Preparation of Graphene Oxide Hydrogels and Their Adsorption Applications toward Various Heavy Metal Ions in Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Graphene Oxide Hydrogels
2.2.1. Preparation of Graphene Oxide
2.2.2. Synthesis of Graphene Oxide Hydrogels
2.3. Material Characterization
2.4. Removal of Cu(II), Pb(II), Zn(II), and Cd(II) Using GO/P (AA-co-AM)
2.5. Adsorption Kinetics Experiments
2.6. Intraparticle Diffusion Model
2.7. Adsorption Isotherms
3. Results and Discussion
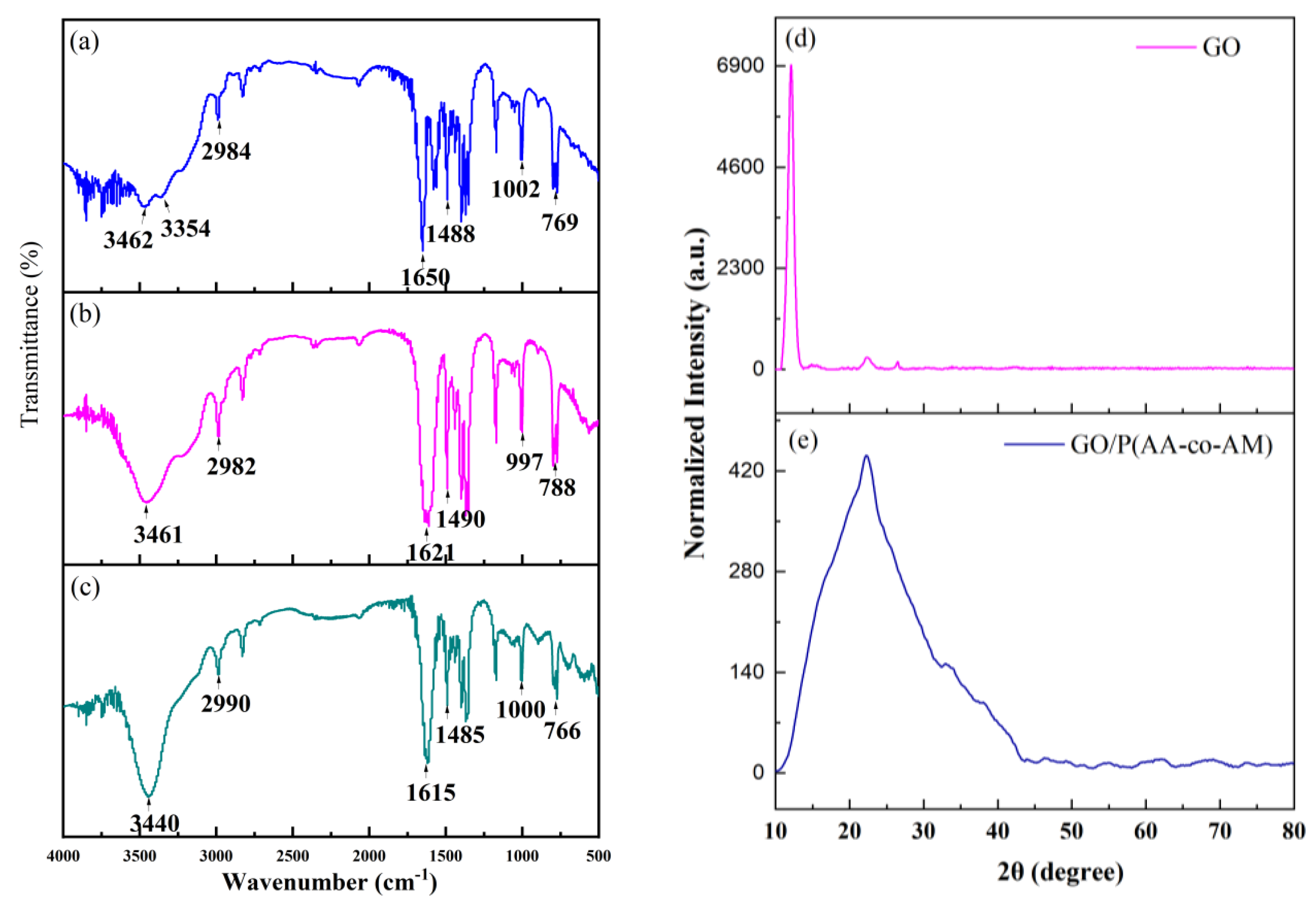
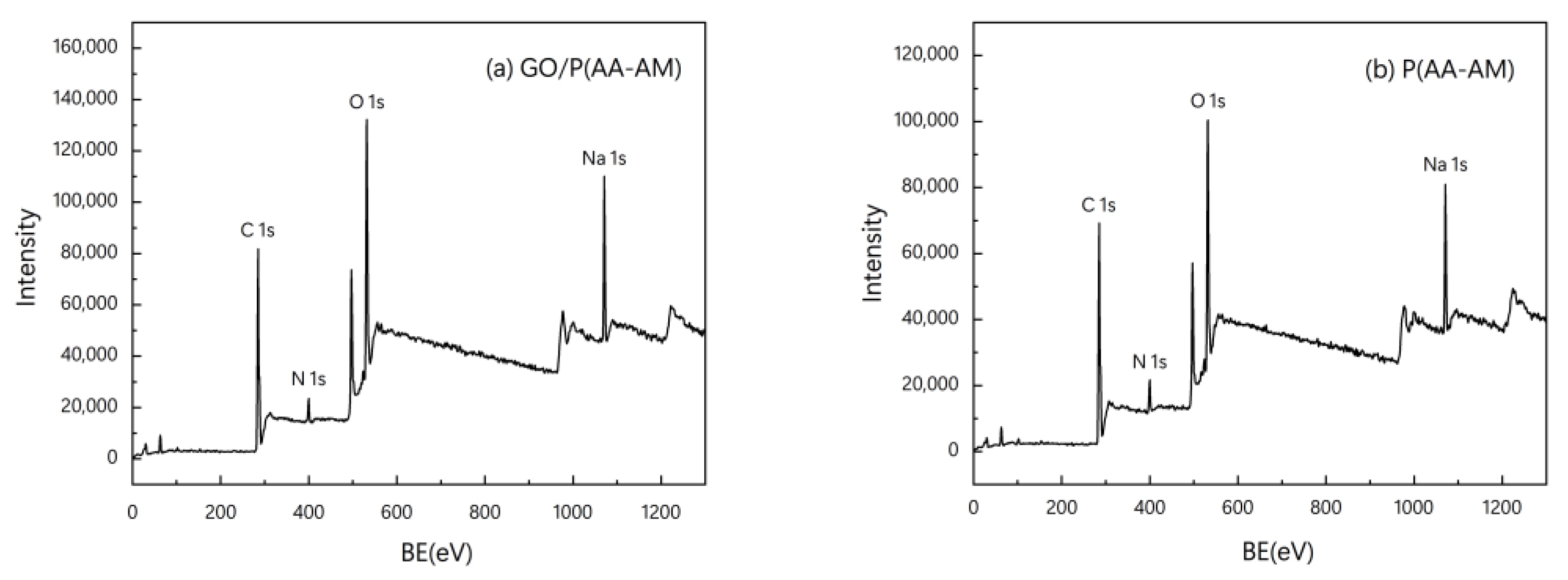
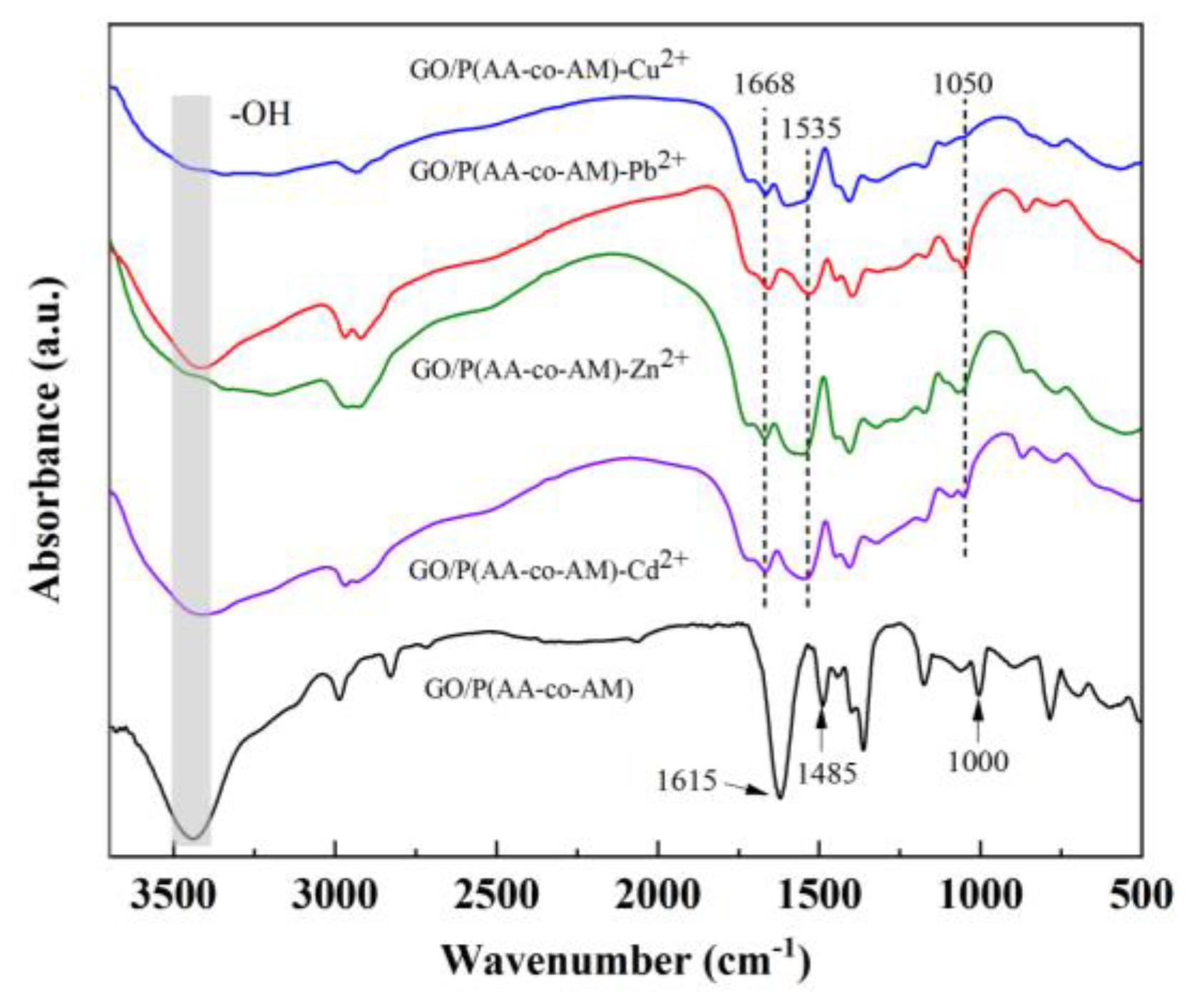
3.1. FTIR, XRD, and XPS Characterization
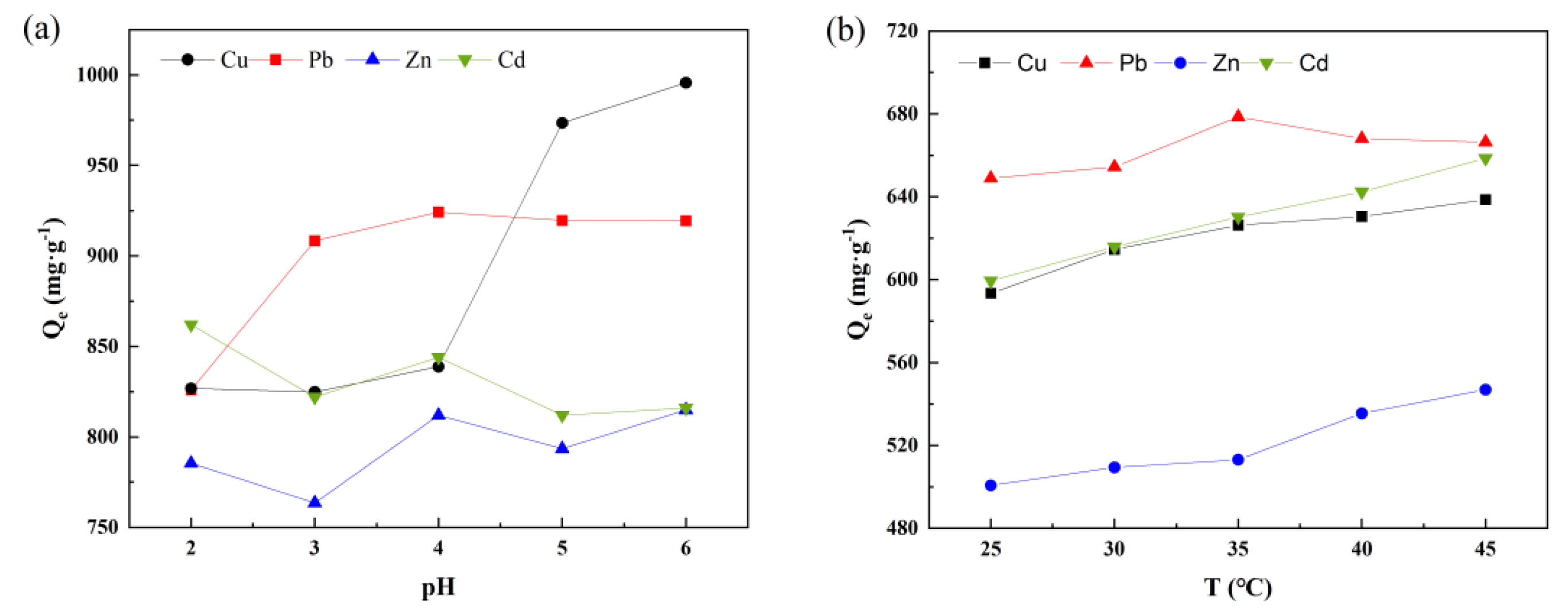
3.2. Exposure to Metal Ions
3.3. Discussion of Adsorption Mechanisms
3.3.1. Adsorption Kinetic Model
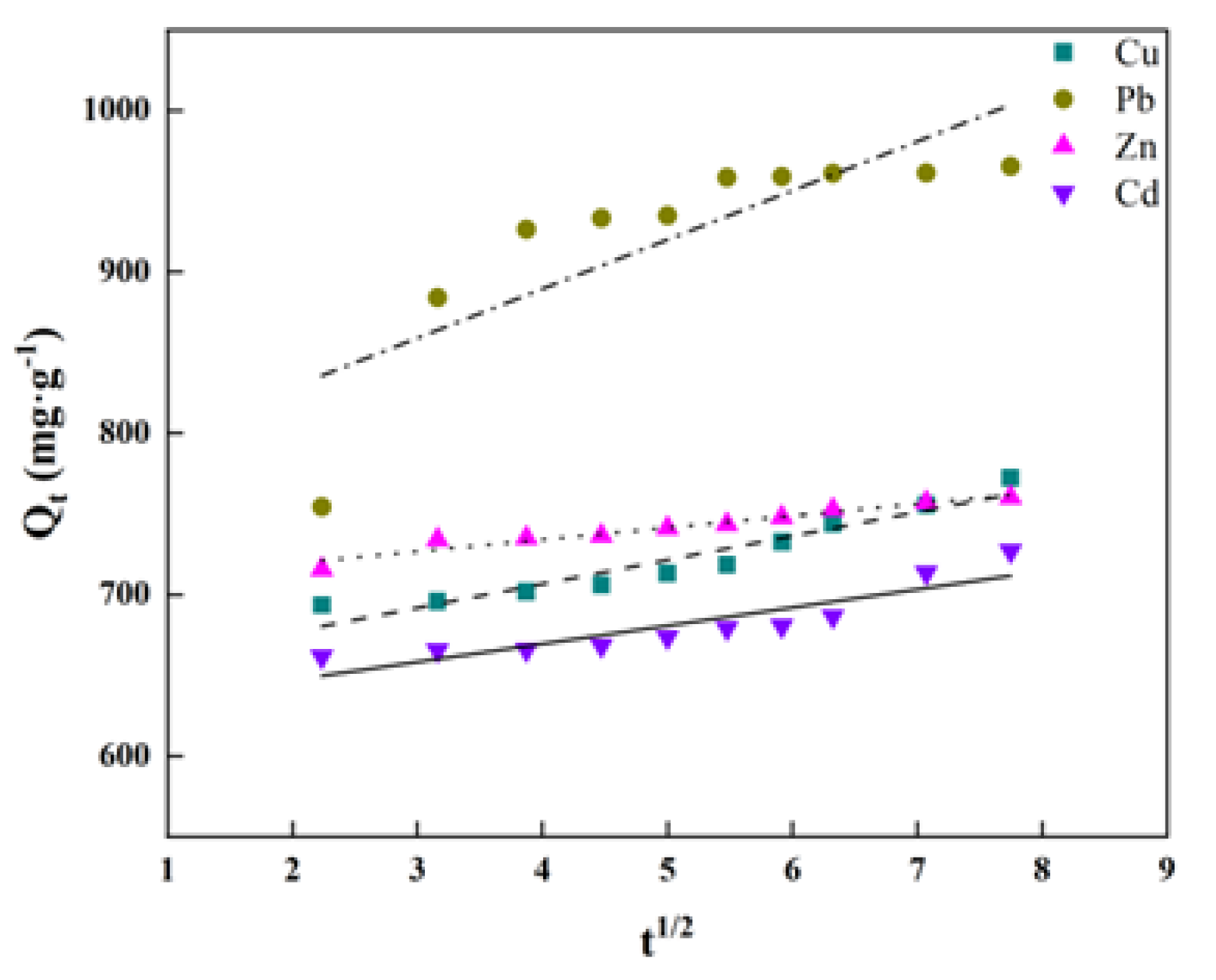
3.3.2. Particle Diffusion Model
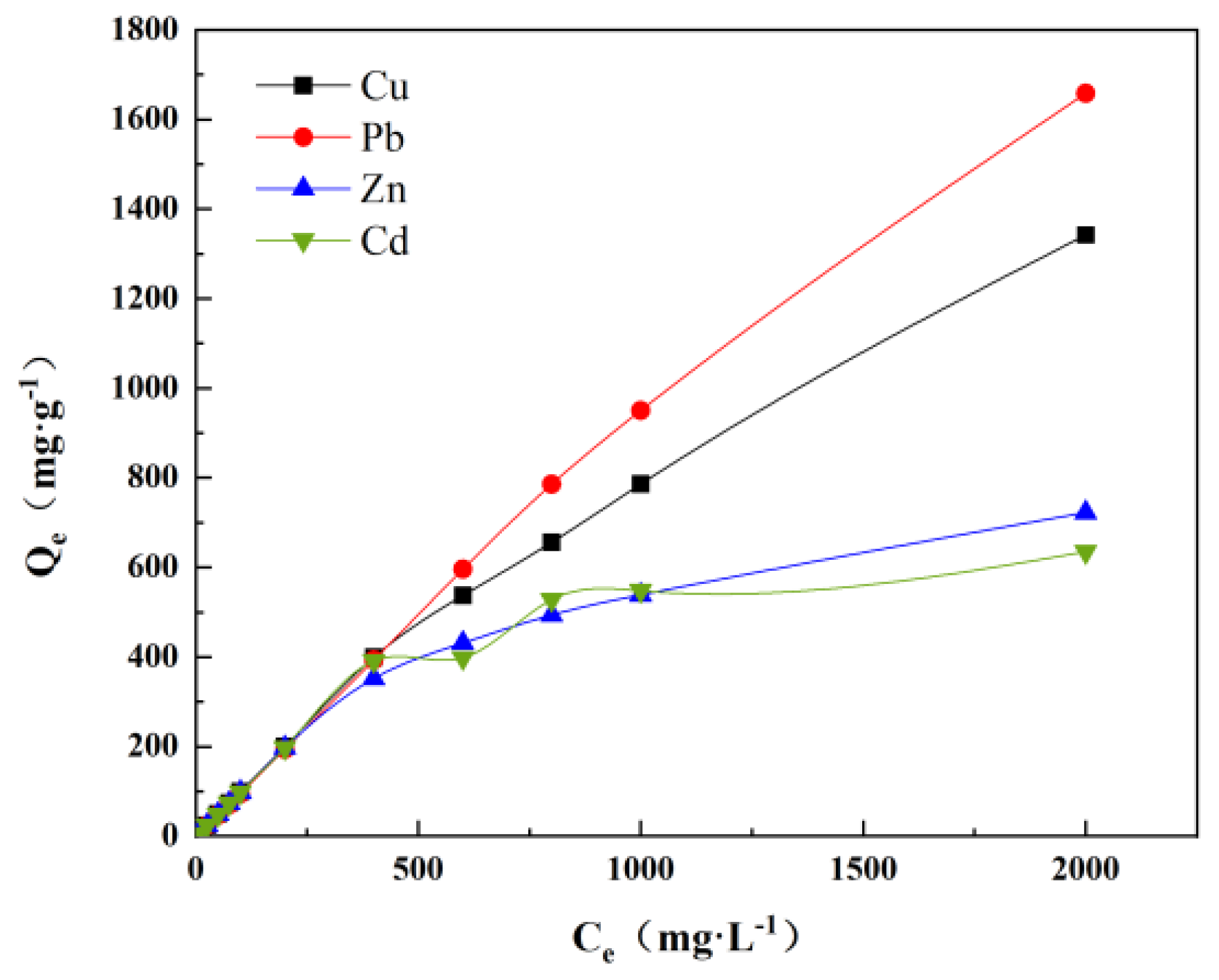
3.3.3. Adsorption Isotherms
3.4. Competitive Adsorption of Four Metal Ions
3.5. Cyclic Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.; Nguyen, K.A.; Vu, C.T.; Senoro, D.; Villanueva, M.C. Contamination levels and potential sources of organic pollution in an Asian river. Water Sci. Technol. 2017, 76, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, T.; Said, M.; Naim, V. Dna replication stress and chromosomal instability: Dangerous liaisons. Genes 2020, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Effects of chromium supplementation on body composition, human