Neuroprotective Potential of Isoquinoline Alkaloids from Glaucium grandiflorum Boiss. and A. Huet subsp. refractum (Nábelek) Mory: Role of NRF2-KEAP1 Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Plant of Study
2.2. Obtaining Alkaloid Extracts from Glaucium grandiflorum
2.3. Spectrophotometric Determination of Total Alkaloid Amount of Alkaloid Extracts
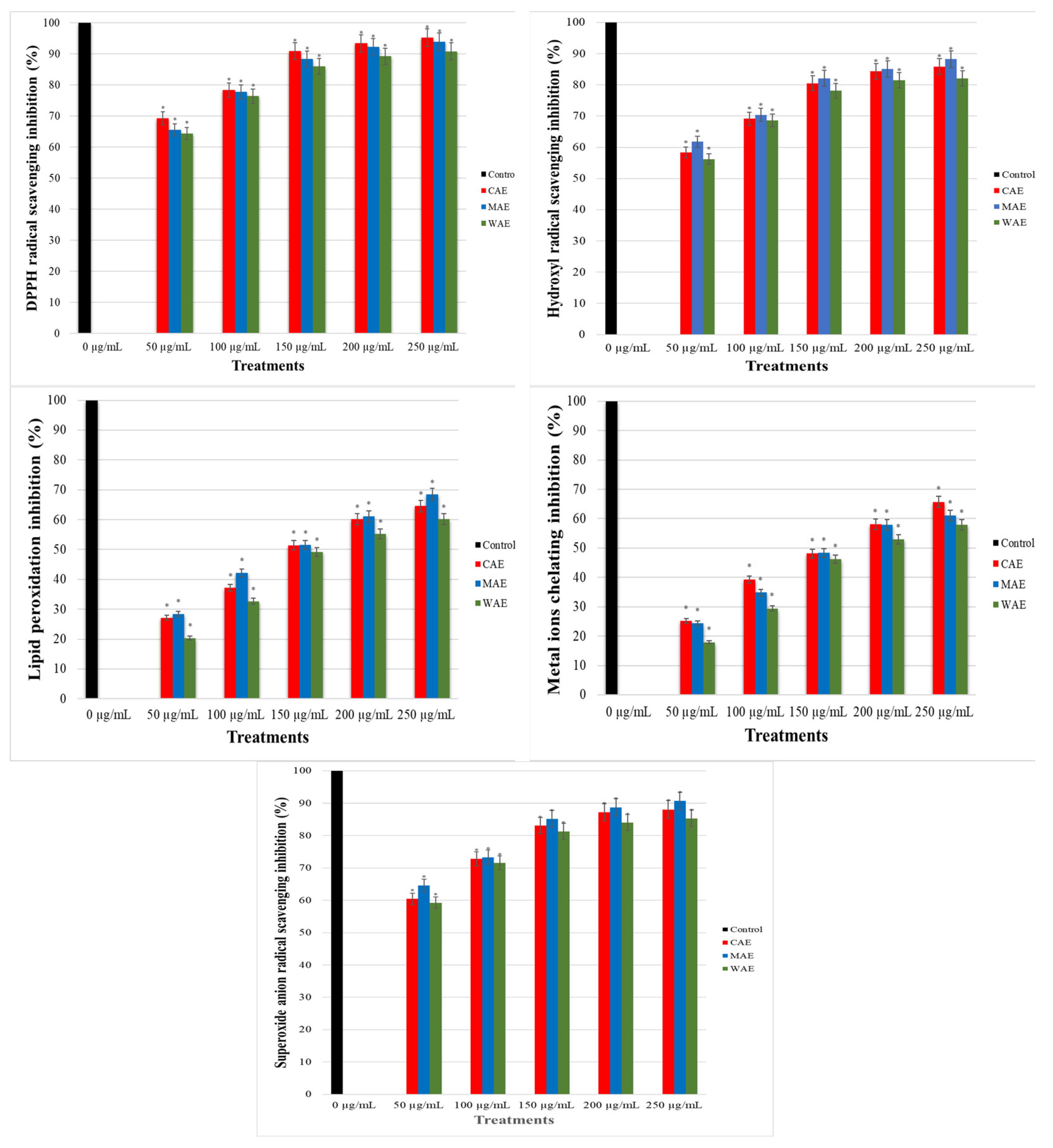
2.4. Spectrophotometric Determination of Antioxidant Activities of Alkaloid Extracts
2.4.1. Metal Ions Chelating Activity
2.4.2. 2,2-Diphenyl-1 Picrylhydrazil (DPPH) Radical Scavenging Activity
2.4.3. Lipid Peroxidation Inhibitory Activity
2.4.4. Hydroxyl Radical Scavenging Activity
2.4.5. Superoxide Anion Radical Scavenging Activity
2.5. Reproduction and Storage of PC-12 Cells
2.6. Cell Viability Test
2.7. The Effect of Alkaloid Extracts on TDH in PC-12 Cells Induced by OS with H2O2
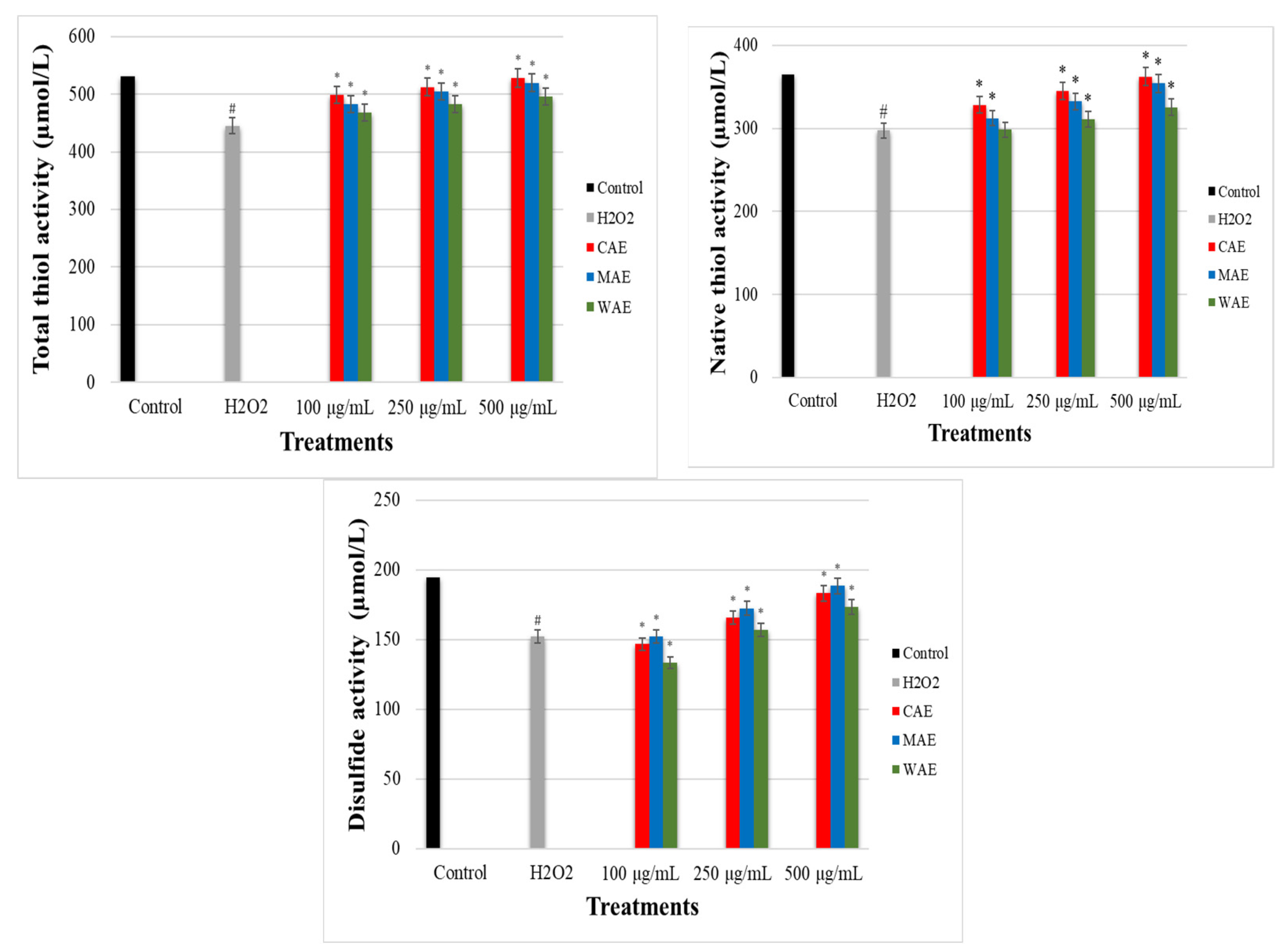
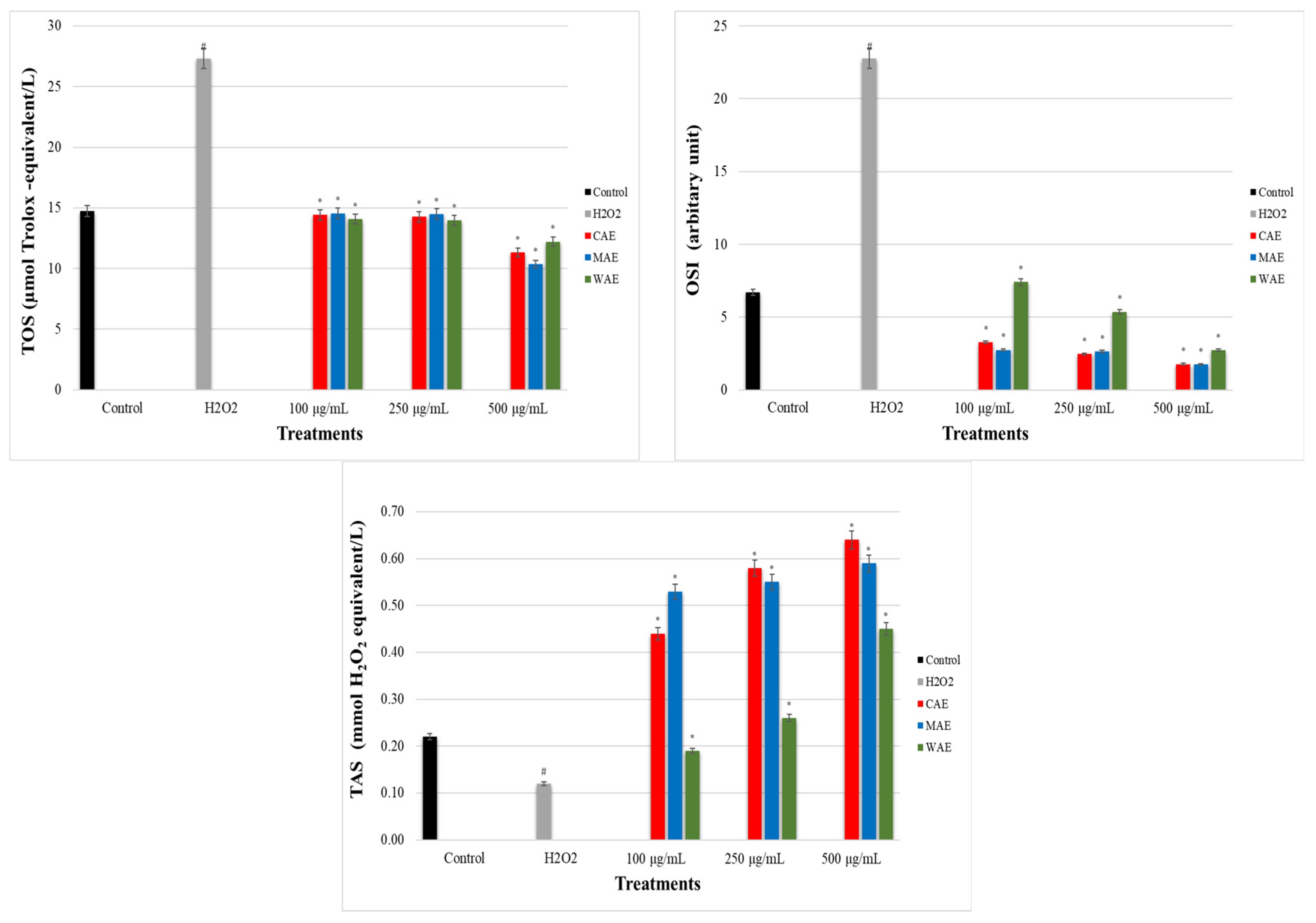
2.8. Determination of the Effect of Alkaloid Extracts on Total Oxidant Status (TOS), Total Antioxidant Status (TAS), and OS Index (OSI) Levels in PC-12 Cells Induced by OS with H2O2
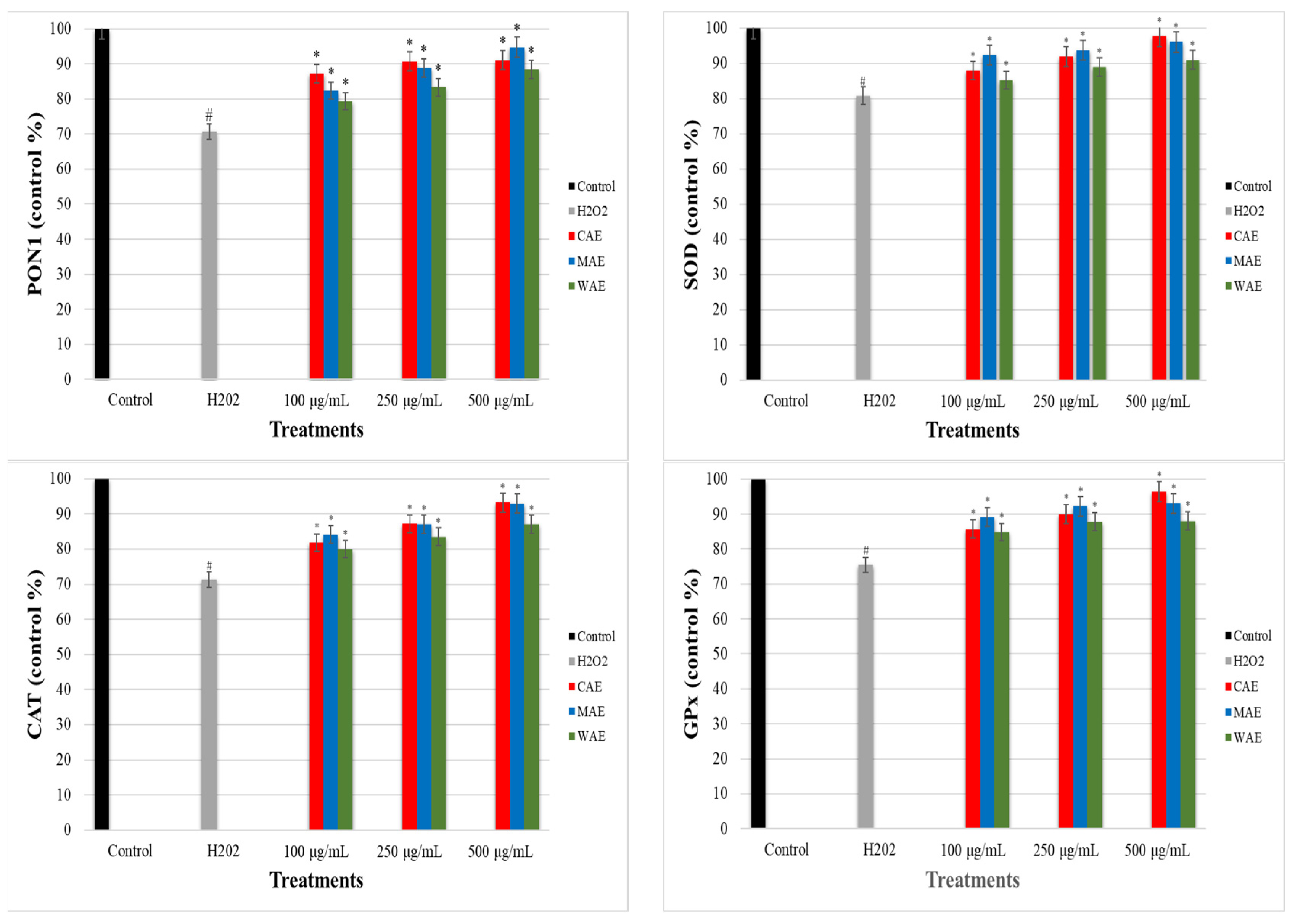
2.9. Determination of the Effect of Alkaloid Extracts on Paraoxanase-1 (PON1), Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione Peroxidase (GPx) Enzyme Levels in PC-12 Cells Induced by OS with H2O2
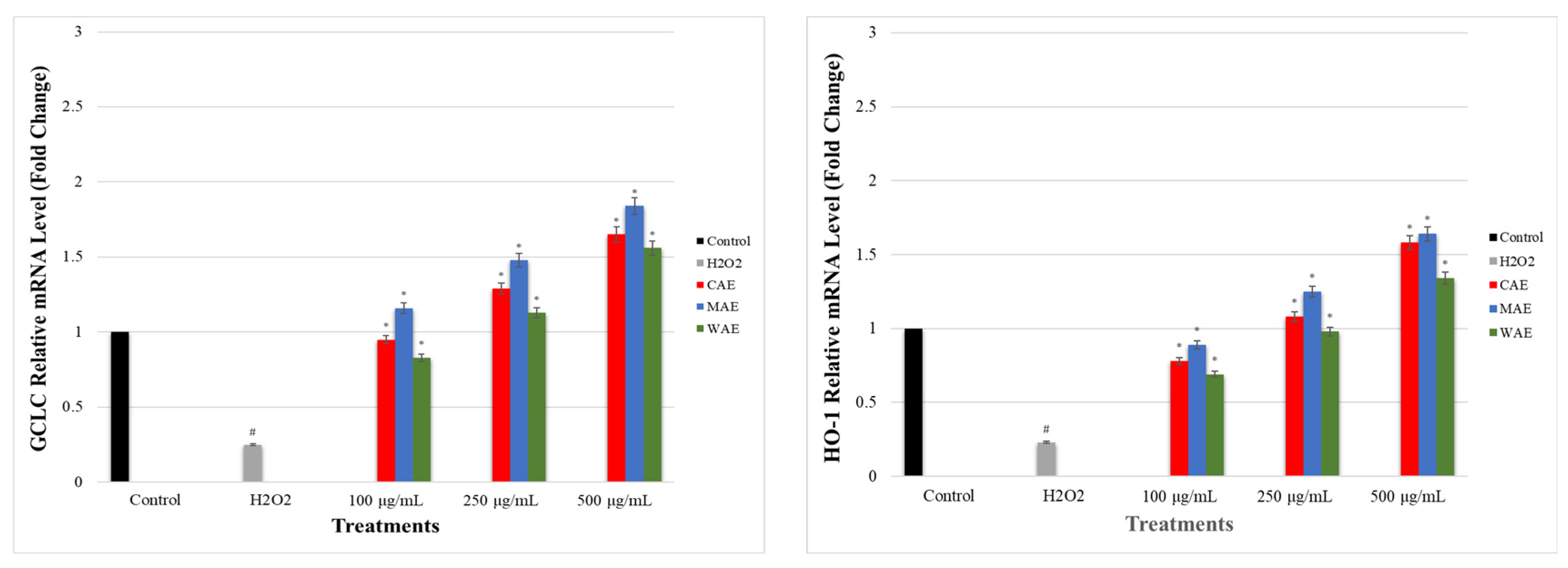
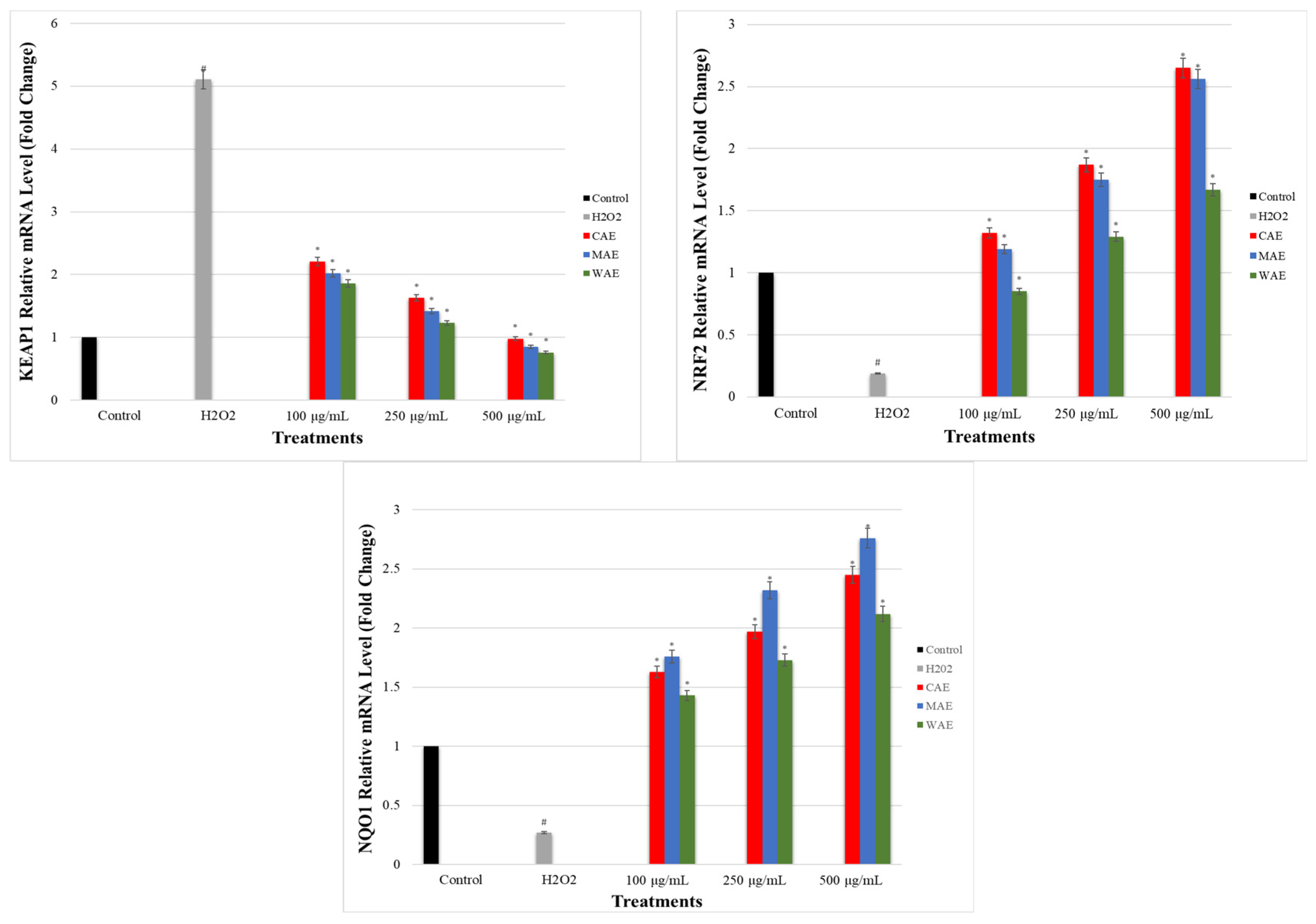
2.10. Determination of the Effect of Alkaloid Extracts on GCLC, HO-1, NRF2, NQO1, and KEAP1 Gene Expression Levels in PC-12 Cells Induced by OS with H2O2 via qRT-PCR
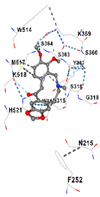
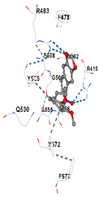
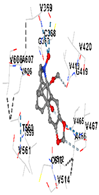
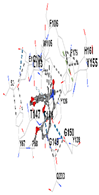
2.11. Molecular Docking
2.12. Statistical Analysis
3. Results and Discussion
3.1. Alkaloid Amount of Extracts
3.2. Antioxidant Activities of Alkaloid Extracts
3.3. Effect of Alkaloid Extracts in PC-12 Cells Induced by OS with H2O2
3.4. Effect of Alkaloid Extracts on TDH in PC-12 Cells Induced by OS with H2O2
3.5. Impact of Alkaloid Extracts on TOS, TAS, and OSI Levels in PC-12 Cells Induced by OS with H2O2
3.6. Impact of Alkaloid Extracts on PON1, SOD, CAT, and GPx Enzyme Levels in PC-12 Cells Induced by OS with H2O2
3.7. Impact of Alkaloid Extracts on GCLC, HO-1, NRF2, NQO1, and KEAP1 Gene Expression Levels in PC-12 Cells Induced by OS with H2O2
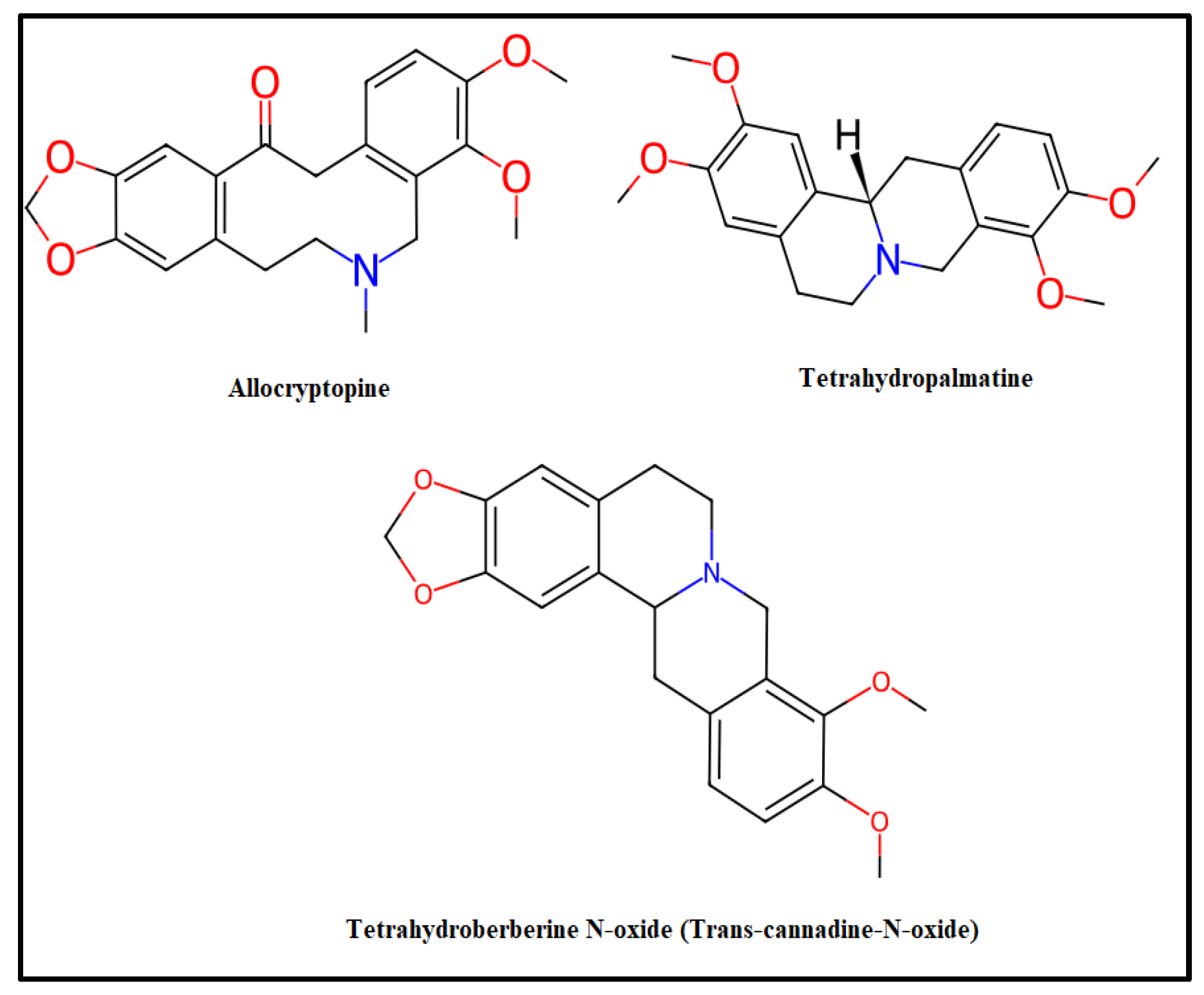
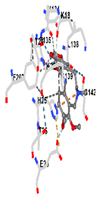
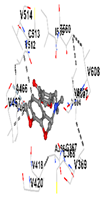
3.8. Molecular Docking Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leyane, T.S.; Jere, S.W.; Hourel, N.N. Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef] [PubMed]
- Dogan, R.; Guler, E.M.; Kocyigit, A.; Çelik, İ.; Senturk, E.; Yenigun, A.; Tugrul, S.; Ozturan, O. Are the oxidative stress levels in the tumor center and tumor boundary different from those in healthy tissue? Eur. Arch. Oto-Rhino-L. 2021, 278, 5013–5020. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, H.; Deveci, C.D.; Alışık, M.; Korkmaz, V.; Kurdoğlu, Z.; Erel, Ö.; Üstün, Y. How do thiol disulfide balance and copper-ceruloplasmin levels change in women using copper intrauterine devices? Gynecol. Endocrinol. 2022, 38, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for prevention and therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Ustay, O. Paraoksanaz-1 enzimi ve koroner kalp hastalıkları ile ilişkisi. Marmara Med. J. 2011, 24, 59–63. [Google Scholar]
