Abstract
Homocyclic aromatics with different degrees of alkylation have been investigated so far as Liquid Organic Hydrogen Carriers (LOHC). A low enthalpy of reaction for the dehydrogenation reaction is generally considered beneficial. Values available for crowded, multi-alkylated aromatics, such as hexamethyl benzene, indicate that these substances could be utilized efficiently as LOHCs. However, no clear trend can be identified in the existing data. The aim of this study is to provide a consistent and comprehensive data set on this substance class to evaluate if multi-alkylation is indeed beneficial. For this purpose, own and literature results from experimental methods such as combustion calorimetry and the transpiration method for measuring the enthalpy of vaporisation were combined with quantum chemical approaches to obtain a validated, consistent data set. This comprehensive study reveals that the positive effect on enthalpy of reaction for dehydrogenation is comparatively weak. A slightly lower enthalpy of reaction is actually observed for crowded alkylbenzenes, but it is most likely not sufficient to reach a significant decrease in temperature for hydrogen release. Nevertheless, the results are of high importance for the further development of LOHC systems with optimal structural motifs.
1. Introduction
The Liquid Organic Hydrogen Carrier (LOHC) technology is a promising approach for the storage of hydrogen [1,2]. A LOHC system consists of a hydrogen-lean form (most commonly an aromatic compound) and a corresponding hydrogenated hydrogen-rich form (typically an alicyclic compound). The hydrogen-lean component of the system can take up hydrogen through catalytic hydrogenation to produce the hydrogen-rich compound. Hydrogen is stored in such systems through covalent bonds in a very safe form. Since the dehydrogenation enthalpies for the aromatic LOHC systems are typically at a high level of about 66 to 68 kJ mol−1/(H2) at ambient temperatures [3,4], accidental and spontaneous hydrogen release is very unlikely and only possible in the presence of catalyst and heat provision at elevated temperatures.
The conditions for hydrogen release are determined by the Gibbs energy of reaction, which can be calculated from the enthalpy and entropy of reaction. The entropy change during the reaction is in the same order of magnitude for most LOHC carrier molecules. In contrast, the enthalpy of reaction for the different molecules can deviate significantly. Thus, studies of the reaction thermodynamics of potential LOHCs focus on the enthalpy of reaction as the first step.
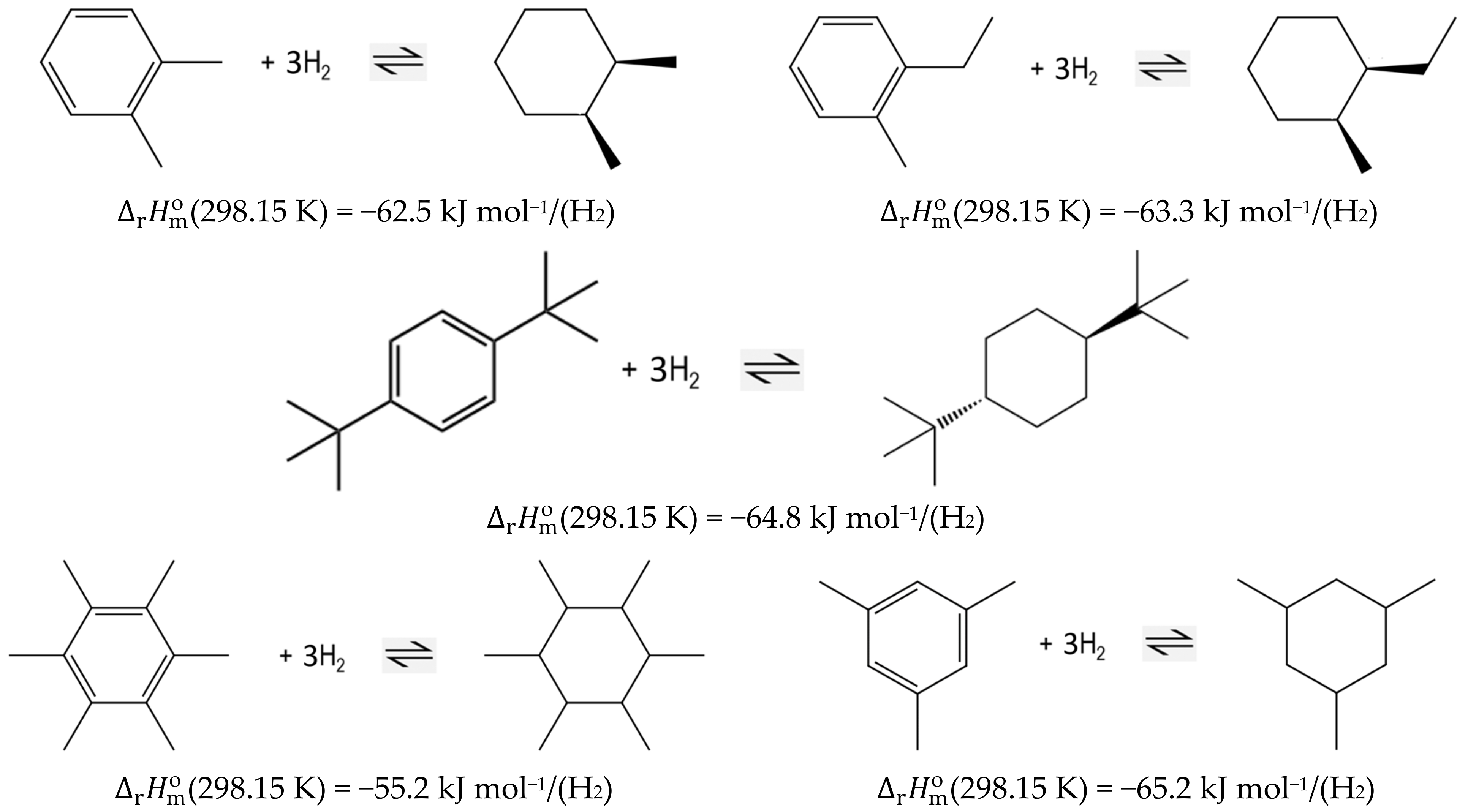
The standard molar reaction enthalpies, , are important fundamentals for process optimization. They are calculated according to Hess’s Law, using the liquid-phase standard molar enthalpies of formation, (liq), of the reactants and products. The enthalpies of the hydrogenation/dehydrogenation reactions are desired to be as low as possible to decrease the energy demand of hydrogen release (without compromising the stable interaction of hydrogen with the organic carrier) [5]. In our recent work [6], the influence of structure on the magnitude of hydrogenation/dehydrogenation enthalpies in the LOHC system based on alkylbenzene/alkyl-cyclohexane component pairs has been analyzed. It has been found that in the case of the sterically hindered ortho-substituted species (e.g., 1,2-dimethyl-benzene and 1-methyl-2-ethyl-benzene, or alkylbenzenes) with branched tert-butyl substituents, the heats of reaction are significantly lower than the usual level for aromatic homocycles (see Figure 1).
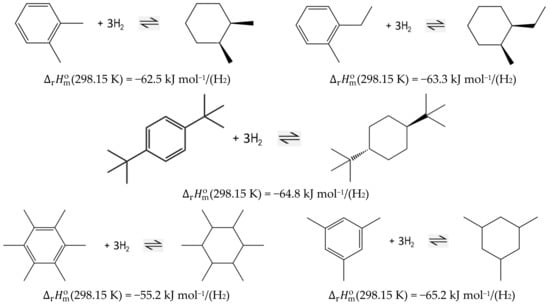
Figure 1.
Liquid-phase reaction enthalpies of hydrogenation (per mole of hydrogen released) for the LOHC alkylbenzene-based system [6].