and animal health, and insulin and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Yeh, G.; Hoang, H.G.; Lin, C.; Bui, X.T.; Tran, H.T.; Shern, C.C.; Vu, C.T. Assessment of heavy metal contamination and adverse biological effects of an industrially affected river. Environ. Sci. Pollut. Res. 2020, 27, 34770–34780. [Google Scholar] [CrossRef]
- Benson, N.U.; Adedapo, A.E.; Fred-Ahmadu, O.H.; Williams, A.B.; Udosen, E.D.; Ayejuyo, O.O.; Olajire, A.A. New ecological risk indices for evaluating heavy metals contamination in aquatic sediment: A case study of the Gulf of Guinea. Reg. Stud. Mar. Sci. 2018, 18, 44–56. [Google Scholar] [CrossRef]
- Vidu, R.; Matei, E.; Predescu, A.M.; Alhalaili, B.; Pantilimon, C.; Tarcea, C.; Predescu, C. Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications. Toxics 2020, 8, 101. [Google Scholar] [CrossRef]
- Czikkely, M.; Neubauer, E.; Fekete, I.; Ymeri, P.; Fogarassy, C. Review of Heavy Metal Adsorption Processes by Several Organic Matters from Wastewaters. Water 2018, 10, 1377. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment-A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Meez, E.; Rahdar, A.; Kyzas, G.Z. Sawdust for the Removal of Heavy Metals from Water: A Review. Molecules 2021, 26, 4318. [Google Scholar] [CrossRef]
- Raninga, M.; Mudgal, A.; Patel, V.K.; Patel, J.; Kumar Sinha, M. Modification of activated carbon-based adsorbent for removal of industrial dyes and heavy metals: A review. Mater. Today Proc. 2022, 77, 286–294. [Google Scholar] [CrossRef]
