Cutibacterium acnes Dysbiosis: Alternative Therapeutics for Clinical Application
Abstract
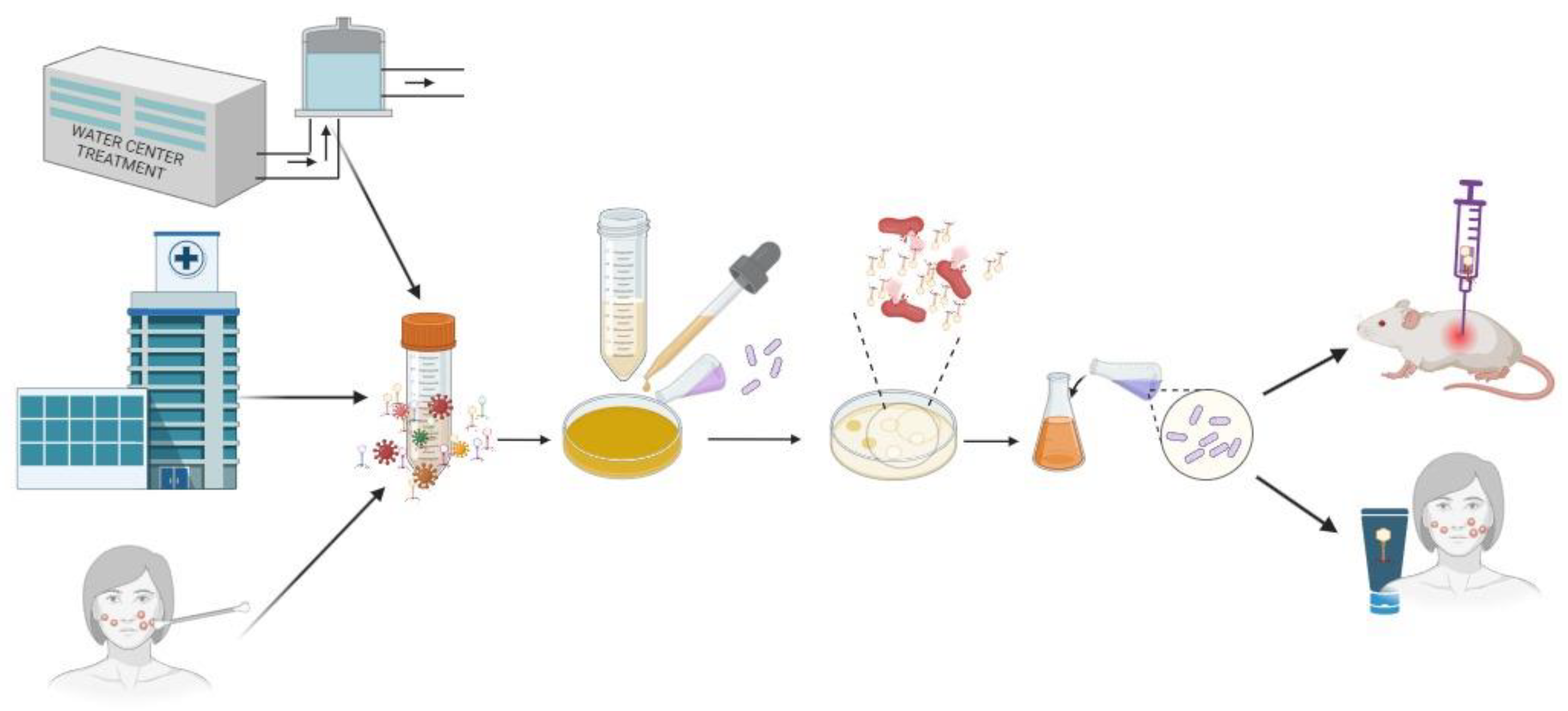
:1. Introduction
1.1. Taxonomy and Nomenclature
1.2. C. acnes Infection and Virulence Factors
1.3. C. acnes Clinical Relevance
Acne Vulgaris (AV)
1.4. C. acnes Antibiotic Treatment
2. New Therapeutic Strategies
3. Conclusions
4. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Corvec, S.; Dagnelie, M.-A.; Khammari, A.; Dréno, B. Taxonomy and phylogeny of Cutibacterium (formerly Propionibacterium) acnes in inflammatory skin diseases. Ann. Dermatol. Venereol. 2019, 146, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Dekio, I.; Asahina, A.; Shah, H.N. Unravelling the eco-specificity and pathophysiological properties of Cutibacterium species in the light of recent taxonomic changes. Anaerobe 2021, 71, 102411. [Google Scholar] [CrossRef] [PubMed]
- Mayslich, C.; Grange, P.A. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the human skin microbiota and its roles in cutaneous diseases. Microb. Cell Fact. 2022, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Falconer, T.M.; Baba, M.; Kruse, L.M.; Dorrestijn, O.; Donaldson, M.J.; Smith, M.M.; Figtree, M.C.; Hudson, B.J.; Cass, B.; Young, A.A. Contamination of the Surgical Field with Propionibacterium acnes in Primary Shoulder Arthroplasty. J. Bone Jt. Surg. Am. 2016, 98, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Makhlouf, Z.; Ahmed, N. The increasing importance of the gut microbiome in acne vulgaris. Folia Microbiol. 2022, 67, 825–835. [Google Scholar] [CrossRef]
- Elston, M.J.; Dupaix, J.P.; Opanova, M.I.; Atkinson, R.E. Cutibacterium acnes (formerly Proprionibacterium acnes) and Shoulder Surgery. Hawai’i J. Health Soc. Welf. 2019, 78 (Suppl. S2), 3–5. [Google Scholar]
- Martin, D.R.; Witten, J.C.; Tan, C.D.; Rodriguez, E.R.; Blackstone, E.H.; Pettersson, G.B.; Seifert, D.E.; Willard, B.B.; Apte, S.S. Proteomics identifies a convergent innate response to infective endocarditis and extensive proteolysis in vegetation components. JCI Insight 2020, 5, e135317. [Google Scholar] [CrossRef]
- Boman, J.; Nilson, B.; Sunnerhagen, T.; Rasmussen, M. True infection or contamination in patients with positive Cutibacterium blood cultures—A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1029–1037. [Google Scholar] [CrossRef]
- Kraaijvanger, R.; Veltkamp, M. The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait? Microorganisms 2022, 10, 1649. [Google Scholar] [CrossRef]
- Ugge, H.; Carlsson, J.; Söderquist, B.; Fall, K.; Andén, O.; Davidsson, S. The influence of prostatic Cutibacterium acnes infection on serum levels of IL6 and CXCL8 in prostate cancer patients. Infect. Agents Cancer 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Radej, S.; Szewc, M.; Maciejewski, R. Prostate Infiltration by Treg and Th17 Cells as an Immune Response to Propionibacterium acnes Infection in the Course of Benign Prostatic Hyperplasia and Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 8849. [Google Scholar] [CrossRef]
