The Surface Free Energy of Resin-Based Composite in Context of Wetting Ability of Dental Adhesives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Materials
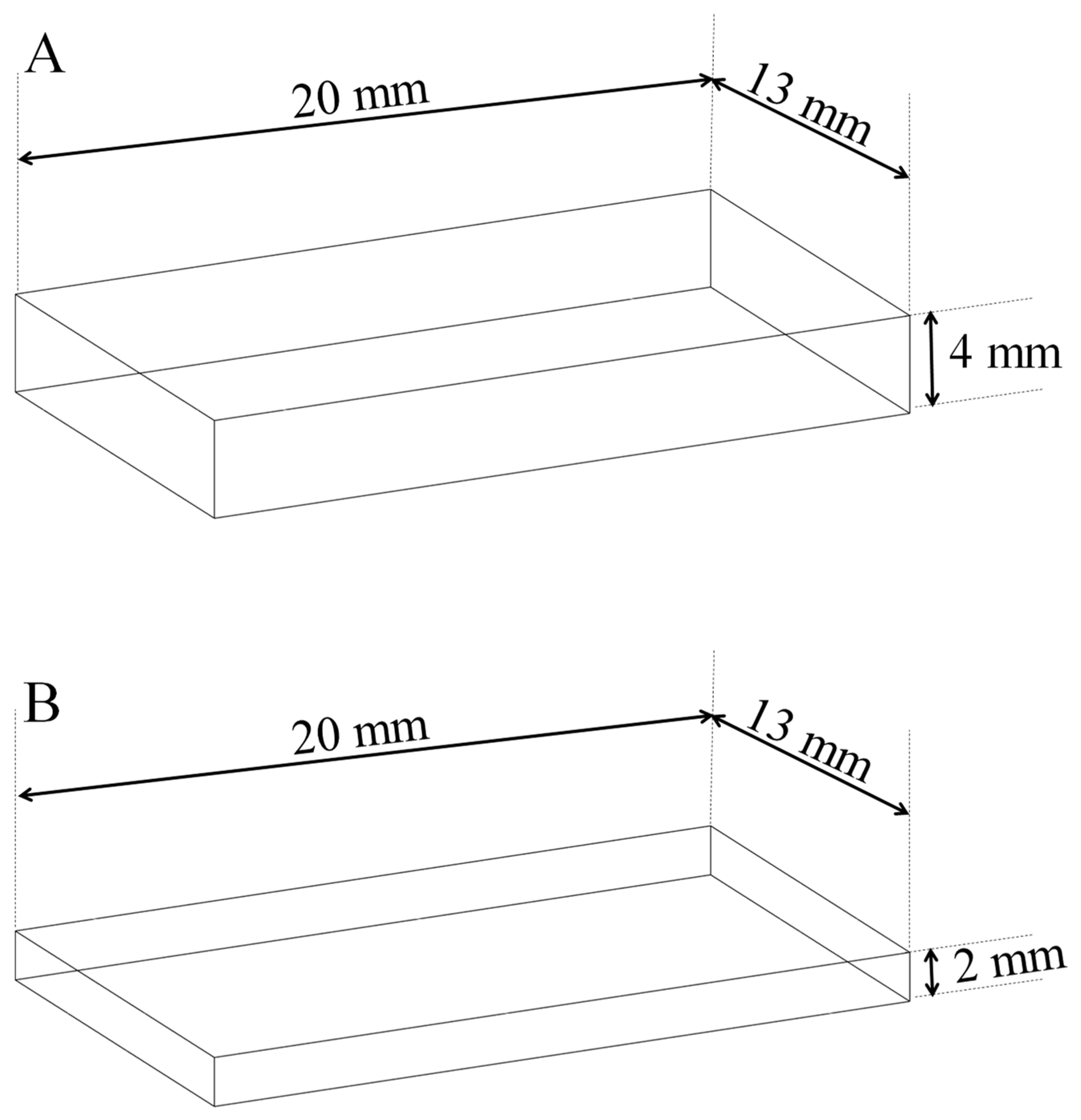
2.2. Preparation of Resin-Based Composite Specimens
2.3. Surface Profilometry Measurements
2.4. Surface Analysis with Scanning Electron Microscopy (SEM)
2.5. Contact Angle (CA) Measurements and Surface Free Energy (SFE) Calculation
2.6. Statistical Analysis
3. Results
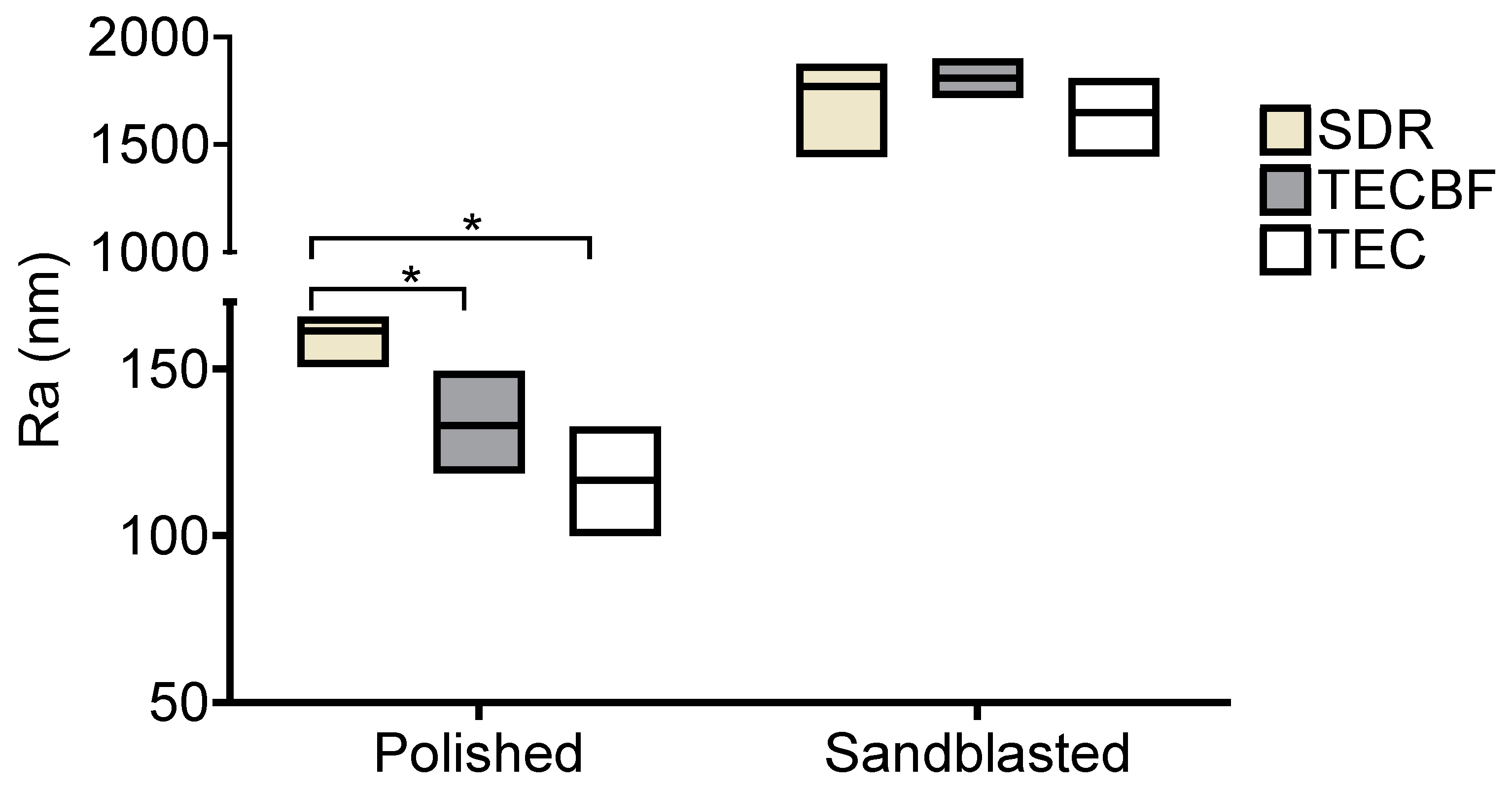
3.1. Results of Profilometry Measurements
3.2. Results of Scanning Electron Microscopy Analysis
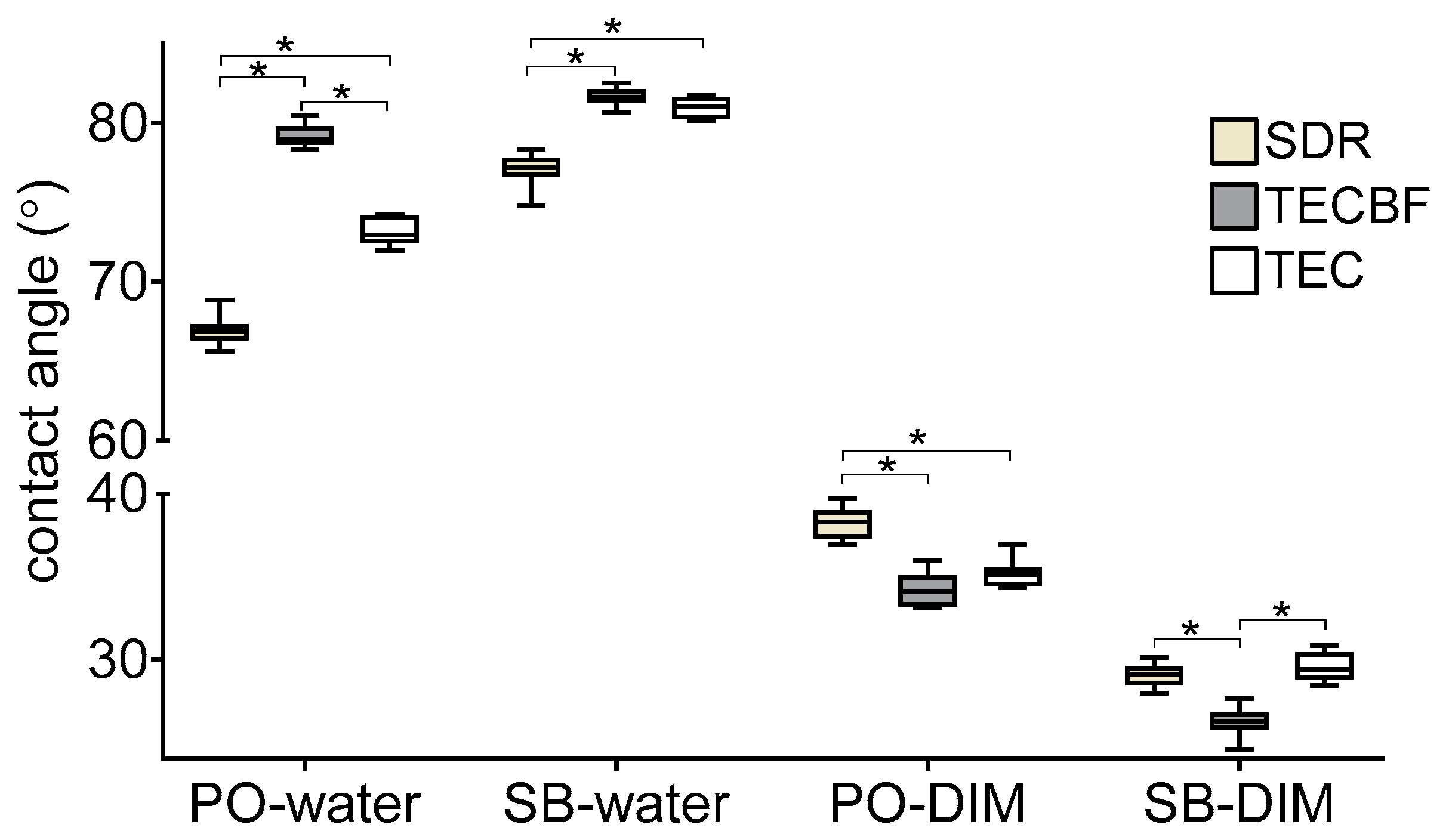
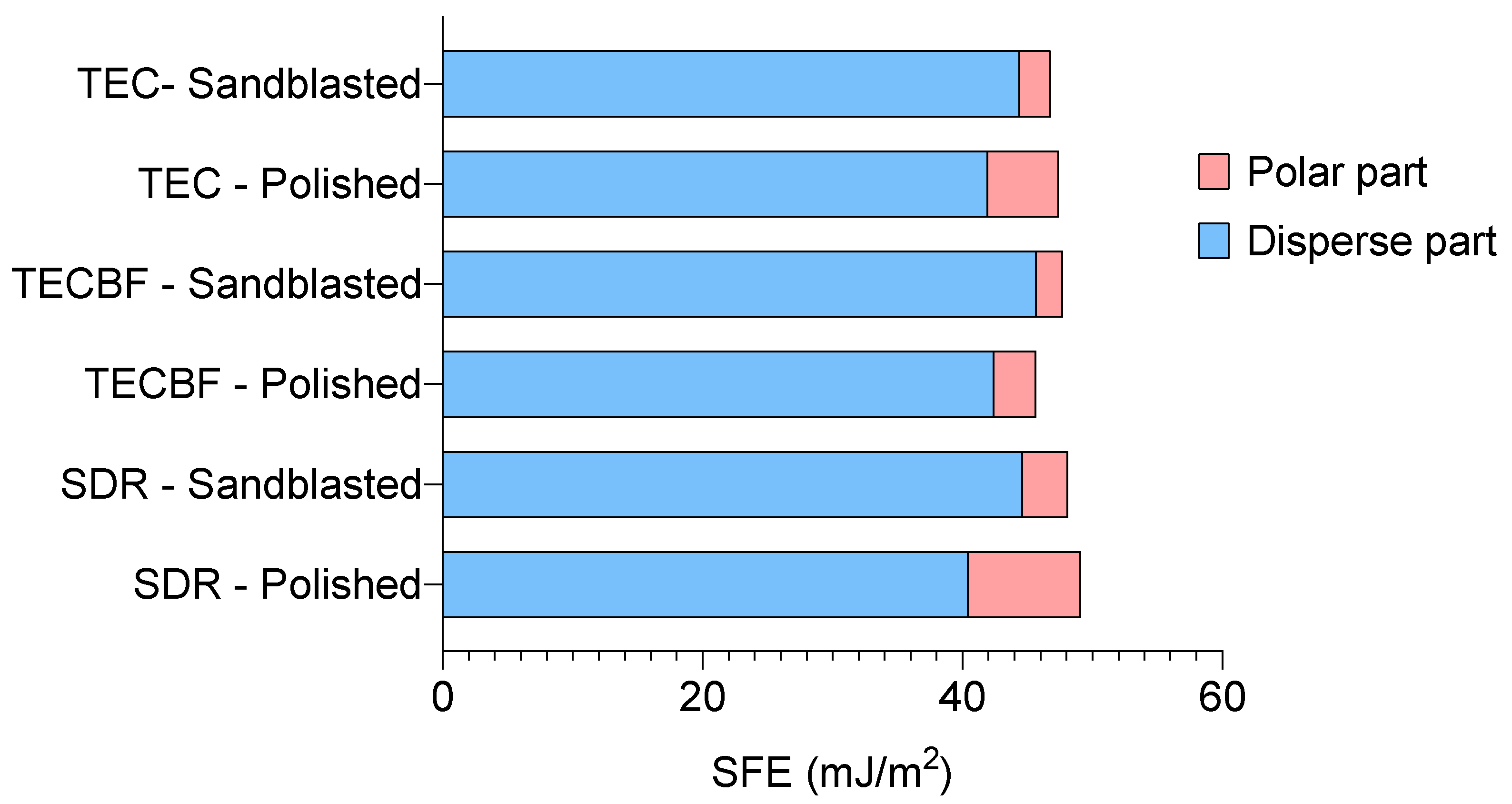
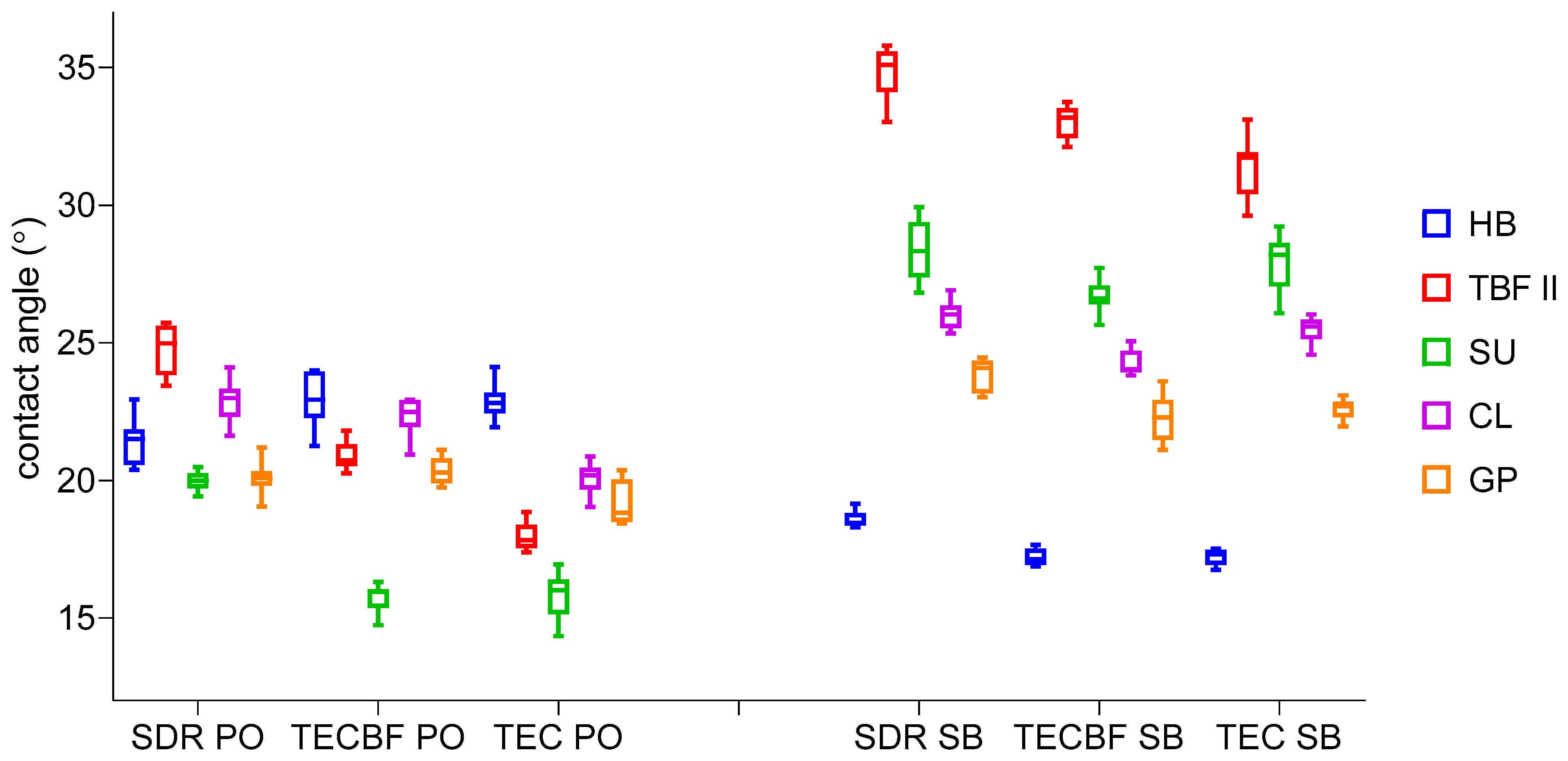
3.3. Results of Contact Angle Measurements and Surface Free Energy Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angerame, D.; De Biasi, M. Do Nanofilled/Nanohybrid Composites Allow for Better Clinical Performance of Direct Restorations Than Traditional Microhybrid Composites? A Systematic Review. Oper. Dent. 2018, 43, E191–E209. [Google Scholar] [CrossRef] [PubMed]
- Furness, A.; Tadros, M.Y.; Looney, S.W.; Rueggeberg, F.A. Effect of Bulk/Incremental Fill on Internal Gap Formation of Bulk-Fill Composites. J. Dent. 2014, 42, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Loomans, B.A.C.; Vivan Cardoso, M.; Roeters, F.J.M.; Opdam, N.J.M.; De Munck, J.; Huysmans, M.C.D.N.J.M.; Van Meerbeek, B. Is There One Optimal Repair Technique for All Composites? Dent. Mater. 2011, 27, 701–709. [Google Scholar] [CrossRef]
