Abstract
Milk is an excellent medium for the growth of several bacteria and other microorganisms and thus, it has been extensively studied. An always current issue in the dairy industry is mastitis, which causes losses in milk volume and profits. In many cases, milk is used raw or treated at low temperatures for further dairy processes while there are quite a few cases in which foodborne-related outbreaks have occurred. Both culture-based methods and PCR were used to assess the presence of certain pathogens related to both contagious and environmental pathogens, especially the emerging pathogenic bacterium Klebsiella pneumoniae, as well as Staphyloccocus aureus and Enterobacter spp., which are associated with mastitis in milk samples from different lactating ruminant species (cows, goats, and sheep) and to further evaluate the significance of the isolated pathogens to public health. Even though significant mastitis contagious pathogens such as Staphylococcus aureus and Staphylococcus epidermidis were not detected, environmental pathogens related to poor hygiene conditions at the farm level (K. pneumoniae, Staphylococcus saprophyticus, and Enterococcus spp.) were detected. In particular, K. pneumoniae and Staphylococcus saprophyticus were present in ovine milk samples while bovine and caprine milk samples were contaminated with Enterococcus spp. The presence of these bacteria underlines the significant role of environmental hygiene especially since Staphylococcus saprophyticus and Enterococcus spp. are related to urinary tract infections and all of the tested pathogens may carry antibiotic resistance genes. More specifically, 20% of the isolated Klebsiella pneumoniae strains were found resistant to carbapenem antibiotics. The presence of emerging K. pneumoniae in ovine milk samples also indicates the need for new policies in terms of safety testing. Suggestions of monitoring processes carried out by the relevant authorities are discussed.
1. Introduction
The dairy industry is among the most developed and lucrative food industries. According to the FAO [1], over 6 million people consume milk at a global level. The vast majority of the milk produced comes from cows (81%), while goat, sheep, and camel milk production combined reach 4% only [2]. In the European Union (EU), the annual milk production in 2021 was 161 million tons, with milk other than cow’s milk representing 5% of the total production even though in Mediterranean countries, non-cow milk represents a higher percentage, up to 56.7% in the case of Greece [3].
Milk ranks highly among other foods and is regarded as the most ideal source of vital nutrients for human beings from infancy to old age since it not only has excellent sensory qualities and all the nutrients needed for rapid growth but also has the potential to prevent or lessen the risk of many diseases caused by nutritional deficiencies. However, due to its unique properties and composition (i.e., high nutritional and moisture content and neutral pH value), milk can serve not only as a unique growth medium for a complex of microbial flora such as lactic acid bacteria (LAB) [4,5] but also as a source of infection such as Propionibacterium, Staphylococcus, Streptococcus, Salmonella, Listeria, Escherichia, Corynebacterium, Brevibacterium, and others [5,6].
According to the European Commission’s Rapid Alert System for Food and Feed (RASFF) [7], for the period of 2020 until mid-2023, most alerts regarding microbiological and (microbiological) toxin risks in milk and dairy products were about not only Listeria, Salmonella, and STEC E. coli but also Enterobacteriaceae, Staphylococcal enterotoxins and Pseudomonas which are regulated by EU legislation. Although data are not available for all notifications, a good share includes cheese from raw or thermized milk, while there are available data for other pathogens that are not monitored yet but are linked to outbreaks related to the intake of milk and dairy.
Scientific interest has been mostly attracted by pathogens, which are related to several diseases such as scarlet fever, gastroenteritis, Q-fever, diphtheria, tuberculosis, and brucellosis which can be transmitted via milk and dairy products [8]. The extensive use of pasteurization and the application of control measures has minimized public health risks. However, there are still outbreaks that are mostly related to the consumption of non-processed milk or dairy products—mostly from small ruminants that have been produced from milk that has not been treated in the appropriate manner (i.e., not pasteurized or heated at low temperature). Even though the use of pasteurized milk is extensive, there are several—mostly traditional—dairy products, including cheese, butter, and cream, which are produced by raw milk, thermized milk, or milk processed with any other heat treatment less effective than pasteurization. Therefore, raw caprine and ovine milk can be potential carriers of foodborne pathogens that could be linked to serious health risks. A recent meta-analysis study showed that the most prevalent genera are Listeria spp., Penicillium spp., Salmonella spp., Staphylococcus spp., and Escherichia spp., but data on the frequency of microbial contamination in cheese from small ruminants’ milk (goat and sheep) are few [9]. The main sources of milk contamination are poor livestock salubriousness and herd health conditions, mastitis ubiquity, and improper milking and conservation practices [10].