Furthermore, the enthalpy of hydrogenation of the highly crowded hexa-methyl-benzene has surprisingly been found to be at the lowest level (−55.2 kJ mol−1/(H2); see Figure 1) ever reported for alkylbenzenes. At the same time, the hydrogenation of 1,3,5-tri-methyl-benzene is nothing special in terms of energy (−65.2 kJ mol−1/(H2); see Figure 1), which is basically the same value as reported for the common LOHC dibenzyl toluene with a value of −65.4 kJ mol−1/(H2) [2]. These observations have prompted a project for a more detailed study of these phenomena: We want to find out how the steric hindrance and congesting of a molecule with branched iso-propyl and tert-butyl substituents could decrease the hydrogenation enthalpies of di-, tri- and tetra-substituted alkylbenzenes. Moreover, we are interested how the bulky iso-propyl and tert-butyl substituents in ortho-substituted alkylbenzenes affect the energetics of the reactions.
From a practical point of view, the di- and tri-substituted iso-propyl- and tert-butyl-benzenes can be prepared using known synthetic routes [7,8], and both the hydrogen-lean and hydrogen-rich counterparts have acceptably low vapour pressures [6], which is one of the criteria for LOHC applications. Thus, if specific structural motifs that allow for a reduction in reaction enthalpy could be revealed, this could support the development of improved LOHC systems. Note that additional alkylation of the aromatic core of a hydrogen-lean LOHC molecule reduces its gravimetric hydrogen capacity. However, this negative effect could be outweighed if such multi-alkylated systems were to exhibit significantly reduced standard molar reaction enthalpies in the hydrogenation and dehydrogenation processes that establish the corresponding hydrogen storage cycle.
In fact, the thermochemical properties of mono-, di- and tri-substituted iso-propyl- and tert-butyl-benzenes as the hydrogen-lean counterparts to the LOHC systems of interest in this paper have already been intensively investigated in our laboratory in the past [9,10,11,12]. In the intervening period, some new data have appeared in the literature and it is important to ensure the consistency of all available data before recommending them for thermochemical calculations leading to the enthalpies of hydrogenation for the alkylbenzenes.
Therefore, two priorities were set in this work. The first is to collect, evaluate and validate the available experimental data. Second, the reliable liquid-phase formation enthalpies for the hydrogen-lean and hydrogen-rich counterparts of the LOHC systems based on the branched alkylbenzenes will be provided in order to calculate their hydrogenation enthalpies and to meaningfully discuss structure–property relationships for them. The evaluation of the experimental data was based on a textbook equation, which relates the thermochemical properties relevant to this work:
where (g) is the gas-phase standard molar enthalpy of formation and is the standard molar enthalpy of vaporisation. The -values are most frequently derived from the vapour-pressure–temperature dependences [13]. The (liq)-values are usually derived from combustion experiments, from solution calorimetry or from chemical equilibrium studies [14].
The data set for the vaporisation enthalpies and the data set for the enthalpies of formation were collected and evaluated separately. The set of vaporisation enthalpies was validated using a complementary transpiration experiment, and empirical and structure–property correlations. The set of enthalpies of formation was validated with quantum chemical calculations. The targeted thermochemical property that is relevant to the energetics of hydrogen storage is the liquid-phase enthalpy of formation, (liq). It was derived using the following equation:
We have extended the symbols for enthalpies to include temperatures, since it is common in thermochemistry to refer all enthalpies given in Equations (1) and (2) to the reference temperature T = 298.15 K. The cross-validation of the two contributors to Equation (2) allows a reasonably accurate determination of the liquid-phase standard molar enthalpies of formation of the hydrogen-lean compounds of given LOHC systems. Along with the carefully evaluated and validated liquid-phase enthalpies of formation of the corresponding alkyl-cyclohexanes (as the hydrogen-rich compounds) [6], the interpretation of the structural effects on the energetics of the hydrogenation/dehydrogenation reactions relies upon the validated data sets and not on individual, possibly inaccurate, data.
2. Materials and Methods
The samples of 1,4-di-tert-butyl-benzene and 1,3,5-tri-tert-butyl-benzene were of commercial origin (TCI) with purities of 98–99% and used as received from the supplier. However, before starting the vapour pressure measurements using the transpiration method, the samples were conditioned in situ in the experimental device, as described in the Electronic Supporting Materials (ESI). Final purities were determined using a gas chromatograph equipped with an HP-5 capillary column and a flame ionization detector. A negligible amount of impurities (below 0.003 mass fraction but heavier in molar mass than the main compound) was found in samples used for vapour pressure measurements.
We used the transpiration method [15,16] to measure the vapour pressure of tert-butyl-benzenes at different temperatures. The standard molar enthalpies of sublimation and standard molar enthalpies of vaporisation, , were derived from the temperature dependencies of the vapour pressures.
Theoretical gas-phase enthalpies of formation of alkylbenzenes were calculated using quantum chemical methods. The Gaussian 16 software package [17] with the composite G3MP2 [18] and G4 [19] methods were utilized for calculations of enthalpies H298 of the most stable conformer of each compound. The H298-values were finally converted to (g, 298.15 K)theor-values and discussed.
3. Results and Discussion
3.1. Vapour Pressures and Vaporisation/Sublimation Enthalpies of Alkylbenzenes
The experimental vapour pressures pi measured in this work (see Table S1) were fitted with the following equation [15,16]:
where R = 8.31446 J·K−1·mol−1 is the molar gas constant, the reference pressure a and b are adjustable parameters, the arbitrary temperature T0 applied in Equation (3) was chosen to be T0 = 298.15 K and is the difference in the molar heat capacities of the gas and the crystal (or liquid) phases, respectively (see Table S2). The vapour pressures measured at different temperatures, T, in this work were used to derive the enthalpies of sublimation/vaporisation using the following equation:
Sublimation entropies at temperatures T were also derived from the temperature dependences of the vapour pressures using Equation (5):
with = 0.1 MPa. The original absolute vapour pressures available in the literature were also treated using Equations (3)–(5) to evaluate sublimation/vaporisation enthalpies at 298.15 K (see Table 1, Table 2, Table 3 and Table 4). Uncertainties in the literature results have been re-assessed. They include uncertainties from the fitting equation and uncertainties from temperature adjustment to T = 298.15 K.
The vaporisation/sublimation enthalpies, which are derived from the experimental vapour-pressure–temperature dependencies according to the Clausius–Clapeyron relation, are referenced to the average temperature, Tav, of the examined interval. For engineering calculations, these enthalpies should be adjusted to the reference temperature T = 298.15 K. Adjustment details are given in ESI. The enthalpies of vaporisation evaluated in this work are given in Table 1 for the iso-propyl-substituted benzenes and in Table 2 for the tert-butyl-substituted benzenes. The enthalpies of sublimation of tert-butyl-benzenes are given in Table 3.

Table 1.
Compilation of the enthalpies of vaporisation, for the iso-propyl-benzene derivates determined in this work and from the data available in the literature.
Table 1.