- Coenye, T.; Spittaels, K.-J.; Achermann, Y. The role of biofilm formation in the pathogenesis and antimicrobial susceptibility of Cutibacterium acnes. Biofilm 2022, 4, 100063. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zohra, F.T.; Sultana, T.; Islam, S.; Nasreen, A. Evaluation of Severity in Patients of Acne Vulgaris by Global Acne Grading System in Bangladesh. Clin. Pathol. Res. J. 2017, 1, 000105. [Google Scholar] [CrossRef]
- Szymańska, A.; Budzisz, E.; Erkiert-Polguj, A. The Anti-Acne Effect of Near-Infrared Low-Level Laser Therapy. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1045–1051. [Google Scholar] [CrossRef]
- Mawardi, P.; Ardiani, I.; Primisawitri, P.P.; Nareswari, A. Dual role of Cutibacterium acnes in acne vulgaris pathophysiology. Bali Med. J. 2021, 10, 486–490. [Google Scholar] [CrossRef]
- Sheffer-Levi, S.; Rimon, A.; Lerer, V.; Shlomov, T.; Coppenhagen-Glazer, S.; Rakov, C.; Zeiter, T.; Nir-Paz, R.; Hazan, R.; Molho-Pessach, V. Antibiotic Susceptibility of Cutibacterium acnes Strains Isolated from Israeli Acne Patients. Acta Derm. Venereol. 2020, 100, adv00295. [Google Scholar] [CrossRef]
- Kuriyama, T.; Karasawa, T.; Williams, D.W. Chapter Thirteen—Antimicrobial Chemotherapy: Significance to Healthcare. In Biofilms in Infection Prevention and Control; Percival, S.L., Williams, D.W., Randle, J., Cooper, T., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 209–244. [Google Scholar] [CrossRef]
- Peckman, B.; Kharel, M.K. Erythromycin. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Alkhawaja, E.; Hammadi, S.; Abdelmalek, M.; Mahasneh, N.; Alkhawaja, B.; Abdelmalek, S.M. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: A cross sectional study. BMC Dermatol. 2020, 20, 17. [Google Scholar] [CrossRef]
- Legiawati, L.; Halim, P.A.; Fitriani, M.; Hikmahrachim, H.G.; Lim, H.W. Microbiomes in Acne Vulgaris and Their Susceptibility to Antibiotics in Indonesia: A Systematic Review and Meta-Analysis. Antibiotics 2023, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D. Topical Antibacterials in Dermatology. Indian J. Dermatol. 2021, 66, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Letzelter, J.; Hill, J.B.; Hacquebord, J. An Overview of Skin Antiseptics Used in Orthopaedic Surgery Procedures. J. Am. Acad. Orthop. Surg. 2019, 27, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, F.; Najafian, J.; Savabi Nasab, S.; Nilforoushzadeh, M.A. Treatment of Acne Vulgaris Using the Combination of Topical Erythromycin and Miconazole. J. Ski. Stem Cell 2014, 1, e23330. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 68553, Miconazole Nitrate. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Miconazole-nitrate (accessed on 18 September 2023).
