Reasons for Removal of Miniplates Used in Fixation of Maxillofacial Bone Fractures: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Language Requirement: Articles had to be in English.
- Full-Text Availability: The full text of the article needed to be accessible.
- Patient Population: The study had to involve patients with fractured jaws who were treated using miniplate fixation and had a minimum follow-up duration of 12 months.
- Intervention and Comparison: The study should investigate rigid internal fixation with miniplates, with or without plate removal.
- Outcome Measure: The primary outcome of interest was the percentage of miniplates that were removed.
- Study Design: Eligible studies included clinical trials, controlled trials, retrospective studies, and case series.
- Language Exclusion: Articles published in languages other than English were excluded.
- Non-Human Studies: Studies conducted on animals or in vitro were not considered.
- Duplicate Publications: Duplicate articles, which could introduce bias, were excluded.
- Insufficient Information: Articles lacking complete and detailed information about miniplate sites and numbers were excluded.
- Publication Types: Editorial letters, case reports, and review articles were excluded.
- Short Follow-Up: Studies with a follow-up duration of less than 12 months were also excluded.
2.2. Information Sources
2.3. Search Strategy
- PubMed: (“facial trauma surgery” OR “jaw bone surgeries”) AND (“plate removal” OR “plates removal” OR “plate failure” OR “plates failure”)
- Scopus: (“facial trauma surgery” OR “jaw bone surgeries”) AND (“plate removal” OR “plates removal” OR “plate failure” OR “plates failure”)
- Embase: (“facial trauma surgery” OR “jaw bone surgeries”) AND (“plate removal” OR “plates removal” OR “plate failure” OR “plates failure”)
- Google Scholar: (“facial trauma surgery” OR “jaw bone surgeries”) AND (“plate removal” OR “plates removal” OR “plate failure” OR “plates failure”)
- ResearchGate: (“facial trauma surgery” OR “jaw bone surgeries”) AND (“plate removal” OR “plates removal” OR “plate failure” OR “plates failure”).
2.4. Selection
2.5. Data Collection Process
2.6. Study Risk of Bias Assessment
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haerle, F.; Champy, M.; Terry, B.C. Atlas of Craniomaxillofacial Osteosynthesis; Thieme: Stuttgart, Germany, 2009. [Google Scholar]
- Champy, M.; Lodde, J.P.; Muster, D.; Wilk, A.; Gastelo, L. Osteosynthesis using miniaturized screws on plates in facial and cranial surgery. Indications and results in 400 cases. Ann. Chir. Plast. 1977, 22, 261–264. [Google Scholar] [PubMed]