Compilation of the enthalpies of vaporisation, for the iso-propyl-benzene derivates determined in this work and from the data available in the literature.
| Compound | M a | T-Range | Ref. | ||
|---|---|---|---|---|---|
| CAS | K | kJ·mol−1 | kJ·mol−1 | ||
| iso-propylbenzene | 45.2 ± 0.2 | [20] | |||
| 2-methyl-iso-propylbenzene | E | 354.3–452.9 | 43.7 ± 1.0 | 49.0 ± 1.5 | [21] |
| 527-84-4 | n/a | 50.6 ± 1.0 | [22] | ||
| Hsol | 48.2 ± 1.0 | Table 6 | |||
| 49.3 ± 0.6 c | |||||
| 3-methyl-iso-propylbenzene | E | 351.9–449.5 | 43.7 ± 1.0 | 48.9 ± 1.4 | [21] |
| 535-77-3 | n/a | 50.0 ± 1.0 | [22] | ||
| Hsol | 49.2 ± 1.0 | Table 6 | |||
| 49.4 ± 0.6 c | |||||
| 4-methyl-iso-propylbenzene | E | 401.4–451.5 | 42.8 ± 1.0 | 49.2 ± 1.6 | [21] |
| 99-87-6 | n/a | 50.3 ± 1.0 | [22] | ||
| n/a | 48.9 ± 1.0 | [23] | |||
| CGC | 49.2 ± 1.0 | [24] | |||
| Hsol | 50.0 ± 1.0 | Table 6 | |||
| 49.6 ± 0.5 c | |||||
| 1,2-di-iso-propylbenzene | E | 388.6–476.9 | 47.4 ± 1.0 | 54.8 ± 1.8 | [25] |
| 577-55-9 | BP | 350.2–477.2 | 48.3 ± 1.3 | 54.6 ± 1.8 | Table S4 |
| Jx | 55.8 ± 1.0 | Table 5 | |||
| Hsol | 54.8 ± 1.0 | Table 6 | |||
| 54.9 ± 0.7 c | |||||
| 1,3-di-iso-propylbenzene | E | 388.1–476.3 | 47.4 ± 1.0 | 54.7 ± 1.8 | [25] |
| 99-62-7 | T | 283.5–318.5 | 56.1 ± 1.5 | 56.2 ± 1.6 | [9] |
| Jx | 55.4 ± 1.0 | Table S4 | |||
| Jx | 54.3 ± 1.0 | Table 5 | |||
| Hsol | 55.8 ± 1.0 | Table 6 | |||
| 55.2 ± 0.6 c | |||||
| 1,4-di-iso-propylbenzene | E | 393.4–483.5 | 47.7 ± 1.0 | 55.2 ± 1.8 | [25] |
| 100-18-5 | E | 328.7–483.7 | 50.7 ± 1.0 | 56.3 ± 1.5 | [26] |
| E | 393.4–484.7 | 47.4 ± 1.0 | 55.3 ± 1.9 | [21] | |
| T | 283.7–318.3 | 56.4 ± 0.6 | 56.5 ± 0.7 | [9] | |
| E | 365.5–529.9 | 47.7 ± 0.1 | 55.7 ± 1.6 | [27] | |
| TFM | 328.7–675.9 | 48.0 ± 1.0 | 57.2 ± 2.1 | [28] | |
| 56.2 ± 0.5 c | |||||
| 1,2,4-tri-iso-propylbenzene | BP | 386–517 | 51.7 ± 1.1 | 64.2 ± 2.7 | Table S4 |
| 648-32-3 | Hsol | 64.7 ± 1.0 | Table 6 | ||
| 64.6 ± 1.1 c | |||||
| 1,2,3-tri-iso-propylbenzene | Hsol | 63.4 ± 1.0 | Table 6 | ||
| 2083-67-2 | |||||
| 1,3,5-tri-iso-propylbenzene | S | 283.0–388.1 | 62.7 ± 0.6 | 66.8 ± 1.0 | [29] |
| 717-74-8 | T | 283.6–323.3 | 64.3 ± 1.2 | 64.6 ± 1.3 | [9] |
| Jx | 64.1 ± 1.0 | Table 5 | |||
| Hsol | 65.5 ± 1.0 | Table 6 | |||
| 65.6 ± 0.6 c | |||||
| 1,2,4,5-tetra-iso-propylbenzene | E | 409.9–574.7 | 56.0 ± 0.1 | 69.7 ± 2.7 | [27] |
| 635-11-0 | Jx | 67.5 ± 1.0 | Table S5 | ||
| 68.3 ± 1.6 c |
a Methods: T = transpiration method; E = ebulliometry; n/a = not available; Jx = from correlation of experimental vaporisation enthalpies with Kovats indices (see text); BP = from boiling points at different temperatures available from the literature [30]; CGC = correlation gas-chromatography; Hsol = derived from results from solution calorimetry. TFM = time flow method [28]; b Vapour pressures available in the literature were treated using Equations (3)–(5) with the help of heat capacity differences from Table S1 to evaluate the enthalpy of vaporisation at 298.15 K. Uncertainties in the vaporisation enthalpies U() are the expanded uncertainty (0.95 level of confidence). They include uncertainties from the fitting equation and uncertainties from temperature adjustment to T = 298.15 K. Uncertainties in the temperature adjustment of vaporisation enthalpies to the reference temperature T= 298.15 K are estimated to account for 20% to the total adjustment; c Weighted mean value (uncertainties were taken as the weighting factor). Values in bold are recommended for further thermochemical calculations.

Table 2.
Compilation of the enthalpies of vaporisation, for the tert-butyl-benzene derivates determined in this work and from the data available in the literature.
Table 2.
Compilation of the enthalpies of vaporisation, for the tert-butyl-benzene derivates determined in this work and from the data available in the literature.
| Compound | M a | T-Range | Ref. | ||
|---|---|---|---|---|---|
| CAS | K | kJ·mol−1 | kJ·mol−1 | ||
| tert-butylbenzene | 47.5 ± 0.4 | [12] | |||
| 2-methyl-tert-butylbenzene | Jx | 50.9 ± 1.0 | Table S6 | ||
| 1074-92-6 | Jx | 51.2 ± 1.0 | Table S7 | ||
| 51.1 ± 0.7 c | |||||
| 3-methyl-tert-butylbenzene | T | 274.4–318.5 | 51.0 ± 0.6 | 51.1 ± 0.6 | [12] |
| 1075-38-3 | Jx | 51.5 ± 1.0 | Table 5 | ||
| Jx | 51.7 ± 1.0 | Table S6 | |||
| Jx | 51.8 ± 1.0 | Table S7 | |||
| 51.4 ± 0.4 c | |||||
| 4-methyl-tert-butylbenzene | T | 273.5–322.9 | 52.2 ± 0.2 | 52.2 ± 0.2 | [12] |
| 98-51-1 | 52.6 ± 1.0 | Table 5 | |||
| 52.3 ± 0.3 c | |||||
| 3,5-di-methyl-tert-butylbezene | S | 253.4–442.9 | 54.9 ± 0.2 | 56.7 ± 0.4 | [29] |
| 98-19-1 | S | 273.7–442.9 | 53.4 ± 0.1 | 56.3 ± 0.7 | [31] |
| T | 283.6–318.2 | 56.5 ± 1.2 | 56.7 ± 1.2 | [10] | |
| 56.6 ± 0.3 c | |||||
| 1,2-di-tert-butylbezene | Sx | 56.6 ± 1.5 | Table 7 | ||
| 1012-76-6 | |||||
| 1,3-di-tert-butylbezene | n/a | 346–374 | 58.0 ± 1.0 | 61.6 ± 1.2 | [32] |
| 1014-60-4 | T | 288.3–333.1 | 58.9 ± 1.0 | 59.6 ± 1.1 | [10] |
| E | 412.7–498.5 | 49.0 ± 0.1 | 58.2 ± 1.8 | [33] | |
| BP | 346.2–497.3 | 52.9 ± 1.9 | 59.6 ± 2.3 | Table S4 | |
| Jx | 59.7 ± 1.0 | Table S7 | |||
| Hsol | 58.8 ± 1.0 | Table 6 | |||
| Sx | 59.0 ± 1.5 | Table 7 | |||
| 59.8 ± 0.5 c | |||||
| 1,4-di-tert-butylbezene | E | 377.0–510.0 | 52.1 ± 0.2 | 60.7 ± 1.7 | [34] |
| 1012-72-2 | S | 353.6–463.1 | 54.4 ± 0.2 | 60.6 ± 1.2 | [31] |
| E | 389.4–517.5 | 51.8 ± 0.1 | 60.7 ± 1.8 | [33] | |
| T | 354.2–383.3 | 56.3 ± 0.9 | 60.5 ± 1.2 | [35] | |
| T | 354.2–383.3 | 56.3 ± 1.1 | 60.4 ± 1.3 | [12] | |
| E | 350.0–510.4 | 53.8 ± 0.1 | 60.9 ± 1.6 | [36] | |
| T | 351.2–384.5 | 56.2 ± 0.6 | 60.3 ± 1.0 | Table S1 | |
| BP | 361.8–514.2 | 52.7 ± 1.1 | 60.9 ± 2.0 | Table S4 | |
| Jx | 60.7 ± 1.0 | Table S6 | |||
| Sx | 59.3 ± 1.5 | Table 7 | |||
| 60.5 ± 0.5 c | |||||
| 1-methyl-3,5-di- | T | 308.3–358.2 | 60.3 ± 0.4 | 62.5 ± 0.6 | [35] |
| tert-butylbezene | E | 379.6–510.6 | 53.2 ± 0.2 | 62.9 ± 1.9 | [35] |
| 15181-11-0 | T | 308.9–338.3 | 61.1 ± 1.8 | 62.7 ± 1.9 | Table S1 |
| BP | 410.2–511.2 | 51.6 ± 1.0 | 62.0 ± 2.3 | Table S4 | |
| 62.5 ± 0.5 c | |||||
| 1,3,5-tri-tert-butylbenzene | S | 343.5–462.7 | 60.9 ± 0.3 | 69.1 ± 1.7 | [31] |