- Kim, S.; Song, H.; Jin, J.S.; Lee, W.J.; Kim, J. Genomic and Phenotypic Characterization of Cutibacterium acnes Bacteriophages Isolated from Acne Patients. Antibiotics 2022, 11, 1041. [Google Scholar] [CrossRef]
- Condrò, G.; Guerini, M.; Castello, M.; Perugini, P. Acne Vulgaris, Atopic Dermatitis and Rosacea: The Role of the Skin Microbiota-A Review. Biomedicines 2022, 10, 2523. [Google Scholar] [CrossRef]
- Ho, H.-H.; Chen, C.-W.; Yi, T.-H.; Huang, Y.-F.; Kuo, Y.-W.; Lin, J.-H.; Chen, J.-F.; Tsai, S.-Y.; Chan, L.-P.; Liang, C.-H. Novel application of a Co-Fermented postbiotics of TYCA06/AP-32/CP-9/collagen in the improvement of acne vulgaris-A randomized clinical study of efficacy evaluation. J. Cosmet. Dermatol. 2022, 21, 6249–6260. [Google Scholar] [CrossRef]
- Han, H.S.; Shin, S.H.; Choi, B.-Y.; Koo, N.; Lim, S.; Son, D.; Chung, M.J.; Park, K.Y.; Sul, W.J. A split face study on the effect of an anti-acne product containing fermentation products of Enterococcus faecalis CBT SL-5 on skin microbiome modification and acne improvement. J. Microbiol. 2022, 60, 488–495. [Google Scholar] [CrossRef]
- Tsai, W.; Chou, C.; Chiang, Y.; Lin, C.; Lee, C. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef]
- Karoglan, A.; Paetzold, B.; Pereira de Lima, J.; Brüggemann, H.; Tüting, T.; Schanze, D.; Güell, M.; Gollnick, H. Safety and Efficacy of Topically Applied Selected Cutibacterium acnes Strains over Five Weeks in Patients with Acne Vulgaris: An Open-label, Pilot Study. Acta Derm. Venereol. 2019, 99, 1253–1257. [Google Scholar] [CrossRef]
- Xuan, G.; Wang, Y.; Wang, Y.; Lin, H.; Wang, C.; Wang, J. Characterization of the newly isolated phage Y3Z against multi-drug resistant Cutibacterium acnes. Microb. Pathog. 2023, 180, 106111. [Google Scholar] [CrossRef]
- Han, M.-H.; Khan, S.A.; Moon, G.-S. Cutibacterium acnes KCTC 3314 Growth Reduction with the Combined Use of Bacteriophage PAP 1-1 and Nisin. Antibiotics 2023, 12, 1035. [Google Scholar] [CrossRef]
- Lam, H.Y.P.; Lai, M.-J.; Chen, T.-Y.; Wu, W.-J.; Peng, S.-Y.; Chang, K.-C. Therapeutic Effect of a Newly Isolated Lytic Bacteriophage against Multi-Drug-Resistant Cutibacterium acnes Infection in Mice. Int. J. Mol. Sci. 2021, 22, 7031. [Google Scholar] [CrossRef] [PubMed]
- Rimon, A.; Rakov, C.; Lerer, V.; Sheffer-Levi, S.; Oren, S.A.; Shlomov, T.; Shasha, L.; Lubin, R.; Zubeidat, K.; Jaber, N.; et al. Topical phage therapy in a mouse model of Cutibacterium acnes-induced acne-like lesions. Nat. Commun. 2023, 14, 1005. [Google Scholar] [CrossRef] [PubMed]
- Golembo, M.; Puttagunta, S.; Rappo, U.; Weinstock, E.; Engelstein, R.; Gahali-Sass, I.; Moses, A.; Kario, E.; Ben-Dor Cohen, E.; Nicenboim, J.; et al. Development of a topical bacteriophage gel targeting Cutibacterium acnes for acne prone skin and results of a phase 1 cosmetic randomized clinical trial. Ski. Health Dis. 2022, 2, e93. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Lee, H.H.; Spina, C.S.; Collins, J.J. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 2013, 499, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Nitayavardhana, S.; Manuskiatti, W.; Cembrano, K.A.G.; Wanitphadeedecha, R. A Comparative Study Between Once-Weekly and Alternating Twice-Weekly Regimen Using Blue (470 nm) and Red 640 nm) Light Combination LED Phototherapy for Moderate-to-Severe Acne Vulgaris. Lasers Surg. Med. 2021, 53, 1080–1085. [Google Scholar] [CrossRef]
- Jain, S.; Yadav, V.; Bhatia, N. Clinical Pharmacokinetics, Safety and Exploratory Efficacy Study of a Topical Bactericidal VB-1953: Analysis of Single and Multiple Doses in a Phase I Trial in Acne Vulgaris Subjects. Clin. Drug Investig. 2020, 40, 259–268. [Google Scholar] [CrossRef]