- Jurcau, A. Insights into the pathogenesis of neurodegenerative diseases: Focus on mitochondrial dysfunction and oxidative stress. Int. J. Mol. Sci. 2021, 22, 11847. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.L.; Moroni, A.D.; Quiroga, S.; Castro, M.M.; Burgueño, A.L.; Genaro, A.M. Immunomodulation induced by central nervous system-related peptides as a therapeutic strategy for neurodegenerative disorders. Pharmacol. Res. Perspect. 2021, 9, e00795. [Google Scholar] [CrossRef]
- Pohl, F.; Kong Thoo Lin, P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [PubMed]
- Allafchian, A.R.; Jalali, S.A.H.; Aghaei, F.; Farhang, H.R. Green synthesis of silver nanoparticles using Glaucium corniculatum (L.) Curtis extract and evaluation of its antibacterial activity. IET Nanobiotechnol. 2018, 12, 574–578. [Google Scholar] [CrossRef]
- Lixia, H.; Jun, C.; Song, H.; FaHu, Y.; Jinwen, T. Neuroprotective effect of (-)-tetrahydropalmatine in Japanese encephalitis virus strain GP-78 infected mouse model. Microb. Pathog. 2018, 114, 197–203. [Google Scholar] [CrossRef]
- Nigdelioglu Dolanbay, S.; Kocanci, F.G.; Aslim, B. Neuroprotective effects of allocryptopine-rich alkaloid extracts against oxidative stress-induced neuronal damage. Biomed. Pharmacother. 2021, 140, 111690. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, J.; Yang, H.; Huo, L.; Gao, J.; Chen, H.; Gao, W. Protective effect of tetrahydropalmatine against D-galactose induced memory impairment in rat. Physiol. Behav. 2016, 154, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Mueen Ahmed, K.K. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kocanci, F.; Hamamcioglu, B.; Aslım, B. The anti-AChE and anti-proliferative activities of Glaucium acutidentatum and Glaucium corniculatum alkaloid extracts. J. Appl. Pharm. Sci. 2017, 7, 191–200. [Google Scholar] [CrossRef]
- Das, A.; Shanker, G.; Nath, C.; Pal, R.; Singh, S.; Singh, H.K. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba anticholinesterase and cognitive enhancing activities. Pharmacol. Biochem. Behav. 2002, 73, 893–900. [Google Scholar] [CrossRef]
- Kocanci, F.G.; Hamamcioglu, B.; Aslim, B. The relationship between neuroprotective activity and antigenotoxic and acetylcholinesterase inhibitory effects of Glaucium corniculatum extracts. Braz. J. Pharm. Sci. 2022, 58, e19472. [Google Scholar] [CrossRef]
- Kocanci, F.G.; Aslim, B. Chemical composition and neurotherapeutic potential of Glaucium corniculatum extracts. Pharmacogn. Mag. 2021, 17, 67. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Orhan, I.; Şener, B.; Choudhary, M.I.; Khali, A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 2004, 91, 57–60. [Google Scholar] [CrossRef]
- Perry, N.S.; Houghton, P.G.; Sampson, J.; Theolad, A.E.; Hart, S.; Lis-balchin, M.; Hoult, J.R.; Evans, P.; Jenner, P.; Perry, E.K. In vitro activities of Salvia lavandulaefolia (Spanish Sage) relevant to treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2001, 53, 1347–1356. [Google Scholar] [CrossRef]
- Perry, N.S.; Houghton, P.G.; Theolad, A.E.; Jenner, P.; Perry, E.K. In vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef]
- Majd, A.; Mehrabian, S.; Khanafari, A. Evaluating antimicrobial effect of Glaucium on oral microflora. Int. J. Dent. Med. 1996, 9, 57–66. [Google Scholar]
- Miroliaei, M.; Zahmatkesh, M. Histopathological evaluation of wound healing by extracts from Glaucium corniculatum (L.) Curt. Cell. Mol. Res. 2018, 31, 58–66. [Google Scholar]
- PC-12, CRL-1721™. Available online: https://www.atcc.org/products/crl-1721 (accessed on 18 September 2023).