| 1460-02-2 | E | 447.9–528.3 | 53.7 ± 0.2 | 69.0 ± 3.1 | [33] |
| T | 352.2–382.7 | 63.1 ± 0.5 | 68.7 ± 1.2 | [35] | |
| T | 348.6–388.7 | 63.3 ± 0.6 | 69.0 ± 1.3 | Table S1 | |
| BP | 381.2–527.4 | 55.9 ± 1.3 | 68.5 ± 2.8 | Table S4 | |
| Sx | 69.7 ± 1.5 | Table 7 | |||
| 69.0 ± 0.7 c | |||||
| 1,2,4-tri-tert-butylbenzene | Sx | 67.7 ± 1.5 | Table 7 | ||
| 1459-11-6 | |||||
| 1,2,3-tri-tert-butylbenzene | Sx | 64.8 ± 1.5 | Table 7 | ||
| 40782-34-1 | |||||
| 1,2,4,5-tetra-tert-butylbenzene | Sx | 73.6 ± 1.5 | Table 7 | ||
| 796-97-4 | |||||
| 1-iso-propyl-2-tert-butyl- | Hsol | 57.4 ± 1.0 | Table 6 | ||
| benzene 20033-11-8 |
a Methods: T = transpiration method; E = ebulliometry; n/a = not available; Jx = from correlation of experimental vaporisation enthalpies with Kovats indices (see text); S = static method; BP = from boiling points at different temperatures available from the literature [30]; Sx = derived from correlation of vaporisation enthalpies with the solvent-accessible surface (see text); b Vapour pressures available in the literature were treated using Equations (3)–(5) with the help of heat capacity differences from Table S1 to evaluate the enthalpy of vaporisation at 298.15 K. Uncertainties in the vaporisation enthalpies U() are the expanded uncertainty (0.95 level of confidence). They include uncertainties from the fitting equation and uncertainties from temperature adjustment to T = 298.15 K. Uncertainties in the temperature adjustment of vaporisation enthalpies to the reference temperature T = 298.15 K are estimated to account for 20% to the total adjustment; c Weighted mean value (uncertainties were taken as the weighting factor). Values in bold are recommended for further thermochemical calculations.

Table 3.
Compilation of the enthalpies of sublimation, for the tert-butyl-benzenes derives determined in this work and from the data available in the literature.
Table 3.
Compilation of the enthalpies of sublimation, for the tert-butyl-benzenes derives determined in this work and from the data available in the literature.
| Compound | M a | T-Range | Ref. | ||
|---|---|---|---|---|---|
| CAS | K | kJ·mol−1 | kJ·mol−1 | ||
| 1,4-di-tert-butylbezene | S | 285.0–325.0 | 83.2 ± 1.5 | 83.4 ± 1.6 | [37] |
| T | 288.2–333.2 | 82.2 ± 0.8 | 82.6 ± 0.9 | [10] | |
| SC | 81.3 ± 2.0 | [38] | |||
| T | 314.3–340.5 | 81.3 ± 0.8 | 82.3 ± 1.1 | Table S1 | |
| PhT | 81.7 ± 0.6 | Table 4 | |||
| 82.1 ± 0.4 c | |||||
| 1-methyl-3,5-di- | T | 274.7–301.7 | 82.4 ± 1.0 | 81.9 ± 1.1 | [10] |
| tert-butylbezene | SC | 81.8 ± 2.0 | [38] | ||
| PhT | 80.7 ± 0.6 | Table 4 | |||
| 81.0 ± 0.5 c | |||||
| 1,3,5-tri-tert-butylbenzene | K | 273.2–315.2 | 79.8 ± 0.8 | 79.5 ± 0.9 | [39] |
| T | 297.7–341.3 | 79.9 ± 0.6 | 81.2 ± 0.7 | [10] | |
| SC | 82.7 ± 2.0 | [38] | |||
| 79.8 ± 0.8 | |||||
| 80.4 ± 0.4 c |
a Methods: T = transpiration method; SC = method based on solution calorimetry; S = static method; PhT = calculated as the difference of phase transitions (see Table 4); K = Knudsen effusion method; b Vapour pressures available in the literature were treated using Equations (3)–(5) with the help of heat capacity differences from Table S1 to evaluate the enthalpy of sublimation at 298.15 K. Uncertainties in the sublimation enthalpies U() are the expanded uncertainty (0.95 level of confidence). They include uncertainties from the fitting equation and uncertainties from temperature adjustment to T = 298.15 K. Uncertainties in the temperature adjustment of sublimation enthalpies to the reference temperature T = 298.15 K are estimated to account for 20% to the total adjustment; c Weighted mean value (uncertainties were taken as the weighting factor). Values in bold are recommended for further thermochemical calculations.
The tables for both iso-propyl-benzenes and tert-butyl-benzenes can create the first impression that these compounds have been sufficiently studied. However, this is not correct as most of the entries come from our validation of structure property relationships described in the next section. In fact, in both series, only di-alkyl-substituted benzenes have been extensively studied, and the tri- and poly-alkyl-substituted benzenes have received significantly less attention. Three main methods have been used for vapour pressure measurements of alkylbenzenes: transpiration, static and ebulliometry. These methods are applied for a specific temperature range: transpiration is generally used below 383 K, the static method can cover a wider temperature range from room temperature up to 450 K (using multiple gauges) and ebulliometry usually works up to boiling temperature. The vaporisation/sublimation enthalpies were not always of interest in the original work and even when these values were derived using the Clausius–Clapeyron equation they were not comparable as they referred to different average temperatures. It should be noted that a systematic development of suitable methods for temperature adjustment of vaporisation enthalpies to the reference temperature T = 298.15 K began in the 1990s [40,41], but even today it is not completely sufficient for proper calculations (see further information in ESI). Since the vaporisation/sublimation enthalpies at the reference temperature are the focus of this study, we carefully analysed the p-T data available in the literature to derive the -values specific to the alkylbenzene series (see Table S2). Our complementary transpiration measurements were intentionally made in the ranges near T = 298.15 K to reduce the influence of the fluctuations on the target property (298.15 K). At long last, our efforts to evaluate specific -values have helped to reconcile the vaporisation/sublimation enthalpies measured using different methods in significantly different temperature ranges with final results that agree very well for each alkylbenzene under study. One of the best examples of this perfect match is 1,4-di-tert-butylbenzene, where transpiration, static and ebulliometry results differ by less than 1 kJ⋅mol−1 (see Table 2). Good agreement at a similar level was obtained for other di- and tri-alkyl substituted alkylbenzenes, which are summarized in Table 1, Table 2 and Table 3.