In domestic animals, despite extensive udder health management and proper milking practices, mastitis is still a hot issue since it is the most prevalent disease of dairy animals, with great economic impact because of its negative effect on the health and welfare of animals and the adverse effect on the yield and quality of the produced milk [11]. Mastitis is induced by bacterial intramammary infection (IMI). Udder tissues host the most frequent pathogens and can be spread from animal to animal (contagious pathogens) or can be present in the livestock environment (known as environmental pathogens), including bedding, manure, or soil. Using conventional culture, more than 140 species were isolated in samples of milk derived from cows affected by mastitis [12]. The most reported etiological sources of mastitis are Staphylococci, and Coliform bacteria (Escherichia coli), independent of the origin of milk (cows, sheep, or goats) [12,13,14]. However, the most prevailing pathogen related to clinical and sub-clinical mastitis is Staphylococcus aureus (S. aureus) [11,15,16], while Klebsiella spp., Enterobacter spp., and Pseudomonas spp. have been associated with environmentally caused mastitis [17]. The detection of such pathogens is considered a definitive diagnosis of mastitis infections [18].
Nowadays, another great concern that has been raised is about the prevalence of multidrug resistance, particularly S. aureus-resistant methicillin (MRSA), which has steadily increased in the EU [19], leading the European Food Safety Authority (EFSA) to suggest increased surveillance of MRSA both in food and livestock intended to be used for food production [16,20]. Additionally, because antibiotics are often and inappropriately used in the medical sector, a broad range of pathogen species have emerged in many cases. Among them, Klebsiella spp. serves as a vehicle for zoonotic pathogen resistance in multiple drugs that the World Health Organization (WHO) has put under surveillance [21]. Klebsiella pneumoniae settles in different environmental recesses in water, plants, and the ground, and it has been extensively detected in dairy cattle mastitis cases [22]. It can be transmitted and invade the mammary epithelial cells in dairy cattle herds mostly through manure, through contiguity with the teats, and via the bedding and equipment used [23]. K. pneumoniae strains which are multidrug resistant and hyper-virulent have been observed [24], leading to several illnesses, including meningitis, urinary tract infections, pneumonia, liver abscesses, and septicemia [25]. Its pathogenesis as a procreator of dairy cattle mastitis has been scarcely studied especially when compared to the extensive studies on other bacterial pathogens [26]. Even more limited data can be retrieved for the occurrence of K. pneumoniae in the milk of small ruminants (mostly caprine and ovine milk) or respective dairy products, as they share a limited segment of the global dairy market, although they significantly participate in the dairy economy of certain South European countries which correspond to major producers of sheep and goat milk.
This preliminary study was performed to assess the presence of certain pathogens (such as S. aureus, K. pneumoniae, and Enterobacter spp.), which are associated with mastitis in samples of milk from various lactating ruminant species, namely cows, goats, and sheep using both conventional microbiological methods and PCR techniques and to further discuss the significance of the isolated pathogens to public health as well as to attract attention to emerging pathogens still not included in monitoring policies.
2. Materials and Methods
2.1. General Work Flow for Milk Samples Derived from Livestock Species
Sixty independent raw milk samples from ruminant species (20 samples per species; sheep, goats, and cows), were collected from local producers in the area of Attica and Viotia (Greece), ten from each area per species. The collection of milk specimens was taken directly from the animals, according to the producer’s milking routine, and the specimens were retained at 4 °C until analysis (maximum 6 h after collection).
A small quantity (100 μL) from each of the examined samples was initially streaked directly onto selective chromogen agar and specifically on CHROMagar Orientation, CHROMagar Enterococcus, and CHROMagar S. aureus, and they were incubated under aerobic conditions at 37 °C for 24 h. Three replicates from each sample were conducted. Gram staining along with motility assays were used for the characterization of the colony-forming isolates of Escherichia coli and Klebsiella pneumoniae. The colonies were also used for further DNA extraction, as described in the respective section.
