Abstract
This study addresses challenges faced by the packaging industry in finding suitable natural and biodegradable materials that can replace plastics while preserving the superior quality and freshness of the items contained within. Chitosan, a biodegradable natural polymer, shows great potential as a matrix for ecofriendly and biodegradable composite materials. In the present study, bioactive substances such as caffeine (CAF) and propolis extract (EP) were used for the enhancement of the bioactivity of chitosan-based films. Two acidic solvents, acetic acid and citric acid, were used to produce chitosan films. The study examined the antioxidant capabilities of the solutions used for film formation; similarly, the characteristics of the resultant films were also examined, encompassing antimicrobial, barrier, and mechanical characteristics. The findings suggested that the use of additives exhibiting antioxidant activity, such as CAF and EP in the chitosan matrix can be an effective method to counteract oxidative stress in food packaging. The study also showed that films produced with citric acid exhibit antimicrobial activity against many strains of bacteria, including foodborne pathogens. In addition, the antimicrobial activity of chitosan/citric acid film can be increased by adding CAF and EP. The results confirmed that both the additives and the acids used affect the mechanical and barrier features of the obtained chitosan-based films. This study suggests that chitosan films supplemented with natural bioactive substances have the potential to serve as viable replacements for traditional plastics in the packaging sector.
1. Introduction
Nowadays, plastics are materials commonly used in the packaging sector. This can be attributed to their easy accessibility, affordability, and exceptional longevity. Unfortunately, the durability of plastics does not necessarily yield positive results. A substantial time investment is necessary to break them down, which is a serious problem if they are not properly disposed of. Due to the observed degradation of the environment associated with excessive consumption and the increase in consumer awareness, the packaging industry is facing the challenge of finding appropriate materials that could replace plastics. This challenge is being addressed by the quest for new, natural and biodegradable materials that will be ecofriendly, while, like plastics, being noted for their advantageous mechanical or optical properties. In addition, these materials, through the use of appropriate additives, can ensure the preservation of high quality and freshness of packaged products [1,2].
The initial purpose of food packaging is to safeguard the enclosed food while it is being transported or stored. Moreover, it is important for the packaging to shield the packaged food from the detrimental impact of external factors such as UV radiation and to exhibit appropriate mechanical or barrier properties. Packaging serves the essential purpose of maximizing the lifespan of food products, preserving their quality, and protecting them from external factors that could accelerate spoilage or compromise their nutritional properties [3]. The greatest food losses during production and transport are caused by microbial transformations. One way to protect finished food products from negative external influences is packaging. However, traditional packaging does not guarantee that food will be durable and fully safe for consumers. This is because many products succumb to microbial contamination during or even after the final stages of production. In such cases, protecting food with traditional packaging is insufficient. This is because a number of undesirable microorganisms, both pathogenic and spoilage-causing, can thrive in contaminated products packaged in traditional packaging. These include pathogenic strains of the genus Listeria, Pseudomonas, Salmonella, Clostridium, Brochothrix and psychrophilic molds, among others [4,5]. Reducing the growth of these microorganisms is possible by replacing traditional packaging, or the so-called passive packaging, with active packaging. One form of active packaging that is attracting increasing interest from consumers and food manufacturers is active antimicrobial packaging. Such packaging can be produced with components that exhibit natural activity, such as, e.g., chitosan, or gain such activity following their physical or chemical modification. Many chemical compounds have proven to be agents through which antimicrobial activity can be imparted to packaging [5].
Packaging materials that are both natural and biodegradable are typically composed of film-forming substances, commonly from proteins, polysaccharides, lipids, and resins, either individually or as composite materials [1,5]. Studies have shown that chitosan is an effective packaging material because it is a biodegradable natural polymer. Chitosan is a polysaccharide that offers various advantages, including lack of toxicity, biocompatibility and ability to break down naturally. Derived from the shells of crustaceans, which are a byproduct of the seafood industry [6]. This versatile material finds applications in several industries, including medicine, agriculture, textiles, food, and environmental protection [7,8]. Also, chitosan exhibits great potential as a biopolymer used for the production of functional and natural packaging [9,10,11]. Therefore, it holds significant promise as a suitable choice for serving as a base material in natural and biodegradable composite materials, thanks to its multitude of advantageous characteristics. While chitosan solutions have demonstrated antimicrobial capabilities against specific pathogenic bacteria, chitosan films produced from these solutions do not seem to retain these properties. This discrepancy is probably attributable to the way chitosan binds within the films, limiting immediate interaction between its molecular chains and microbial cells [12]. Therefore, researchers are exploring the addition of natural components, for example, essential oils or botanical extracts, to enhance the microbial activity of chitosan films [13,14].
Propolis is a natural material gathered by honeybees from resinous and balsamic plant materials, including flowers, pollen, branches and tree exudates [15]. Throughout history, bee glue has been employed in traditional medicinal practices because of the various advantageous qualities found in its extracts. These include antibacterial, antifungal, anticancer, anti-inflammatory, and antioxidant properties, inter alia [16,17]. The diverse biological effects of propolis extract are linked to their intricate chemical composition. According to the literature data, propolis samples from diverse geographic sources encompass phenolic compounds (flavonoids, aromatic acids, and their esters), alcohols, terpenes, enzymes, vitamins, amino acids, sugars, and minerals [16,18]. Propolis extracts exhibit activity against a wide spectrum of bacterial strains, including microorganisms that cause foodborne illnesses, such as Staphylococcus aureus, Listeria monocytogenes, Bacillus cereus, Salmonella Enteritidis, Escherichia coli and Pseudomonas fluorescens [19,20,21]. As a result, propolis extracts have been incorporated as additives in films composed of biopolymers, among others, chitosan [9,22,23,24,25,26].
Caffeine is an organic chemical compound (1,3,7-trimethylxanthine) found in coffee, tea, energy drinks and some carbonated beverages [27]. It stands as one of the most widely recognized and frequently consumed stimulants on a global scale [28,29]. Caffeine is a chemical compound that exhibits antimicrobial and antioxidant properties, making it a potentially important ingredient in many pharmaceutical and cosmetic products [29,30,31]. Caffeine displays potent antioxidant characteristics, shielding the body from harmful free radicals and potentially lowering the likelihood of diseases linked to oxidative stress [32]. Coffee extracts exhibit antimicrobial activity against Gram-positive bacteria (S. aureus, L. monocytogenes, Streptococcus mutans), Gram-negative bacteria (E. coli) and yeast (Candida albicans) [33,34,35]. The literature data show that caffeine as an additive can be used in the production of natural biopolymer films and affect their mechanical or antioxidant properties [36].