3.2. Consistency of Phase Transition Enthalpies of Alkylbenzenes
Some of alkylbenzenes, 1,2,4,5-tetra-iso-propylbenzene, 1,4-di-tert-butylbenzene, 1-methyl-3,5-di-tert-butylbenzene and 1,3,5-tri- tert-butylbenzene are solids at room temperature and their enthalpies of fusion, (Tfus), are known from the literature. The enthalpies of fusion of 1,4-di-tert-butylbenzene were reported several times and agree well (see Table 4). To provide more confidence, weighted average values of (Tfus) were calculated using experimental uncertainty as a weighting factor.
The thermodynamics of their solid–liquid, liquid–gas and solid–gas phase transitions can be easily reconciled and validated using the following fundamental relationship:
Since the thermochemical calculations are commonly carried out at the reference temperature T = 298.15 K, the enthalpies of fusion have to be adjusted to this temperature. The adjustment of (Tfus) was performed with the help of the equation [41]:
where and were taken from Table S2. With this adjustment, the molar enthalpies of fusion, (298.15 K), for four alkylbenzenes were calculated (see Table 4). Uncertainties in the temperature adjustment of fusion enthalpy from Tfus to the reference temperature were estimated to account for 30 % of the total adjustment [42].

Table 4.
Phase transition thermodynamics of alkylbenzenes (in kJ⋅mol−1) a.
Table 4.
Phase transition thermodynamics of alkylbenzenes (in kJ⋅mol−1) a.
| Compounds | Tfus, K | |||||
|---|---|---|---|---|---|---|
| WC b | 298.15 K | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 1,2,4,5-tetra-iso-propylbenzene | 393.0 | 19.6 ± 0.5 [27] | 49.9 | 18.8 ± 0.6 | 68.3 ± 1.6 f | 87.1 ± 1.7 |
| 1,4-di-tert-butylbezene | 350.8 | 22.5 ± 0.4 [34] | ||||
| 350.5 | 21.8 ± 0.2 [10] | |||||
| 350.5 | 22.6 ± 0.1 [36] | |||||
| 22.4 ± 0.1f | 63.9 | 21.2 ± 0.4 | 60.5 ± 0.5 | 81.7 ± 0.6 | ||
| 1-methyl-3,5-di-tert-butylbezene | 307.6 | 18.4 ± 0.2 [10] | 59.8 | 18.2 ± 0.3 | 62.5 ± 0.5 | 80.7 ± 0.6 |
| 1,3,5-tri-tert-butylbezene | 343.2 | 11.9 ± 0.2 [10] | 34.7 | 10.9 ± 0.4 | 69.0 ± 0.7 | 79.9 ± 0.8 |
| 52.1 ± 6.5f |
a Uncertainties are presented as expanded uncertainties (0.95 level of confidence with k = 2); b The Walden’s Constant (WC) [43,44] calculated according to Equation (S9) in J·mol−1·K−1 (for details see ESI); c The experimental enthalpies of fusion measured at Tfus were adjusted to T = 298.15 K with the help of Equation (7); d The values of vaporisation enthalpies evaluated in Table 1 and Table 2 (given in bold); e Calculated according to the equation: (298.15 K) = (298.15 K) + (298.15 K) as the sum of columns 5 and 6 in this table f Average value.
Apparently, the vaporisation enthalpies of alkylbenzenes have been measured more frequently (see Table 1 and Table 2) than the sublimation enthalpies (see Table 3). It therefore makes sense to derive additional sublimation enthalpies according to Equation (6) using the evaluated (298.15 K)-values from Table 1 and Table 2. The sublimation enthalpies calculated in this way are denoted by PhT (calculated as the difference in Phase Transitions) in Table 4. As it can be seen in Table 4, these values for all three compounds are hardly distinguishable from experimental results, proving consistency of the solid–liquid, solid–gas and liquid–gas phase-transition enthalpies evaluated in the current study.
3.3. Validation of Vaporisation Enthalpies Using Structure–Property Relationships
Examination of the data compiled in Table 1 and Table 2 shows that for 1,2-di-iso-propyl-benzene, 1,2,4,5-tetra-iso-propyl-benzene and 2-methyl-, 3-methyl- and 4 -methyl-tert-butyl-benzene, the experimental vaporisation enthalpies are only known from a single source. Their vaporisation enthalpies are not necessarily questionable, but it is important to consider how these data correlate with data known for other members of the corresponding series. Therefore, different structure–property correlations are usually used to check internal data consistency within the series of structurally similar compounds. In addition, such correlations allow the prediction of the missing data for compounds that have not yet been studied.
3.3.1. Empirical Correlations: Vaporisation Enthalpies versus Kovats Retention Indices
The Kovats index, Jx, represents the retention time of a compound in the gas-chromatographic (GC) column [45]. The retention time reflects the intensity of absorption/desorption of the molecule in the stationary phase. Since the retention times of structurally related molecules are measured under identical experimental conditions (stationary phase, temperature), the Jx-values model the relative differences in the intermolecular interactions of the pure compounds, and the Jx-values are indirectly related to the enthalpies of vaporization. As a rule, the vaporisation enthalpies (298.15 K) and Kovats indices correlate linearly within the series of structurally similar compounds [11]. Kovats retention indices were taken from the original literature [46,47,48,49]. To increase reliability, it is better to correlate the vaporisation enthalpies of alkylbenzenes with the Jx-values measured on a specific GC column. We did not find a comprehensive Jx-set containing all the alkylbenzenes of interest for this work; instead we used a few sets of Kovats indices containing the required molecules. For example, Kovats indices of different iso-propyl- and tert-butyl-substituted benzenes were measured on the non-polar column OV-101 [46]. Results for correlations of experimental enthalpies of vaporisation versus Kovats retention indices on this column are shown in Table 5.

Table 5.
Correlation of the vaporisation enthalpies, (298.15 K), of alkyl-substituted benzenes with their Kovats indices on (Jx) on OV-101.
Table 5.
Correlation of the vaporisation enthalpies, (298.15 K), of alkyl-substituted benzenes with their Kovats indices on (Jx) on OV-101.
| Jxa | Δ d | |||
|---|---|---|---|---|
| Compound | kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | |
| iso-propylbenzene | 920 | 45.2 | 44.7 | 0.5 |
| 2-Me-iso-propylbenzene | 1032 | 49.3 | 50.0 | −0.7 |
| 3-Me-iso-propylbenzene | 1012 | 49.4 | 49.1 | 0.3 |
| 4-Me-iso-propylbenzene | 1018 | 49.6 | 49.4 | 0.2 |
| 1,2-di-iso-propylbenzene | 1152 | - | 55.8 | - |
| 1,3-di-iso-propylbenzene | 1143 | - | 55.4 | - |
| 1,4-di-iso-propylbenzene | 1162 | 56.2 | 56.3 | −0.1 |
| 1,3,5-tri-iso-propylbenzene | 1325 | - | 64.1 | - |
| tert-butylbenzene | 988 | 47.5 | 47.9 | −0.4 |
| tert-amylbenzene | 1086 | 52.3 | 52.6 | −0.3 |
| 3-Me-tert-butylbenzene | 1062 | - | 51.5 | - |
| 4-Me-tert-butylbenzene | 1085 | 52.2 | 52.6 | −0.4 |
| 3,5-di-Me-1-tert-butylbenzene | 1152 | 56.6 | 55.8 | 0.8 |
a Kovats indices, Jx, on the standard non-polar column OV-101 [46]; b Evaluated experimental data (given in bold in Table 1 and Table 2); c Calculated using Equation (7) with the assessed uncertainty of ±1.0 kJ·mol−1 (expanded uncertainty 0.95 level of confidence); d Difference between columns 3 and 4 in this table.
The vaporisation enthalpies, (298.15 K), and Kovats indices, Jx, on OV-101 correlate according to the following equation:
The ‘empirical’ vaporisation enthalpies of alkylbenzenes according to Equation (8) are denoted as Jx in Table 1 and Table 2 and agree well with the experimental values obtained with conventional experimental methods. Similarly, the retention indices of alkylbenzenes on GC columns containing squalane (see Table S5), SPB-5 (see Table S6) and CP-SIL (see Table S7) were correlated with experimental enthalpies of vaporisation and successfully compared with experimental values obtained with conventional methods.