- Khamtache-Abderrahim, S.; Lequart-Pillon, M.; Gontier, E.; Gaillard, I.; Pilard, S.; Mathiron, D.; Djoudad-Kadji, H.; Maiza-Benabdesselam, F. Isoquinoline alkaloid fractions of Fumaria officinalis: Characterization and evaluation of their antioxidant and antibacterial activities. Ind. Crops Prod. 2016, 94, 1001–1008. [Google Scholar] [CrossRef]
- Sarikaya, B.B.; Somer, N.U.; Kaya, G.I.; Onur, M.A.; Bastida, J.; Berkov, S. GC-MS investigation and acetylcholinesterase inhibitory activity of Galanthus rizehensis. Z. Naturforsch C 2013, 68, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Ajanal, M.; Gundkalle, M.B.; Nayak, S.U. Estimation of total alkaloid in Chitrakadivati by UV-spectrophotometer. Anc. Sci. Life 2012, 31, 198–201. [Google Scholar] [CrossRef] [PubMed]
- John, B.; Sulaiman, C.T.; George, S.; Reddy, V.R.K. Spectrophotometric estimation of total alkaloids in selected Justicia species. Int. J. Pharm. Pharm. Sci. 2014, 6, 647–648. [Google Scholar]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food. Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Oke, F.; Aslim, B.; Ozturk, S.; Altunda, S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem. 2009, 112, 874–879. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, M.A.; Ruiz-Torres, A. Homeostasis between lipid peroxidation and antioxidant enzyme activities in healthy human aging. Mech. Ageing Dev. 1992, 66, 213–222. [Google Scholar] [CrossRef]
- Leung, R.; Venus, C.; Zeng, T.; Tsopmo, A. Structure-function relationships of hydroxyl radical scavenging and chromium-VI reducing cysteine-tripeptides derived from rye secalin. Food Chem. 2018, 254, 165–169. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, J.; Luo, Z.; Zeng, W.; Wei, Z.; Spinney, R.; Hu, W.P.; Dionysiou, D.D. Experimental and theoretical insight into hydroxyl and sulfate radicals-mediated degradation of carbamazepine. Environ. Pollut. 2020, 257, 113498. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, W.; Yuan, Q.; Ye, H.; Sun, Y.; Zhang, H.; Zeng, X. Box–Behnken design for extraction optimization, characterization and in vitro antioxidant activity of Cicer arietinum L. hull polysaccharides. Carbohydr. Polym. 2016, 147, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kolla, N.; Wei, Z.; Richardson, J.S.; Li, X.M. Amitriptyline and fluoxetine protect PC12 cells from cell death induced by hydrogen peroxide. J. Psychiatry. Neurosci. 2005, 30, 196–201. [Google Scholar]
- Shen, K.; Wang, Y.; Zhang, Y.; Zhou, H.; Song, Y.; Cao, Z.; Kou, J.; Yu, B. Cocktail of four active components derived from Sheng Mai San inhibits hydrogen peroxide-induced PC12 cell apoptosis linked with the caspase-3/ROCK1/MLC pathway. Rejuvenation Res. 2015, 18, 517–527. [Google Scholar] [CrossRef]
- Wu, Y.; Shang, Y.; Sun, S.; Liang, H.; Liu, R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3β/caspase-3 mediated signaling pathway. Apoptosis 2007, 12, 1365–1375. [Google Scholar] [CrossRef]
- Kumar, S.; Seal, C.J.; Howes, M.J.R.; Kite, G.C.; Okello, E.J. In vitro protective effects of Withania somnifera (L.) dunal root extract against hydrogen peroxide and β-amyloid (1–42)-induced cytotoxicity in differentiated PC12 cells. Phytother. Res. 2010, 24, 1567–1574. [Google Scholar] [CrossRef]
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Ip, S.P.; Su, Z.R.; Lai, X.P. Protective effect of isorhynchophylline against β-amyloid-induced neurotoxicity in PC12 cells. Cell Mol. Neurobiol. 2012, 32, 353–360. [Google Scholar] [CrossRef]
- Kim, E.; Jeon, I.S.; Kim, J.W.; Kim, J.; Jung, H.S.; Lee, S.J. An MTT-based method for quantification of periodontal ligament cell viability. Oral Dis. 2017, 13, 495–499. [Google Scholar] [CrossRef]