- Kuldeyev, E.; Seitzhanova, M.; Tanirbergenova, S.; Tazhu, K.; Doszhanov, E.; Mansurov, Z.; Azat, S.; Nurlybaev, R.; Berndtsson, R. Modifying Natural Zeolites to Improve Heavy Metal Adsorption. Water 2023, 15, 2215. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G.; Calina, I.C. Sodium Alginate-g-acrylamide/acrylic Acid Hydrogels Obtained by Electron Beam Irradiation for Soil Conditioning. Int. J. Mol. Sci. 2023, 24, 104. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Sharma, P.P.; Yadav, V.; Gupta, H.; Kulshrestha, V. Synthesis and characterization of different metal oxide and GO composites for removal of toxic metal ions. Sep. Sci. Technol. 2019, 54, 426–433. [Google Scholar] [CrossRef]
- Jian, M.; Zhang, Y.; Liu, Z. Graphene Fibers: Preparation, Properties, and Applications. Wuli Huaxue Xuebao/Acta Phys. Chim. Sin. 2021, 38, 2007093. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, L.; Bai, H.; Hong, W.; Li, C.; Shi, G. Chemically converted graphene induced molecular flattening of 5,10,15,20-tetrakis(1-methyl-4-pyridinio)porphyrin and its application for optical detection of cadmium(II) ions. J. Am. Chem. Soc. 2009, 131, 13490–13497. [Google Scholar] [CrossRef]
- Swidan, M.M.; Essa, B.M.; Sakr, T.M. Pristine/folate-functionalized graphene oxide as two intrinsically radioiodinated nano-theranostics: Self/dual in vivo targeting comparative study. Cancer Nanotechnol. 2023, 14, 6. [Google Scholar] [CrossRef]