2.2. DNA Isolation from Isolated Colonies
The magnetic beads Method was performed for the extraction of the nucleic acids from the isolated colonies using a Zybio EXM 3000 (Chongqing, China) automatic extractor according to the manufacturer’s instructions.
2.3. DNA/RNA Isolation from Animal Milk Samples
All of the samples derived from each species were homogenized and centrifuged for 10 min at 12,000× g. The supernatant was removed. Approximately 10 μL of internal control was added to the sample/lysis mixture for DNA/RNA isolation using the MagCore® (Bangalore, Karnataka) cultured Cells DNA Kit without using the RNAase treatment and following the manufacturer’s instructions.
2.4. Confirmation of Colonies by the Conventional Polymerase Chain Reaction
Approximately 1 mM of each gene-specific pair of primers (Table 1) [27] along with 10 μL of eluted DNA was diluted to 50 μL volume by ddH2O (molecular grade) in order to perform the PCR assay using GoTaq Hot Start Master Mix (Promega Gmbh, Mannheim, Germany). An Applied Biosystems thermal cycler was used for the conduction of the PCR reactions. The conditions of amplification included a 5 min denaturation step at 95 °C at the beginning combined with 35 s denaturation at 94 °C (40 cycles) and annealing of 60 s at 54 °C with an extension of 60 s at 72 °C before the final extension step of 10 min at 72 °C. Approximately 0.5 μg/mL of ethidium bromide was used to stain 2% agarose gel, [28,29], which was further used for the separation of the PCR products. The negative control was a Salmonella enteritis’ (clinical strain) genomic DNA.

Table 1.
Primer sequences used for the detection of pathogens related to mastitis using conventional PCR.
2.5. Phenotypic Analysis of the Antibiotic Resistance of Klebsiella pneumoniae
During phenotypic analysis, the common carbapenemase families in the bacterial colonies, namely OXA-48-like, IMP, VIM, KPC, and NDM, were detected and differentiated using an in vitro multiplex immunoassay NG-Test Carba 5 (NG Biotech, Guipry, France) as per the manufacturer’s instructions.
2.6. Real-Time PCR for the Amplification of Seven Bacterial Pathogens in the Milk Samples
An RT-PCR assay was developed in order to qualitatively identify and successfully differentiate Shigella spp. E. coli, Salmonella spp., Campylobacter spp., Adenovirus F, Rotavirus A, No-rovirus 2 genotype, and Astrovirus, as per the Saccace Biotechnology Kit (Acute Intestinal Infection-A.I.I.-Screen Real-TM) instructions. Four reaction mixes had to be prepared in new sterile tubes for each one of the samples (PCR-mix-1 Shigella spp./Salmonella spp., PCR-mix-1 Campylobacter spp./Adenovirus, PCR-mix-1 Rotavirus/Astrovirus, and PCR-mix-1 Norovirus/IC). The interpretation of the results is achieved (software included in the device) when the fluorescence curve crosses the threshold line as follows: PCR-mix-1 Norovirus/IC is used to detect the internal control (IC) on the (Green) FAM channel along with Norovirus on the (Yellow) JOE channel. On the same channels, Rotavirus A and Astrovirus are detected when PCR-mix-1 Rotavirus/Astrovirus is used, respectively. On the FAM channel Shigella spp. summer. E. coli was also detected with PCR-mix-1 Shigella spp./Salmonella spp., while Salmonella spp. was detected on the JOE channel. Finally, with PCR-mix-1 Campylobacter spp./Adenovirus on the FAM channel, there was the detection of Campylobacter spp., while Adenovirus was detected on the JOE channel. A Ct value less than 40 is indicative of a positive sample.
Analytical specificity: The negative samples validated the analytical specificity of the primers and probes, as they did not generate any signal with the above-mentioned tested microorganisms. The A.I.I. kit’s specificity was 100%.
Analytical sensitivity: With the A.I.I., the specific microorganisms can be detected in all tests (100%) with a minimum sensitivity of 500–1000 copies/mL. Detection was performed using the control standard while dilutions were used for the negative samples.