- Yaremchuk, M.J.; Posnick, J.C. Resolving controversies related to plate and screw fixation in the growing craniofacial skeleton. J. Craniofac. Surg. 1995, 6, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; Van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; De Visscher, J.G.A.M.; Hoppenreijs, T.J.M.; Bergsma, J.E.; Van Minnen, B.; Stegenga, B.; Bos, R.R.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: A multicenter randomized controlled trial. PLoS ONE 2017, 12, e0177152. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Matsusue, Y.; Horita, S.; Murakami, K.; Sugiura, T.; Kirita, T. Routine removal of the plate after surgical treatment for mandibular angle fracture with a third molar in relation to the fracture line. Ann. Maxillofac. Surg. 2015, 5, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, J.; Kinnunen, J.; Bondestam, S.; Majola, A.; Rokkanen, P.; Törmälä, P. Bone changes after experimental osteotomies fixed with absorbable self-reinforced poly-l-lactide screws or metallic screws studied by plain radiographs, quantitative computed tomography and magnetic resonance imaging. Biomaterials 1995, 16, 1353–1358. [Google Scholar] [CrossRef]
- Pihlajamäki, H.; Böstman, O.; Tynninen, O.; Laitinen, O. Long-term tissue response to bioabsorbable poly-l-lactide and metallic screws: An experimental study. Bone 2006, 39, 932–937. [Google Scholar] [CrossRef]
- Acero, J.; Calderon, J.; Salmeron, J.I.; Verdaguer, J.J.; Concejo, C.; Somacarrera, M.L. The behaviour of titanium as a biomaterial: Microscopy study of plates and surrounding tissues in facial osteosynthesis. J. Craniomaxillofac. Surg. 1999, 27, 117–123. [Google Scholar] [CrossRef]
- Seino, D.; Fukunishi, S.; Yoshiya, S. Late foreign-body reaction to PLLA screws used for fixation of acetabular osteotomy. J. Orthop. Traumatol. 2007, 8, 188–191. [Google Scholar] [CrossRef][Green Version]
- Jeon, H.B.; Kang, D.H.; Gu, J.H.; Oh, S.A. Delayed Foreign Body Reaction Caused by Bioabsorbable Plates Used for Maxillofacial Fractures. Arch. Plast. Surg. 2016, 43, 40–45. [Google Scholar] [CrossRef]
- Sadiq, A.; Khan, M.A. Rational for Maxillofacial Fracture Plate Removal. Pak. J. Med. Health Sci. 2021, 15, 276–279. [Google Scholar]
- Brown, J.S.; Trotter, M.; Cliffe, J.; Ward-Booth, R.P.; Williams, E.D. The fate of miniplates in facial trauma and orthognathic surgery: A retrospective study. Br. J. Oral. Maxillofac. Surg. 1989, 27, 306–315. [Google Scholar] [CrossRef]
- Islamoglu, K.; Coskunfirat, O.K.; Tetik, G.; Ozgentas, H.E. Complications and removal rates of miniplates and screws used for maxillofacial fractures. Ann. Plast. Surg. 2002, 48, 265–268. [Google Scholar] [CrossRef]
- Murthy, A.S.; Lehman, J.A. Jr Symptomatic plate removal in maxillofacial trauma: A review of 76 cases. Ann. Plast. Surg. 2005, 55, 603–607. [Google Scholar] [CrossRef]
- Rallis, G.; Mourouzis, C.; Papakosta, V.; Papanastasiou, G.; Zachariades, N. Reasons for miniplate removal following maxillofacial trauma: A 4-year study. J. Craniomaxillofac. Surg. 2006, 34, 435–439. [Google Scholar] [CrossRef]
- Dae-Kyun, P.; Yoo, S.-C.; Park, S.-H.; Koo, S.-H. The Removal of Plates after Craniomaxillofacial Surgery: A Retrospective Study. Arch. Plastic. Surg. 2007, 34, 186–190. [Google Scholar]