- Sadighian, S.; Tozihi, M. Synthesis, Characterization, and Dye Removal Applications of Graphene Oxide-Gold Nanocomposite. Biointerface Res. Appl. Chem. 2023, 13, 385. [Google Scholar] [CrossRef]
- Bulin, C. Combination mechanism of the ternary composite based on Fe3O4-chitosan-graphene oxide prepared by solvothermal method. Int. J. Biol. Macromol. 2023, 231, 123337. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z.; Kabiri, F. Preparation and Investigation on Swelling and Drug Delivery Properties of a Novel Silver/Salep-g-Poly(Acrylic Acid) Nanocomposite Hydrogel. Bull. Korean Chem. Soc. 2012, 33, 2635–2641. [Google Scholar] [CrossRef]
- Bal, A.; Cepni, F.E.; Cakir, O.; Acar, I.; Guclu, G. Synthesis and characterization of copolymeric and terpolymeric hydrogel-silver nanocomposites based on acrylic acid, acrylamide and itaconic acid: Investigation of their antibacterial activity against gram-negative bacteria. Braz. J. Chem. Eng. 2015, 32, 509–518. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.G.; Zhao, Y.L.; Bai, H.Y.; Huang, M.Y.; Zhang, T.T.; Song, S.X. High-performance two-dimensional montmorillonite supported-poly(acrylamide-co-acrylic acid) hydrogel for dye removal. Environ. Pollut. 2020, 257, 113574. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, H.Y.; Yang, Q.Y.; Zhou, W.N.; Sun, C.B. Adsorption of Pb2+and Cd2+on reduced graphene oxide hydrogel prepared from natural cryptocrystalline graphite. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 642, 128630. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Sand, A.; Vyas, A.; Gupta, A.K. Graft copolymer based on (sodium alginate-g-acrylamide): Characterization and study of Water swelling capacity, metal ion sorption, flocculation and resistance to biodegradability. Int. J. Biol. Macromol. 2016, 90, 37–43. [Google Scholar] [CrossRef]
- Couto da Feira, J.M.; Klein, J.M.; de Camargo Forte, M.M. Ultrasound-assisted synthesis of polyacrylamide-grafted sodium alginate and its application in dye removal. Polim. Cienc. E Tecnol. 2018, 28, 139–146. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Fatinathan, S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Wan, S.; He, F.; Wu, J.; Wan, W.; Gu, Y.; Gao, B. Rapid and highly selective removal of lead from water using graphene oxide-hydrated manganese oxide nanocomposites. J. Hazard. Mater. 2016, 314, 32–40. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, J.; Tang, M.; He, Q.; Long, A.; Luo, S.; Sun, S.; Wan, J.; Gao, Y.; Zhou, L.; et al. Physically-crosslinked activated CaCO3/polyaniline-polypyrrole-modified GO/alginate hydrogel sorbent with highly efficient removal of copper(II) from aqueous solution. Chem. Eng. J. 2022, 431, 133375. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Yang, L.-Z.; Yu, M.; Liu, M. Preparation of Poly(Acrylate-Acrylamide) Hydrogel and Its Adsorption Performance to Heavy Metal Ions. Chin. J. Anal. Chem. 2016, 44, 707–715. [Google Scholar] [CrossRef]