- Neshatia, V.; Matin, M.M.; Iranshahi, M.; Bahrami, A.R.; Behravan, J.; Mollazadeh, S.; Rassouli, F.B. Cytotoxicity of vincristine on the 5637 cell line is enhanced by combination with conferone. Z. Für Naturforschung C 2009, 64, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Küçükgül, A. The antioxidant effects of Ziziphus jujuba on neurodegeneration. J. Am. Vet. Med. 2016, 27, 108–112. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Yumru, M.; Savas, H.A.; Kalenderoglu, A.; Bulut, M.; Celik, H.; Erel, O. Oxidative imbalance in bipolar disorder subtypes: A comparative study. Prog. Neuropsychopharmacol. 2009, 33, 1070–1074. [Google Scholar] [CrossRef]
- Ustunova, S.; Meral, I.; Koçyiğit, A.; Kilic, A.; Zeybek, O.; Üyüklü, M.; Meydan, S. Effects of Nigella sativa on plasma oxidative stress, and some apoptotic protein markers in cerebrum and hippocampus in pentylenetetrazol induced-kindling rats. Eur. J. Biol. 2020, 79, 124–131. [Google Scholar]
- Wu, H.; Xuan, R.; Li, Y.; Zhang, X.; Wang, Q.; Wang, L. Effects of cadmium exposure on digestive enzymes, antioxidant enzymes, and lipid peroxidation in the freshwater crab Sinopotamon henanense. Environ. Sci. Pollut. Res. 2013, 20, 4085–4092. [Google Scholar] [CrossRef]
- Moharana, M.; Pattanayak, S.K.; Khan, F. Molecular recognition of bio-active triterpenoids from Swertia chirayita towards hepatitis delta antigen: A mechanism through docking, dynamics simulation, Gibbs free energy landscape. J. Biomol. Struct. Dyn. 2023, 1, 1–14. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, C.; Cao, X.; Zhou, H.; Zhang, H.; Pan, Y. Preparative counter-current chromatography isolation of liensinine and its analogues from embryo of the seed of Nelumbo nucifera GAERTN. using upright coil planet centrifuge with four multilayer coils connected in series. J. Chromatogr. A 2004, 1041, 153–162. [Google Scholar] [CrossRef]
- Rachmaniah, O.; Wilson, E.G.; Choi, Y.H.; Witkamp, G.J.; Verpoorte, R. Pressurized natural deep eutectic solvent extraction of galanthamine and related alkaloids from Narcissus pseudonarcissus. Planta Med. 2022, 88, 814–825. [Google Scholar] [CrossRef]
- Doncheva, T.; Kostova, N.; Yordanova, G.; Saadi, H.; Akrib, F.; Dimitrov, D.; Philipov, S. Comparison of alkaloid profile from Glaucium corniculatum (Papaveraceae) of Algerian and Bulgarian origin. Biochem. Syst. Ecol. 2014, 56, 278–280. [Google Scholar] [CrossRef]
- Hashim, F.J.; Vichitphan, S.; Boonsiri Vichitphan, K. Neuroprotective assessment of Moringa oleifera leaves extract against oxidative-stress-induced cytotoxicity in SHSY5Y neuroblastoma cells. Plants 2021, 10, 889. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen extracts from Cetrarioid clade provide neuroprotection against hydrogen peroxide-induced oxidative stress. Molecules 2022, 27, 6520. [Google Scholar] [CrossRef]
- Rojas, J.; Buitrago, A. Antioxidant activity of phenolic compounds biosynthesized by plants and its relationship with prevention of neurodegenerative diseases. In Bioactive Compounds; Woodhead Publishing: Cambridge, UK, 2019; pp. 3–31. [Google Scholar] [CrossRef]
- Pottera, O.M. Antioxidants and free radical scavengers of natural origin. Curr. Org. Chem. 1997, 1, 415–440. [Google Scholar] [CrossRef]
- Dung, N.T.; Thanh, D.M.; Huong, N.T.; Thuy, P.T.; Hoan, N.T.; Thanh, D.T.M.; Trang, N.V.; Son, N.T. Quinolone and isoquinolone alkaloids: The structural-electronic effects and the antioxidant mechanisms. Struct. Chem. 2020, 31, 2435–2450. [Google Scholar] [CrossRef]