In this investigation, films made from chitosan obtained from crabs were produced, incorporating both propolis extract (EP) and caffeine (CAF). Two solvents, acetic acid and citric acid, were employed to formulate solutions for film production. The primary objective of this research was to assess the functional attributes of these materials, which encompass mechanical properties, oxygen and water vapor barrier properties, structural features, and antibacterial activity, with the aim of evaluating appropriateness for application in the food packaging sector.
2. Materials and Methods
2.1. Preparation of Chitosan Films
The film-forming solutions were produced based on methods described in our previous paper [37]. In this study, 4 g of chitosan from crab shells (Sigma Aldrich, Darmstadt, Germany) was dissolved in 400 mL of a 3% acetic acid solution (Avantor Performance Materials, Gliwice, Poland) and a 3% citric acid solution (Avantor Performance Materials, Gliwice, Poland). The chitosan solutions (400 mL) were blended with caffeine (CAF) (Sigma Aldrich, Darmstadt, Germany) to achieve the CAF concentration of 1% and combined with 1.5 mL of Tween-20 (Sigma Aldrich, Darmstadt, Germany). Subsequently, the chitosan–CAF solutions (400 mL) were combined with an ethanolic extract of propolis (EP) (PROP-MAD, Poznań, Poland) to achieve an EP concentration of 1%. The solutions were thoroughly mixed using a homogenizer and then poured into Petri dishes lined with Teflon. Finally, the films were left to dry at room temperature.
Following the experiment, a total of six distinct film samples were produced, and their labels are detailed in Table 1.

Table 1.
Symbols of film samples.
2.2. Antiradical Properties
The antiradical activity of all film-forming solutions was assessed using the DPPH (2,2-diphenyl-1-picrylhydrazyl, Sigma Aldrich, Darmstadt, Germany) radical scavenging assay.
Each chitosan-based solution (3 µL) was added to 297 µL of deionized water and vortexed. Subsequently, 300 µL of a 0.1 mM DPPH ethanol solution was added to each sample. The samples were vortexed and incubated for 30 min at room temperature in the dark. After incubation, the samples were vortexed, and the absorbance values of the solutions were measured at a wavelength of 517 nm using a BioMateTM 160 UV-Visible spectrophotometer (Thermo Scientific, Waltham, MA, USA). Each sample was replicated three times, and each experiment was performed thrice. Trolox (Sigma Aldrich, Darmstadt, Germany) was used as the reference antioxidant. The antiradical properties of the chitosan-based solutions and Trolox were calculated using the equation:
where A0 is the absorbance value of the control sample, and A1 is the absorbance value of the test sample. The results (n = 9) are presented as mean values ± standard deviation (SD).
2.3. Antibacterial Activity
The antimicrobial activities of chitosan films were determined via the diffusion method. The indicator stains used were pathogenic or potentially pathogenic bacteria strains: Bacillus subtilis (food isolate), Enterococcus faecalis (food isolate), Listeria monocytogenes (ATCC 15313), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 10536), Pseudomonas aeruginosa (ATCC 15443), Pseudomonas fluorescens (food isolate), Salmonella enterica (clinical isolate), and probiotic bacteria strains: Lacticaseibacillus rhamnosus GG (ATCC 53103), Lactiplantibacillus plantarum 299v (isolated from Sanprobi IBS, Sczecin, Poland) and Lacticaseibacillus paracasei (CNCM I-1572). The study films (discs with a diameter of 10 mm) were applied to Petri dishes with Mueller–Hilton Agar (Graso, Jabłkowo, Poland) inoculated with 107 CFU/mL indicator strains (24 h culture in a nutrient broth with the addition of 2% glucose). After 24 h incubation at 35 ± 2 °C, the diameters of the zones of inhibition formed around the discs of chitosan films were measured in millimeters using a Computer Scanning System (MultiScaneBase v14.02).
2.4. Oxygen Transmission Rate (OTR)
The oxygen transmission rate (OTR) of the prepared films was determined using the Oxysense 325i apparatus equipped with special OTR measurement chambers (Industrial Physics, Boston, MA, USA). The measurements were performed according to ASTM F3136—15 standard [38]. Test samples were shaped into squares (6.5 cm on each side). The test conditions were as follows: a temperature of 23 °C and a relative humidity (RH) of 65%. The OTR test was performed three times for each sample variant.
2.5. Water Vapor Transmission Rate (WVTR)
The water vapor transmission rate (WVTR) of the films was measured according to the standard ISO 2528:2017 [39]. To conduct the measurements, vessels containing 10 g of anhydrous calcium chloride (Avantor Performance Materials, Gliwice, Poland) were covered with a film sample, sealed tightly, and placed inside a desiccator that contained a saturated solution of sodium chloride (Avantor Performance Materials, Gliwice, Poland) with a relative humidity (RH) of 65% and a temperature of 23 °C. The vessels were then weighed accurately to 0.001 g. The water vapor transmission rate (WVTR) was calculated using the following Formula (2):
where WVTR is the water vapor transmission rate (g/m2·24 h), m (g) is the mass increase and A (m2) is the water vapor transmission area.
2.6. Sorption Experiments
Before the sorption experiment, a set of produced chitosan film samples (10 mm in diameter) was stored in a desiccator over phosphorus pentoxide (P2O5) (Sigma Aldrich, Darmstadt, Germany). Equilibrium moisture content (EMC) measurements were conducted using a dynamic vapour sorption apparatus (DVS Advantage 2, Surface Measurement Systems, London, UK) at a temperature of 25 °C. The schedule of the sorption experiment consists of an initial phase of sample equilibration in dry nitrogen at a flow rate of 150 cm3·s−1 to obtain a dry state. The initial mass of each sample was 10 ± 1 mg. The EMCs were determined in the adsorption mode from 0 to 0.80 relative humidity (RH). A sample was considered to have reached equilibrium at a given RH when the change in mass was less than 0.0005%·min−1 over a 10 min window. The EMC data were used to determine experimental adsorption isotherms.
2.7. Sorption Modelling
Adsorption isotherms were calculated using the generalized D’Arcy and Watt (GDW) sorption model [40,41,42], given by the formula:
where m is the maximum amount of monolayer water content (kg/kg), K and k are the kinetic constant related to sorption on primary and secondary sorption centers, respectively, w is the ratio of water molecules bound to primary sorption centers and converted into secondary sorption centers, and RH is the air relative humidity (-). The coefficients of the GDW model were estimated using the Levenberg–Marquardt approach. Calculations were performed using STATISTICA 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA).
2.8. Scanning Electron Microscopy (SEM)
The morphology of the chitosan-based films was measured using an Evo 40 scanning electron microscope (Zeiss, Oberkochen, Germany) equipped with a secondary electron detector.