- Chottawornsak, N.; Chongpison, Y.; Asawanonda, P. Topical 2% ketoconazole cream monotherapy significantly improves adult female acne: A double-blind, randomized. J. Dermatol. 2019, 46, 1184–1189. [Google Scholar] [CrossRef]
- Nawarathne, N.W.; Wijesekera, K.; Mudiyanselage, W.; Gaya, D.; Wijayaratne, B.; Napagoda, M. Development of Novel Topical Cosmeceutical Formulations from Nigella sativa L. with Antimicrobial Activity against Acne-Causing Microorganisms. Sci. World J. 2019, 2019, 5985207. [Google Scholar] [CrossRef]
- Weber, N.; Schwabe, K.; Schempp, C.M. Effect of a botanical cleansing lotion on skin sebum and erythema of the face: A randomized controlled blinded half-side comparison. J. Cosmet. Dermatol. 2018, 18, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Park, C.K.; Koo, B.; Lee, K.Y. Anti-acne properties of hydrophobic fraction of red ginseng (Panax ginseng C.A. Meyer) and its active components. Phyther. Res. 2018, 33, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Seo, J.; Kim, K. Comparative study of the bactericidal effects of indocyanine green-and methyl aminolevulinate-based photodynamic therapy on Propionibacterium acnes as a new treatment for acne. J. Dermatol. 2018, 45, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kerrouche, N. Combination of benzoyl peroxide 5% gel with liquid cleanser and moisturizer SPF 30 in acne treatment results in high levels of subject satisfaction, good adherence and favorable tolerability. J. Dermatolog. Treat. 2018, 29, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Martin, R.; Moyal, D.; Henley, J.B.; Khammari, A.; Seité, S. Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp. Dermatol. 2017, 26, 798–803. [Google Scholar] [CrossRef]
- Kawashima, M.; Nagare, T.; Katsuramaki, T. Open-label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long-term use in patients with acne vulgaris: A secondary publication. J. Dermatol. 2017, 44, 635–643. [Google Scholar] [CrossRef]
- Richter, C.; Trojahn, C.; Dobos, G.; Kottner, J. Follicular fluorescence quantity to characterize acne severity: A validation study. Ski. Res. Technol. 2016, 22, 451–459. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Q.; Liu, Y.; Wang, Q.; Huang, Z.; Xiang, L. Photodiagnosis and Photodynamic Therapy Effects of 5-aminolevulinic acid photodynamic therapy on TLRs in acne lesions and keratinocytes co-cultured with P. acnes. Photodiagnosis Photodyn. Ther. 2016, 15, 172–181. [Google Scholar] [CrossRef]
- Hassan, N.; Shady, M.; Ibrahim, M. Intense pulsed light versus photodynamic therapy using liposomal methylene blue gel for the treatment of truncal acne vulgaris: A comparative randomized split body study. Arch. Dermatol. Res. 2016, 308, 263–268. [Google Scholar] [CrossRef]
- Pan-in, P.; Wongsomboon, A.; Kokpol, C. Depositing a-mangostin nanoparticles to sebaceous gland area for acne treatment. J. Pharmacol. Sci. 2015, 129, 226–232. [Google Scholar] [CrossRef]
- Pezza, M.; Carlomagno, V. Inositol in women suffering from acne and PCOS: A randomized study. Glob. Dermatol. 2017, 4, 1–4. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Pak, S.C. Effects of cosmetics containing purified honeybee (Apis mellifera L.) venom on acne vulgaris. J. Integr. Med. 2013, 11, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, M.; Tokura, Y.; Shimada, S.; Furukawa, F.; Noguchi, N.; Ito, T. Clinical and bacteriological evaluation of adapalene 0.1% gel plus nadifloxacin 1% cream versus adapalene 0.1% gel in patients with acne vulgaris. J. Dermatol. 2013, 40, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Symonds, T.; Grant, A.; Doma, K.; Hinton, D.; Wilkinson, M.; Morse, L. The efficacy of topical preparations in reducing the incidence of Cutibacterium acnes at the start and conclusion of total shoulder arthroplasty: A randomized controlled trial. J. Shoulder Elb. Surg. 2022, 31, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Unterfrauner, I.; Phm, M.B.A.; Wieser, K.; Catanzaro, S.; Bouaicha, S. Acne cream reduces the deep Cutibacterium acnes tissue load before elective open shoulder surgery: A randomized controlled pilot trial. J. Shoulder Elb. Surg. Board. Trust. 2022, 31, 897–905. [Google Scholar] [CrossRef]
- Cotter, E.J.; Cotter, L.M.; Franczek, E.B.; Godfrey, J.J.; Hetzel, S.J.; Safdar, N.; Dai, T.; Arkin, L.; Grogan, B.F. Efficacy of combinational therapy using blue light and benzoyl peroxide in reducing Cutibacterium acnes bioburden at the deltopectoral interval: A randomized controlled trial. J. Shoulder Elb. Surg. 2021, 30, 2671–2681. [Google Scholar] [CrossRef]
- Grewal, G.; Polisetty, T.; Boltuch, A.; Colley, R.; Tapia, R.; Levy, J.C. Does application of hydrogen peroxide to the dermis reduce incidence of Cutibacterium acnes during shoulder arthroplasty: A randomized controlled trial. J. Shoulder Elb. Surg. 2021, 30, 1827–1833. [Google Scholar] [CrossRef]
- Steffen, V.; Moor, B.K.; Bertrand, L.; Troillet, N.; Emonet, S.; Gallusser, N. Subcutaneous tissue disinfection significantly reduces Cutibacterium acnes burden in primary open shoulder surgery. J. Shoulder Elb. Surg. Board. Trust. 2021, 30, 1537–1543. [Google Scholar] [CrossRef]
- Scheer, J.H.; Serrander, L.; Kal, A. Benzoyl peroxide treatment decreases Cutibacterium acnes in shoulder surgery, from skin incision until wound closure. J. Shoulder Elb. Surg. Board. Trust. 2021, 30, 1316–1323. [Google Scholar] [CrossRef]
- Dörfel, D.; Maiwald, M.; Daeschlein, G.; Müller, G.; Hudek, R.; Assadian, O.; Kampf, G.; Kohlmann, T.; Harnoss, J.C.; Kramer, A. Comparison of the antimicrobial efficacy of povidone-iodine-alcohol versus chlorhexidine-alcohol for surgical skin preparation on the aerobic and anaerobic skin flora of the shoulder region. Antimicrob. Resist. Infect. Control. 2021, 10, 17. [Google Scholar] [CrossRef]
- Hsu, J.E.; Whitson, A.J.; Woodhead, B.M.; Napierala, M.A.; Gong, D.; Iii, F.A.M. Randomized controlled trial of chlorhexidine wash versus benzoyl peroxide soap for home surgical preparation: Neither is effective in removing Cutibacterium from the skin of shoulder arthroplasty patients. Int. Orthop. 2020, 44, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Van Diek, F.M.; Pruijn, N.; Spijkers, K.M.; Mulder, B.; Kosse, N.M.; Dorrestijn, O. The presence of Cutibacterium acnes on the skin of the shoulder after the use of benzoyl peroxide: A placebo-controlled, double-blinded, randomized trial. J. Shoulder Elb. Surg. 2020, 29, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Stull, J.D.; Nicholson, T.A.; Davis, D.E.; Namdari, S. Addition of 3% hydrogen peroxide to standard skin preparation reduces Cutibacterium acnes—positive culture rate in shoulder surgery: A prospective randomized controlled trial. J. Shoulder Elb. Surg. 2019, 29, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, P.N.; Beck, L.; Stertz, I.; Tashjian, R.Z. Hydrogen peroxide skin preparation reduces Cutibacterium acnes in shoulder arthroplasty: A prospective, blinded, controlled trial. J. Shoulder Elb. Surg. 2019, 28, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Hancock, D.S.; Rupasinghe, S.L.; Elkinson, I.; Bloom, M.G.; Larsen, P.D. Benzoyl peroxide+chlorhexidine versus chlorhexidine alone skin preparation to reduce Propionibacterium acnes: A randomized controlled trial. ANZ J. Surg. 2018, 88, 1182–1186. [Google Scholar] [CrossRef]
- Smith, M.L.; Gotmaker, R.; Hoy, G.A.; Ek, E.T.; Carr, A.; Flynn, J.N.; Evans, M.C. Minimizing Propionibacterium acnes contamination in shoulder arthroplasty: Use of a wound protector. ANZ J. Surg. 2018, 88, 1178–1181. [Google Scholar] [CrossRef]