- Orozco-Guareño, E.; Santiago-Gutiérrez, F.; Morán-Quiroz, J.L.; Hernandez-Olmos, S.L.; Soto, V.; de la Cruz, W.; Manríquez, R.; Gomez-Salazar, S. Removal of Cu(II) ions from aqueous streams using poly(acrylic acid-co-acrylamide) hydrogels. J. Colloid Interface Sci. 2010, 349, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Y.; Luo, J.M.; Liu, C.B.; Chu, L.; Ma, J.H.; Tang, Y.H.; Zeng, Z.B.; Luo, S.L. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016, 89, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jiang, L.P.; Zhu, L.X.; Wang, A.Q. Novel Covalently Cross-Linked Attapulgite/Poly(acrylic acid-co-acrylamide) Hybrid Hydrogels by Inverse Suspension Polymerization: Synthesis Optimization and Evaluation as Adsorbents for Toxic Heavy Metals. Ind. Eng. Chem. Res. 2014, 53, 4277–4285. [Google Scholar] [CrossRef]
- Jiang, L.P.; Liu, P. Covalently crosslinked fly ash/poly(acrylic acid-co-acrylamide) composite microgels as novel magnetic selective adsorbent for Pb2+ ion. J. Colloid Interface Sci. 2014, 426, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ohnaka, K.; Fujita, S.; Kishi, M.; Yuchi, A. Effects of the Spaces Available for Cations in Strongly Acidic Cation-Exchange Resins on the Exchange Equilibria by Quaternary Ammonium Ions and on the Hydration States of Metal Ions. Anal. Chem. 2011, 83, 7480–7485. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, M.; Xue, D. Solution-phase electronegativity scale: Insight into the chemical behaviors of metal ions in solution. J. Phys. Chem. A 2012, 116, 4192–4198. [Google Scholar] [CrossRef]
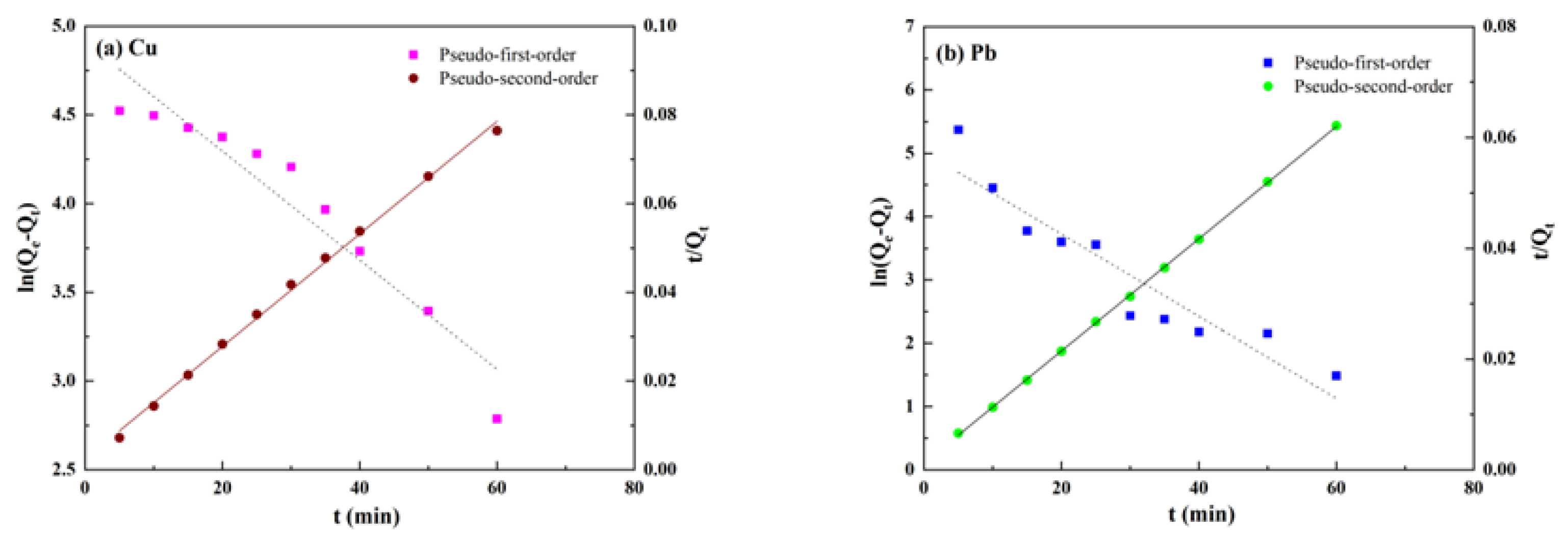
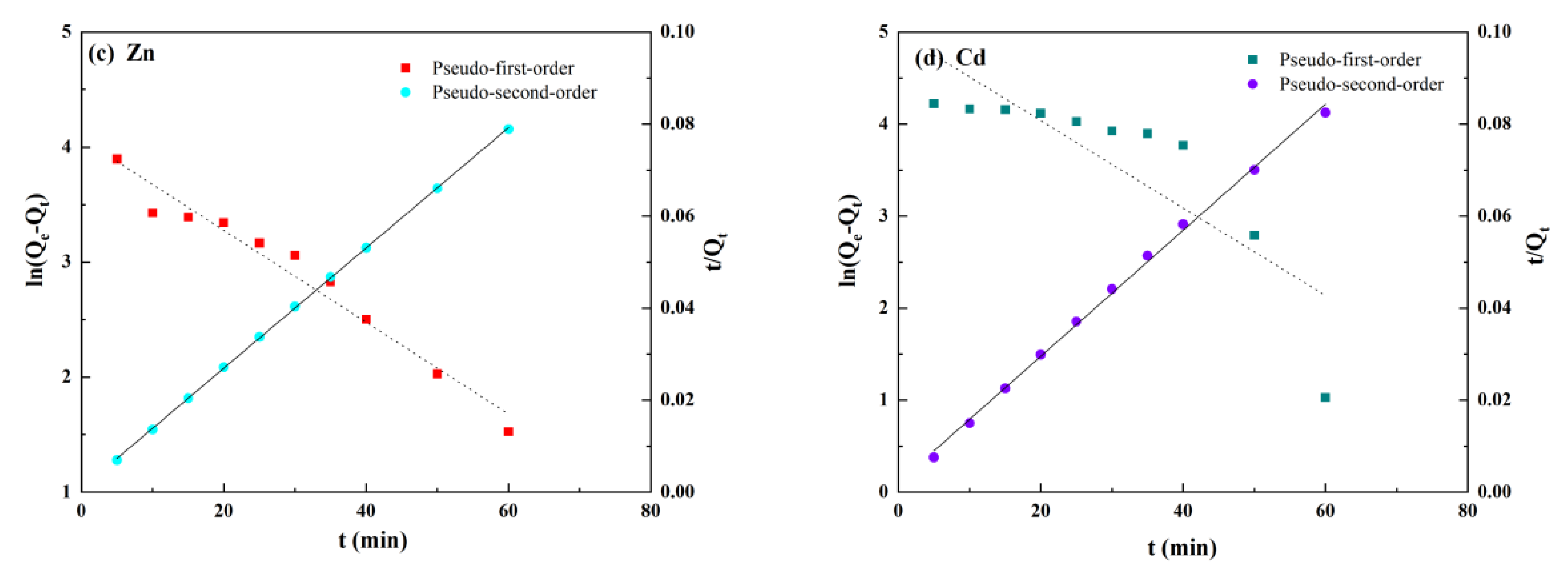
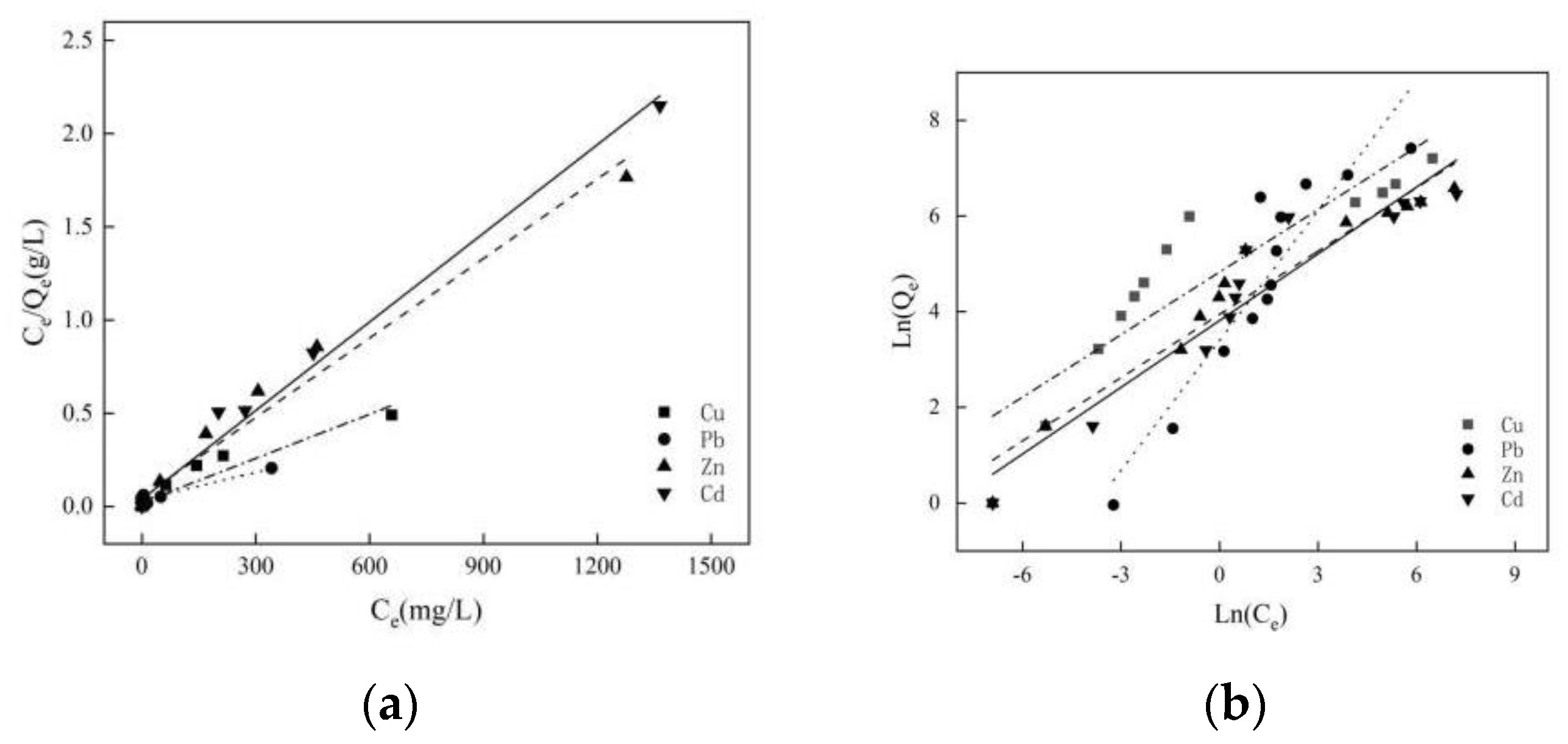
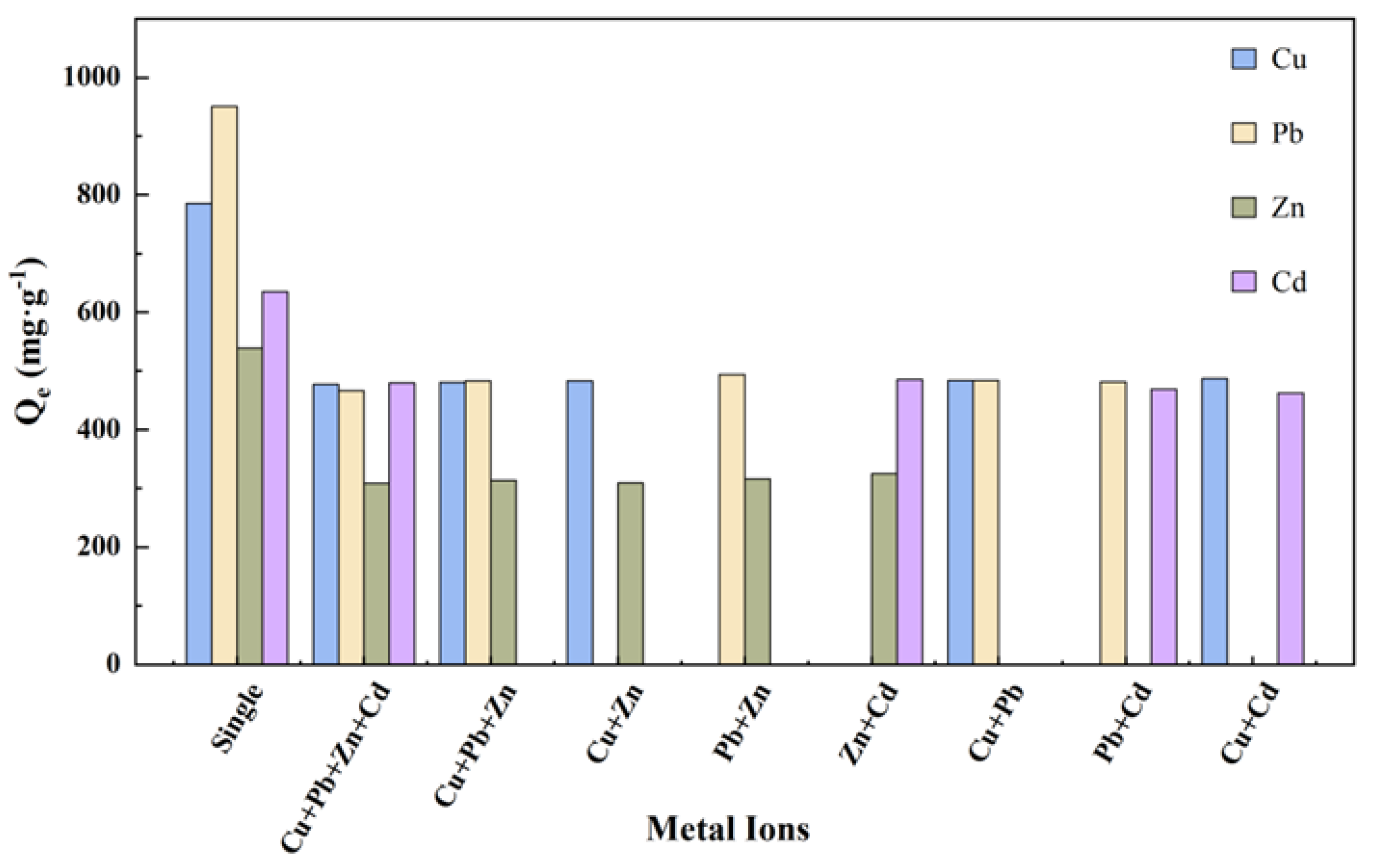
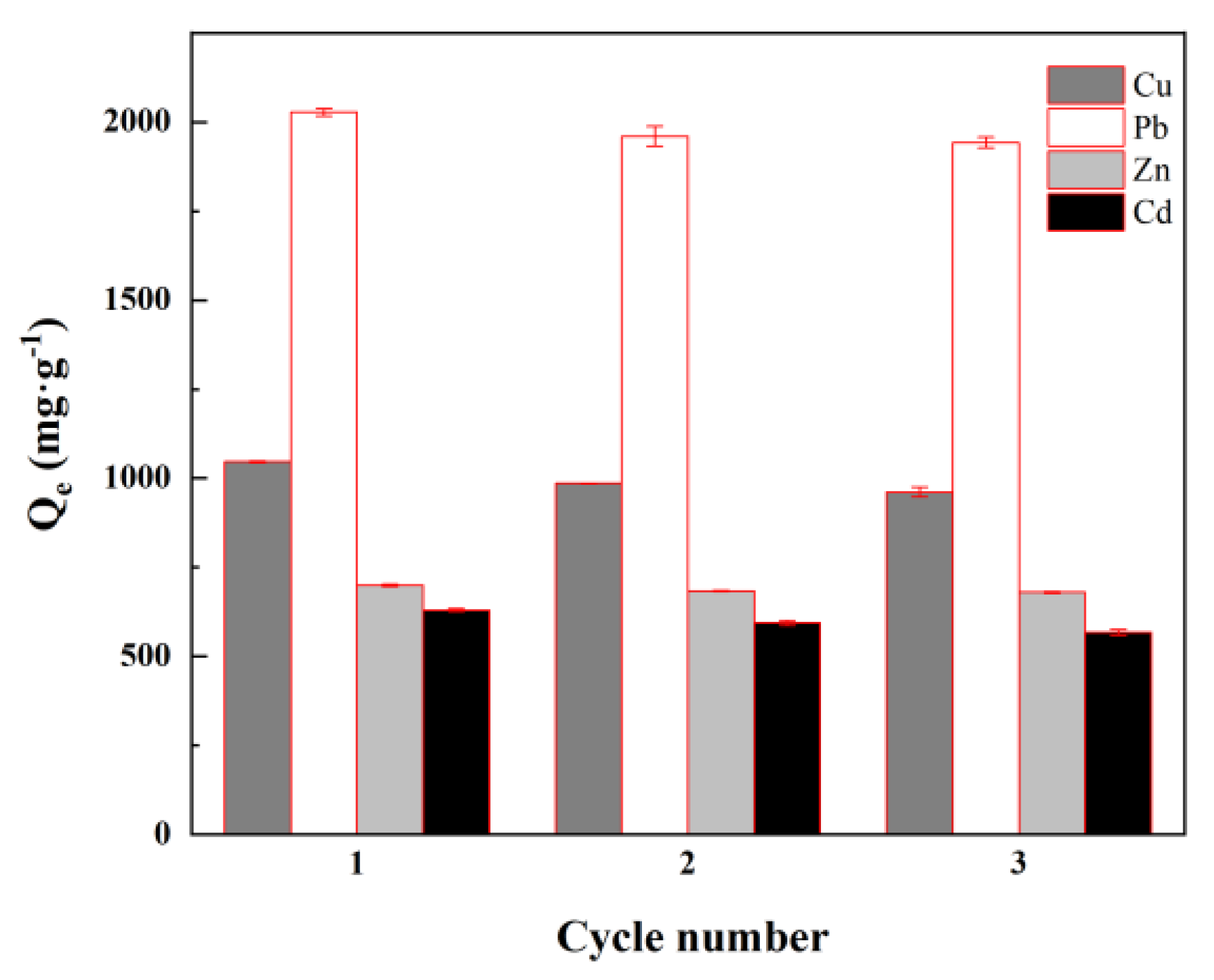
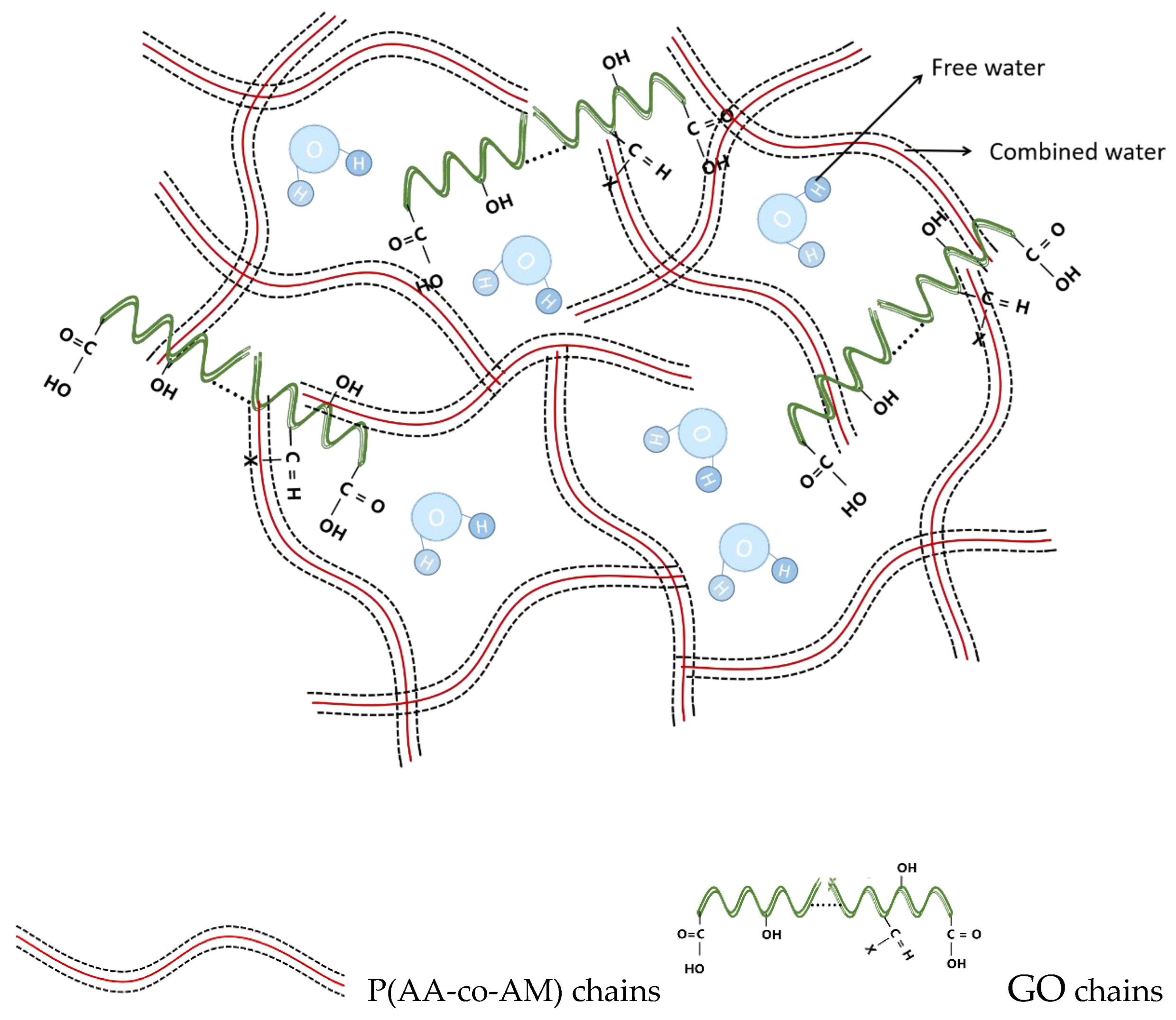
| Ions | Qe/mg·g−1 | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|
| Qe/mg·g−1 | k1 | R1 | Qe/mg·g−1 | k2 | R2 | ||
| Cu2+ | 785.72 | 661.41 | 0.03073 | 0.907 | 787.4 | 0.000661 | 0.997 |
| Pb2+ | 970.03 | 950.24 | 0.06489 | 0.876 | 990.09 | 0.000843 | 0.999 |
| Zn2+ | 764.96 | 695.31 | 0.03993 | 0.960 | 763.36 | 0.001361 | 0.999 |
| Cd2+ | 729.89 | 687.83 | 0.04752 | 0.667 | 729.93 | 0.000885 | 0.998 |
| Adsorbent | Qm/mg·g−1 | Report | |||
|---|---|---|---|---|---|
| Cu2+ | Pb2+ | Zn2+ | Cd2+ | ||
| GO/P(AA-AM) | 787.4 | 990.09 | 763.36 | 729.93 | |
| P(AA-AM) | 185.73 | 587.99 | 208.42 | 402.86 | [30] |
| P(AA/AM) | 69.81 | - | - | - | [31] |
| P(AA/MMA) | 153.8 | 216.1 | - | - | [32] |
| ATP/P(AA/AM) | 156.3 | 84.03 | - | - | [33] |
| FA/P(AA/AM) | - | 103.8 | - | - | [34] |
| Ions | Kd | α | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu2+ | Pb2+ | Zn2+ | Cd2+ | Pb2+/Cu2+ | Pb2+/Zn2+ | Pb2+/Cd2+ | Cu2+/Zn2+ | Cu2+/Cd2+ | Zn2+/Cd2+ | Cd2+/Zn2+ | |
| Cu2+ + Pb2++ Zn2+ + Cd2+ | 0.275 | 0.270 | 0.178 | 0.277 | 0.982 | 1.517 | 0.975 | 1.545 | 0.993 | 0.643 | 1.555 |
| Cu2+ + Pb2+ + Zn2+ | 0.376 | 0.378 | 0.245 | - | 1.005 | 1.543 | - | 1.535 | - | - | - |