2.7. Statistical Analysis
The tested samples were statistically analyzed with Stata 15.0 software (Stata Corp., College Station, TX, USA) using descriptive statistics. The chi-square test was performed for the comparison of the categorical variables. Statistical significance was indicated by a p-value < 0.05.
3. Results and Discussion
Sixty milk samples from three species, namely cows, sheep, and goats, were tested for a number of pathogens by both conventional culture-based methods as well as by the real-time PCR method. The isolated bacteria from the milk samples are shown in Table 2. Overall, K. pneumoniae strains were detected in all analyzed unpasteurized ovine milk samples (n = 20), whereas Enterococcus strains were detected in all of the examined bovine (n = 20) and caprine (n = 20) milk samples, therefore comprising 66.6% of the total analyzed milk samples (Table 2). Staphylococcus saprophyticus was identified only in the analyzed ovine milk samples (n = 20; 33.3%). In regard to the rest of the examined pathogens (Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli), they were not identified in any of the examined samples, independently of the species. The presence of K. pneumoniae, which belongs to the pathogens that cause mastitis, has been reported mostly in dairy cows [30,31] and it is regulated by good hygiene practices as its presence has been associated with feces and manure treatment, wood, and inorganic bedding. K. pneumoniae can act opportunistically as a pathogen in patients under immunosuppression and as such has been extensively studied in human infection through the incidence of severe hospital outbreaks. It is a current issue for public health since many K. pneumoniae strains are resistant to antibiotics of beta-lactam, which are hydrolyzed by the enzyme beta-lactamases produced by the bacteria [31].

Table 2.
Isolated bacteria in animal milk samples verified by chromogen agar and conventional PCR.
The isolates that were confirmed as K. pneumoniae were then further analyzed for antibiotic resistance with the NG-Test Carba 5 kit, which detects the presence of five carbapenemases. Strains of Klebsiella pneumoniae that are resistant to the antibiotics carbapenems were first identified in 1996 and are considered important nosocomial pathogens causing serious infections associated with increased mortality [32]. The Carbapenem-resistant Klebsiella pneumoniae have genes most commonly carried in plasmids that encode for the production of enzymes called carbapenemases that have the ability to hydrolyze the carbapenems. Carbapenemases are enzymes that belong to the family of β-lactamases but are extremely versatile and can recognize almost all hydrolyzable β-lactams, and most importantly, they are resistant to inhibition by β-lactamase inhibitors [33]. In this study, the detection of the most common carbapenemases was performed such as: KPC (“Klebsiella pneumoniae carbapenemase”), OXA (“oxacillin-hydrolyzing β-lactamases”), IMP (“active on imipenem”), VIM (“Verona integron-encoded metallo-lactamase”), and NDM (“New Delhi Metallo-β-Lactamase”).
As presented in Figure 1, the results of the in vitro multiplex immunoassay showed that at least one carbapenemase was produced by four (20.0%) isolates. In particular, NDM carbapenemase production was observed in two K. pneumoniae isolates (10%) while an equal number of isolates produced OXA-48 carbapenemase. This is a significant finding because as of the year 2005, there were no cases of non-susceptible Klebsiella in carbapenem at a European level but in the following years, carbapenem-resistant K. pneumoniae occurred in the Balkans and other South European countries, including Greece [34]. Moreover, the Centers for Disease Control and Prevention (CDC) has classified the Carbapenem-resistant Klebsiella pneumoniae as one of the most “urgent public health threats” requiring immediate and effective actions [35]. The prevalence of invasive Carbapenem-resistant Klebsiella pneumoniae increased on a European level by +31% in 2020, and in 2021, it increased by another +20% according to the annual report on antimicrobial resistance in Europe of the European Centre for Disease Prevention and Control [36]. According to the same report, Greece has reported 73.7% of Carbapenem-resistant Klebsiella pneumoniae isolates, the highest % percentage of resistant isolates among all European countries in 2021 and an increase from 2020 (66.3%) and 2019 (58.3%).