- O’Connell, J.; Murphy, C.; Ikeagwuani, O.; Adley, C.; Kearns, G. The fate of titanium miniplates and screws used in maxillofacial surgery: A 10 year retrospective study. Int. J. Oral. Maxillofac. Surg. 2009, 38, 731–735. [Google Scholar] [CrossRef]
- Pan, Z.; Patil, P.M. Titanium osteosynthesis hardware in maxillofacial trauma surgery: To remove or remain? A retrospective study. Eur. J. Trauma Emerg. Surg. 2013, 40, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Llandro, H.; Langford, R. Reasons for plate removal after treatment of orbitozygomatic complex fractures. J. Craniomaxillofac. Surg. 2015, 43, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Gorrela, H. Policy towards Removal of Mini Plates in Maxillofacial Trauma—A Follow up Study of 234 Patients. J. Surg. Proceed Case Rep. 2019, 1, 102. [Google Scholar] [CrossRef]
- Sukegawa, S.; Masui, M.; Sukegawa-Takahashi, Y.; Nakano, K.; Takabatake, K.; Kawai, H.; Nagatsuka, H.; Furuki, Y. Maxillofacial Trauma Surgery Patients with Titanium Osteosynthesis Miniplates: Remove or Not? J. Craniofac. Surg. 2020, 31, 1338–1342. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Janaphan, K.; Hashem, I.; Smith, H.; Holmes, S.; Chatzopoulou, D. Periodontal disease as a primary cause of surgical site infection in fractures of the mandible: Is smoking a confounding variable? Br. J. Oral. Maxillofac. Surg. 2022, 60, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.A.; Abdullah, A.A.B.; Dar-Odeh, N.; Altaweel, A.A. Intraoral Wound Dehiscence After Open Reduction Internal Fixation of Mandibular Fractures: A Retrospective Cohort Study. Wounds 2021, 33, 60. [Google Scholar]
- Fernandez-Gonzalez, F.; Monje, F.; Insua, A.; de Vicente, J.C.; Burgueno, M. Infection after removal of miniplates in maxillofacial surgery: A retrospective study. Med. Oral. Patol. Oral. Cir. Bucal 2018, 23, 96–102. [Google Scholar]
- Mosbah, M.R.; Oloyede, D.; Koppel, D.A.; Moos, K.F.; Stenhouse, D. Miniplate removal in trauma and orthognathic surgery—A retrospective study. Int. J. Oral. Maxillofac. Surg. 2003, 32, 148–151. [Google Scholar] [CrossRef]
- Bhatt, V.; Chhabra, P.; Dover, M.S. Removal of miniplates in maxillofacial surgery: A follow-up study. J. Oral. Maxillofac. Surg. 2005, 63, 756–760. [Google Scholar] [CrossRef]
- Cole, P.; Kaufman, Y.; Hollier, L.H. Jr Managing the pediatric facial fracture. Craniomaxillofac. Trauma Reconstr. 2009, 2, 77–84. [Google Scholar] [CrossRef]
- Park, H.C.; Kim, S.G.; Oh, J.S.; You, J.S.; Kim, W.G. Mini-plate removal in maxillofacial trauma patients during a five-year retrospective study. J. Korean Assoc. Oral. Maxillofac. Surg. 2016, 42, 182–186. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, J.Y.; Park, C.J. Complications after miniplate fixation of facial bone fractures. Arch. Craniofac. Surg. 2017, 18, 96–102. [Google Scholar]