2.9. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
The Nicolet iS5 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a Fourier transform was employed to record the spectra relating to chitosan-based film samples. The spectra were obtained with a resolution of 4 cm−1, covering a range from 4000 to 600 cm−1, and recorded by averaging 32 scans.
2.10. Mechanical Properties
The mechanical properties of the produced films were evaluated with an Instron Universal Testing Machine (Model 5965, Instron, Norwood, MA, USA) in accordance with the standard (ASTMD882-12) [43]. Tensile strength (TS) and elongation at break (EB) were among the mechanical properties measured, and the average results from ten samples were reported.
2.11. Statistical Analysis
Statistical analyses encompassed a factorial one-way ANOVA, which was followed by Tukey’s honest significant difference (HSD) test at a significance level (α) of 0.05. These analyses were conducted using TIBCO Software Inc. Statistica version 13.3 (Palo Alto, CA, USA).
3. Results and Discussion
3.1. Antiradical Activity of Film-Forming Solutions
Examining the antioxidant properties of materials with potential applications in food packaging is of significant importance, especially in the context of their ability to mitigate food oxidation during storage [3]. In order to prevent food oxidation, new solutions which will improve the antioxidant properties of packaged food are sought in the packaging industry. The solution may be provided by packaging materials made of natural polymers with additives showing proven antioxidant properties [44,45,46].
The antiradical activity was investigated in the chitosan-based solution with CAF and EP prepared with two acids, acetic and citric, and the results are presented in Figure 1.
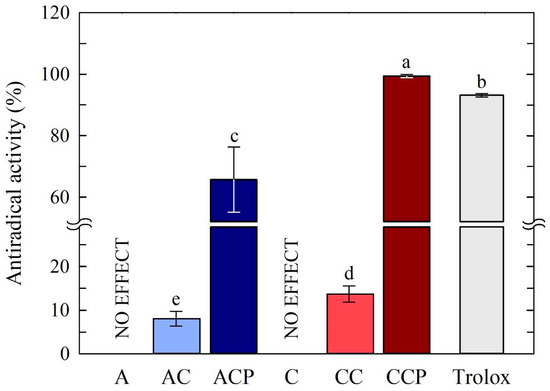
Figure 1.
Antiradical activity of chitosan-based solutions. Different letters indicate samples that were significantly different (p < 0.05).
The outcomes of the antiradical activity tests revealed that chitosan solutions lacking the inclusion of natural active components (EP and CAF) exhibited no ability to capture free radicals, regardless of the acid used. The addition of CAF resulted in a minor elevation in the antioxidant activity of the tested film-forming solutions—sample AC (8.07%) and sample CC (17.47%). The value of antiradical activity in chitosan solutions was found to increase after the addition of CAF and EP to the solutions. Moreover, when comparing the activity of the chitosan-based solutions with both natural additives, it was observed that the acid used to dissolve the chitosan also affected the antioxidant activity of the final solutions. Statistical analysis indicates that the greatest antiradical properties were exhibited by a solution consisting of chitosan prepared with citric acid and the addition of CAF and EP. This ability to scavenge free radicals was higher (99.42%) than for standard antioxidant Trolox (92.82%). This high antiradical activity of the chitosan/citric acid film with propolis and caffeine may be due to the well-known antioxidant properties of caffeine and propolis. The high antioxidant activity of propolis extracts has been confirmed in numerous literature reports [47,48,49]. The antioxidant effect of caffeine is related to its ability to neutralize free radicals [28,32], while ethanolic propolis extract (EP) comprises numerous compounds, notably flavonoids and phenolic acids, known for their potent antioxidant properties [47,50,51,52].
The use of additives such as caffeine and propolis extract exhibiting antioxidant effects to chitosan-forming solutions may be an effective method to mitigate oxidative stress in food packaging.
3.2. Antibacterial Activity of Films
One of the primary factors leading to the hastened deterioration of food is its contamination with microorganisms during processing or transportation [53]. Hence, there is a quest for innovative and environmentally friendly solutions to facilitate food packaging while simultaneously shielding against the detrimental impact of microorganisms. In this context, the use of films featuring antimicrobial properties seems to be a good solution, with chitosan films being promising examples.
The effectiveness of chitosan-based films in inhibiting the growth of pathogenic and probiotic bacterial strains was assessed, and the findings are outlined in Table 2.

Table 2.
The antibacterial activity of chitosan-based films.
The obtained results showed that the films were characterized by varied activity against pathogenic bacteria. All films, also with natural additives, obtained using acetic acid to dissolve chitosan, showed no antimicrobial activity against any tested strains of bacteria. On the other hand, films with citric acid used as a chitosan solvent showed activity against all the tested strains of pathogenic bacteria—the inhibition zone ranged from 17 to >28 mm. The exception was the chitosan film without additives, which was not active against S. aureus. The findings indicated that utilizing citric acid as a solvent positively impacted the antimicrobial effectiveness of the resulting films. None of the films made from chitosan exhibited any activity against probiotic bacterial strains. This is a very favorable result and shows that the obtained films can be used in packaging functional food which contains such strains, and their elimination would be unfavorable. Chitosan-based films can also serve as a beneficial option for encapsulating probiotic strains. The results obtained align with existing literature, affirming the potential utility of chitosan materials for encapsulating viable probiotic bacteria, among other applications [54,55].
Literature data show that caffeine exhibits antibacterial activity against P. fluorescens, E. coli and P. aeruginosa [30,31]. However, the antibacterial activity of the caffeine-chitosan films was comparable and, in the case of P. aeruginosa and P. fluorescens, even lower than that of films prepared with chitosan alone. The films with the most substantial antibacterial effectiveness were those produced using chitosan dissolved in citric acid, along with the inclusion of CAF and EP. Numerous literature data indicate that propolis extract has antibacterial activity against many strains of pathogenic bacteria, mainly Gram-positive, such as S. aureus, B. subtilis, B. cereus and E. faecalis. However, it is less effective against Gram-negative bacteria, including E. coli or P. aeruginosa [19,20,56]. The antagonistic activity of Polish propolis extracts against such bacterial strains as S. aureus, Salmonella epidermidis, Klebsiella pneumoniae, E. coli, P. aeruginosa and L. monocytogenes has already been demonstrated [57,58]. Literature reports indicate that the antimicrobial activity of poplar-type propolis may be associated with the high content of phenolic compounds [59,60,61]. The results described by Siripatrawan and Vitchayakitti [9] indicated that chitosan films containing propolis extract showed activity against S. aureus, S. Enteritidis, E. coli and P. aeruginosa in contrast to films obtained from chitosan alone. In turn, propolis–chitosan nanoparticle films strongly inhibited the growth of L. monocytogenes, E. coli and S. Enteritidis [62]. Furthermore, packaging materials based on cellulose and containing the active combination of chitosan and propolis complex exhibited antibacterial properties against L. innocua and S. aureus [24]. The literature data indicated that films based on high molecular weight chitosan with propolis extract showed activity against Gram-positive bacteria: B. cereus, L. monocytogenes and S. aureus [26].