| Cu2+ + Zn2+ | 0.610 | - | 0.390 | - | - | - | - | 1.564 | - | - | - |
| Pb2+ + Zn2+ | - | 0.610 | 0.390 | - | - | 1.564 | - | - | - | 0.669 | - |
| Zn2+ + Cd2+ | - | - | 0.401 | 0.599 | - | - | - | - | - | - | 1.494 |
| Cu2+ + Pb2+ | 0.500 | 0.500 | - | - | 1.000 | - | - | - | - | - | - |
| Pb2+ + Cd2+ | - | 0.506 | - | 0.494 | - | - | 1.024 | - | - | - | - |
| Cu2+ + Cd2+ | 0.513 | - | - | 0.487 | - | - | - | - | 1.530 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, Y.; Wu, Y.; Liu, C.; Liu, X. Preparation of Graphene Oxide Hydrogels and Their Adsorption Applications toward Various Heavy Metal Ions in Aqueous Media. Appl. Sci. 2023, 13, 11948. https://doi.org/10.3390/app132111948
Liu M, Wang Y, Wu Y, Liu C, Liu X. Preparation of Graphene Oxide Hydrogels and Their Adsorption Applications toward Various Heavy Metal Ions in Aqueous Media. Applied Sciences. 2023; 13(21):11948. https://doi.org/10.3390/app132111948
Chicago/Turabian StyleLiu, Miao, Yi Wang, Yingjun Wu, Chunyang Liu, and Xin Liu. 2023. "Preparation of Graphene Oxide Hydrogels and Their Adsorption Applications toward Various Heavy Metal Ions in Aqueous Media" Applied Sciences 13, no. 21: 11948. https://doi.org/10.3390/app132111948
APA StyleLiu, M., Wang, Y., Wu, Y., Liu, C., & Liu, X. (2023). Preparation of Graphene Oxide Hydrogels and Their Adsorption Applications toward Various Heavy Metal Ions in Aqueous Media. Applied Sciences, 13(21), 11948. https://doi.org/10.3390/app132111948