- Macáková, K.; Afonso, R.; Saso, L.; Mladěnka, P. The influence of alkaloids on oxidative stress and related signaling pathways. Free Radic. Biol. Med. 2019, 134, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kim, H.Y.; Kang, K.S.; Yokozawa Park, J.H. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch. Pharm. Res. 2009, 32, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Shirwaikar, A.; Shirwaikar, A.; Rajendran, K.; Punitha, I.S.R. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 2006, 29, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Rackova, L.; Oblozinsky, M.; Kostalova, D.; Kettmann, V.; Bezakova, L. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J. Inflamm. 2007, 4, 15. [Google Scholar] [CrossRef]
- Takahashi, S.; Matsunaga, T.; Hasegawa, C.; Saito, H.; Fujita, D.; Kiuchi, F.; Tsuda, Y. Martefragin A, a novel indole alkaloid isolated from red alga, inhibits lipid peroxidation. Chem. Pharm. Bull. 1998, 46, 1527–1529. [Google Scholar] [CrossRef]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, J.; Peng, S.; Li, X.; Fang, J. Securinine disturbs redox homeostasis and elicits oxidative stress-mediated apoptosis via targeting thioredoxin reductase. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.N.; Rau, M.R.; Fett-Neto, A.G. Oxidative stress and production of bioactive monoterpene indole alkaloids: Biotechnological implications. Biotechnol. Lett. 2014, 36, 191–200. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Alkaloids in the nature: Pharmacological applications in clinical practice of berberine and mate tea. Curr. Top. Med. Chem. 2014, 14, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Pesce, M.; Patruno, A.; Moradi, S.Z.; Iranpanah, A.; Farzaei, M.H.; Sobarzo-Sánchez, E. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules 2020, 25, 4926. [Google Scholar] [CrossRef] [PubMed]
- Nigdelioglu Dolanbay, S.; Şirin, S.; Aslim, B. Cocktail of three isoquinoline alkaloids derived from Glaucium grandiflorum Boiss. & A. Huet subsp. refractum (Nábelek) Mory inhibits the production of LPS-induced ROS, pro-inflammatory cytokines, and mediators through the down-regulation of p38 MAPK in BV-2 cells. Fitoterapia 2023, 170, 105652. [Google Scholar] [CrossRef]
- Tufan, Z.K.; Hasanoglu, I.; Kolgelier, S.; Alisik, M.; Ergin, M.; Yilmaz, G.R.; Tasyaran, M.A.; Erel, O.; Guner, R. A retrospective controlled study of thiol disulfide homeostasis as a novel marker in Crimean Congo hemorrhagic fever. Redox Rep. 2017, 22, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Requejo, R.; Hurd, T.R.; Costa, N.J.; Murphy, M.P. Cysteine residues exposed on protein surfaces are the dominant intramitochondrial thiol and may protect against oxidative damage. FEBS J. 2010, 277, 1465–1480. [Google Scholar] [CrossRef]
- Puri, S.; Hsu, S.T.D. Cross-over loop cysteine C152 acts as an antioxidant to maintain the folding stability and deubiquitinase activity of UCH-L1 under oxidative stress. J. Mol. Biol. 2021, 433, 166879. [Google Scholar] [CrossRef] [PubMed]
- Gumusyayla, S.; Vural, G.; Bektas, H.; Deniz, O.; Neselioglu, S.; Erel, O. A novel oxidative stress marker in patients with Alzheimer’s disease: Dynamic thiol–disulphide homeostasis. Acta Neuropsychiatr. 2016, 28, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.N.; Pessano, N.T.; Leal, L.; Roos, D.H.; Folmer, V.; Punte, G.O.; Rocha, J.B.T.; Aschner, M.; Avila, D.S.; Puntel, R.L. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Res. Bull. 2012, 87, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Verma, P.K.; Sood, S.; Pankaj, N.K.; Agarwal, S.; Rain, R. Neuroprotective potential of hydroethanolic hull extract of Juglans regia L. on isoprenaline induced oxidative damage in brain of Wistar rats. Toxicol. Rep. 2021, 8, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Adamczyk-Sowa, M. New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxid. Med. Cell. Longev. 2016, 2016, 1973834. [Google Scholar] [CrossRef] [PubMed]