- Armencea, G.; Gheban, D.; Onisor, F.; Mitre, I.; Manea, A.; Trombitas, V.; Lazar, M.; Baciut, G.; Baciut, M.; Bran, S. Histological Change in Soft Tissue Surrounding Titanium Plates after Jaw Surgery. Materials 2019, 12, 3205. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Li, Z.; Zeng, X.; Xu, Y.; Zhao, S.; Hu, H.; Zhang, Y.; Ren, T. Tribological behavior of Ti-6Al-4V against cortical bone in different bio lubricants. J. Mech. Behav. Biomed. Mater. 2019, 90, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Bessho, K.; Fujimura, K.; Iizuka, T. Experimental longterm study of titanium ions eluted from pure titanium miniplates. J. Biomed. Mater. Res. 1995, 29, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V Titanium Alloy Leads to Redox Abnormalities, Oxidative Stress, and Oxidative Damage in Patients Treated for Mandible Fractures. Oxid. Med. Cell Longev. 2018, 2018, 3714725. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Bandl, W.; Maier, S.; Summer, B.; Przybilla, B. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: Case report and review of the literature. Contact Dermat. 2006, 55, 199–202. [Google Scholar] [CrossRef]
- Egusa, H.; Ko, N.; Shimazu, T.; Yatani, H. Suspected association of an allergic reaction with titanium dental implants: A clinical report. J. Prosthet. Dent. 2008, 100, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Matthew, I.R.; Frame, J.W.; Browne, R.M.; Millar, B.G. In vivo surface analysis of titanium and stainless steel miniplates and screws. Int. J. Oral. Maxillofac. Surg. 1996, 25, 463–468. [Google Scholar] [CrossRef]
- Torgersen, S.; Gjerdet, N.R. Retrieval study of stainless steel and titanium miniplates and screws used in maxillofacial surgery. J. Mater. Sc. Mater. Med. 1994, 5, 256–262. [Google Scholar] [CrossRef]
- Hayes, J.; Richards, R. The use of titanium and stainless steel in fracture fixation. Expert. Rev. Med. Devices 2010, 7, 843–853. [Google Scholar] [CrossRef]
- Deepak, S.; Manjula, S. Comparison of Titanium bone plates and screws vs. stainless steel bone plates and screws in the management of mandibular fractures—A long term clinical study. Int. J. Clin. Dent. Sci. 2011, 2, 38–43. [Google Scholar]
- Frost, D.E.; El-Attar, A.; Moos, K.F. Evaluation of metacarpal bone plates in the mandibular fracture. Br. J. Oral. Surg. 1983, 21, 214–221. [Google Scholar] [CrossRef]
- Khandelwal, P.; Rai, B.A.; Bulgannawar, B.; Vakaria, N.; Sejani, H.; Hajira, N. Miniplate removal in operated cases of maxillofacial region in a dental institute in Rajasthan, India. Med. Pharm. Rep. 2019, 92, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Meningaud, J.P.; Poupon, J.; Bertrand, J.C.; Chenevier, C.; Galliot-Guilley, M.; Guilbert, F. Dynamic study about metal release from titanium miniplates in maxillofacial surgery. Int. J. Oral. Maxillofac. Surg. 2001, 30, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Matthew, I.R.; Frame, J.W. Policy of consultant oral and maxillofacial surgeons towards removal of miniplate components after jaw fracture fixation: Pilot study. Br. J. Oral. Maxillofac. Surg. 1999, 37, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Rosa, J.; Villanueva, N.L.; Sanati-Mehrizy, P.; Factor, S.H.; Taub, P.J. Review of Maxillofacial Hardware Complications and Indications for Salvage. Craniomaxillofac. Trauma Reconstr. 2016, 9, 134–140. [Google Scholar] [CrossRef]
- Kennedy, M.C.; Tucker, M.R.; Lester, C.E.; Buckley, M.J. Stress shielding effect of rigid internal fixation plates on mandibular bone grafts. A photon absorption densitometry and quantitative computerized tomographic evaluation. Int. J. Oral. Maxillofac. Surg. 1989, 18, 307–310. [Google Scholar] [CrossRef]
- Iizuka, T.; Lindqvist, C. Rigid internal fixation of mandibular fractures. An analysis of 270 fractures treated using the AO/ASIF method. Int. J. Oral. Maxillofac. Surg. 1992, 21, 65–69. [Google Scholar] [CrossRef]
- Moberg, L.E.; Nordenram, A.; Kjellman, O. Metal release from plates used in jaw fracture treatment. A Pilot Study. Int. J. Oral. Maxillofac. Surg. 1989, 18, 311–314. [Google Scholar] [CrossRef]
- Rosenberg, A.; Grätz, K.W.; Sailer, H.F. Should titanium miniplates be removed after bone healing is complete? Int. J. Oral. Maxillofac. Surg. 1993, 22, 185–188. [Google Scholar] [CrossRef]
- Gareb, B.; van Bakelen, N.; Dijkstra, P.; Vissink, A.; Bos, R.; van Minnen, B. Biodegradable versus titanium osteosynthesis in maxillofacial traumatology: A systematic review with meta-analysis and trial sequential analysis. Int. J. Oral. Maxillofac. Surg. 2020, 49, 914–931. [Google Scholar] [CrossRef]
- Steffen, C.; Sellenschloh, K.; Polster, V.; Heyland, M.; Vollmer, M.; Morlock, M.; Heiland, M.; Huber, G.; Rendenbach, C. Biomechanical comparison of polylactide-based versus titanium miniplates in mandible reconstruction in vitro. J. Stomatol. Oral. Maxillofac. Surg. 2020, 121, 377–382. [Google Scholar] [CrossRef]
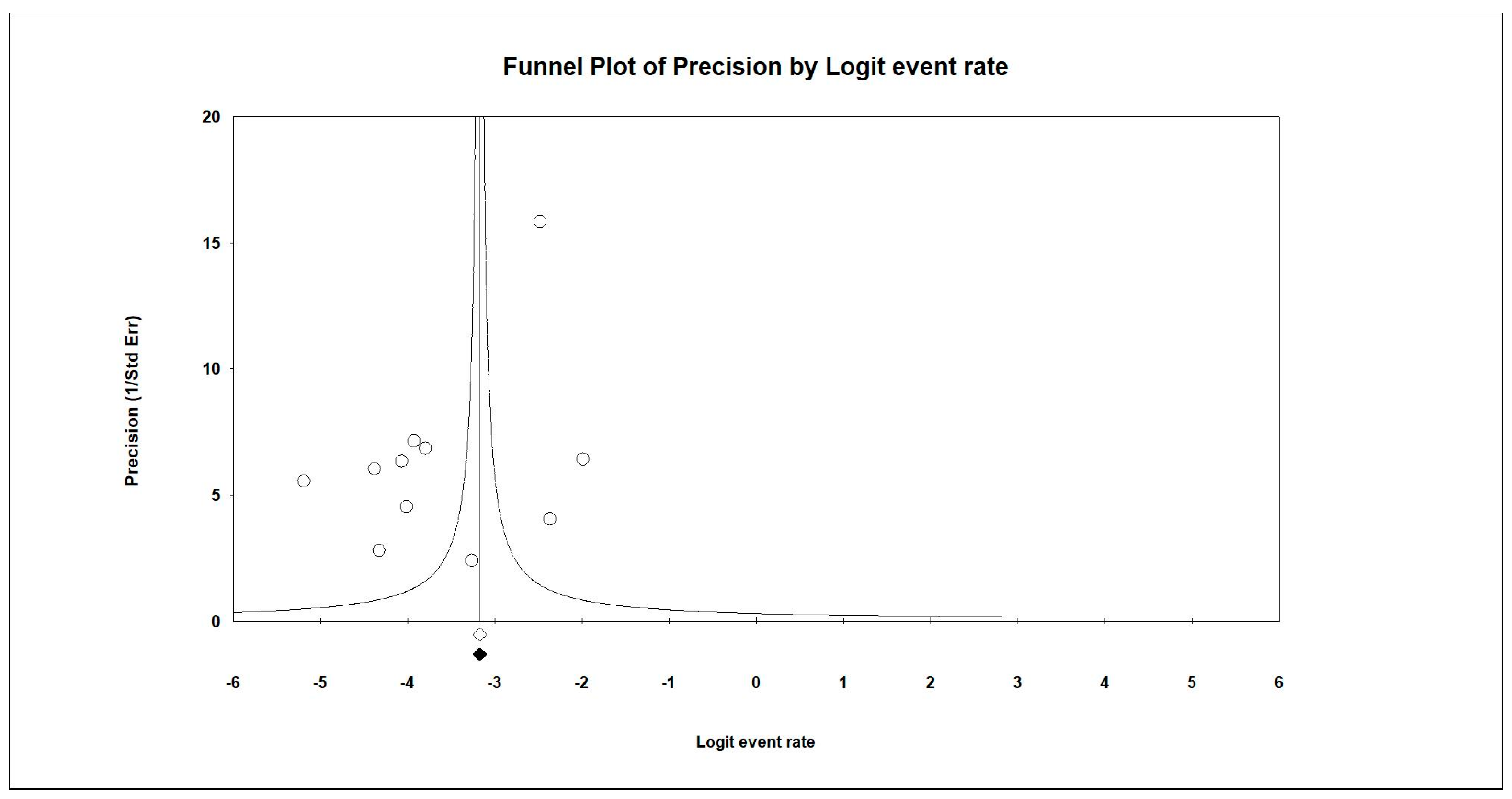
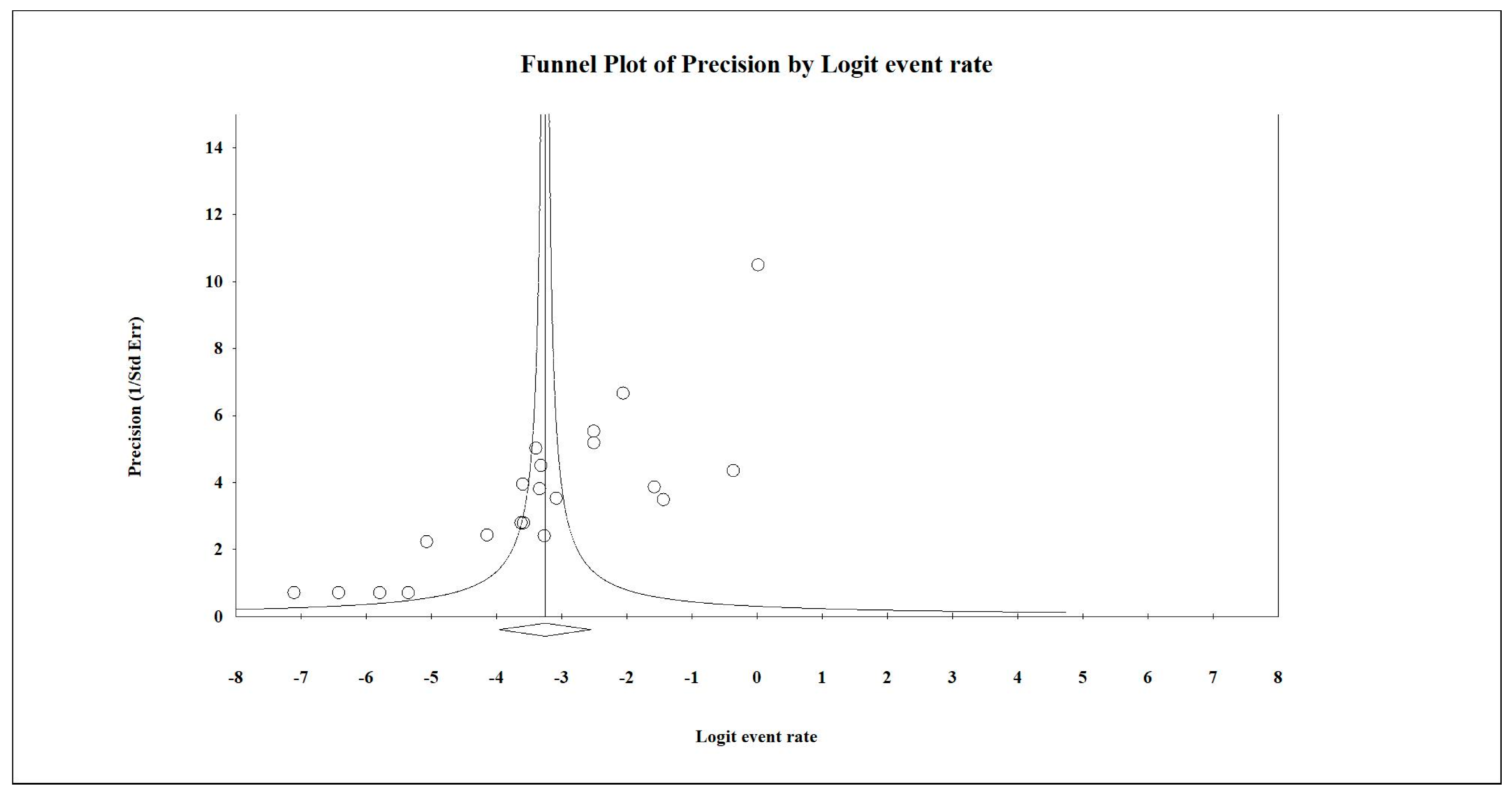

| Author/Year | Selection | Comparability | Outcomes | Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | ||
| Brown et al., 1989 [12] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Islamoglu et al., 2002 [13] | ★ | ☆ | ☆ | ★ | ★☆ | ★ | ★ | ★ | 6 |
| Murthy et al., 2005 [14] | ★ | ★ | ★ | ★ | ★☆ | ★ | ☆ | ★ | 7 |
| Rallis et al., 2006 [15] | ★ | ☆ | ★ | ★ | ☆☆ | ★ | ★ | ★ | 7 |
| Dae-Kyun et al., 2007 [16] | ★ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 8 |
| O’Connell et al., 2009 [17] | ☆ | ★ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Pan et al., 2013 [18] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Liandro et al., 2015 [19] | ★ | ★ | ★ | ☆ | ★☆ | ★ | ★ | ★ | 8 |
| Gorrela et al., 2019 [20] | ★ | ☆ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 |