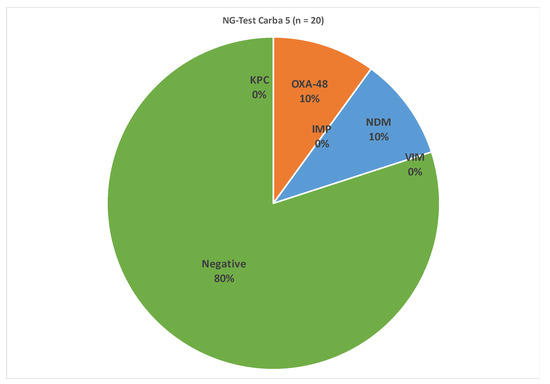
Figure 1.
Results of NG-Test Carba 5 of positive isolates of Klebsiella pneumoniae.
Furthermore, although microbiological analysis for testing K.pneumoniae in milk is not mandatory for safety reasons, this study has shown the presence of viable K.pneumoniae cells in ovine raw milk samples. This fact reflects the importance of monitoring milk samples of various origins not only for the most common bacterial pathogens but also for some of the emerging pathogens that may have serious virulent activity. It should also be noted that, as shown in Figure 1, some strains of K.pneumoniae showed resistance to antibiotics, therefore rendering their treatment more difficult and also their monitoring having to be conducted in a more elaborate manner. Also, it is essential to apply measures for the prevention and control of dairy animal mastitis in order to limit the spread of these emerging bacterial pathogens to the food chain. Although certain hygiene practices should be followed by farmers, there are quite a few cases where this is not implemented, especially if a hand milking routine is applied rather than using milking machines. This is typically the case in Greece, as machine milking is not extensively used for small ruminants. Bedding materials, if used, should be stored in clean and dry places. Pre-milking disinfection of teats reduces bacteria loads, but if the teats are dirty, the presence of the pathogen cannot be eliminated. Another treatment method should focus on the separation of animals with Klebsiella mastitis as a way to prevent contaminating the surroundings and equipment with high bacteria loads. The other two identified bacteria are also associated with poor hygiene conditions at the farm level and show resistance to antibiotics. In addition, both of them (Enterococcus spp. and Staphylococcus saprophyticus) are also associated with urinary tract infections [37]. When it comes to lactic acid bacteria, Enterococcus spp. are the most commonly identified bacteria in unprocessed milk. The Gram-positive bacteria of the Enterococci genus partially form the microbiome in both human and animal intestines. Certain species, such as Enterococcus faecium and Enterococcus faecalis, are considered human pathogens and are involved in foodborne illness outbreaks [38]. They can enter milk from the farm environment and through poor hygiene management. They may also be used as microbiological process hygiene standards for food and drinking water as indicators of fecal contamination in organic matrices such as food [39]. The same authors reported positive detection of E. faecalis and E. faecium in more than 50% of the analyzed milk samples that had been pasteurized. Therefore, even pasteurized milk can be a source of potentially resistant Enterococcus spp. However, since Enterococci are also considered lactic acid bacteria, specific species and strains could have a beneficial role in foods, especially since the growth of enterococci is actively connected to the ripening process as well as the flavor development in various kinds of cheese [40].
On the other hand, Staphylococcus saprophyticus is considered to be a common urinary tract pathogen. Poor hygiene conditions in the farm environment as well as at the farm level could have resulted in the infection of milk with a certain pathogen. A previous study reports the contamination of approximately 16% of various food samples by Staphylococcus saprophyticus [41], without milk participating in the examined samples, however. This particular pathogen can be transferred by hand contact to food and, also, through it being transferred more readily than other pathogens [41].
The number of positive results between the bovine and caprine samples were statistically similar (p = 0.19), while the positive percentages between the bovine, caprine, and ovine samples were statistically different (p < 0.005). Concerning the PCR analysis results, there was agreement between them and those determined by culture-based tests in all analyzed samples.
Further to our analyses, all of the samples were tested for seven well-known pathogens related to public health: Shigella spp. E. coli, Salmonella spp., Campylobacter spp., Adenovirus F, Rotavirus A, Norovirus 2 genotype, and Astrovirus (analyzed with the kit of Saccace) were negative.