More research is required to explain the reasons for the loss of antibacterial activity by both CAF and EP in the environment of chitosan and acetic acid.
3.3. Barrier Properties of Films
The barrier properties of packaging are pivotal in the preservation of products during storage. Attributes like the barrier characteristics of active films and edible coatings are closely tied to their capacity to safeguard food, often gauged through water vapor permeability [63]. Furthermore, it is essential to restrict the ingress of water vapor and oxygen through packaging to hinder the growth of microorganisms and the degradation of specific active components [64]. Hence, the water vapor transmission rate (WVTR) and oxygen transmission rate (OTR) of the chitosan-based films produced were assessed, and the findings are outlined in Table 3.

Table 3.
Water vapor transmission rate (WVTR) and oxygen transmission rate (OTR) of chitosan-based films.
The application of citric and acetic acids influenced the water vapor permeability parameter of the obtained films. Chitosan films prepared with the application of acetic acid as a chitosan solvent were characterized by a higher water vapor transmission rate (WVTR) amounting to 159.09 g/m2·24 h than films where citric acid was used (108.79 g/m2·24 h). The addition of caffeine to the chitosan film where acetic acid was used reduced WVTR to 110.91 g/m2·24 h, while in the case of the chitosan film with citric acid, the WVTR reduction was greater and amounted to 80.91 g/m2·24 h. The addition of propolis extract to both chitosan–caffeine solutions (with both acids) resulted in an even greater decrease in the WVTR parameter. This effect is due to the crosslinking reaction, which can restrict the movement of molecules and thus reduce the moisture transmission rate [53].
Modifications and supplements to films based on chitosan are unquestionably of significant importance and impact on their barrier properties. Literature data indicated that factors affecting the WVTR of chitosan films include molecular weight and the deacetylation degree of chitosan, the type and number of added plasticizers, and the conditions of sample preparation [65]. The findings obtained align with existing literature data confirming that the addition of propolis extracts to chitosan films resulted in a reduction in their water vapor permeability [9,26]. The results presented by Correa-Pacheco et al. [62] showed that propolis–chitosan nanoparticle films were characterized by a lower value of the water vapor diffusion coefficient (WVDC) than chitosan films without propolis. Moreover, a higher concentration of propolis extract added to chitosan film resulted in a decrease in WVDC, which was connected with the lipophilic nature of propolis [62]. Additionally, Velickova et al. [66] found that the incorporation of other hive products, the incorporation of beeswax into edible chitosan films, led to a reduction in the permeability of the initial chitosan films.
The films were also characterized by varied oxygen transmission rates (OTRs), as presented in Table 3. The film based on chitosan prepared with citric acid and caffeine (sample CC) was the greatest oxygen barrier material, with the lowest OTR at 3.3 cm3/m2 · 24 h. This is mainly because citric acid contains three carboxyl groups. Thus, the reaction caused the formation of more hydrogen bonds between the chitosan and the acid, thus leading to the formation of a dense structure that reduced the barrier of the material. Furthermore, caffeine caused additional crosslinking and consequently provided an enhanced oxygen barrier. Therefore, the crosslinked film formed after the addition of caffeine can be recognized as an effective method to enhance the barrier properties of chitosan films.
3.4. Sorption Experiments
The experimental EMC data and fitted adsorption isotherms of the examined film samples are presented in Figure 2. The adsorption isotherm for these chitosan-based films demonstrated a minor increase in EMC below RH 0.50, followed by a notable surge in EMC in the range of RH exceeding 0.50. This occurrence has been documented in prior studies [67,68,69,70]. The results showed a positive effect of caffeine and caffeine-propolis on reducing the hygroscopicity of chitosan films. The characteristic feature of tested chitosan-based created using the studied additives is a negligibly low EMC (less than 0.01 kg/kg) in the RH range below 0.35. A similar effect of lowering EMC in the described RH range was observed in earlier studies of films created using chitosan and kombucha solutions derived from coffee as well as three different types of tea [71].
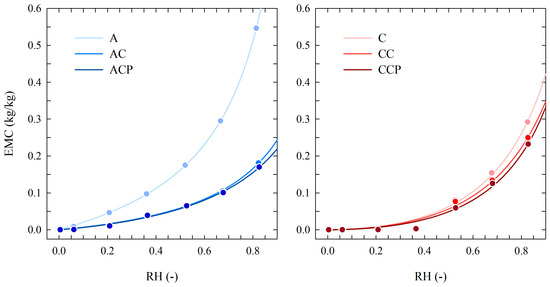
Figure 2.
Adsorption isotherms of chitosan-based film samples including caffeine and caffeine-propolis solution (chitosan dissolved in acetic acid—left plot; chitosan dissolved in citric acid—right plot; dots represent experimental data; solid lines—fitting with the GDW sorption model, temperature of 25 °C).
The EMC of pure chitosan film (sample A) produced with acetic acid is clearly higher in the whole RH range. In turn, the application of citric acid as a solvent in the production process of crab chitosan-based films (pure chitosan) results in a greater reduction in their hygroscopic properties than in the case of acetic acid. When exposed to air at a temperature of 25 °C RH below 0.80, the maximum value of equilibrium moisture content (EMC) of the tested samples of the C-type film did not exceed 0.292 kg/kg. Under analogous conditions, the observed EMC for samples of A film was almost 2-fold greater (0.546 kg/kg). The results of the sorption experiment shown in Figure 2 confirmed that the hygroscopicity of the tested film samples based on crab chitosan may be significantly reduced by the addition of CAF and CAF with EP. The proposed modification variants for chitosan-based films, i.e., an addition of CAF and CAF with EP, cause a further decrease in EMC within the RH range below 0.80. An advantageous effect of EP on the reduction in hygroscopicity of film based on crab chitosan confirmed the results of earlier studies [26].
The results of the conducted sorption experiments confirmed that a greater range of EMC reduction is found for film samples produced using acetic acid (A-type samples). The application of an addition of caffeine or caffeine–propolis in the case of samples obtained using citric acid (C type samples) causes only a slight reduction in the hygroscopicity of the produced chitosan films.
The estimated coefficients of the GDW model for the investigated chitosan-based films, taking into account the caffeine and caffeine with propolis extract used, are presented in Table 4.

Table 4.
Estimated coefficients of the sorption GDW model at 25 °C for chitosan-based films.