- Özcan, M.; Pekkan, G. Effect of Different Adhesion Strategies on Bond Strength of Resin Composite to Composite-Dentin Complex. Oper. Dent. 2013, 38, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Rudawska, A.; Danczak, I.; Müller, M.; Valasek, P. The Effect of Sandblasting on Surface Properties for Adhesion. Int. J. Adhes. Adhes. 2016, 70, 176–190. [Google Scholar] [CrossRef]
- Puleio, F.; Rizzo, G.; Nicita, F.; Lo Giudice, F.; Tamà, C.; Marenzi, G.; Centofanti, A.; Raffaele, M.; Santonocito, D.; Risitano, G. Chemical and Mechanical Roughening Treatments of a Supra-Nano Composite Resin Surface: SEM and Topographic Analysis. Appl. Sci. 2020, 10, 4457. [Google Scholar] [CrossRef]
- de Souza, M.O.; Leitune, V.C.B.; Rodrigues, S.B.; Samuel, S.M.W.; Collares, F.M. One-Year Aging Effects on Microtensile Bond Strengths of Composite and Repairs with Different Surface Treatments. Braz. Oral Res. 2017, 31, e4. [Google Scholar] [CrossRef]
- Rathke, A.; Tymina, Y.; Haller, B. Effect of Different Surface Treatments on the Composite-Composite Repair Bond Strength. Clin. Oral Investig. 2009, 13, 317–323. [Google Scholar] [CrossRef]