According to the results of the present study, pathogens related to mastitis in animals and humans were identified in milk samples intended for further human consumption either as pasteurized milk or processed for the production of other dairy products. Both implemented techniques (the traditional culture-based approach and PCR) showed similar results and, in some cases, an uncommon level of 100% detection per milk sample. Interestingly, common contagious mastitis-causing pathogens were not isolated but rather pathogens related to environmental mastitis. The presence of such pathogens is related to poor hygiene conditions. Even though great progress in terms of milk quality in Greece over the last three decades has been reported [42], the need for further improvements including hygiene conditions is ever present. However, it is important to note that the identified pathogens are related to resistance to antibiotics, which may further raise serious concerns about public health. Antibiotic-resistant pathogens are involved in the spread of antibiotic resistance traits in populations of bacteria of the same species or other species belonging to the same family [35]. Therefore, in farming practice, it is crucial to eliminate the use of antibiotics without authorization. In addition, pasteurization seems unable to eliminate Enterococci at a zero level, as previous studies have reported. Thus, a possible strategy could be potentially increasing the applied temperature or the extension of the holding time. More extensive monitoring of these pathogens, at the farming level which does not belong to the mandatory conducted analyses of milk intended for human consumption, can also be considered by responsible authorities. Possibly, animals and livestock could probably serve as a reservoir for which food (animal products like milk) is the vehicle for their transmission into the human environment.
4. Conclusions
The analysis of milk samples from various ruminant species with both culture-based tests and PCR analysis showed the presence of bacteria which are associated with mastitis but are mostly considered as environmental factors. Although the limitation of this study is the relatively small sample size at a preliminary level, it is important that it has revealed the spread of the serious pathogen Klebsiella pneumoniae in small-scale ruminant farms near the capital area of Greece. Also, the presence of viable bacterial cells of Klebsiella pneumoniae in ovine milk indicates that pathogens that are not included in microbiological criteria could also pose a significant risk to public and animal health and this should be taken into consideration, especially since many economically important products such as Feta cheese (PDO) are produced with ovine milk. Moreover, most of these pathogens are considered antibiotic resistant, and therefore, further mitigating measures have emerged. In this study, 20% of the isolated Klebsiella pneumoniae strains were found resistant to carbapenems. It is of utmost importance to monitor the levels of this serious pathogen and to characterize the isolated strains for antimicrobial resistance at the farm level in order to prevent the wide and uncontrollable spread of multidrug-resistant (MDR) and in some cases pandrug-resistant (PDR) bacteria in the food chain. Moreover, as all culture-based results were confirmed by PCR, it was revealed that the implementation of PCR analysis is a fast and dependable method for the determination of these bacteria in a routine base. The authorities and, generally, stakeholders could improve monitoring processes and implement further additional measurements for consumers’ protection. In addition, according to the results, further policies could be decided not only at the consumer level but also at the livestock (farm) level.
Author Contributions
Conceptualization, D.H., A.E.T. and E.T.; methodology, D.H. and A.G.T.; validation, P.H. and G.P.L.; formal analysis, D.H., A.B. and G.P.L.; investigation, D.H., A.B., A.E.T. and E.T.; resources, D.H., A.E.T. and E.T.; writing—original draft preparation, D.H. and E.T.; writing—review and editing, D.H., G.P.L., A.G.T., A.E.T., A.B. and E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agricultural Organization of the United Nations (FAO). Gateway to Dairy Production and Products: Milk and Milk Products. Available online: https://www.fao.org/dairy-production-products/products/en/ (accessed on 24 December 2022).
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO: Rome, Italy; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Eurostat: Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Milk_and_milk_product_statistics (accessed on 24 December 2022).
- Frank, J.F. Milk and dairy products. In Food Microbiology—Fundamental and Frontiers; Doyle, P., Beuchat, R., Montville, J., Eds.; ASM Press: Herndon, VA, USA, 1997. [Google Scholar]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int. J. Food Microbiol. 2021, 150, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Blaiotta, G.; Ercolini, D.; Moschetti, G. Molecular evaluation of microbial diversity occurring in different types of Mozzarella cheese. J. Appl. Microbiol. 2001, 90, 414–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- European Commission. RASFF—Food and Feed Safety Alerts. 2023. Available online: https://ec.europa.eu/food/safety/rasff_en (accessed on 28 July 2023).
- Vasavada, P.C. Pathogenic Bacteria in Milk—A Review. J. Dairy Sci. 1987, 71, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.; Falzon, O.; Camilleri, K.; Valdramidis, V.P. Bacterial and fungal contaminants in caprine and ovine cheese: A meta-analysis assessment. Food Res. Int. 2020, 137, 109445. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Ordoñez, V.; Valladares-Carranza, B.; Tenorio-Borroto, E.; Talavera-Rojas, M.; Varela-Guerrero, J.A.; Acosta-Dibarrat, J.; Puigvert, F.; Grille, L.; Revello, Á.G.; Pareja, L. Microbial Contamination in Milk Quality and Health Risk of the Consumers of Raw Milk and Dairy Products. In Nutrition in Health and Disease—Our Challenges Now and Forthcoming Time; Mózsik, G., Figler, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Taponen, S.; McGuinness, D.; Hiitiö, H.; Simojoki, H.; Pyorala, S. Bovine milk microbiome: A more complex issue than expected. Vet. Res. 2019, 50, 44. [Google Scholar] [CrossRef]
- Bergonier, D.; de Crémoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef]
- Menzies, P.I.; Ramanoon, S.Z. Mastitis of sheep and goats. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 333–358. [Google Scholar] [CrossRef]
- Vasudevan, P.; Nair, M.K.M.; Annamalai, T.; Venkitanarayanan, K.S. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 2003, 92, 179–185. [Google Scholar] [CrossRef]
- Titouche, Y.; Akkou, M.; Houali, K.; Auvray, F.; Hennekinne, J.A. Role of milk and milk products in the spread of methicillin-resistant Staphylococcus aureus in the dairy production chain. J. Food Sci. 2022, 87, 3699–3723. [Google Scholar] [CrossRef]
- Klaas, I.C.; Zadoks, R. An update on environmental mastitis: Challenging perceptions. Transbound. Emerg. Dis. Suppl. 2017, 65, 166–185. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Rohani, S.M.; Ayremlou, N. Detection of Staphylococcus aureus in milk by PCR. Comp. Clin. Path. 2010, 19, 91–94. [Google Scholar] [CrossRef]
- Tomao, P.; Pirolo, M.; Agnoletti, F.; Pantosti, A.; Battisti, A.; Di Martino, G.; Visaggio, D.; Monaco, M.; Franco, A.; Pimentel de Araujo, F.; et al. Molecular epidemiology of methicillin-resistant Staphylococcus aureus from dairy farms in North-eastern Italy. Int. J. Food Microbiol. 2020, 332, 108817. [Google Scholar] [CrossRef] [PubMed]
- Aerts, M.; Battisti, A.; Hendriksen, R.; Kempf, I.; Teale, C.; Tenhagen, B.A.; Veldman, K.; Wasyl, D.; Guerra, B.; Liébana, E.; et al. Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA J. 2013, 17, 5709. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 10 September 2023).