Such a good agreement can be considered evidence of good consistency of the experimental data included in correlations. The results given in Table 5 and Tables S5–S7 indicate that differences between experimental vaporisation enthalpies and “empirical” values are mostly below 1.0 kJ·mol−1. Therefore, the uncertainties in the enthalpies of vaporisation, which are estimated from the empirical correlation of (298.15 K) with the Kovats indices, are evaluated with an uncertainty of ±1.0 kJ·mol−1.
3.3.2. Empirical Correlations: Vaporisation Enthalpies versus Solution Enthalpies
Another empirical method based on the GC technique is to correlate enthalpies (298.15 K) with enthalpies of solution, Hsol, of a compound of interest in the GC stationary phase. The measurable value in this method is also the retention time and the enthalpy of solution results from the temperature dependence of the retention times [50,51]. We found a comprehensive compilation of the experimental solution enthalpies in the non-polar stationary phase OV-101 [52] and used these data (see Table 6) in this work to correlate with the vaporisation enthalpies (298.15 K).

Table 6.
Correlation of the vaporisation enthalpies, (298.15 K), of alkyl-substituted benzenes with their solution enthalpies (Hsol).
Table 6.
Correlation of the vaporisation enthalpies, (298.15 K), of alkyl-substituted benzenes with their solution enthalpies (Hsol).
| Hsola | [20] | Δ c | ||
|---|---|---|---|---|
| Compound | kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | |
| benzene | 27.2 | 33.9 | 34.2 | −0.3 |
| toluene | 31.2 | 38.1 | 37.8 | 0.3 |
| ethyl-benzene | 36.4 | 42.3 | 42.5 | −0.2 |
| n-propyl-benzene | 40.6 | 46.2 | 46.3 | −0.1 |
| n-butyl-benzene | 47.0 | 51.4 | 52.1 | −0.7 |
| tert-butyl-benzene | 41.3 | 47.5 d | 46.9 | 0.6 |
| 1,2-dimethyl-benzene | 36.3 | 43.5 | 42.4 | 1.1 |
| 1,3-dimethy-benzene | 37.0 | 42.4 | 43.0 | −0.6 |
| 1,4-dimethyl-benzene | 37.8 | 42.7 | 43.8 | −1.1 |
| 1,3,5-trimethyl-benzene | 41.4 | 47.5 | 47.0 | 0.5 |
| 2-Me-iso-propyl-benzene | 42.7 | 48.2 | ||
| 3-Me-iso-propyl-benzene | 43.8 | 49.2 | ||
| 4-Me-iso-propyl-benzene | 44.7 | 50.0 | ||
| 1,2-di-iso-propyl-benzene | 50.0 | 54.8 | ||
| 1,3-di-iso-propyl-benzene | 51.1 | 55.8 | ||
| 1,4-di-iso-propyl-benzene | 51.3 | 56.2 d | 56.0 | 0.2 |
| 1-iso-propyl-2-tert-butyl-benzene | 52.9 | 57.4 | ||
| 1-iso-propyl-3-tert-butyl-benzene | 53.1 | 57.6 | ||
| 1-iso-propyl-4-tert-butyl-benzene | 53.3 | 57.8 | ||
| 1,3-di-iso-propyl-benzene | 54.4 | 58.8 | ||
| 1,4-di-iso-propyl-benzene | 56.4 | 60.5 d | 60.6 | −0.1 |
| 1,3,5-trii-iso-propyl-benzene | 61.8 | 65.5 | ||
| 1,2,4-tri-iso-propyl-benzene | 61.0 | 64.7 | ||
| 1,2,3-tri-iso-propyl-benzene | 59.5 | 63.4 |
a Solution enthalpies, Hsol, (in kJ·mol−1) of solutes in the non-polar stationary phase OV-101 [52]; b Calculated using Equation (9) with the assessed uncertainty of ±1.0 kJ·mol−1 (expanded uncertainty 0.95 level of confidence); c Difference between columns 3 and 4 in this table; d Evaluated experimental data (given in bold in Table 1).
The vaporisation enthalpies, (298.15 K), and solution enthalpies, Hsol, on OV-101 correlate according to the following equation:
3.3.3. Empirical Correlations: Vaporisation Enthalpies versus Solvent-Accessible Surfaces
The vaporisation enthalpies for the most interesting extremely strained di-tert-butyl-, tri-tert-butyl- and tetra-tert-butylbenzenes were estimated using the correlation of vaporisation enthalpies versus solvent-accessible surfaces, Sx. The solvent-accessible surface area is the surface area of a molecule calculated using the “rolling ball” algorithm suggested by Connolly [53]. This algorithm uses a sphere of a suitable radius to develop the surface, Sx, of the molecule. In our earlier work, we propose Sx as a measure of the intermolecular forces related to the enthalpy of vaporisation [54]. As a test, these solvent-accessible surface areas were calculated with the Connolly program [55] for a range of aromatics and alkyl-substituted aromatics using a 250 pm probe sphere. A good linear correlation between (298.15 K) and Sx was found. In this work, we calculated the surface areas of di-tert-butyl, tri-tert-butyl, and tetra-tert-butylbenzenes (see Table 7) using the same algorithm.

Table 7.
Correlation of the vaporisation enthalpies, (298.15 K), of tert-butyl-substituted benzenes with their solvent-accessible surfaces (Sx).
Table 7.
Correlation of the vaporisation enthalpies, (298.15 K), of tert-butyl-substituted benzenes with their solvent-accessible surfaces (Sx).
| Sxa | Δ d | |||
|---|---|---|---|---|
| Compound | kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | |
| tert-butyl-benzene | 153.0 | 47.5 | 48.1 | −0.6 |
| 1,2-di-tert-butylbenzene | 201.6 | 56.6 | - | |
| 1,3-di-tert-butylbenzene | 214.9 | 59.0 | 59.0 | 0.0 |
| 1,4-di-tert-butylbenzene | 216.9 | 60.5 | 59.3 | 1.2 |
| 1,3,5-tri-tert-butylbenzene | 276.0 | 69.0 | 69.7 | −0.7 |
| 1,2,4-tri-tert-butylbenzene | 264.7 | 67.7 | - | |
| 1,2,3-tri-tert-butylbenzene | 248.2 | 64.8 | ||
| 1,2,4,5-tetra-tert-butylbenzene | 298.6 | 73.6 | - |
a Solvent-accessible surfaces, Sx, calculated using the Connolly method [53,55]; b Evaluated experimental data (given in bold in Table 2); c Calculated using Equation (10): (298.15 K)/(kJ·mol−1) = 21.3 + 0.1752 × Sx with (R2 = 0.991), with the assessed uncertainty of ±1.5 kJ·mol−1 (expanded uncertainty 0.95 level of confidence); d Difference between columns 3 and 4 in this table.
The vaporisation enthalpies, (298.15 K), and surfaces, Sx, correlate according to the following equation:
The ‘empirical’ vaporisation enthalpies of alkylbenzenes according to Equation (10) are denoted as Sx in Table 2. The vaporisation enthalpies of 1,3-di-tert-butyl-benzene, 1,4-di-tert-butyl-benzene, and 1,3,5-tri-tert-butyl-benzene estimated with Equation (10) are indistinguishable from the experimental values obtained using conventional experimental methods, providing confidence in the estimates for 1,2-di-tert-butylbenzene, 1,2,4-tri-tert-butylbenzene, 1,2,3-tri-tert-butylbenzene and 1,2,4,5-tetra-tert-butylbenzene (see Table 7).
3.3.4. Experimental Enthalpies of Formation of Iso-Propyl- and Tert-Butyl-Benzenes
The thermochemistry of iso-propyl- and tert-butyl-benzenes is a long-standing interest of our laboratory [9,10,11,12]. The available data on the condensed state enthalpies of formation are compiled in Table 8.