- Pilo, R.; Brosh, T.; Geron, V.; Levartovsky, S.; Eliades, G. Effect of Silane Reaction Time on the Repair of a Nanofilled Composite Using Tribochemical Treatment. J. Adhes. Dent. 2016, 18, 125–134. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Matsumoto, T.; Minagi, S.; Osaka, A.; Van Landuyt, K.; Van Meerbeek, B. HEMA Inhibits Interfacial Nano-Layering of the Functional Monomer MDP. J. Dent. Res. 2012, 91, 1060–1065. [Google Scholar] [CrossRef]
- Carrilho, E.; Cardoso, M.; Ferreira, M.M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability-A Systematic Review. Material 2019, 12, 790. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Q.; Zhao, J.; Zhao, B.; Ma, Z.; Zhang, C. Adhesion of Teeth. Front. Mater. 2021, 7, 615225. [Google Scholar] [CrossRef]
- da Costa, T.R.F.; Serrano, A.M.; Atman, A.P.F.; Loguercio, A.D.; Reis, A. Durability of Composite Repair Using Different Surface Treatments. J. Dent. 2012, 40, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Namen, F.; Galan, J., Jr.; de Oliveira, J.F.; Cabreira, R.D.; Costa e Silva Filho, F.; Souza, A.B.; de Deus, G. Surface Properties of Dental Polymers: Measurements of Contact Angles, Roughness and Fluoride Release. Mater. Res. 2008, 11, 239–243. [Google Scholar] [CrossRef]
- Meiron, T.S.; Marmur, A.; Saguy, I.S. Contact Angle Measurement on Rough Surfaces. J. Colloid Interface Sci. 2004, 274, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef] [PubMed]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 2008, 2, 66–81. [Google Scholar] [CrossRef]