- Blonna, D.; Allizond, V.; Bellato, E.; Banche, G.; Cuffini, A.M.; Castoldi, F.; Rossi, R. Single versus Double Skin Preparation for Infection Prevention in Proximal Humeral Fracture Surgery. Biomed. Res. Int. 2018, 2018, 7. [Google Scholar] [CrossRef]
- Kolakowski, L.; Lai, J.K.; Duvall, G.T.; Jauregui, J.J.; Dubina, A.G.; Jones, D.L.; Williams, K.M.; Hasan, S.A.; Iii, R.F.H.; Gilotra, M.N. Neer Award 2018: Benzoyl peroxide effectively decreases preoperative Cutibacterium acnes shoulder burden: A prospective randomized controlled trial. J. Shoulder Elb. Surg. 2018, 27, 1539–1544. [Google Scholar] [CrossRef]
- Patzer, T.; Petersdorf, S.; Krauspe, R.; Verde, P.E.; Henrich, B.; Hufeland, M. Prevalence of Propionibacterium acnes in the glenohumeral compared with the subacromial space in primary shoulder arthroscopies. J. Shoulder Elb. Surg. Board. Trust. 2017, 27, 771–776. [Google Scholar] [CrossRef]
- Yamakado, K. Propionibacterium acnes Suture Contamination in Arthroscopic Rotator Cuff Repair: A Prospective Randomized Study. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 34, 1151–1155. [Google Scholar] [CrossRef]
- Dizay, H.H.; Lau, D.G.; Nottage, W.M. Benzoyl peroxide and clindamycin topical skin preparation decreases Propionibacterium acnes colonization in shoulder arthroscopy. J. Shoulder Elb. Surg. 2017, 26, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Hailer, N.P.; Rahme, H. High incidence of periprosthetic joint infection with propionibacterium acnes after the use of a stemless shoulder prosthesis with metaphyseal screw fixation—A retrospective cohort study of 241 patients propionibacter infections after eclipse TSA. BMC Musculoskelet. Disord. 2017, 18, 203. [Google Scholar] [CrossRef]
- Marecek, G.S.; Weatherford, B.M.; Fuller, E.B.; Saltzman, M.D. The effect of axillary hair on surgical antisepsis around the shoulder. J. Shoulder Elb. Surg. 2014, 24, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Van Oosten, M.; Bijker, K.L.B.; Boiten, K.E.; Salomon, E.N.; Rosema, S.; Rossen, J.W.A.; Natour, E.; Douglas, Y.L.; Kampinga, G.A.; et al. Sonication of heart valves detects more bacteria in infective endocarditis. Sci. Rep. 2018, 8, 12967. [Google Scholar] [CrossRef]
- Ploeger, M.M.; Jacobs, C.; Gathen, M.; Kaup, E.; Randau, T.M.; Friedrich, M.J.; Hischebeth, G.T.; Wimmer, M.D. Fluid collection bags pose a threat for bacterial contamination in primary total hip arthroplasty: A prospective, internally controlled, non-blinded trial. Arch. Orthop. Trauma. Surg. 2018, 138, 1159–1163. [Google Scholar] [CrossRef]
- Machado, I.; Baptista, D.C.; Canato, F.; Giacomelli, G.; Eduardo, C.; Falchete, R.; Carneiro, M. Colonization of oropharynx and lower respiratory tract in critical patients: Risk of ventilator-associated pneumonia. Arch. Oral. Biol. 2018, 85, 64–69. [Google Scholar] [CrossRef]
- Litrico, S.; Recanati, G.; Gennari, A.; Maillot, C.; Saffarini, M.; Le Huec, J.C. Single-use instrumentation in posterior lumbar fusion could decrease incidence of surgical site infection: A prospective bi-centric study. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 21–26. [Google Scholar] [CrossRef]
- Falk-Brynhildsen, K.; Söderquist, B.; Friberg, Ö.; Nilsson, U.G. Bacterial recolonization of the skin and wound contamination during cardiac surgery: A randomized controlled trial of the use of plastic adhesive drape compared with bare skin. J. Hosp. Infect. 2013, 84, 151–158. [Google Scholar] [CrossRef]
- Rieger, U.M.; Mesina, J.; Kalbermatten, D.F.; Haug, M.; Frey, H.P.; Pico, R.; Frei, R.; Pierer, G.; Lüscher, N.J.; Trampuz, A. Bacterial biofilms and capsular contracture in patients with breast implants. Br. J. Surg. 2013, 100, 768–774. [Google Scholar] [CrossRef]