Table 8.
Thermochemical data for iso-propyl- and tert-butyl-benzenes at T = 298.15 K (p°= 0.1 MPa, in kJ·mol−1) a.
Most of the entries in Table 8 were measured in our laboratory using combustion calorimetry and we took this opportunity to update the uncertainties of the enthalpies of formation reported in our previous work [9,10] following Olofsson’s [58] recommendation. These recalculated values are now given in Table S8. Furthermore, for this work, we re-evaluated the liquid-phase enthalpies of formation derived from chemical equilibrium studies reported in literature [10,59]. The reactions and the liquid-phase reaction enthalpies, (liq), are given in Table 9.

Table 9.
Results of chemical equilibrium studies of interconversions of alkylbenzenes at T = 298.15 K (p° = 0.1 MPa, in kJ·mol−1) a.
These reaction enthalpies were used to re-calculate the (liq)-values of 1,4-di-iso-propyl-benzene (from R1), 3-methyl-tert-butyl-benzene (from R2), 1,3-di-tert-butyl-benzene (from R3), 1,3-di-tert-butyl-benzene (from R4) and 1,3,5-tri-tert-butyl-benzene (from R5). The re-calculated data are given in Tables S9–S11. The re-evaluated and updated experimental data on (cr, liq) of iso-propyl- and tert-butyl-benzenes were used together with the vaporisation and sublimation enthalpies evaluated in Table 1, Table 2 and Table 3 to calculate the experimental gas-phase enthalpies of formation of alkylbenzenes (see Table 8 column 4). These values will be utilised for validation of quantum-chemical methods as follows.
3.3.5. Quantum Chemistry: Theoretical Enthalpies of Formation of Iso-Propyl- and Tert-Butyl-Benzenes
Since experimental data are not available for all alkylbenzenes of interest for this work, especially not for crowded tert-butylbenzenes, they were calculated using quantum chemistry (QC). Are the high-level methods G3MP2 and G4 good enough to accurately calculate the gas-phase formation enthalpies of large molecules such as tri- and tetra-tert-butylbenzenes? It is common practice to test QC methods against a set of reliable experimental data. There are fourteen reliable gas-phase formation enthalpies of di- and tri-alkyl substituted benzenes (see Table 8) that might help answer this question.
This data set was utilized to validate the results of the composite methods G3MP2 and G4. The most stable conformers for each alkylbenzene were found using a computer code named CREST (conformer-rotamer ensemble sampling tool) [60] and optimised with the B3LYP/6-31g(d,p) method [61]. The energies E0 and the enthalpies H298 of the most stable conformers were calculated using the G3MP2 and G4 methods implemented in the Gaussian 16 Software.
The enthalpies, H298, from the output files were converted into the theoretical gas-phase enthalpies of formation of alkylbenzenes using an atomisation (AT) reaction [62], as well as using the well-balanced reactions (WBR) given in Table S12. The comparison of the G3MP2 results from the atomisation reaction with the experimental results is given in Table S13. The comparison of the G4 results from the atomisation reaction with the experimental results is given in Table S14.
It was found that the enthalpies of formation calculated using AT are slightly but systematically more negative for both methods. Such a feature of AT reactions is known [63], but this disadvantage can be easily removed by correcting the calculated results. The latter correction was derived from the correlation between experimental enthalpies of formation and the (g, AT)-values directly calculated via atomisation reaction. The following correction equations were derived:
The theoretical (g)theor-values of alkylbenzenes “corrected” in this way for both methods agree well with the values derived from the well-balanced reactions given in Table S12. For the sake of brevity, all quantum chemical results are summarized in Table 10.

Table 10.
Calculation of the theoretical gas-phase enthalpies of alkylbenzenes at T = 298.15 K (p° = 0.1 MPa, in kJ·mol−1) a.
Since the results from the G3MP2 and G4 methods agree well, the theoretical average values, (g)theor, were calculated in Table 10, column 6 for all alkylbenzenes and these values were used for comparison with the experimental results in Table 8, column 5. As can be seen from Table 8, the theoretical results agree, within experimental uncertainties, with the reliable experimental enthalpies of formation. Such good agreement could convince that the QC results for the tri- and tetra-tert-butylbenzenes can be considered reliable. However, from our experience, the QC results for large molecules with 20–25 “heavy” atoms should be viewed with caution. For this reason we decided to examine the applicability of the QC results for 1,2-di-tert-butylbenzene, 1,2,4-tri-tert-butylbenzene and 1,2,4,5-tetra-tert-butylbenzene, where the experimental enthalpies of formation in the condensed state are available from the literature [57,64,65]. The algorithm of validation is given in Table 11.

Table 11.
Calculation of the crystal-phase enthalpies of formation of tert-butyl-benzenes at T = 298.15 K (in kJ.mol−1) a.
We have taken the theoretical gas-phase enthalpies of formation of these three crowded tert-butylbenzenes from Table 10, column 6. Their vaporisation enthalpies were evaluated in Table 2. Their enthalpies of fusion were estimated using Walden’s rule in Table S15. The sum of the latter values resulted in the required sublimation enthalpies (see Table 11, column 3). The difference between columns 2 and 3 in this table provides the theoretical (cr)theor-values, which are compared to the available experimental (cr)exp data in column 5. From Table 11 it can be seen that the agreement for 1,2-di-tert-butylbenzene and 1,2,4,5-tetra-tert-butylbenzene is good. The agreement for 1,2,4-tri-tert-butylbenzene is quite fair and could be considered acceptable as no details on combustion experiments and purity of the sample were reported [64]. This additional validation provides confidence that the QC results for the di-, tri- and tetra-tert-alkylbenzenes can be considered reliable.
3.3.6. Liquid-Phase Enthalpies of Formation and Thermodynamic Analysis of the Hydrogenation/Dehydrogenation of the Alkylbenzene-Based LOHC Systems
Having established a consistent data sets for the gas-phase enthalpies of formation and vaporisation enthalpies for alkylbenzenes, reliable liquid-phase enthalpies of formation for di-, tri-, and tetra-substituted iso-propyl- and tert-butyl-benzenes can now be derived using Equation (2). These results can be utilized to calculate the enthalpies of hydrogenation for reactions given in Figure 1 and for other alkylbenzenes listed in Table 12.

Table 12.
Calculation of the liquid-phase enthalpies of formation, (liq), of the hydrogen-lean (HL) and hydrogen-reach (HR) counterparts of the LOHC systems based on alkylbenzenes (at T = 298.15 K, p° = 0.1 MPa, in kJ·mol−1) a.
The liquid-phase enthalpies of formation, (liq), of the hydrogen-lean (HL) compounds given in Table 12 were calculated as shown in Table S16. The gas-phase enthalpies of formation of the hydrogen-reach (HR) counterparts of the LOHC systems were calculated using the G3MP2 method (for details see Ref. [6]) and the results are given in Table S17. The vaporisation enthalpies of the alkyl-cyclohexanes related to this work are also given in Table S16 and the resulting liquid-phase enthalpies of formation of the hydrogen-rich compounds are listed in Table S16, column 7. The final (liq)-values for the HL and HR compounds are summarized in Table 12 and were used to calculate the reaction enthalpies according to Hess’s Law (see Table 12, column 4). The enthalpies of reaction per mole of hydrogen are given in Table 12, column 5 and help to analyse the influence of structure and degree of crowding on the hydrogenation enthalpies of alkylbenzenes.
The results of this study on the enthalpies of hydrogenation of branched alkylbenzenes turned out to be less spectacular than expected. As can be seen from Table 12, the /H2-values for all iso-propyl-substituted benzenes are at the level of −64 to −66 kJ mol−1/(H2), which is hardly distinguishable within experimental uncertainties. The only exception is evident for 1,3,5-tri-iso-propyl-benzene with (298.15 K) = −59.1 kJ mol−1/(H2).