| Sukegawa et al., 2020 [21] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Sadiq et al., 2021 [11] | ☆ | ★ | ☆ | ☆ | ★☆ | ★ | ★ | ★ | 5 |
| Author/ Year | Country | # of pts | Age Range (yrs.) | M:F Ratio | Site of Plate Placement | Type of Plates | # of Plates | Surgical Approach | Reasons for Plate Removal | Duration between Insertion and Removal | Site of Plate Removal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O’Connell et al., 2009 [17] | Ireland | 434 | 3–72 | 4:1 |
| Titanium miniplates | 800 | Intraoral |
| 2.5–68 months average: 19 months |
|
| Brown et al., 1989 [12] | UK | 62 | 15–80 | - |
| Stainless steel | 105 | Intraoral |
| 3–24 months | |
| Islamoglu et al., 2002 [13] | Turkey | 66 | 6–64 | 51:15 (17:5) | Mandible
| Titanium | 296 | Intraoral Extraoral |
| 3–14 months | |
| Llandro et al., 2015 [19] | UK | 216 | 17–69 | 91:9 |
| Titanium | 307 | Intraoral extraoral |
| 111–972 days 180 days |
|
| Murthy et al., 2005 [14] | USA | 76 | 14–71 | 62:14 (31:7) | Mandible
| Titanium | 163 | Intraoral extraoral |
| No average |
|
| Pan et al., 2013 [18] | China /India | 156 | 12–60 | 128:28 (32:7) | Mandibular
| Titanium | 384 | Intraoral extraoral |
| 0–36 months |
|
| Rallis et al., 2006 [15] | Greece | 280 | 17–75 | 20:7 | Maxilla and midface
| Titanium | 599 | Extraoral |
| 0.5–36 months Average 11.5 months | |
| Sukegawa et al., 2020 [21] | Japan | 158 | 8–91 | 112:46 (56:2) | Maxilla and midface
| Titanium | 440 | Intraoral and extraoral |
| 258 days |
|
| Gorrela et al., 2019 [20] | India | 234 | 14–59 | 172:62 (86:3) | Mandible
| Stainless steel | 437 | Intraoral |
| - | |
| Dae-Kyun et al., 2007 [16] | Korea | 419 | Mean: 41.4 | 340:79 | Titanium Absorbable | 609 | Intraoral |
| <3 months–>3 years |
| |
| Sadiq et al., 2021 [11] | Pakistan | 139 | 21–63 | 30:2 (15:1) | Mandible
| Titanium | 78 | Intraoral Extraoral |
| 3–36 months | - |
| Total | - | 2240 | 6–91 | - | - | 4218 | - | - | 0.5 months–>3 yrs | - |
| Author/Year | Number of Plates | Indications for Plate Removal | Total No (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection | Tooth Extraction | Plate Exposure | Prosthetic Rehabilitation | Pain | Plate Fracture | Palpable Plate | Loose Fixation Device | Patients Preference | Other Reasons | |||
| Brown et al., 1989 [12] | 105 | 14 | - | - | - | 4 | - | - | - | - | - | N = 18 17.14% |
| Islamoglu et al., 2002 [13] | 296 | 6 | - | - | - | 3 | 1 | - | - | - | Malunion: 2 Extrusion: 5 Facial deformity: 4 | N = 21 7.095% |
| Murthy et al., 2005 [14] | 163 | 6 | - | - | - | - | - | - | - | - | - | N = 6 3.680% |
| Rallis et al., 2006 [15] | 599 | 9 | - | 8 | - | - | - | 7 | - | 8 | Re-operation: 2 Non-union: 2 Plate displaced: 1 | N = 37 6.18% |
| Dae-Kyun et al., 2007 [16] | 609 | 26 | - | - | - | - | - | 5 | - | 4 | 2nd operation: 6 | N = 41 6.73% |