- Craig, J.P.; Cruzat, A.; Cheung, I.M.Y.; Watters, G.A.; Wang, M.T.M. Randomized masked trial of the clinical efficacy of MGO Manuka Honey microemulsion eye cream for the treatment of blepharitis. Ocul. Surf. 2020, 18, 170–177. [Google Scholar] [CrossRef]
- Saxena, R.; Mittal, P.; Clavaud, C.; Dhakan, D.B.; Roy, N.; Breton, L.; Misra, N.; Sharma, V.K. Longitudinal study of the scalp microbiome suggests coconut oil to enrich healthy scalp commensals. Sci. Rep. 2021, 11, 7220. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.R.; Feazel, L.M.; Gitomer, S.A.; Ir, D.; Robertson, C.E.; Daniel, N. The Microbiome of the Middle Meatus in Healthy Adults. PLoS ONE 2013, 8, e85507. [Google Scholar] [CrossRef] [PubMed]

| Virulence Factors | Function |
|---|---|
| Lipases | Enzymes involved in the metabolization of sebum and free fatty acid release and triglycerides. |
| Polyunsaturated Fatty Acid Isomerase | Catalyzation and isomerization of linoleic acid. |
| Hyaluronate Lyase | Promotes the degradation of hyaluronic acid andolaolablablaother glycosaminoglycans, such as chondroitin-4-sulfate, chondroitin-6-sulfate, and dermatan sulfate, of the extracellular matrix in the epidermis and dermis. |
| Glycosidase | Disruption of carbohydrate and glycan structures that constitute the eukaryotic host glycolipids and glycoproteins. |
| Sialidase | Discard the sialic acid from sialoglycoconjugates. |
| Radical oxygenase | Reduction of the oxygen free radicals. |
| Sortase F | Capacity to covalently attach to various proteins, including adhesion factors. |
| Porphyrin | Fluorescent molecules that can stimulate inflammatory host reactions. |
| Biofilm | Matrix that provides bacterial resistance to adverse compounds, such as antibiotics |
| Adhesin dermatan-sulfate protein | Molecular surface components that recognize adhesive molecules of the matrix. |
| Christie–Atkins–Munch–Petersen Factors | Promote the formation of pores in host cells membranes. |
| Score | Acne Severity Denomination | Type of Lesions Observed |
|---|---|---|
| 0 | Clear skin | No lesions observed. |
| 1 | The skin is almost unchanged | Few comedones and less, or 1, small inflammatory lesion. |
| 2 | Mild severity | 12 comedones and less or equal severe inflammatory lesions. |
| 3 | Moderate severity | Many comedones and more several inflammatory lesions and less, or 1, nodule. |
| 4 | Severe severity | Many comedones and inflammatory lesions, less or equal several nodules and cysts. |
| Global Score | Lesions Count |
| None: 0 Mild: 1 to 18 Moderate: 31 to 38 Very severe: >39 | |
| Local score = Factor × Grade (0–4) | |
| Factor (1–3) | Grade (0–4) |
| Nose/chin: 1 Forehead/right cheek/left cheek: 2 Chest and upper back: 3 | No lesions: 0 One or more comedone: 1 One or more papule: 2 One or more pustule: 3 One or more nodule: 4 |
| Compound | Antibacterial Mechanism | Positive Factors | Negative Factors |
|---|---|---|---|
| Benzoyl peroxide (BPO) | The discharge of reactive oxygen intermediates oxidizes the proteins in the bacterial cell membrane. | No bacterial resistance to BPO has emerged despite decades of use. Keratolytic and anti-inflammatory properties are an additional component of BPO. | BPO is expensive and is a skin irritant, especially in darker skin types. |
| Clindamycin | Inhibits the bacterial 50S ribosome-mediated protein production. | Has a synergetic effect when used with BPO. Fox–Fordyce illness, folliculitis, periorificial face dermatitis, and rosacea have all been treated successfully with topical clindamycin, according to reports. | C. acnes isolates was shown to be resistant to clindamycin. Topical clindamycin side effects generally take the shape of dryness, stinging, burning, and erythema. |