- Özyiğit, F.; Değer, A.; Altıkat, S.; Zeren, S.; Bayhan, Z.; Kavasoğlu, M.; Şimşek, H.; Arık, Ö.; Değer, H. Dalak iskemi reperfüzyon hasarı oluşturulan sıçanlarda polydatin’in koruyucu etkisi. In Proceedings of the Turkish Society of Physiological Sciences 42nd National Physiology Congress, Düzce, Turkey, 5–8 September 2016; Volume 218, pp. 34–35. [Google Scholar]
- Arikanoglu, A.; Akil, E.; Varol, S.; Yucel, Y.; Yuksel, H.; Cevik, M.U.; Palanci, Y.; Unan, F. Relationship of cognitive performance with prolidase and oxidative stress in Alzheimer disease. Neurol. Sci. 2013, 34, 2117–2121. [Google Scholar] [CrossRef]
- Copoglu, U.S.; Virit, O.; Kokacya, M.H.; Orkmez, M.; Bulbul, F.; Erbagci, A.B.; Semiz, M.; Alpak, G.; Unal, A.; Ari, M.; et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry. Res. 2015, 229, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Koolen, H.H.; Pral, E.M.; Alfieri, S.C.; Marinho, J.V.; Serain, A.F.; Hernández-Tasco, A.J.; Andreazza, N.L.; Salvador, M.J. Antiprotozoal and antioxidant alkaloids from Alternanthera littoralis. Phytochemistry 2017, 134, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Račková, L.; Májeková, M.; Koštálová, D.; Štefek, M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg. Med. Chem. 2004, 12, 4709–4715. [Google Scholar] [CrossRef]
- Hasanein, P.; Ghafari-Vahed, M.; Khodadadi, I. Effects of isoquinoline alkaloid berberine on lipid peroxidation, antioxidant defense system, and liver damage induced by lead acetate in rats. Redox Rep. 2017, 22, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Arora, S. Alkaloids-important therapeutic secondary metabolites of plant origin. J. Crit. Rev. 2015, 2, 1–8. [Google Scholar]
- Kazaz, I.O.; Mentese, A.; Demir, S.; Kerimoglu, G.; Colak, F.; Bodur, A.; Alver, A.; Kutlu, O.; Turedi, S. Berberine inhibits the ischemia-reperfusion induced testicular injury through decreasing oxidative stress. Am. J. Emerg. Med. 2020, 38, 33–37. [Google Scholar] [CrossRef]
- Colovic, M.B.; Vasic, V.M.; Djuric, D.M.; Krstic, D.Z. Sulphur-containing amino acids: Protective role against free radicals and heavy metals. Curr. Med. Chem. 2018, 25, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress: A review. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Muzykantov, V.R. Delivery of antioxidant enzyme proteins to the lung. Antioxid. Redox Signal. 2001, 3, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Hanna, D.N.; Cyr, S.; Baechle, J.J.; Kuravi, S.; Balusu, R.; Rathmell, K.; Baregamian, N. Glutathione peroxidase 4 inhibition induces ferroptosis and mTOR pathway suppression in thyroid cancer. Sci. Rep. 2022, 12, 19396. [Google Scholar] [CrossRef] [PubMed]
- Mao, P. Oxidative stress and its clinical applications in dementia. J. Neurodegener. Dis. 2013, 2013, PMC4437276. [Google Scholar] [CrossRef]
- Romani, A.; Trentini, A.; van der Flier, W.M.; Bellini, T.; Zuliani, G.; Cervellati, C.; Teunissen, C.E. Arylesterase activity of paraoxonase-1 in serum and cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Antioxidants 2020, 9, 456. [Google Scholar] [CrossRef]