| O’Connell et al., 2009 [17] | 800 | 12 | 7 | 2 | 2 | - | 1 | 1 | - | - | Fibrous union: 2 Temp conduction: 1 Prior to orthg. surgery: 1 Peds patient: 2 | N = 31 3.88% |
| Pan et al., 2013 [18] | 384 | 16 | - | 1 | 6 | 6 | - | 1 | - | - | Cold intolerance: 1 Plate Failure: 2 Dental/nerve damage: 2 | N = 35 9.11% |
| Llandro et al., 2015 [19] | 307 | 3 | - | 5 | - | - | - | - | - | - | - | N = 8 2.60% |
| Gorrela et al., 2019 [20] | 437 | 15 | - | - | - | - | - | 5 | 8 | 5 | Wound Dehiscence: 12 Non-union: 3 | N = 48 10.98% |
| Sukegawa et al., 2020 [21] | 440 | 12 | 39 | 2 | 6 | - | - | 4 | 6 | 199 | Growth facilitation: 3 Unknown: 1 (not presented) | N = 272 61.81% |
| Sadiq Ali et al., 2021 [11] | 78 | 20 | - | - | - | 14 | 2 | 8 | - | - | Cancer phobia: 3 | N = 47 60.26% |
| Total | 4218 | 139 | 46 | 18 | 14 | 27 | 4 | 31 | 14 | 216 | 55 | 564 |
| Author/ Year | No and % of Miniplates Placed (Maxilla and Mandible) | No and % of Miniplates Removed (Maxilla and Mandible) | No and Location of Miniplates Placed in Mandible | No and Location of Miniplates Removed from Mandible No (%) | No and Location of Miniplates Placed (Maxilla and Zygoma) | Location of Miniplates Removed from Maxilla No (%) |
|---|---|---|---|---|---|---|
| Brown et al., 1989 [12] | 105 | 18 (17.14%) | Angle: 29 Mental area: 21 Symphysis: 46 Ramus: 2 Total = 98 | Angle: 2 Mental area: 6 Symphysis: 10 Ramus: 0 Total = 18 (17.14%) | Zygomatic Buttress: 5 Piriform fossa: 2 Total = 7 | Zygomatic Buttress: 0 Piriform fossa: 0 Total = 0 |
| Islamoglu et al., 2002 [13] | 296 | 21 (7.09%) | Body: 56 Symphysis: 20 Angle: 22 Total = 98 | Body: 3 Symphysis: 10 Angle: 0 Total = 13 (4.39%) | Maxillary: 96 Other: 102 Inferior orbital rim Zygomatic region Total = 198 | Maxilla: 8 Other:0 Total = 8 (2.70%) |
| Murthy et al., 2005 [14] | 163 | 6 (3.68%) | Mand ramus: 6 Mand angle: 15 Mand body: 7 Mand symphysis: 25 Total = 53 | Mand ramus: 0 Mand angle: 3 Mand body: 0 Mand symphysis: 3 Total = 6 (3.68%) | Frontal: 4 Nasal: 8 Frontozyg suture: 30 Zygoma: 4 Infraorbital rim: 23 Piriform area: 10 Zyg maxillary buttress: 31 Total = 110 | Frontal Nasal Frontozyg suture Zygoma Infraorbital rim Piriform area Zyg maxillary buttress Total = 0 |
| Rallis et al., 2006 [15] | 599 | 37 (6.18%) | Ramus: 28 Ext oblique ridge: 22 Angle: 16 Body: 68 Mental area: 169 Total = 303 | Ramus: Ext oblique ridge: 3 Angle: 1 Body: 9 Mental area: 8 Total = 21 (3.50%) | Frontal: 8 Frontozyg suture: 129 Frontonasal suture: 6 Infraorbital rim: 73 Zygomatic arch: 1 Ant. wall of antrum: 39 Zyg buttress: 40 Total = 296 | Frontal Frontozyg suture: 2 Frontonasal: 2 Infraorbital rim: 4 Zygomatic arch: Ant. wall of antrum: 6 Zyg buttress: 2 Total = 16 (2.67%) |
| Dae-Kyun et al., 2007 [16] | 609 | 41 (6.73%) | Mandible = 222 | Mandible = 11 (1.8%) | Maxilla = 387 | Maxilla = 30 (4.93%) |
| O’Connell et al., 2009 [17] | 800 | 31 (3.88%) | Mandible: 402 | Mandible: 26 Total 26 (3.25%) | Maxilla: 92 Orbitozygomatic: 306 Total = 398 | Maxilla: 3 Orbitozygomatic: 2 Total = 5 (0.63%) |