| Micozanole Nitrate (MN) | Antifungal drug that affects the integrity of fungal cell membranes. | Annihilates Malassezia furfur, a fungus that provides an optimal environment for the growth of C. acnes. | May provoke allergic reactions, skin irritation such as erythema, pruritus, and occasionally exudation. |
| Hydrogen Peroxide (HP) | Is known by its powerful antiseptic activity against the vast known microorganisms in the skin. It can be used in the concentrations of 3 to 6% of (v/v). Even though the precise mechanism of action of hydrogen peroxide is unknown, it is widely thought that it is connected to its oxidizing activity. | No cases of acquired bacterial resistance to HP have been reported. PVP–I and HP interact positively. | HP concentrated solutions (20–30% or more) are extremely irritating to the skin and mucous membranes and should be handled carefully. |
| Chlorhexidine (CHX) | CHX has an antibacterial activity by affecting the integrity of cell membranes. | Being a highly safe topical medication, chlorhexidine is also commonly found in wound dressings and central line catheters. Chlorhexidine has a broad spectrum of activity and persistent residual effects. | Associated with poor efficacy, chlorhexidine side effects are uncommon but include minor skin irritation and, less often, allergic responses such as severe anaphylaxis. |
| Povidone-iodine (PVP-I) | It is hypothesized that PVP–I mechanisms include the inhibition of the electron transportation and cellular and inhibiting protein synthesis. | It is considered, among the antiseptics, the one with the broadest spectrum of activity against viruses, bacteria, molds, fungi, yeasts, and protozoa. | Low solubility, poor chemical stability, and shows local toxicity if not used in a soluble polymer matrix. PVP–I should not be used in patients with thyroid diseases and applicated iodine radiotherapy and it is also contraindicated to pregnant women or during lactation, and to newborns, and to young children. |
| Isopropanol | It is hypothesized that alcohols promote the protein denaturation or inhibition of mRNA and protein synthesis. | Rapid bacterial activity and broad spectrum of activity (vegetative bacteria, including mycobacteria, viruses, fungi, but not against bacterial spores). No reported allergic reactions. | Alcohols’ antimicrobial properties are brief, so they are commonly combined with compounds such as chlorhexidine, which keep working after the alcohol has evaporated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, S.; Fernandes, R.; Gestoso, Á.; Macedo, J.M.; Martins-Mendes, D.; Pereira, A.C.; Baylina, P. Cutibacterium acnes Dysbiosis: Alternative Therapeutics for Clinical Application. Appl. Sci. 2023, 13, 12086. https://doi.org/10.3390/app132112086
Sá S, Fernandes R, Gestoso Á, Macedo JM, Martins-Mendes D, Pereira AC, Baylina P. Cutibacterium acnes Dysbiosis: Alternative Therapeutics for Clinical Application. Applied Sciences. 2023; 13(21):12086. https://doi.org/10.3390/app132112086
Chicago/Turabian StyleSá, Sara, Ruben Fernandes, Álvaro Gestoso, José Mário Macedo, Daniela Martins-Mendes, Ana Cláudia Pereira, and Pilar Baylina. 2023. "Cutibacterium acnes Dysbiosis: Alternative Therapeutics for Clinical Application" Applied Sciences 13, no. 21: 12086. https://doi.org/10.3390/app132112086
APA StyleSá, S., Fernandes, R., Gestoso, Á., Macedo, J. M., Martins-Mendes, D., Pereira, A. C., & Baylina, P. (2023). Cutibacterium acnes Dysbiosis: Alternative Therapeutics for Clinical Application. Applied Sciences, 13(21), 12086. https://doi.org/10.3390/app132112086