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Ezabadi, A.; Peeri, M.; Azarbayjani, M.A.; Hosseini, S.A. The effects of resistance training and berberine chloride supplementation on oxidative stress markers in the cerebellum tissue of diazinon-poisoned rats. Middle East J. Rehabil. Health Stud. 2019, 6, 92870. [Google Scholar] [CrossRef]
- Jia, W.; Su, Q.; Cheng, Q.; Peng, Q.; Qiao, A.; Luo, X.; Zhang, J.; Wang, Y. Neuroprotective effects of palmatine via the enhancement of antioxidant defense and small heat shock protein expression in Aβ-transgenic caenorhabditis elegans. Oxid. Med. Cell Longev. 2021, 2021, 9966223. [Google Scholar] [CrossRef]
- Hussein, J.S.; Medhat, D.; Abdel-Latif, Y.; Morsy, S.; Gaafar, A.A.; Ibrahim, E.A.; Al-kashef, A.S.; Nooman, M.U. Amelioration of neurotoxicity induced by esfenvalerate: Impact of Cyperus rotundus L. tuber extract. Comp. Clin. Path. 2021, 30, 1–10. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An overview of the Nrf2/ARE pathway and its role in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, B.; Liu, J.; Qiao, C.; Liu, Y.; Li, S.; Lv, H. Isoorientin exerts a protective effect against 6-OHDA-induced neurotoxicity by activating the AMPK/AKT/Nrf2 signalling pathway. Food Func. 2020, 11, 10774–10785. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ding, H.; Li, D.; Lu, J. Curcumin protects trabecular meshwork cells against hydrogen peroxide-induced oxidative stress and apoptosis via Nrf2-Keap1 pathway. Trop. J. Pharm. Res. 2018, 17, 2169–2176. [Google Scholar] [CrossRef]
- Ying, C.L.Y.; Hao, J.C.; Shang, B.; Zhao, C.; Wang, L.J.; Yang, K.L.; He, X.Z.; Tian, Q.Q.; Wang, Z.L.; Jing, H.L.; et al. Neuroprotective effects of aucubin on hydrogen peroxide-induced toxicity in human neuroblastoma SH-SY5Y cells via the Nrf2/HO-1 pathway. Phytomedicine 2021, 87, 153577. [Google Scholar] [CrossRef]
- Bao, F.; Tao, L.; Zhang, H. Neuroprotective effect of natural alkaloid fangchinoline against oxidative glutamate toxicity: Involvement of keap1-Nrf2 axis regulation. Cell. Mol. Neurobiol. 2019, 39, 1177–1186. [Google Scholar] [CrossRef]
- Wang, L.; Pu, Z.; Li, M.; Wang, K.; Deng, L.; Chen, W. Antioxidative and antiapoptosis: Neuroprotective effects of dauricine in Alzheimer’s disease models. Life Sci. 2020, 243, 117237. [Google Scholar] [CrossRef]
- Gaillard, T. Evaluation of AutoDock and AutoDock Vina on the CASF-2013 benchmark. J. Chem. Inf. Model 2018, 58, 1697–1706. [Google Scholar] [CrossRef]
| Gene Name | Forward Primer 5′→3′ | Reverse Primer 5′→3′ |
|---|---|---|
| GCLC | GTGGACACCCGATGCAGTAT | TCATCCACCTGGCAACAGTC |
| HO-1 | GCTCTATCGTGCTCGCATGA | AATTCCCACTGCCACGGTC |
| KEAP | TGGGCGTGGCAGTGCTCAAC | GCCCATCGTAGCCTCCTGCG |
| NRF2 | GCTGCCATTAGTCAGTCGCTCTC | ACCGTGCCTTCAGTGTGCTTC |
| NQO1 | ACATCACAGGGGAGCCGAAGGACT | GGCACCCCAAACCAATACAATG |
| GAPDH | CAACTCCCTCAAGATTGTCAGCAA | GGCATGGACTGTGGTCATGA |
| Solvents | Extract a (mg/g Plant) | Alkaloid b (mg/g Extract) |
|---|---|---|
| Chloroform | 4.04 ± 0.40 * | 133.43 ± 4.42 * |
| Methanol | 5.03 ± 0.22 * | 153.21 ± 6.21 * |
| Water | 3.01 ± 0.51 * | 80.19 ± 7.14 * |
| Ligand | Allocryptopine | ||||
|---|---|---|---|---|---|
| Target Protein | GCLC | HO-1 | KEAP1 | NRF2 | NQO1 |
| Vina Score (kkal/mol) | −7.9 | −8.0 | −8.0 | −8.1 | −9.7 |
| Contact Residues and Bonds | Chain A: ASN215 PHE252 TRP314 ASN315 SER318 GLY319 LYS359 SER360 TYR362 SER363 SER364 TRP514 MET517 LYS518 HIS521 | Chain A: LYS18 THR21 LYS22 HIS25 THR26 GLU29 TYR134 THR135 LEU138 GLY139 SER142 PHE207 | Chain X: ALA366 GLY367 CYS368 VAL369 VAL418 VAL420 VAL465 ALA466 VAL467 VAL512 CYS513 VAL514 ILE559 THR560 VAL604 VAL606 ALA607 VAL608 | Chain A: TYR334 PHE335 ARG336 TYR572 HIS575 THR576 PHE577 Chain B: TYR334 ARG336 GLN337 ASN382 SER383 PRO384 Chain C: GLY76 GLY81 GLU82 | Chain M: HIS11 SER16 PRO102 LEU103 GLN104 TRP105 PHE106 THR147 THR148 GLY149 GLY150 TYR155 HIS161 Chain N: ILE50 PHE65 GLN66 TYR67 PRO68 GLU117 TYR126 TYR128 PHE178 |