Generally, the estimated parameter m (monolayer water content) is lower than 1 for all the investigated chitosan-based films since the water molecules absorbed on primary sites do not undergo a complete transformation into secondary sorption sites. Moreover, the use of caffeine and caffeine with propolis extract as additives to produce a chitosan film affected the monolayer water content. Nevertheless, while in samples of film A and C (pure chitosan) samples, the value of the described parameter varies slightly (i.e., 0.5609 vs. 0.5419, respectively), in the case of chitosan-based films produced with the addition of acetic acid it was significantly reduced.
As a result of the use of caffeine and caffeine with propolis extract, the accessibility of the primary sorption sites is limited. The value of m for the caffeine (AC) and caffeine–propolis (ACP) variants, i.e., 0.1216 and 0.0984, represents, respectively, 21.7 and 17.5% of the corresponding value determined for the chitosan film produced with acetic acid (0.5609). Consequently, the anticipated impact of incorporating the examined additives into the chitosan-based films could include diminishing their moisture-absorbing characteristics, enhancing their stability, and potentially improving mechanical properties such as tensile strength (TS) and elongation at break (EB).
3.5. Scanning Electron Microscopy (SEM)
The surface attributes of the films, as examined via scanning electron microscopy, are presented in Figure 3.
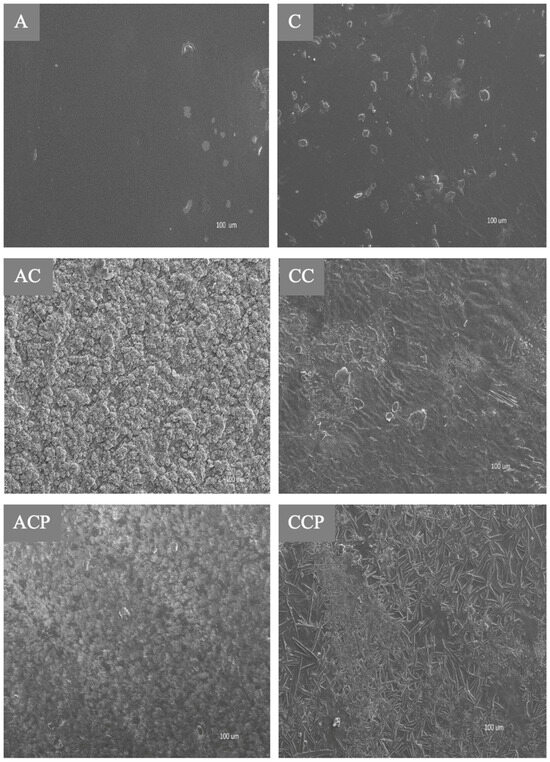
Figure 3.
SEM images of the chitosan-based films samples: (A) (acetic acid/no additives), (AC) (acetic acid/CAF), (ACP) (acetic acid/CAF/EP), (C) (citric acid/no additives), (CC) (citric acid/CAF), (CCP) (citric acid/CAF/EP).
The studies revealed that the film samples A (chitosan prepared in acetic acid) and C (chitosan prepared in citric acid) were characterized by a smooth surface structure in most areas, without obvious holes or bubbles, with all major areas being smooth and homogeneous. This is evidence for a great dispersion of chitosan in both used acids. A homogenous appearance is an indication of the structural stability of the chitosan/acid systems. The inclusion of CAF in the tested chitosan film samples (AC and CC) caused the emergence of unequally sized irregular clusters, probably due to the aggregation of caffeine particles on the surface. In the case of samples AC and CC, agglomeration caused a decrease in the sample elasticity (decrease in elongation). Also, the addition of EP to chitosan films caused the emergence of irregular clusters on the surface (samples ACP and CCP). This agglomeration reduced the mechanical properties of samples ACP and CCP and caused a deterioration of the OTR, as presented in Section 3.3.
3.6. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
In order to evaluate the interaction among chitosan and used natural additives—caffeine and propolis extract—an FTIR infrared study was carried out, and the outcomes in the form of spectra are illustrated in Figure 4.
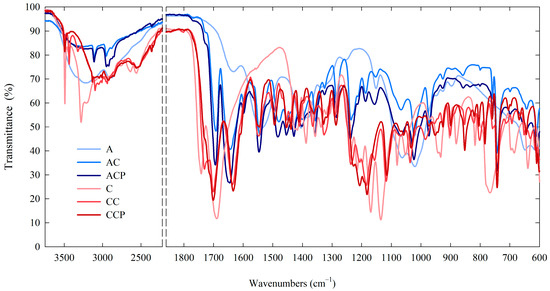
Figure 4.
ATR-FTIR spectra of chitosan-based films.
The spectra of all the films exhibited a pattern akin to that of chitosan films devoid of additives, with the majority of peaks displaying chitosan characteristics. However, there were variations in transmittance intensities at specific peaks. The peaks in the range of 3500–3000 cm−1 and at 2880 cm−1 are related to the -OH bonds and aliphatic C–H stretching vibration [62,72]. The peaks at 1650, 1557 and 1371 cm−1 can be assigned to C–O stretching in amide I, N–H bending in amide II and C–N stretching in amide III [9,62]. The peak at 1025 cm−1 is attributed to C–O stretching in the alcohol group [72].
In the spectra of films containing caffeine, distinctive bands corresponding to the caffeine molecule were observed, specifically at 1700 and 1650 cm−1, which can be attributed to the stretching vibrations of C=O bonds within the acetamide groups of amide I, as well as at 1550 and 765 cm−1, indicative of the stretching vibrations of N–H and/or C–N groups of amide II [73,74,75]. On the other hand, in the spectra of films containing both caffeine and propolis extracts, distinctive bands originating from propolis, primarily associated with phenolic compounds, can be discerned. The bands at 2924 and 2850 cm−1 are ascribed to C–H stretching vibrations and confirm the presence of long-chain alkyl compounds in the bee glue extract. The peak at 1732 cm−1 corresponds to C–O stretching vibration. The stretching and bending bands at 1634 (C=O), 1515 and 1451(C=C) cm−1 may be assigned mainly to aromatic rings of phenolic compounds. Moreover, the wide band around 3420 cm−1 corresponding to the stretching vibrations of the O–H band also confirms the presence of phenolic compounds from propolis [62,76,77]. Alterations, such as shifting and broadening of certain bonds observed in the FTIR spectra, may be attributed to the presence of intermolecular interactions between the -OH and -NH2 groups within chitosan and the phenolic compounds found in propolis. This aligns with information from the existing literature [9,62,72].
3.7. Tensile Properties
Mechanical testing holds significance in terms of assessing the practicality of the fabricated materials. Hence, the tensile strength (TS) and elongation at break (EB) of the chitosan-based films under investigation have been assessed, and the outcomes are illustrated in Figure 5 and Figure 6, respectively.
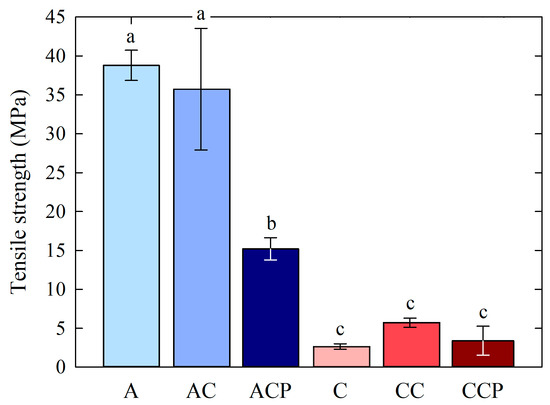
Figure 5.
Tensile strength (TS) for prepared chitosan films. Different letters indicate samples that were significantly different (p < 0.05).
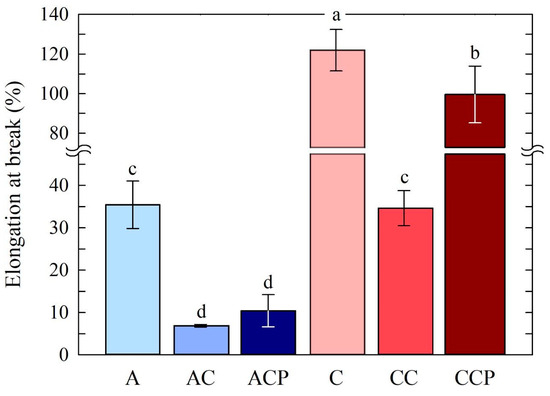
Figure 6.
Elongation at break (EB) for prepared chitosan films. Different letters indicate samples that were significantly different (p < 0.05).
The films obtained had various values of tensile strength, whereby the highest values were found in the samples with acetic acid. The TS for sample A was 38.80 MPa, and for sample AC, it was 35.73 MPa. Sample ACP, which involved the use of additional caffeine and EP, had a TS of 15.22 MPa. Samples where citric acid was used were characterized by the following tensile strengths. Sample C, with pure chitosan in citric acid, had a TS of 2.65 MPa. The addition of caffeine resulted in a minor tensile strength of 5.72 MPa. On the other hand, sample CCP, which is a combination of chitosan dissolved in citric acid, caffeine and EP, obtained a value of 3.40 MPa. The mere addition of caffeine had no major effect on changes in tensile strength. However, the solvent used was an important factor. The selection of a natural carboxylic acid is essential for evaluating the potential utility of food packaging materials. Furthermore, investigating the interplay between the structure and properties of chitosan films in conjunction with the type of acid can optimize the attributes of the base film without the need for additional additives. This can offer substantial insights into film selection to meet the requirements of food packaging [78].
Regarding elongation at break, an opposite relationship to tensile strength (TS) can be observed. Samples C, CC and CCP are characterized by the highest elongation at break (EB) for the prepared chitosan films, while samples ACP and CCP are marked by the highest value of elongation at break at 121.87 and 99.4%, respectively. Therefore, the use of citric acid as a solvent has a positive effect on elongation at break and, thus, the flexibility of the sample. For sample CC, where caffeine was applied, there was a decrease in elongation to 34.71%. Samples in which acetic acid was used were marked by lesser elongation. For pure sample A, EB was 35.5%, while samples AC and ACP had elongation values of 6.92 and 10.46%, respectively.
The research on the mechanical properties of the prepared films demonstrated that the solvent had a key impact on the results. As is known, acetic acid is a common solvent that acts as a proton donor, creating a dense chitosan film. The study demonstrated no effect of acetic acid on the deterioration of tensile strength in the tested films. The samples with acetic acid were characterized by greater tensile strength than the samples with citric acid. As reported in the literature [78], acidic structure affects the properties of the resulting films through electrostatic interactions, hydrogen bonds, and hydrophobic interactions. Therefore, acids with more carboxyl groups can improve film elongation by means of ionic crosslinking. In this study, citric acid improved the elongation at break of the obtained films. Elongation for the samples with citric acid was significantly greater than for the film where acetic acid was used. Citric acid is recognized for containing three carboxyl groups, which leads to a greater number of hydroxyl groups in the film matrix. This increase in hydroxyl groups promotes enhanced chain mobility and reduces intermolecular forces, resulting in an augmentation of the elongation at break while simultaneously causing a decrease in the tensile strength of the tested samples [79].
4. Conclusions
Chitosan solutions supplemented with caffeine and propolis extract have been demonstrated to display strong antioxidant properties, contributing to the antioxidant efficacy of chitosan-based solutions. Among the chitosan-based solutions tested, the highest antioxidant properties were observed in solution prepared with citric acid with the addition of CAF and EP, showing a greater free radical scavenging capacity than the standard antioxidant Trolox. Furthermore, the study demonstrated that chitosan-based films dissolved in citric acid exhibited strong antimicrobial activity against harmful bacterial strains, and this effect was further heightened with the inclusion of propolis extract and caffeine. The addition of acids, caffeine, and propolis extract can alter the barrier properties of chitosan-based films, consequently influencing their water vapor and oxygen permeability. This modification renders them well-suited for application in the food packaging sector. The choice of solvent was observed to influence the mechanical characteristics of chitosan films, with citric acid resulting in higher elongation at break but lower tensile strength in comparison to acetic acid. This underscores the significance of selecting appropriate natural acids for potential food packaging applications.
The findings indicate that natural additives like propolis extract and caffeine possess the capacity to enhance the antioxidant, antimicrobial, and barrier characteristics of chitosan-based materials, rendering them a promising choice for food packaging applications.