- St-Pierre, L.; Martel, C.; Crépeau, H.; Vargas, M. Influence of Polishing Systems on Surface Roughness of Composite Resins: Polishability of Composite Resins. Oper. Dent. 2019, 44, E122–E132. [Google Scholar] [CrossRef]
- Aytac, F.; Karaarslan, E.S.; Agaccioglu, M.; Tastan, E.; Buldur, M.; Kuyucu, E. Effects of Novel Finishing and Polishing Systems on Surface Roughness and Morphology of Nanocomposites. J. Esthet. Restor. Dent. 2016, 28, 247–261. [Google Scholar] [CrossRef]
- Martos, R.; Hegedüs, V.; Szalóki, M.; Blum, I.R.; Lynch, C.D.; Hegedüs, C. A Randomised Controlled Study on the Effects of Different Surface Treatments and Adhesive Self-Etch Functional Monomers on the Immediate Repair Bond Strength and Integrity of the Repaired Resin Composite Interface. J. Dent. 2019, 85, 57–63. [Google Scholar] [CrossRef]
- Martos, R.; Szalóki, M.; Gáll, J.; Csík, A.; Hegedűs, C. Comparative Analysis of Bond Strength Durability of 10-Methacryloyloxydecyl Dihydrogen Phosphate-Containing Adhesives on a Low-Viscosity Bulk-Fill Composite Surface. J. Adhes. Dent. 2022, 24, 427–434. [Google Scholar] [CrossRef]
- Blum, I.R.; Martos, R.; Szalóki, M.; Lynch, C.D.; Hegedűs, C. Effects of Different Surface Treatments and Adhesive Self-Etch Functional Monomers on the Repair of Bulk Fill Composites: A Randomised Controlled Study. J. Dent. 2021, 108, 103637. [Google Scholar] [CrossRef] [PubMed]
- Silikas, N.; Kavvadia, K.; Eliades, G.; Watts, D. Surface Characterization of Modern Resin Composites: A Multitechnique Approach. Am. J. Dent. 2005, 18, 95–100. [Google Scholar] [PubMed]