- Abdalhamed, A.M.; Zeedan, G.S.G.; Abou Zeina, H.A.A. Isolation and identification of bacteria causing mastitis in small ruminants and their susceptibility to antibiotics, honey, essential oils, and plant extracts. Vet. World 2018, 11, 355–362. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, J.; Han, B.; Barkema, H.W.; Cobo, E.R.; Kastelic, J.P.; Zhou, M.; Shi, Y.; Wang, J.; Yang, R.; et al. Klebsiella pneumoniae isolated from bovine mastitis is cytopathogenic for bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 3493–3504. [Google Scholar] [CrossRef]
- Juan, C.H.; Chuang, C.; Chen, C.H.; Li, L.; Lin, Y.T. Clinical characteristics, antimicrobial resistance and capsular types of community-acquired, healthcare-associated, and nosocomial Klebsiella pneumoniae bacteremia. Antimicrob. Resist. Infect. Control 2019, 8, 1. [Google Scholar] [CrossRef]
- Marr, C.M.; Russo, T.A. Hypervirulent Klebsiella pneumoniae: A new public health threat. Expert Rev. Anti Infect. Ther. 2018, 17, 71–73. [Google Scholar] [CrossRef]
- Zheng, Z.; Gorden, P.J.; Xia, X.; Zheng, Y.; Li, G. Whole-genome analysis of Klebsiella pneumoniae from bovine mastitis milk in the US. Environ. Microbiol. 2022, 24, 1183–1199. [Google Scholar] [CrossRef]
- Ke, D.; Picard, F.J.; Martineau, F.; Ménard, C.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Development of a PCR assay for rapid detection of enterococci. J. Clin. Microbiol. 1999, 37, 3497–3503. [Google Scholar] [CrossRef]
- Sunagar, R.; Deore, S.N.; Deshpande, P.V.; Rizwan, A.; Sannejal, A.D.; Sundareshan, S.; Rawoo, D.B.; Barbuddhe, S.B.; Jhala, M.K.; Bannalikar, A.S.; et al. Differentiation of Staphylococcus aureus and Staphylococcus epidermidis by PCR for the fibrinogen binding protein gene. J. Dairy Sci. 2013, 96, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Morot-Bizot, S.C.; Talon, R.; Leroy, S. Development of a multiplex PCR for the identification of Staphylococcus genus and four staphylococcal species isolated from food. J. Appl. Microbiol. 2004, 97, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.A.; Ahlström, C.; Rauch, B.J.; Zadoks, R.N. Fecal shedding of Klebsiella pneumoniae by dairy cows. J. Dairy Sci. 2006, 89, 3425–3430. [Google Scholar] [CrossRef] [PubMed]
- Enferad, E.; Mahdavi, S. Antibiotic resistance pattern and frequency of some beta lactamase genes in Klebsiella pneumoniae isolated from raw milk samples in Iran. J. Hellenic Vet. Med. Soc. 2021, 71, 2455–2462. [Google Scholar] [CrossRef]
- Maraki, S.; Mavromanolaki, V.E.; Magkafouraki, E.; Moraitis, P.; Stafylaki, D.; Kasimati, A.; Scoulica, E. Epidemiology and in vitro activity of ceftazidime–avibactam, meropenem–vaborbactam, imipenem–relebactam, eravacycline, plazomicin, and comparators against Greek carbapenemase-producing Klebsiella pneumoniae isolates. Infection 2022, 50, 467–474. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Antimicrobial Resistance Threats Report. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 13 September 2023).
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe-Annual Epidemiologic Report for 2021 of the European Antimicrobial Resistance Surveillance Network (EARS-Net). 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2021 (accessed on 14 September 2023).
- Svanbong, C. Urinary Tract Infections. In Encyclopedia of Immunology; Delves, P.J., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 1998; pp. 2452–2454. [Google Scholar]
- Grispoldi, L.; Karama, M.; El-Ashram, S.; Saraiva, C.; García-Díez, J.; Chalias, A.; Cenci-Goga, B. Evolution and antimicrobial resistance of enterococci isolated from Pecorino and goat cheese manufactured on-farm in an area facing constraints as per EU Regulation 1305/2013 in Umbria, Italy. Ital. J. Food Saf. 2022, 11, 10070. [Google Scholar] [CrossRef]
- Nasiri, M.; Hanifian, S. Enterococcus faecalis and Enterococcus faecium in pasteurized milk: Prevalence, genotyping, and characterization of virulence traits. LWT 2022, 153, 112452. [Google Scholar] [CrossRef]
- Dapkevicius, M.D.; Sgardioli, B.; Câmara, S.P.A.; Poeta, P.; Malcata, F.X. Current Trends of Enterococci in Dairy Products: A Comprehensive Review of Their Multiple Roles. Foods 2021, 10, 821. [Google Scholar] [CrossRef]
- Hedman, P.; Ringertz, O.; Eriksson, B.; Kvarnfors, P.; Andersson, M.; Bengtsson, L.; Olsson, K. Staphylococcus saprophyticus found to be a common contaminant of food. J. Infect. 1990, 21, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lianou, D.T.; Michael, C.K.; Fthenakis, G.C. Data on Mapping 444 Dairy Small Ruminant Farms during a Countrywide Investigation Performed in Greece. Animals 2023, 13, 2044. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).