| Pan et al., 2013 [18] | 384 | 35 (9.11%) | Body: 92 Symph/parasymph:75 Angle: 38 Condyle: 31 Total = 236 | Body:15 Symphysis/Para symphysis:12 Angle: 1 Condyle: 1 Total = 29 (7.55%) | Zygomatic buttress: 44 Frontozygomatic: 33 Infraorbital rim: 42 NOE/frontal bone: 14 Piriform aperture: 15 Total = 148 | Zygomatic buttress: 2 Frontozygomatic: 2 Infraorbital rim: 2 NOE/frontal bone: Piriform aperture: Total = 6 (1.56%) |
| Llandro et al., 2015 [19] | 307 | 8 (2.61%) | - | - | Buttress: 192 Zygomatico-frontal: 86 Infraorbital: 28 Left zygomatic arch: 1 Total = 307 | Buttress: 7 Zygomatico-frontal: Infraorbital: 1 Left zygomatic arch: Total = 8 (2.61%) |
| Gorrela et al., 2019 [20] | 437 | 48 (10.98%) | Para symphysis: 126 Angle: 52 Condyle: 25 Body: 42 Total = 245 | Para symphysis: 12 Angle: 12 Condyle: Body: 9 Total = 33 (7.50%) | Maxilla:140 Zygomatic complex:52 Total = 192 | Maxilla: 6 Zygomatic complex: 9 Total = 15 (3.43%) |
| Sukegawa et al., 2020 [21] | 440 | 272 (61.82%) | Condyle: 98 Symphysis: 79 Angle: 62 Para symphysis: 48 Body: 30 Total = 317 | Condyle: 59 Symphysis: 59 Angle: 53 Para symphysis: 37 Body: 14 Total = 222 (50.45%) | Zygomatic buttress: 41 Pyriform aperture: 34 Infraorbital rim: 30 Frontozygomatic: 18 Total = 123 | Zygomatic buttress: 22 Pyriform aperture: 20 Infraorbital rim: 3 Frontozygomatic: 5 Total = 50 (11.36%) |
| Sadiq et al., 2021 [11] | 78 | 47 (60.26%) | Angle: 21 Symphysis: 15 Para symphysis: 7 Body: 8 Condyle: 4 Total = 55 | Angle: 16 Symphysis: 8 Para symphysis: 4 Body: 3 Condyle: 1 Total = 32 (41.0%) | Maxilla: 7 Zygoma: 16 Total = 23 | Maxilla: 6 Zygoma: 9 Total = 15 (19.2%) |
| Total | 4218 | 564 (13.37%) | 2029 | 411 | 2189 | 153 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaber, M.; Abouseif, N.; Ibrahim, N.; Hassan, M.; El-Ameen, A.M. Reasons for Removal of Miniplates Used in Fixation of Maxillofacial Bone Fractures: Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 11899. https://doi.org/10.3390/app132111899
Jaber M, Abouseif N, Ibrahim N, Hassan M, El-Ameen AM. Reasons for Removal of Miniplates Used in Fixation of Maxillofacial Bone Fractures: Systematic Review and Meta-Analysis. Applied Sciences. 2023; 13(21):11899. https://doi.org/10.3390/app132111899
Chicago/Turabian StyleJaber, Mohamed, Nadin Abouseif, Noor Ibrahim, Mawada Hassan, and Alaa Mohamed El-Ameen. 2023. "Reasons for Removal of Miniplates Used in Fixation of Maxillofacial Bone Fractures: Systematic Review and Meta-Analysis" Applied Sciences 13, no. 21: 11899. https://doi.org/10.3390/app132111899
APA StyleJaber, M., Abouseif, N., Ibrahim, N., Hassan, M., & El-Ameen, A. M. (2023). Reasons for Removal of Miniplates Used in Fixation of Maxillofacial Bone Fractures: Systematic Review and Meta-Analysis. Applied Sciences, 13(21), 11899. https://doi.org/10.3390/app132111899