- Erdman, N.; Bell, D.C.; Reichelt, R. Scanning Electron Microscopy. In Springer Handbook of Microscopy; Springer Handbooks; Hawkes, P.W., Spence, J.C.H., Eds.; Springer: Cham, Switzerland, 2019; pp. 229–318. [Google Scholar] [CrossRef]
- Ryba, T.M.; Dunn, W.J.; Murchison, D.F. Surface Roughness of Various Packable Composites. Oper. Dent. 2002, 27, 243–247. [Google Scholar]
- Marghalani, H.Y. Effect of Filler Particles on Surface Roughness of Experimental Composite Series. J. Appl. Oral Sci. 2010, 18, 59–67. [Google Scholar] [CrossRef]
- Ishii, R.; Takamizawa, T.; Tsujimoto, A.; Suzuki, S.; Imai, A.; Barkmeier, W.W.; Latta, M.A.; Miyazaki, M. Effects of Finishing and Polishing Methods on the Surface Roughness and Surface Free Energy of Bulk-Fill Resin Composites. Oper. Dent. 2020, 45, E91–E104. [Google Scholar] [CrossRef]
- Tulumbaci, F.; Korkut, E.; Ozer, H.; Özcan, M. Evaluation of Wettability Characteristics and Adhesion of Resin Composite to Photo-Polymerized Pulp-Capping Materials with and without Bioactive Glass. Braz. Dent. Sci. 2019, 22, 335–343. [Google Scholar] [CrossRef]
- Namen, F.M.; Ferrandini, E.; Galan, J. Surface Energy and Wettability of Polymers Light-Cured by Two Different. J. Appl. Oral Sci. 2011, 19, 517. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, E.; Peutzfeldt, A. Resin Composites: Strength of the Bond to Dentin versus Surface Energy Parameters. Dent. Mater. 2005, 21, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.A.; Farina, M.; Soares, G.A.; Anselme, K. Surface Energy of Hydroxyapatite and Beta-Tricalcium Phosphate Ceramics Driving Serum Protein Adsorption and Osteoblast Adhesion. J. Mater. Sci. Mater. Med. 2008, 19, 2307–2316. [Google Scholar] [CrossRef] [PubMed]



| Code | SDR | TECBF | TEC |
|---|---|---|---|
| Full name | Smart Dentin Replacement | Tetric EvoCeram Bulk-Fill | Tetric EvoCeram |
| Manufacturer | Dentsply Sirona, Milford, DE, USA | Ivoclar Vivadent, Shaan, Lichtenstein | Ivoclar Vivadent, Shaan, Lichtenstein |
| LOT number | 1806000584 | X25116 | LOT Y22007 |
| Composition | SDR Patented UDMA, TEGDMA, Bis-EMA, CQ, BHT, UV stabilizer, titanium dioxide, and iron oxide pigments fluorescent agent fillers: nanoparticles 68 wt% 44 vol% Ba-Al-F-B-silicate glass, Sr-Al-F-silicate glass 4.2 μm agglomerate of 0.8 μm [22] | Resin: 20–21 wt% dimethacrylates (Bis-GMA, UDMA, Bis-EMA), Filler: Barium-alumino-fluoro-silica, YbF3, spherical mixed oxide, glass filler, prepolymer fillers, filler 80 wt% (17% prepolymer) 60 vol% 40–3000 nm, average: 550 nm Additional contents are additives, catalysts, stabilizers, and pigments (<1.0% weight) [22] | Resin: 17–18% weight Bis-GMA, UDMA, Bis- EMA and CQ + Lucirin TPO photoinitiators, stabilizers Filler: 48.5 wt% Barium aluminum silicate glass fillers with size between 40–3000 nm mean particle size of 550 nm 34 wt% ground prepolymers with fillers, YbF3, mixed oxide (macro-filler dimension) [20] |
| Applied thickness | 4 mm | 4 mm | 2 mm |
| Code | HB | TBF II | SU | CL | GP |
|---|---|---|---|---|---|
| Full name | Heliobond | Tokuyama Bond Force II | Scotchbond Universal | Clearfil Universal Bond Quick | G-Premio Bond |
| Manufacturer | Ivoclar Vivadent, Shaan, Lichtenstein | Tokuyama Dental, Tokyo, Japan | 3M Oral Care, St Paul, MN, USA | Kuraray Noritake, Tokyo, Japan | GC, Tokyo, Japan |
| LOT number | X10508 | 097 | 80409A | 3K0206 | 1906121012687 |
| Composition | Bis-GMA 59.5 wt%, TEGDMA 39.7 wt%, CQ, stabilizers and catalysts 0.8 wt% [21] | 3D-SR phosphate monomer, HEMA, Bis-GMA, TEGDMA, water, alcohol, CQ, catalyst [21] | 10-MDP, Bis-GMA, DCDMA, EDMAB, MPTMS, DMAEMA, VCP, HEMA, ethanol, water, CQ, treated silica [21] | 10-MDP, Bis-GMA, HEMA, hydrophilic amid methacrylate, MPTMS, colloidal silica, NaF, CQ, ethanol, and water [21] | 10-MDP, 4-MET, MDTP, methacrylic acid ester, silica, catalyst, photoinitiator, acetone, water [21] |
| Surface Treatment | |||
|---|---|---|---|
| Polished | HB | SDR | TECBF |
| TECBF | 0.003 | ||
| TEC | 0.003 | 0.999 | |
| TBF II | SDR | TECBF | |
| TECBF | 0.017 | ||
| TEC | ˂0.001 | 0.017 | |
| SU | SDR | TECBF | |
| TECBF | ˂0.001 | ||
| TEC | 0.001 | 0.999 | |
| CL | SDR | TECBF | |
| TECBF | 0.334 | ||
| TEC | ˂0.001 | 0.002 | |
| GP | SDR | TECBF | |
| TECBF | 0.582 | ||
| TEC | 0.046 | 0.004 | |
| Sandblasted | HB | SDR | TECBF |