An opposite trend than expected was observed for the tert-butyl-substituted alkylbenzenes. Indeed, for the crowded 1,2-di-tert-butyl-, 1,2,4-tri-tert-butyl-, 1,2,3-tri-tert-butyl- and 1,2,4,5-tetra-tert-butyl-benzenes, the enthalpy of hydrogenation increased dramatically up to -85 kJ mol−1/(H2), as shown in Table 12. Interestingly, the reaction enthalpy of 1-iso-propyl-2-tert-butyl-benzene does not follow this trend and exhibits an anomalously low value of -61.4 kJ mol−1/(H2).
The reaction enthalpies for the methyl-tert-butyl- and di-tert-butyl-benzenes are very close at the common level of −64 to −66 kJ mol−1/(H2). However, a positive finding of this study is that 1,3,5-tri-substituted alkylbenzenes (except for 1,3,5-tri-methyl-benzene) exhibit remarkably low hydrogenation enthalpies at the level of -60 kJ mol−1/(H2) as shown in bold in Table 12. The findings of this study indicate that the low absolute values for enthalpy of reaction for crowded aromatic rings, which could be expected from data published so far, cannot be backed in a comprehensive and consistent thermochemical study. As a consequence, it should not be expected that alkylation will lead to a strong decrease in heat demand for hydrogen release. However, a slight decrease in the enthalpy of dehydrogenation due to alkylation can be observed. The reason for this is most likely the lowest possible strain of the perhydrogenated 1,3,5-trisubstituted alkyl-cyclohexanes as a hydrogen-rich LOHC compound. Hence, such a low enthalpy of reaction could significantly improve the reaction thermodynamics and enable lower dehydrogenation temperatures in such 1,3,5-trialkyl-substituted LOHC systems.
4. Conclusions
This work systematically evaluated the thermochemical properties of multi-alkylated aromatic components of LOHCs. Values for enthalpies of reactions for the (de)hydrogenation of these compounds calculated from different references in the literature indicated that crowded alkylbenzenes could be beneficial in terms of hydrogen release. However, the comprehensive study conducted in this work reveals that the positive effect on reaction enthalpy of dehydrogenation is comparatively weak. The results confirm that a lower enthalpy of reaction is actually achieved with crowded alkylbenzenes, but it is most likely not sufficient to reach a significant decrease in temperature for hydrogen release.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13020953/s1, Transpiration method; Table S1: Results of transpiration method: absolute vapor pressures p, standard molar vaporisation/sublimation enthalpies and standard molar vaporisation/sublimation entropies; Table S2: Compilation of data on molar heat capacities (cr or liq) and heat capacity differences at T = 298.15 K (in J.K−1.mol−1); Table S3: Compilation of data on molar heat capacities (liq) and heat capacity differences at T = 298.15 K (in J.K−1.mol−1); Adjustment of vaporisation/sublimation enthalpies to the reference temperature T = 298.18 K; Table S4: Vapor pressures, pi, at different temperatures compiled from the literature, standard molar vaporisation enthalpies, , and standard molar vaporisation entropies, ; Empirical correlations: vaporisation enthalpies versus Kovats retention indices; Table S5: Correlation of the vaporisation enthalpies, (298.15 K), of alkyl-substituted benzenes with their Kovats indices (Jx) on Squalane; Table S6: Correlation of the vaporisation enthalpies, (298.15 K), of alkyl-substituted benzenes with their Kovats indices (Jx) on SPB-5; Table S7: Correlation of the vaporisation enthalpies, (298.15 K), of alkyl-substituted benzenes with their Kovats’s indices (Jx) on CP-SIL; Table S8: Enthalpies of combustion and enthalpies of formation of alkyl-substituted benzenes reported in our earlier work and their uncertainties re-calculated in this paper (at T = 298.15 K (p° = 0.1 MPa, in kJ·mol−1); Table S9: Thermochemical data for alkyl-substituted biphenyls at T = 298.15 K (p° = 0.1 MPa, in kJ·mol−1); Table S10: Thermochemical data for alkyl-substituted biphenyls at T = 298.15 K (p° = 0.1 MPa, in kJ·mol−1); Table S11: Thermochemical data for alkyl-substituted biphenyls at T = 298.15 K (p° = 0.1 MPa, in kJ·mol−1); Table S12: Calculation of the quantum-chemical gas-phase enthalpies of formation using the well-balance reactions (WBR) at T = 298.15 K (in kJ.mol−1); Table S13: Calculation of the G3MP2 corrected gas-phase enthalpies of alkyl-benzenes at T = 298.15 K (in kJ.mol−1); Table S14: Calculation of the G4 corrected gas-phase enthalpies of alkyl-benzenes at T = 298.15 K (in kJ.mol−1); Estimation of fusion enthalpies according to the Walden’s Rule; Table S15: Calculation of the sublimation enthalpies, (298.15 K), of tert-butyl-substituted benzenes in kJ·mol−1); Table S16: Calculation of the liquid phase enthalpies of formation, (liq), of the hydrogen-lean (HL) and hydrogen-reach (HR) counterparts of the LOHC systems based on alkyl-benzenes (at T = 298.15 K, p° = 0.1 MPa, in kJ·mol−1); Table S17: Calculation of the G3MP2 corrected gas-phase enthalpies of formation of alkyl-cyclohexanes at T = 298.15 K (in kJ.mol−1); Table S18: Correlation of vaporisation enthalpies, (298.15 K), of alkyl-cyclohexanes with their normal boiling temperatures (Tb). [6,9,10,11,12,16,18,19,20,27,29,30,31,34,36,40,43,44,45,47,48,49,56,57,64,66,67,68,69,70,71,72,73,74,75,76,77,78].
Author Contributions
Conceptualization, S.P.V.; methodology, S.P.V., P.W. and K.M.; software, A.A.S. and V.V.T.; validation, S.V.V., V.V.T. and S.P.V.; formal analysis, P.W., K.M. and S.P.V.; investigation, S.V.V.; resources, P.W. and K.M.; data curation, P.W., K.M. and S.P.V.; writing—original draft prepara-tion, S.P.V., P.W. and K.M.; writing—review and editing, S.P.V. and V.V.T.; visualization, S.P.V. and A.A.S.; supervision, S.P.V.; project administration, S.P.V.; funding acquisition, S.P.V. All authors have read and agreed to the published version of the manuscript.
Funding
S.P.V. acknowledges financial support from the German Science Foundation in the frame of SPP 1807 “Control of London Dispersion Interactions in Molecular Chemistry”, grant VE 265-9/2. This paper has been supported by the Kazan Federal University Strategic Academic Leadership Program (“PRIORITY-2030”). The work was supported by the Ministry of Science and Higher Education of the Russian Federation (theme No. AAAA- A12-1111100072-9) as part of the state task of the Samara State Technical University (creation of new youth laboratories). A.A.S acknowledges gratefully a research scholarship from the DAAD (Deutscher Akademischer Austauschdienst).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data used in this paper are given in the main text or in the electronic Supplementary Materials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen Storage Technologies for Future Energy Systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Stark, K.; Emelyanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Benzyl- and Dibenzyl-toluene Derivatives. Ind. Eng. Chem. Res. 2015, 54, 7967–7976. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Safronov, S.P.; Samarov, A.A.; Vostrikov, S.V. Hydrogen storage: Thermodynamic analysis of alkyl-quinolines and alkyl-pyridines as potential liquid organic hydrogen carriers (LOHC). Appl. Sci. 2021, 11, 11758. [Google Scholar] [CrossRef]
- Konnova, M.E.; Li, S.; Bösmann, A.; Müller, K.; Wasserscheid, P.; Andreeva, I.V.; Turovtzev, V.V.; Zaitsau, D.H.; Pimerzin, A.A.; Verevkin, S.P. Thermochemical Properties and Dehydrogenation Thermodynamics of Indole Derivates. Ind. