, especially reducing midstance phase compensatory knee flexion, in this population.
2. Materials and Methods
The study was approved by our university hospital’s research review board. Subjects were given a clear explanation of the study protocol and provided informed consent prior to inclusion.
We have previously published a study comparing the gait parameters between IGT subjects and controls using the same methods [15]. To address the objective of this study, a Stanish protocol was carried out in the IGT group after the first gait analysis, and another gait analysis was performed using the same method at the end of the Stanish protocol.
2.1. Inclusion/Exclusion Criteria
Subjects aged between 18 and 65 years who were able to walk without experiencing pain were recruited. To ensure measurement accuracy and eliminate potential confounding factors, we excluded subjects with pathologies leading to secondary equinus or pathologies that could affect gait parameters, such as neurologic disorders, clubfoot pathologies, or any other conditions related to the spine, hip, knee, ankle, and/or foot. Furthermore, participants exhibiting an asymmetric Silfverskiöld test (one lower limb with a positive test and one with a negative test), hindfoot valgus exceeding 10 degrees, hindfoot varus, genu recurvatum greater than 10 degrees, or genu flexum were also excluded to minimize confounding biases during the analysis phase [22,23,24].
The IGT group consisted of lower limbs that were identified as positive for the Silfverskiöld test, indicating a lack of ankle dorsiflexion when the knee was fully extended and an increase of at least 10 degrees in dorsiflexion when the knee was flexed at 90 degrees [22,25]. In order to ensure precise measurements, the investigator manually performed angular assessments using a fixed system attached to the leg and connected to a dynamometer, which provided consistent and reproducible goniometry. A controlled traction force of 10 newtons was applied along the limb axis, and great care was taken to avoid any contraction of the tibialis anterior muscle. The methods and device were previously published [15]. The control group consisted of lower limbs that tested negative on the Silfverskiöld test, and the measurement procedure used for this group was exactly the same as that employed for the IGT group.
2.2. Stanish Protocol
The program used in the IGT group was previously published by Stanish et al. [20,21], including stretching and eccentric exercises. The protocol was implemented during 4 weeks, five times a week, with rest days incorporated for muscle recovery and adaptation. Throughout the duration of the protocol, close monitoring of participants was conducted, and modifications were made if needed to ensure safety. Participants were advised to adhere to the prescribed program consistently and seek guidance from healthcare professionals or physical therapists if any discomfort was experienced. The control group did not complete the Stanish protocol and was used only as a baseline comparison.
2.3. Examinations
Within the laboratory setting, two distinct systems were employed for motion analysis. The first system, a ZEBRIS baropodometer, consisted of two ZEBRIS FDM platforms measuring a total of 628 × 62 × 2.5 cm (Zebris Medical GmbH, Isny, Germany). These platforms were equipped with a collective number of 34,048 receptors, with each receptor responsible for analyzing the distribution of plantar pressure. The second system was a VICON 3D motion analysis system, which utilized a configuration of 12 infrared cameras (6 VICON Bonita and 6 VICON Vantage, Oxford Metrics, UK). These cameras accurately recorded the positions of reflective markers on the patient’s body placed according to the plug-in gait lower body model. A set of 20 markers with a diameter of 14 mm was strategically positioned [26].
Calibration procedures were consistently applied to ensure precision and accuracy. Patients underwent the calibration process, during which markers were placed in specific anatomical positions, establishing neutral positions for both the knee (0 degrees of extension) and ankle (0 degrees of dorsiflexion). This meticulous calibration process allowed for the acquisition of reliable absolute angle data. Gait analysis was conducted based on each patient’s natural walking rhythm and speed, excluding the recording of acceleration and deceleration phases.
2.4. Assessment Criteria
The range of motion of the knee and ankle (in degrees) was recorded with the VICON system. According to our previous protocol, maximum ankle dorsiflexion during the midstance phase of the gait and the corresponding knee flexion angles at that very moment were collected [15]. Maximum forefoot pressure (newton/cm2/kg) and rearfoot contact time (% stance phase) were recorded with the ZEBRIS system. We did not collect these parameters during the initial contact phase, as this does not correspond to current knowledge on IGT pathogenesis and how it affects gait. Indeed, during the initial contact, the body weight is not applied to the foot, unlike during the midstance phase.
All assessment criteria were consistently collected during the same walking analysis and subsequently analyzed by an impartial observer. For each lower limb, a total of six gait passages, each approximately 12 m in length, were recorded. To ensure statistical validity, the mean value derived from the six passages was utilized for the analysis of each criterion and gait passage.
2.5. Statistics
Normality and heteroskedasticity of continuous data were assessed with the Shapiro–Wilk and Levene’s tests, respectively. Continuous outcomes were compared with the unpaired Student t-test, Welch t-test, or Mann–Whitney U test, according to the data distribution. Discrete outcomes were compared with the chi-squared or Fisher’s exact test accordingly. Effect sizes were assessed using Cohen’s d. Spearman’s coefficient was used to assess the correlation between the improvement in knee flexion induced by the Stanish protocol and the age. The alpha risk was set to 5%, and two-tailed tests were used. Statistical analysis was performed with EasyMedStat (version 3.25; www.easymedstat.com, accessed on 1 October 2023).
3. Results
Recruitment ran from 1 January 2016 to 1 January 2019. A total of 20 subjects was enrolled in the study: 10 individuals with asymptomatic bilateral IGT and 10 controls. Each lower limb in both groups was individually analyzed.
The two groups were comparable in age and weight but not gender (p = 0.02) (Table 1). The Silfverskiöld test confirmed that the study group had IGT and the control group did not (Table 1).

Table 1.
Demographics and Silfverskiöld test.
Prior to the Stanish protocol, the IGT group showed greater knee flexion compared with the control group during the midstance phase (respectively, 8.9 +/− 4.6 vs. 3.4 +/− 2.3, p < 0.001). There was no difference in maximum ankle dorsiflexion, maximum forefoot pressure, or rearfoot contact time between the groups at this time (Table 2).

Table 2.
Group comparison before the Stanish protocol.
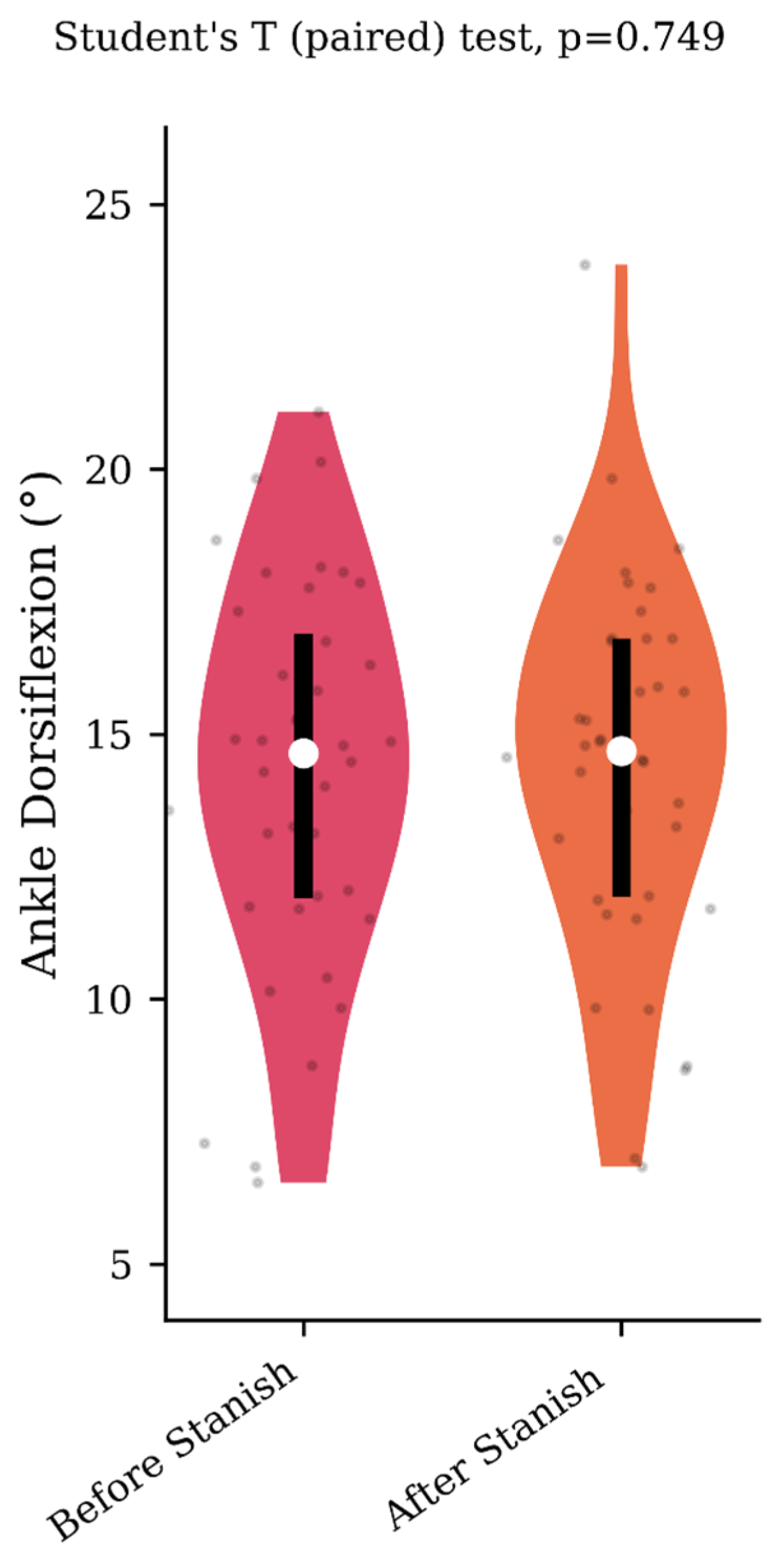
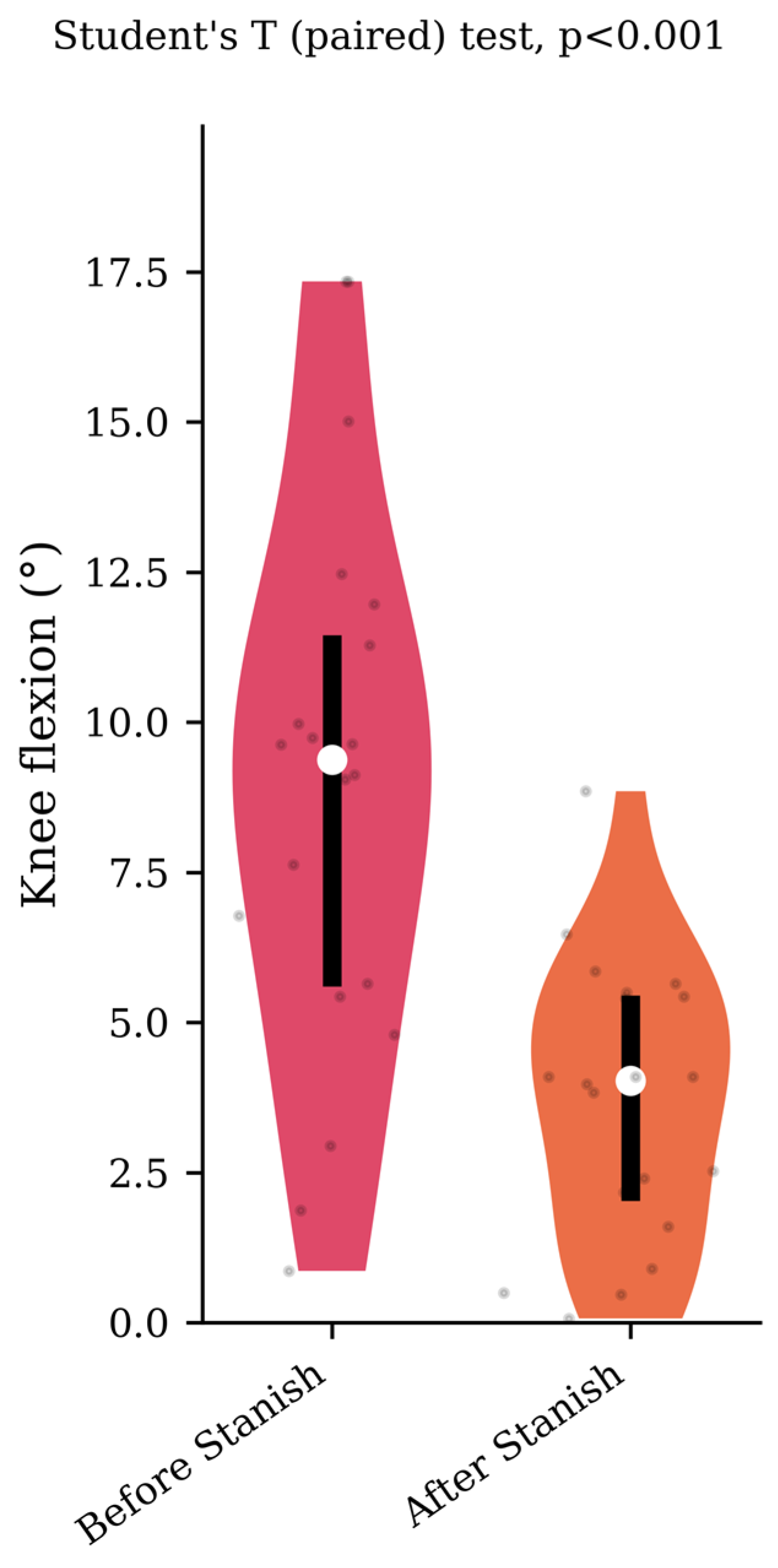
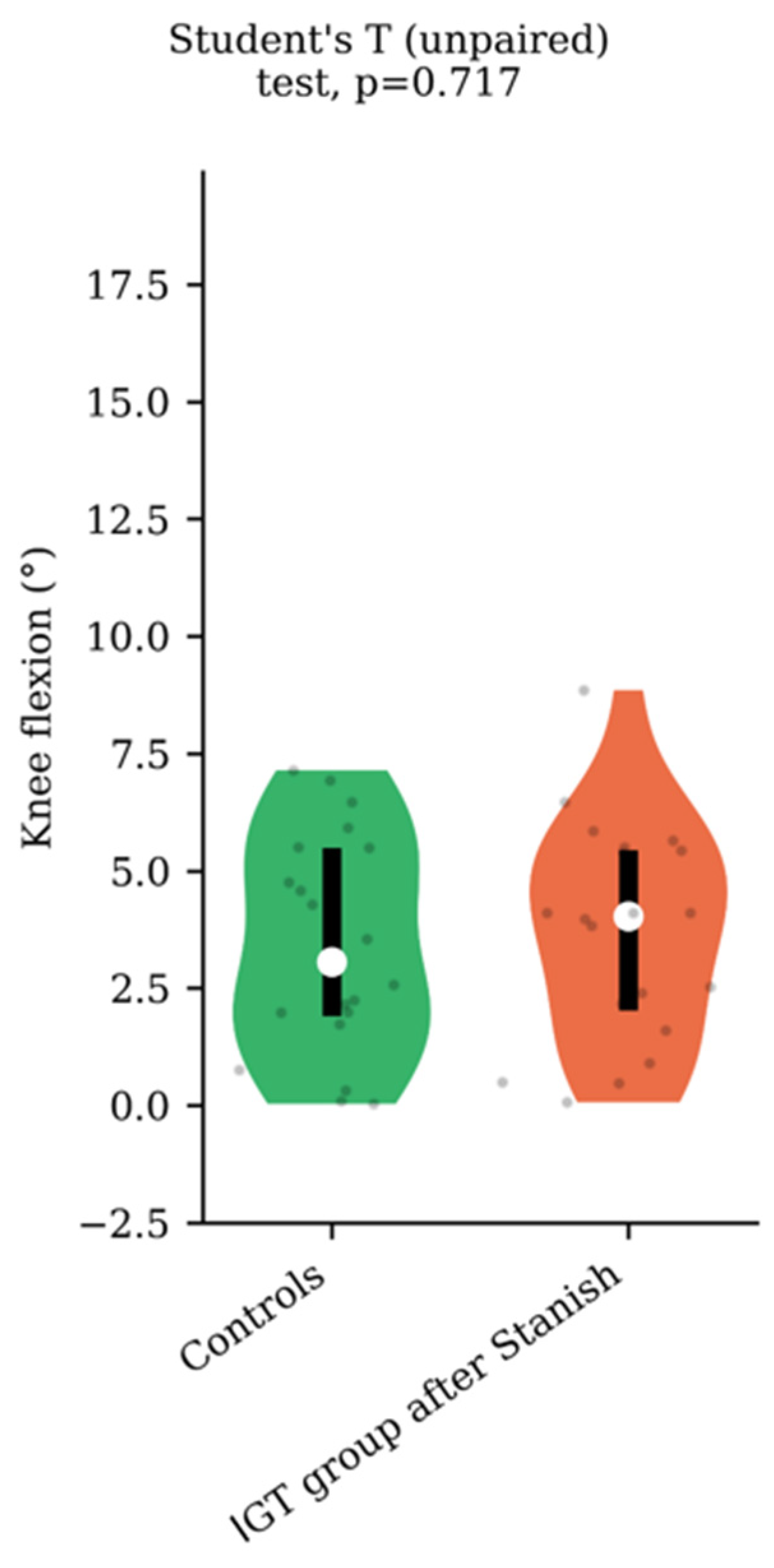
Regarding the range of motion analysis, the Stanish protocol did not change the maximum ankle dorsiflexion in the IGT group (13.5 +/− 4.1 to 13.8 +/− 4 degrees, p = 0.75, d = 0.07) (Figure 1), whereas it did change the corresponding knee flexion angle (8.9 +/− 4.6 to 3.7 +/− 2.3 degrees, p < 0.001, d = 1.43) (Figure 2). No correlation was found between the improvement in knee flexion induced by the Stanish protocol and the age (ρ = −0.17; r2 = 0.033; p = 0.484). Comparing the IGT knee flexion after Stanish with that of the control group showed no significant difference (3.7 +/− 2.3 degrees in IGT vs. 3.4 +/− 2.3 degrees in controls, p = 0.72, d = 0.13) (Figure 3).
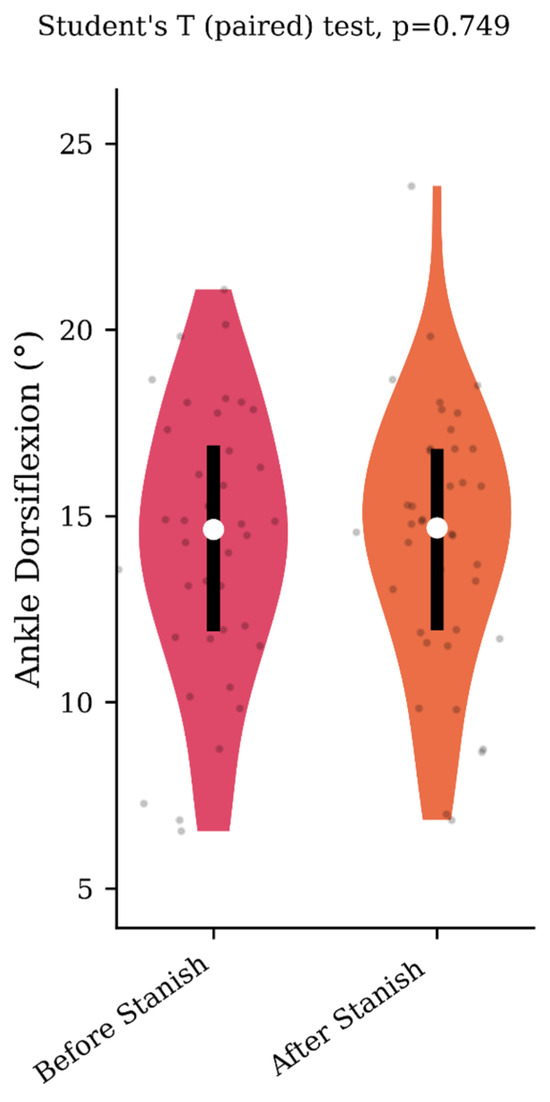
Figure 1.
Maximum ankle dorsiflexion during the midstance phase before and after a Stanish protocol in the IGT group (n = 20).
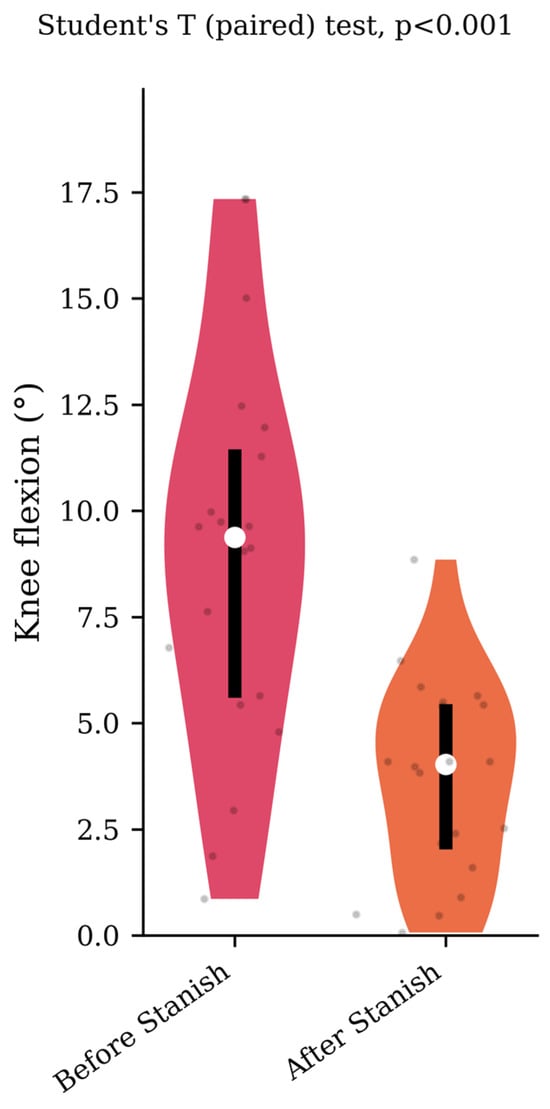
Figure 2.
Corresponding knee flexion before and after a Stanish protocol in the IGT group (n = 20).
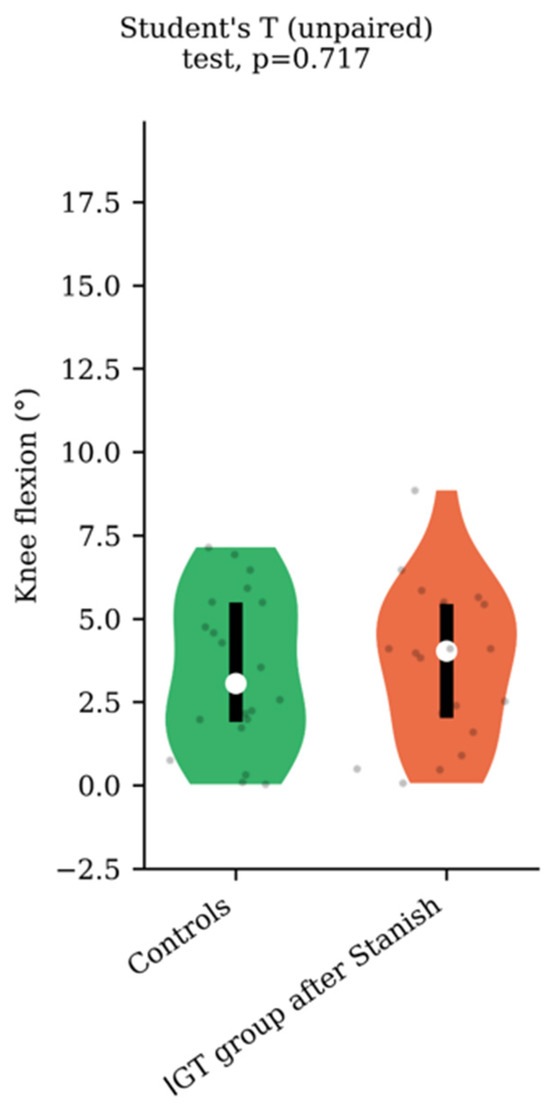
Figure 3.
Knee flexion comparison between IGT after Stanish protocol (n = 20) and controls (n = 20).
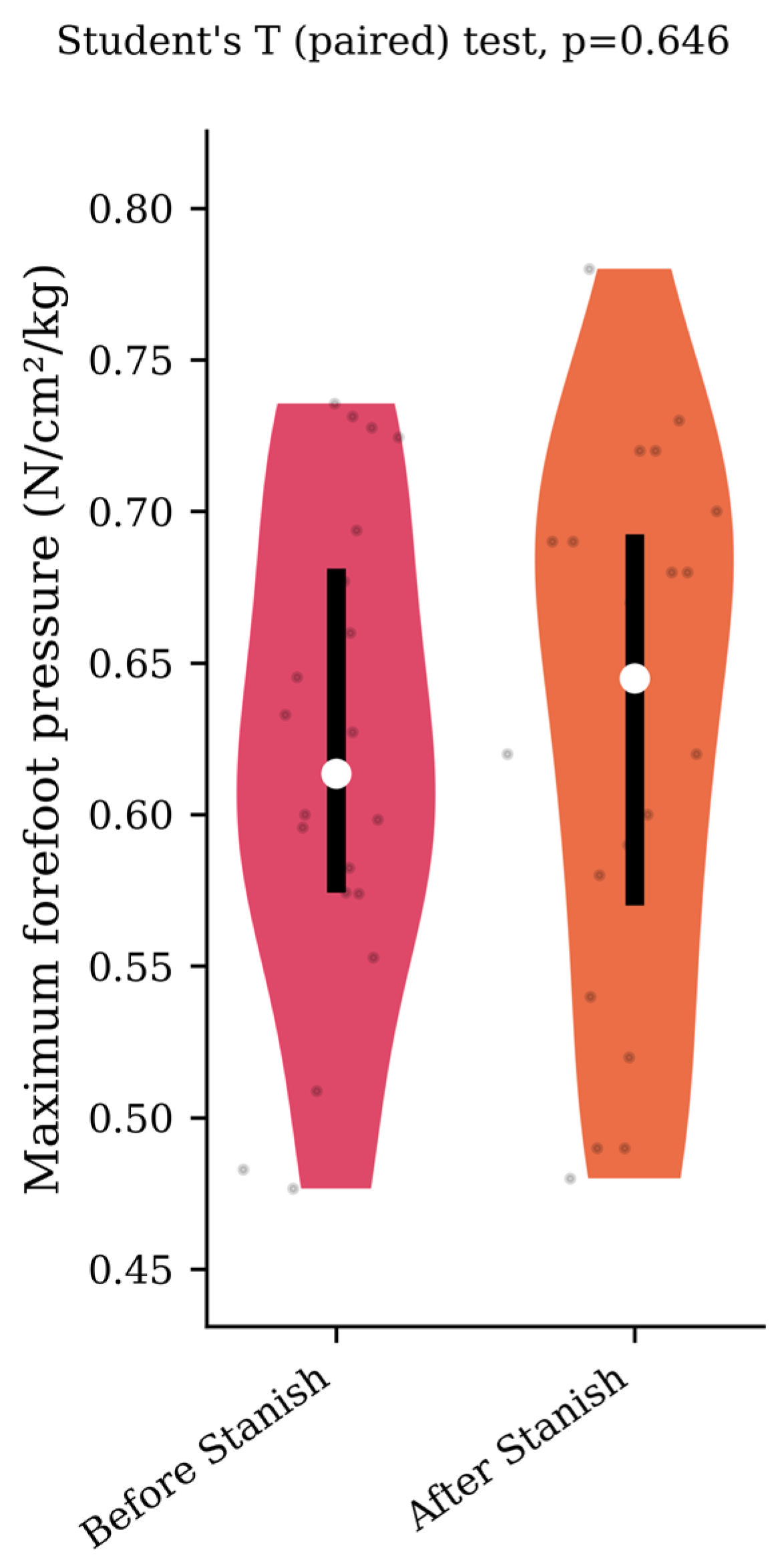
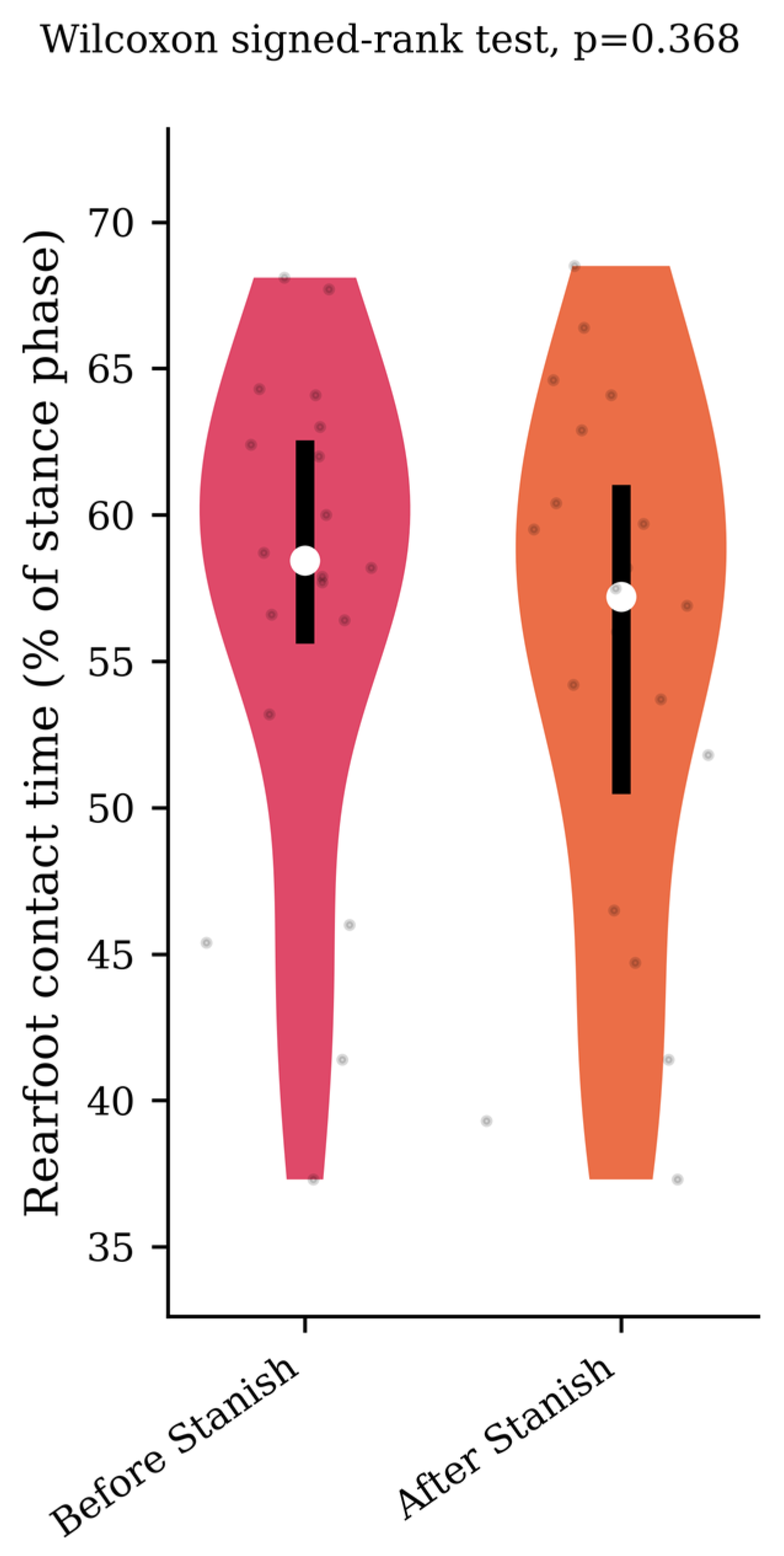
Regarding the plantar pressure analysis, the Stanish protocol did not change the maximum forefoot pressure (0.62 +/− 0.08 to 0.63 +/− 0.09 N/cm2/kg, p = 0.65, d = 0.12) (Figure 4), nor the rearfoot contact time (57 +/− 8.5 to 55.2 +/− 9.1 percent of the stance phase, p = 0.37, d = 0.2) (Figure 5) in the IGT group.
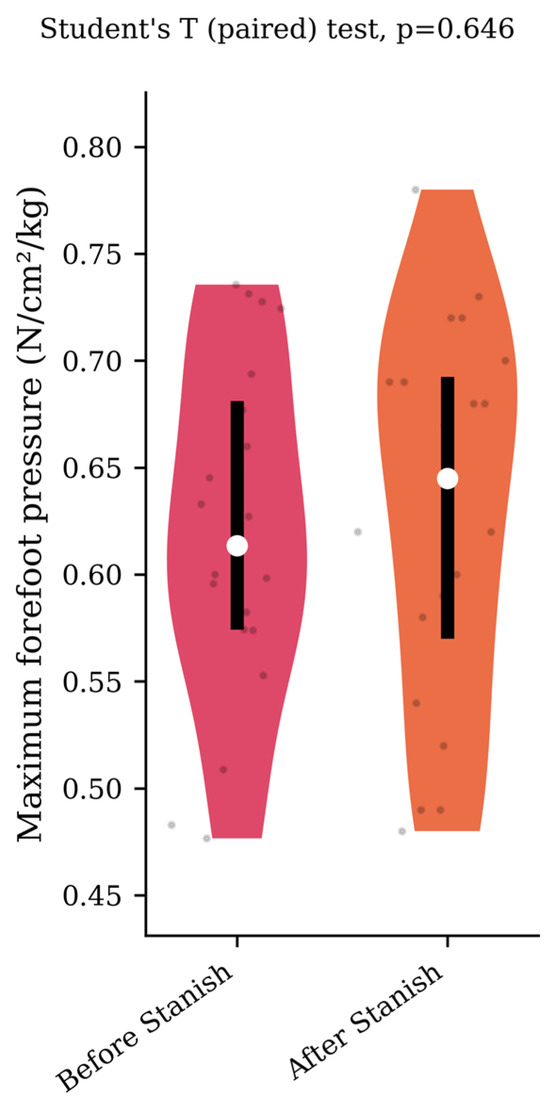
Figure 4.
Maximum forefoot pressure before and after a Stanish protocol in the IGT group (n = 20).
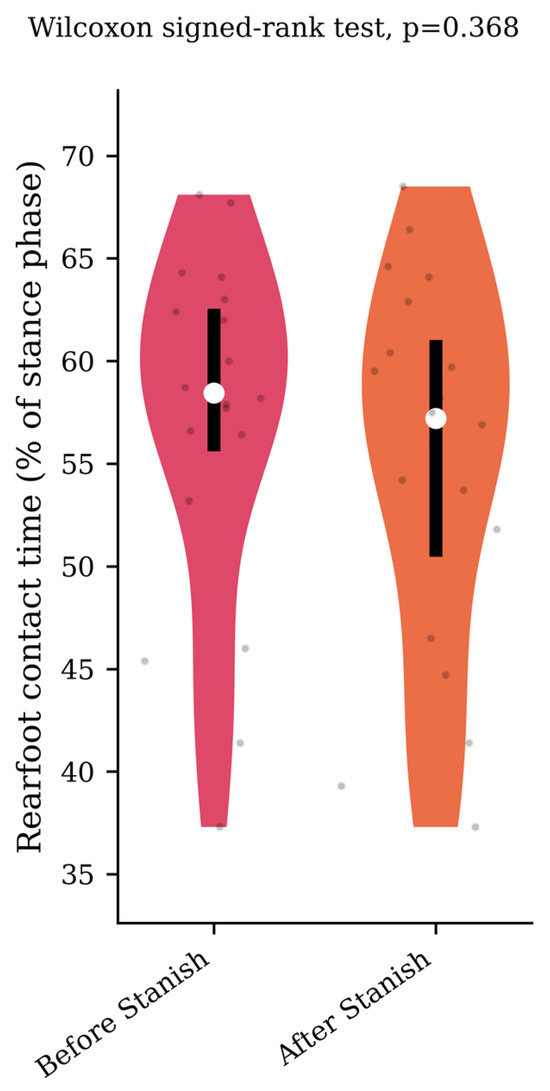
Figure 5.
Rearfoot contact time before and after a Stanish protocol in the IGT group (n = 20).
4. Discussion
One of the main findings of our study was the significant reduction in knee flexion observed in the IGT subjects following stretching and eccentric exercises performed according to the Stanish protocol. Going further, our study did not show any significant difference between the controls and IGT after the Stanish protocol, thus considering their gait to be normalized. Our hypothesis was therefore confirmed.
This phenomenon suggests that the protocol not only addresses gastrocnemius tightness but also contributes to a substantial improvement in gait parameters [20,21]. Our results are consistent with the clinical efficacy of the Stanish protocol as a self-management technique for IGT [27,28]. The reduction in knee flexion during gait observed in our study participants after the Stanish protocol indicates a potential corrective mechanism that aligns with the protocol’s objectives. Increased knee flexion might be a compensatory strategy to alleviate excessive pressure on the forefoot in individuals with IGT [15,29]. By targeting the gastrocnemius muscle, the Stanish protocol appears to mitigate the need for this compensatory knee flexion, resulting in a more normalized gait pattern.
Gastrocnemius tightness can contribute to a range of foot pathologies, and its impact on gait abnormalities has been the subject of various studies [13,14,15,30]. Our results provide empirical evidence that a Stanish protocol can not only mitigate the effects of gastrocnemius tightness on gait but also restore a more physiologically sound walking pattern. This has significant implications for the clinical management of IGT, emphasizing the importance of noninvasive self-management techniques that address the underlying biomechanical issues. Our findings also contribute to the understanding of the intricate interplay between muscle flexibility, joint mobility, and gait dynamics. These results underscore the protocol’s potential as a valuable tool in gait rehabilitation, particularly for individuals with IGT. Consequently, IGT-related foot and ankle pathologies, such as metatarsalgia, hallux valgus or rigidus, ankle instability, Achilles tendinopathy, or fasciitis, could benefit from the Stanish protocol [31,32,33,34]. However, the persistence of these effects over time and the potential for long-term gait improvement warrant further investigation.
Several limitations need to be acknowledged in the context of this study. Firstly, it is important to note that the individuals in our study did not present with painful pathologies such as metatarsalgia, hallux valgus, or Achilles tendinopathy, which are often associated with IGT. This absence of painful pathologies may have contributed to a potentially lower degree of tightness in our study participants compared with those experiencing associated discomfort. However, including individuals with painful conditions might have introduced complexities in analyzing gait parameters, making it challenging to isolate the effects of IGT. Another limitation relates to the method used to assess tightness. We employed a device that applies force, which is different from assessing IGT under weight-bearing conditions. The precise threshold distinguishing pathological IGT from nonpathological IGT remains scientifically unknown. Consequently, our study does not assert a direct comparison between patients with pathological IGT and controls; rather, it focuses on patients with varying degrees of IGT and their comparison with controls. Furthermore, it is important to emphasize that our study is based on a population with bilateral IGT. Drawing conclusions regarding a unilateral IGT population would be premature, as the relationship between limbs in such a context remains uncharted territory. Additionally, there was variation in the walking rhythms among our participants. We did not impose a specific walking rhythm, allowing participants to adopt their own comfortable walking speeds. This flexibility may have introduced selection bias and baseline intergroup differences, which should be considered when interpreting the results. However, we systematically asked the subjects to walk at their natural pace and speed. Because we carried out a before-and-after comparison of the IGT group, we can assume that each subject should not have changed his or her natural walking speed between the two tests. In terms of data collection, while reflective markers were positioned consistently by the same investigator using a standardized protocol and the system was calibrated daily, variations in joint centers could still occur. Although the measuring range of motion helped mitigate this bias, it is worth noting that knee flexion during the early stance phase, influenced by loading response, was more pronounced than the knee flexion during the midstance phase, which was the primary focus of our study in assessing gastrocnemius tightness. It is important to highlight the disparity in gender distribution between the IGT and control groups. Recognizing such discrepancies is important as they may introduce confounding variables, thereby potentially influencing the interpretation of our findings. No power calculations were performed in this study, which could limit the conclusions, particularly in the event of negative results. It would have been difficult to define a precise sample size for this study, as each method of quantitative gait analysis is unique to each team. This would not have changed the results of the fact that a Stanish protocol reduced knee flexion during the stance phase in IGT subjects, but it may have influenced our conclusions on the normalization and it should be noted that increasing the number of subjects may result in finding a small significant difference between the groups. However, we do not believe that this would substantially change the conclusions of this study. Finally, the VICON Plugin Gait lower body model provides ankle angles by considering the foot as a single, rigid segment. Compensation by other mid- and rearfoot joints might introduce bias in ankle angle measurements.
5. Conclusions
In conclusion, our study showed a normalization in gait patterns in IGT subjects following stretching and eccentric exercises performed as part of a Stanish protocol. This supports the efficacy of this protocol as a self-management technique for IGT-related foot and ankle pathologies. This approach could also be valuable in a prophylactic context, preventing decompensation in asymptomatic IGT individuals. However, future longitudinal studies are needed to determine the durability of these effects.
Author Contributions
Conceptualization, F.B. and M.L.; methodology, J.B., E.H. and M.L.; investigation, A.H., L.M. and R.S.; statistics, T.A.; writing, A.H. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Rouen University Hospital, France (#E2017-26).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DiGiovanni, C.W.; Kuo, R.; Tejwani, N.; Price, R.; Hansen, S.T.J.; Cziernecki, J.; Sangeorzan, B.J. Isolated Gastrocnemius Tightness. JBJS 2002, 84, 962. [Google Scholar] [CrossRef] [PubMed]
- Jastifer, J.R.; Marston, J. Gastrocnemius Contracture in Patients With and Without Foot Pathology. Foot Ankle Int. 2016, 37, 1165–1170. [Google Scholar] [CrossRef]
- Antończak, P.P.; Hartman-Petrycka, M.; Garncarczyk, A.; Adamczyk, K.; Wcisło-Dziadecka, D.; Błońska-Fajfrowska, B. The Effect of Callus and Corns Removal Treatments on Foot Geometry Parameters, Foot Pressure, and Foot Pain Reduction in Women. Appl. Sci. 2023, 13, 4319. [Google Scholar] [CrossRef]
- Chan, O.; Malhotra, K.; Buraimoh, O.; Cullen, N.; Welck, M.; Goldberg, A.; Singh, D. Gastrocnemius Tightness: A Population Based Observational Study. Foot Ankle Surg. 2019, 25, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, C. Rôle pathogénique de la brièveté du gastrocnémien dans les métatarsalgies. Médecine Chir. Pied 2004, 20, 3–5. [Google Scholar] [CrossRef]
- Hill, R.S. Ankle Equinus. Prevalence and Linkage to Common Foot Pathology. J. Am. Podiatr. Med. Assoc. 1995, 85, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Cazeau, C.; Stiglitz, Y. Effects of Gastrocnemius Tightness on Forefoot During Gait. Foot Ankle Clin. 2014, 19, 649–657. [Google Scholar] [CrossRef]
- Chen, G.; Liao, Q.; Luo, W.; Li, K.; Zhao, Y.; Zhong, D. Triceps-Sparing versus Olecranon Osteotomy for ORIF: Analysis of 67 Cases of Intercondylar Fractures of the Distal Humerus. Injury 2011, 42, 366–370. [Google Scholar] [CrossRef]