 |  |  | 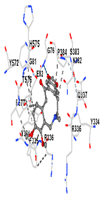 |  | |
| Ligand | Tetrahydropalmatine | ||||
| Target Protein | GCLC | HO-1 | KEAP1 | NRF2 | NQO1 |
| Vina Score | −7.8 | −7.7 | −8.1 | −8.7 | −8.8 |
| Contact Residues | Chain A: CYS213 MET214 ASN215 TRP314 ASN315 SER318 GLY319 ASP322 ARG324 PRO358 LYS359 SER360 SER363 SER364 TRP514 MET517 LYS518 HIS521 | Chain A: HIS25 PHE33 MET34 PHE37 PHE47 VAL50 MET51 LEU54 TYR134 THR135 ARG136 LEU138 GLY139 ASP140 SER142 GLY143 GLY144 LEU147 PHE166 PHE167 PHE207 ASN210 PHE214 | Chain X: LEU365 ALA366 GLY367 CYS368 VAL369 ILE416 GLY417 VAL418 VAL463 GLY464 VAL465 ALA466 ALA510 VAL512 CYS513 VAL514 LEU557 GLY558 ILE559 THR560 VAL561 VAL604 GLY605 VAL606 ALA607 VAL608 | Chain B: LEU365 ALA366 GLY367 CYS368 ILE416 VAL418 GLY419 VAL420 GLY462 VAL463 GLY464 VAL465 GLY509 ALA510 GLY511 VAL512 CYS513 ALA556 LEU557 GLY558 ILE559 THR560 GLY603 VAL604 GLY605 VAL606 | Chain I: ILE50 PHE65 GLN66 TYR67 PRO68 TYR126 PHE178 Chain J: HIS11 SER12 SER16 PRO102 LEU103 GLN104 TRP105 PHE106 THR148 GLY149 TYR155 |
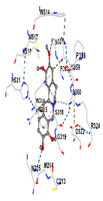 | 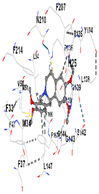 |  |  | 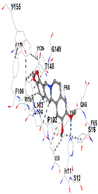 | |
| Ligand | Tetrahydroberberine N-oxide (Trans-cannadine-N-oxide) | ||||
| Target Protein | GCLC | HO-1 | KEAP1 | NRF2 | NQO1 |
| Vina Score | −8.1 | −7.0 | −7.8 | −7.2 | −9.7 |
| Contact Residues | Chain A: PHE46 TRP47 GLY48 ASP49 GLU50 HIS94 THR105 PRO106 ALA107 SER108 PRO109 THR270 PHE271 GLN272 PRO465 ARG468 | Chain A: LEU141 GLN145 LYS148 PHE167 THR168 PHE169 PRO170 ILE172 Chain B: ALA173 ALA175 Chain B: ALA94 GLY98 PRO99 GLU162 | Chain X: ARG415 GLY462 PHE478 ARG483 SER508 GLY509 TYR525 GLN530 SER555 ALA556 TYR572 PHE577 | Chain B: GLY367 CYS368 VAL369 VAL418 GLY419 VAL420 VAL465 ALA466 VAL467 VAL512 CYS513 VAL514 ILE559 THR560 VAL561 VAL606 ALA607 VAL608 | Chain H: GLN233 Chain I: ILE50 TYR67 PRO68 GLU117 PHE120 TYR126 TYR128 PHE178 Chain J: LEU103 GLN104 TRP105 PHE106 THR147 THR148 GLY149 GLY150 TYR155 HIS161 |
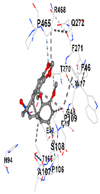 | 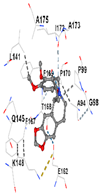 |  |  |  | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niğdelioğlu Dolanbay, S.; Şirin, S.; Aslim, B. Neuroprotective Potential of Isoquinoline Alkaloids from Glaucium grandiflorum Boiss. and A. Huet subsp. refractum (Nábelek) Mory: Role of NRF2-KEAP1 Pathway. Appl. Sci. 2023, 13, 11205. https://doi.org/10.3390/app132011205
Niğdelioğlu Dolanbay S, Şirin S, Aslim B. Neuroprotective Potential of Isoquinoline Alkaloids from Glaucium grandiflorum Boiss. and A. Huet subsp. refractum (Nábelek) Mory: Role of NRF2-KEAP1 Pathway. Applied Sciences. 2023; 13(20):11205. https://doi.org/10.3390/app132011205
Chicago/Turabian StyleNiğdelioğlu Dolanbay, Serap, Seda Şirin, and Belma Aslim. 2023. "Neuroprotective Potential of Isoquinoline Alkaloids from Glaucium grandiflorum Boiss. and A. Huet subsp. refractum (Nábelek) Mory: Role of NRF2-KEAP1 Pathway" Applied Sciences 13, no. 20: 11205. https://doi.org/10.3390/app132011205
APA StyleNiğdelioğlu Dolanbay, S., Şirin, S., & Aslim, B. (2023). Neuroprotective Potential of Isoquinoline Alkaloids from Glaucium grandiflorum Boiss. and A. Huet subsp. refractum (Nábelek) Mory: Role of NRF2-KEAP1 Pathway. Applied Sciences, 13(20), 11205. https://doi.org/10.3390/app132011205