Author Contributions
Conceptualization, K.S., M.W., R.D. and I.R.; methodology, K.S., J.M., A.S., L.M. and W.K.; formal analysis, K.S., J.M., A.S., L.M. and W.K.; writing—original draft preparation, K.S. and M.W.; visualization, K.S. and J.M.; supervision, R.D. and I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Porta, R.; Sabbah, M.; Di Pierro, P. Biopolymers as Food Packaging Materials. Int. J. Mol. Sci. 2020, 21, 4942. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, S.; Manzocco, L.; Anese, M.; Nicoli, M.C. Shelf-Life Assessment of Food Undergoing Oxidation–A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan Based Antimicrobial Films for Food Packaging Applications. e-Polymers 2008, 8, 93. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; De La Caba, K. Functional Properties of Chitosan-Based Films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving Functional Properties of Chitosan Films as Active Food Packaging by Incorporating with Propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and Characterization of Edible Chitosan/Olive Oil Emulsion Films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Van Den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan Films and Blends for Packaging Material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.J.R.; Butt, J. Chitosan Films Are NOT Antimicrobial. Biotechnol. Lett. 2011, 33, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Stefanowska, K.; Woźniak, M.; Dobrucka, R.; Ratajczak, I. Chitosan with Natural Additives as a Potential Food Packaging. Materials 2023, 16, 1579. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Navarro, A.; Yamul, D.; Libonatti, C.; Tabera, A.; Basualdo, M. The Use of Propolis as a Functional Food Ingredient: A Review. Trends Food Sci. Technol. 2021, 115, 297–306. [Google Scholar] [CrossRef]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical Diversity and Challenges in Quality Control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the Propolis Chemical Composition between 2013 and 2018: A Review. eFood 2020, 1, 24–37. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a Novel Antibacterial Agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef]
- Petruzzi, L.; Corbo, M.R.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Żubernik, J.; Witrowa-Rajchert, D.; Gniewosz, M. Growth Biocontrol of Foodborne Pathogens and Spoilage Microorganisms of Food by Polish Propolis Extracts. Molecules 2019, 24, 2965. [Google Scholar] [CrossRef] [PubMed]
- Torlak, E.; Sert, D. Antibacterial Effectiveness of Chitosan-Propolis Coated Polypropylene Films against Foodborne Pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef]
- Ismail, M.I.; Roslan, A.; Saari, N.S.; Hashim, K.H.; Kalamullah, M.R. Ethanolic Extract of Propolis for Biodegradable Films Packaging Enhanced with Chitosan. AIP Conf. Proc. 2017, 1885, 020231. [Google Scholar] [CrossRef]
- Rollini, M.; Mascheroni, E.; Capretti, G.; Coma, V.; Musatti, A.; Piergiovanni, L. Propolis and Chitosan as Antimicrobial and Polyphenols Retainer for the Development of Paper Based Active Packaging Materials. Food Packag. Shelf Life 2017, 14, 75–82. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antifungal Properties of Hydroxypropylmethylcellulose Based Films Containing Propolis as Affected by Moisture Content. Carbohydr. Polym. 2010, 82, 1174–1183. [Google Scholar] [CrossRef]
- Stanicka, K.; Dobrucka, R.; Woźniak, M.; Sip, A.; Majka, J.; Kozak, W.; Ratajczak, I. The Effect of Chitosan Type on Biological and Physicochemical Properties of Films with Propolis Extract. Polymers 2021, 13, 3888. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- Ramanavièienë, A.; Mostovojus, V.; Bachmatova, I.; Ramanavièius, A. Anti-Bacterial Effect of Caffeine on Escherichia Coli and Pseudomonas fluorescens. Acta Med. Litu. 2003, 10, 185–188. [Google Scholar]
- Chakraborty, P.; Dastidar, D.G.; Paul, P.; Dutta, S.; Basu, D.; Sharma, S.R.; Basu, S.; Sarker, R.K.; Sen, A.; Sarkar, A.; et al. Inhibition of Biofilm Formation of Pseudomonas aeruginosa by Caffeine: A Potential Approach for Sustainable Management of Biofilm. Arch. Microbiol. 2020, 202, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and Neuroprotective Effects of Caffeine against Alzheimer’s and Parkinson’s Disease: Insight into the Role of Nrf-2 and A2AR Signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef]
- Monente, C.; Bravo, J.; Vitas, A.I.; Arbillaga, L.; De Peña, M.P.; Cid, C. Coffee and Spent Coffee Extracts Protect against Cell Mutagens and Inhibit Growth of Food-Borne Pathogen Microorganisms. J. Funct. Foods 2015, 12, 365–374. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Salameh, M.M.; Phetsomphou, S.; Yang, H.; Seo, C.W. Application of Caffeine, 1,3,7-Trimethylxanthine, to Control Escherichia Coli O157:H7. Food Chem. 2006, 99, 645–650. [Google Scholar] [CrossRef]
- Almeida, A.A.P.; Naghetini, C.C.; Santos, V.R.; Antonio, A.G.; Farah, A.; Glória, M.B.A. Influence of Natural Coffee Compounds, Coffee Extracts and Increased Levels of Caffeine on the Inhibition of Streptococcus mutans. Food Res. Int. 2012, 49, 459–461. [Google Scholar] [CrossRef]
- Bajer, D.; Burkowska-But, A. Innovative and Environmentally Safe Composites Based on Starch Modified with Dialdehyde Starch, Caffeine, or Ascorbic Acid for Applications in the Food Packaging Industry. Food Chem. 2022, 374, 131639. [Google Scholar] [CrossRef]
- Stefanowska, K.; Woźniak, M.; Sip, A.; Mrówczyńska, L.; Majka, J.; Kozak, W.; Dobrucka, R.; Ratajczak, I. Characteristics of Chitosan Films with the Bioactive Substances—Caffeine and Propolis. J. Funct. Biomater. 2023, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- ASTM F3136-15; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Dynamic Accumulation Method. ASTM International: West Conshohocken, PA, USA, 2015.
- ISO 2528:2017; Sheet Materials—Determination of Water Vapour Transmission Rate (WVTR)—Gravimetric (Dish) Method. International Organization for Standarization: Geneva, Switzerland, 2017.
- Furmaniak, S.; Terzyk, A.P.; Gauden, P.A. Some Remarks on the Classification of Water Vapor Sorption Isotherms and Blahovec and Yanniotis Isotherm Equation. Dry. Technol. 2011, 29, 984–991. [Google Scholar] [CrossRef]
- Furmaniak, S.; Terzyk, A.P.; Gauden, P.A.; Rychlicki, G. Applicability of the Generalised D’Arcy and Watt Model to Description of Water Sorption on Pineapple and Other Foodstuffs. J. Food Eng. 2007, 79, 718–723. [Google Scholar] [CrossRef]
- Furmaniak, S.; Terzyk, A.P.; Gołembiewski, R.; Gauden, P.A.; Czepirski, L. Searching the Most Optimal Model of Water Sorption on Foodstuffs in the Whole Range of Relative Humidity. Food Res. Int. 2009, 42, 1203–1214. [Google Scholar] [CrossRef]
- ASTMD 882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2015.
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; Da Silva, J.A.L.; Coimbra, M.A. Chitosan–Caffeic Acid–Genipin Films Presenting Enhanced Antioxidant Activity and Stability in Acidic Media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tian, F.; Feng, Z.; Fan, X.; Pan, Z.; Zhou, J. Antioxidant Activity and Physicochemical Properties of Chitosan Films Incorporated with Lycium Barbarum Fruit Extract for Active Food Packaging. Int. J. Food Sci. Technol. 2015, 50, 458–464. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical Properties and Antioxidant Activity of an Active Film from Chitosan Incorporated with Green Tea Extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of Bioactive Compounds Potential and Antioxidant Activity of Brown, Green and Red Propolis from Brazilian Northeast Region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F.; Zooprofilattico, I.; Delle Venezie, S. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic Composition and Antioxidant Activity of Propolis from Various Regions of Poland. Curr. Bioact. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Miłek, M.; Ciszkowicz, E.; Tomczyk, M.; Sidor, E.; Zaguła, G.; Lecka-Szlachta, K.; Pasternakiewicz, A.; Dżugan, M. The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells. Molecules 2022, 27, 725. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. The Role of Seasonality on the Chemical Composition, Antioxidant Activity and Cytotoxicity of Polish Propolis in Human Erythrocytes. Rev. Bras. Farmacogn. 2019, 29, 301–308. [Google Scholar] [CrossRef]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Citric Acid-Incorporated Fish Gelatin/Chitosan Composite Films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, G.J.E.; Chorianopoulos, N. Probiotic Incorporation in Edible Films and Coatings: Bioactive Solution for Functional Foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Giannopoulou, E.; Skalicka-Wózniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bȩben, K.; Antosiewicz, B.; et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Sip, A.; Mrówczyńska, L.; Broniarczyk, J.; Waśkiewicz, A.; Ratajczak, I. Biological Activity and Chemical Composition of Propolis from Various Regions of Poland. Molecules 2023, 28, 141. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.S.; De Mendonc ßa, S.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Netto, A.A.L.; Lira, I.S.; Opez, G.-C.L.; Negrao, V.; Marcucci, M.C. Artepillin C and Phenolic Compounds Responsible for Antimicrobial and Antioxidant Activity of Green Propolis and Baccharis Dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef]
- Yilmaz, S.; Sova, M.; Microbiology, S.E.-J.A. Antimicrobial Activity of Trans-cinnamic Acid and Commonly Used Antibiotics against Important Fish Pathogens and Nonpathogenic Isolates. J. Appl. Microbiol. 2018, 125, 1714–1727. [Google Scholar] [CrossRef]
- Uzel, A.; Önçağ, Ö.; Çoğulu, D.; Research, Ö.G.-M. Chemical Compositions and Antimicrobial Activities of Four Different Anatolian Propolis Samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Ramos-García, M.d.L.; Martínez-González, M.d.C.; Hernández-Romano, J. Physicochemical Characterization and Antimicrobial Activity of Edible Propolis-Chitosan Nanoparticle Films. Prog. Org. Coat. 2019, 137, 105326. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Analysis of Film-Forming Properties of Chitosan with Different Molecular Weights and Its Adhesion Properties with Different Postharvest Fruit Surfaces. Food Chem. 2022, 395, 133605. [Google Scholar] [CrossRef]
- Zehra, A.; Wani, S.M.; Bhat, T.A.; Jan, N.; Hussain, S.Z.; Naik, H.R. Preparation of a Biodegradable Chitosan Packaging Film Based on Zinc Oxide, Calcium Chloride, Nano Clay and Poly Ethylene Glycol Incorporated with Thyme Oil for Shelf-Life Prolongation of Sweet Cherry. Int. J. Biol. Macromol. 2022, 217, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and Characterization of Biocomposite Film Based on Chitosan and Kombucha Tea as Active Food Packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of Chitosan-Beeswax Edible Coatings on the Quality of Fresh Strawberries (Fragaria ananassa Cv Camarosa) under Commercial Storage Conditions. LWT 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Wiles, J.L.; Vergano, P.J.; Barron, F.H.; Bunn, J.M.; Testin, R.F. Water Vapor Transmission Rates and Sorption Behavior of Chitosan Films. J. Food Sci. 2000, 65, 1175–1179. [Google Scholar] [CrossRef]
- Cervera, M.F.; Karjalainen, M.; Airaksinen, S.; Rantanen, J.; Krogars, K.; Heinämäki, J.; Colarte, A.I.; Yliruusi, J. Physical Stability and Moisture Sorption of Aqueous Chitosan-Amylose Starch Films Plasticized with Polyols. Eur. J. Pharm. Biopharm. 2004, 58, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, P.C.; Ramesh, M.N.; Tharanathan, R.N. Effect of Plasticizers and Fatty Acids on Mechanical and Permeability Characteristics of Chitosan Films. Food Hydrocoll. 2007, 21, 1113–1122. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of Equilibrium Moisture Content on Barrier, Mechanical and Thermal Properties of Chitosan Films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef]
- Stefanowska, K.; Woźniak, M.; Majka, J.; Sip, A.; Mrówczyńska, L.; Waśkiewicz, A.; Kozak, W.; Dobrucka, R.; Ratajczak, I. A New Approach to Obtain Chitosan Films–Characteristics of Films Prepared with Tea and Coffee Kombucha as Natural Chitosan Solvents. Ind. Crops Prod. 2023, 197, 116634. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sankari, G.; Ponnusamy, S. Vibrational Spectral Investigation on Xanthine and Its Derivatives—Theophylline, Caffeine and Theobromine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 117–127. [Google Scholar] [CrossRef]
- Belscak-Cvitanovic, A.; Komes, D.; Karlović, S.; Djaković, S.; Špoljarić, I.; Mršić, G.; Ježek, D. Improving the Controlled Delivery Formulations of Caffeine in Alginate Hydrogel Beads Combined with Pectin, Carrageenan, Chitosan and Psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Morrish, C.; Whitehead, F.; Istivan, T.; Kasapis, S. The Effect of Trisodium Phosphate Crosslinking on the Diffusion Kinetics of Caffeine from Chitosan Networks. Food Chem. 2022, 381, 132272. [Google Scholar] [CrossRef] [PubMed]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and Effective Evaluation of the Antioxidant Capacity of Propolis Extracts Using DPPH Bleaching Kinetic Profiles, FT-IR and UV-Vis Spectroscopic Data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Wu, Y.W.; Sun, S.Q.; Zhao, J.; Li, Y.; Zhou, Q. Rapid Discrimination of Extracts of Chinese Propolis and Poplar Buds by FT-IR and 2D IR Correlation Spectroscopy. JMoSt 2008, 883–884, 48–54. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Q.; Shen, J.; Gao, P.; Yu, D.; Xu, Y.; Xia, W. The Role of Organic Acid Structures in Changes of Physicochemical and Antioxidant Properties of Crosslinked Chitosan Films. Food Packag. Shelf Life 2022, 31, 100792. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Benjakul, S.; Prodpran, T.; Nilsuwan, K.; Huda, N.; de la Caba, K. Composite Films Based on Chitosan and Epigallocatechin Gallate Grafted Chitosan: Characterization, Antioxidant and Antimicrobial Activities. Food Hydrocoll. 2021, 111, 106384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).