- Crisco, J.J.; Halilaj, E.; Moore, D.C.; Patel, T.; Weiss, A.-P.C.; Ladd, A.L. In Vivo Kinematics of the Trapeziometacarpal Joint during Thumb Extension-Flexion and Abduction-Adduction. J. Hand Surg. 2015, 40, 289–296. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Boulton, A.J.M. Ankle Equinus Deformity and Its Relationship to High Plantar Pressure in a Large Population with Diabetes Mellitus. J. Am. Podiatr. Med. Assoc. 2002, 92, 479–482. [Google Scholar] [CrossRef]
- El-Hawary, R.; Karol, L.A.; Jeans, K.A.; Richards, B.S. Gait Analysis of Children Treated for Clubfoot with Physical Therapy or the Ponseti Cast Technique. J. Bone Joint Surg. Am. 2008, 90, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Lofterød, B.; Fosdahl, M.A.; Terjesen, T. Can Persistent Drop Foot After Calf Muscle Lengthening Be Predicted Preoperatively? J. Foot Ankle Surg. 2009, 48, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Chimera, N.J.; Castro, M.; Davis, I.; Manal, K. The Effect of Isolated Gastrocnemius Contracture and Gastrocnemius Recession on Lower Extremity Kinematics and Kinetics during Stance. Clin. Biomech. 2012, 27, 917–923. [Google Scholar] [CrossRef] [PubMed]
- You, J.-Y.; Lee, H.-M.; Luo, H.-J.; Leu, C.-C.; Cheng, P.-G.; Wu, S.-K. Gastrocnemius Tightness on Joint Angle and Work of Lower Extremity during Gait. Clin. Biomech. 2009, 24, 744–750. [Google Scholar] [CrossRef]
- Lalevée, M.; Menez, C.; Roussignol, X.; Hue, A.G.; Dujardin, F.; Dodelin, D.; Dechelotte, B.; Lintz, F. A Comparative Study between Isolated Gastrocnemius Tightness Patients and Controls by Quantitative Gait Analysis and Baropodometry. Foot Ankle Surg. 2021, 27, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, C.C.; Phisitkul, P.; Belatti, D.A.; Glazebrook, M.A.; DiGiovanni, C.W. Gastrocnemius Recession for Foot and Ankle Conditions in Adults: Evidence-Based Recommendations. Foot Ankle Surg. 2015, 21, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Knapik, D.M.; LaTulip, S.; Salata, M.J.; Voos, J.E.; Liu, R.W. Impact of Routine Gastrocnemius Stretching on Ankle Dorsiflexion Flexibility and Injury Rates in High School Basketball Athletes. Orthop. J. Sports Med. 2019, 7, 2325967119836774. [Google Scholar] [CrossRef] [PubMed]
- Barouk, P. Technique, Indications, and Results of Proximal Medial Gastrocnemius Lengthening. Foot Ankle Clin. 2014, 19, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Riiser, M.O.; Husebye, E.E.; Hellesnes, J.; Molund, M. Outcomes After Proximal Medial Gastrocnemius Recession and Stretching vs Stretching as Treatment of Chronic Plantar Fasciitis at 6-Year Follow-Up. Foot Ankle Int. 2023, 10711007231205559, Epub ahead of print. [Google Scholar] [CrossRef]
- Stanish, W.D.; Rubinovich, R.M.; Curwin, S. Eccentric Exercise in Chronic Tendinitis. Clin. Orthop. 1986, 208, 65–68. [Google Scholar] [CrossRef]
- Fyfe, I.; Stanish, W.D. The Use of Eccentric Training and Stretching in the Treatment and Prevention of Tendon Injuries. Clin. Sports Med. 1992, 11, 601–624. [Google Scholar] [CrossRef] [PubMed]
- Barouk, P.; Barouk, L.S. Clinical Diagnosis of Gastrocnemius Tightness. Foot Ankle Clin. 2014, 19, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Silfverskiold, N. Reduction of the Uncrossed Two-Joints Muscles of the Leg to One-Joint Muscles in Spastic Conditions. Acta Chir Scandinav 1924, 56, 315–330. [Google Scholar]
- Lalevée, M.; Barbachan Mansur, N.S.; Schmidt, E.; Carvalho, K.; Vandelune, C.; Bernasconi, A.; Wilken, J.; de Cesar Netto, C. Does Tibialis Posterior Dysfunction Correlate with a Worse Radiographic Overall Alignment in Progressive Collapsing Foot Deformity? A Retrospective Study. Foot Ankle Surg. Off. J. Eur. Soc. Foot Ankle Surg. 2022, 28, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Lalevée, M.; Beldame, J.; L’Hermette, M.; Brunel, H.; Dujardin, F.; Billuart, F. Reliability of a New Computerized Equinometer Based on Silfverskiöld Test to Measure Gastrocnemius Tightness. PLoS ONE 2023, 18, e0284279. [Google Scholar] [CrossRef]
- Lower Body Modeling with Plug-in Gait-Nexus 2.5 Documentation-Vicon Documentation. Available online: https://docs.vicon.com/display/Nexus25/Lower+body+modeling+with+Plug-in+Gait (accessed on 25 June 2023).
- Zvetkova, E.; Koytchev, E.; Ivanov, I.; Ranchev, S.; Antonov, A. Biomechanical, Healing and Therapeutic Effects of Stretching: A Comprehensive Review. Appl. Sci. 2023, 13, 8596. [Google Scholar] [CrossRef]
- Stecco, C.; Pirri, C.; Fede, C.; Yucesoy, C.A.; De Caro, R.; Stecco, A. Fascial or Muscle Stretching? A Narrative Review. Appl. Sci. 2021, 11, 307. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Yang, C.-M.; Kim, J.-J. Effectiveness of Combined Stretching and Strengthening Exercise Using Rehabilitation Exercise System with a Linear Actuator and MR Damper on Static and Dynamic Sitting Postural Balance: A Feasibility Study. Appl. Sci. 2021, 11, 7329. [Google Scholar] [CrossRef]
- Marouvo, J.; Sousa, F.; Fernandes, O.; Castro, M.A.; Paszkiel, S. Gait Kinematics Analysis of Flatfoot Adults. Appl. Sci. 2021, 11, 7077. [Google Scholar] [CrossRef]
- Steinberg, N.; Tenenbaum, G.; Zeev, A.; Witchalls, J.; Waddington, G. The Relationship between the Ability to Cope with Unexpected Perturbations and Mechanical and Functional Ankle Instability. Appl. Sci. 2022, 12, 11119. [Google Scholar] [CrossRef]
- Lalevée, M.; Anderson, D.D.; Wilken, J.M. Current Challenges in Chronic Ankle Instability. Foot Ankle Clin. 2023, 28, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Križaj, L.; Kozinc, Ž.; Šarabon, N. The Outcomes of Conservative Nonpharmacological Treatments for Achilles Tendinopathy: An Umbrella Review. Appl. Sci. 2022, 12, 12132. [Google Scholar] [CrossRef]
- Agostini, F.; Mangone, M.; Finamore, N.; Di Nicola, M.; Papa, F.; Alessio, G.; Vetrugno, L.; Chiaramonte, A.; Cimbri, G.; Bernetti, A.; et al. The Efficacy of Instrumental Physical Therapy through Extracorporeal Shock Wave Therapy in the Treatment of Plantar Fasciitis: An Umbrella Review. Appl. Sci. 2022, 12, 2841. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).