| TECBF | ˂0.001 | ||
| TEC | ˂0.001 | 0.999 | |
| TBF II | SDR | TECBF | |
| TECBF | 0.031 | ||
| TEC | ˂0.001 | 0.021 | |
| SU | SDR | TECBF | |
| TECBF | 0.001 | ||
| TEC | 0.813 | 0.009 | |
| CL | SDR | TECBF | |
| TECBF | ˂0.001 | ||
| TEC | 0.156 | 0.008 | |
| GP | SDR | TECBF | |
| TECBF | ˂0.001 | ||
| TEC | 0.001 | 0.813 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalóki, M.; Szabó, Z.; Martos, R.; Csík, A.; Szőllősi, G.J.; Hegedűs, C. The Surface Free Energy of Resin-Based Composite in Context of Wetting Ability of Dental Adhesives. Appl. Sci. 2023, 13, 12061. https://doi.org/10.3390/app132112061
Szalóki M, Szabó Z, Martos R, Csík A, Szőllősi GJ, Hegedűs C. The Surface Free Energy of Resin-Based Composite in Context of Wetting Ability of Dental Adhesives. Applied Sciences. 2023; 13(21):12061. https://doi.org/10.3390/app132112061
Chicago/Turabian StyleSzalóki, Melinda, Zsófia Szabó, Renáta Martos, Attila Csík, Gergő József Szőllősi, and Csaba Hegedűs. 2023. "The Surface Free Energy of Resin-Based Composite in Context of Wetting Ability of Dental Adhesives" Applied Sciences 13, no. 21: 12061. https://doi.org/10.3390/app132112061
APA StyleSzalóki, M., Szabó, Z., Martos, R., Csík, A., Szőllősi, G. J., & Hegedűs, C. (2023). The Surface Free Energy of Resin-Based Composite in Context of Wetting Ability of Dental Adhesives. Applied Sciences, 13(21), 12061. https://doi.org/10.3390/app132112061








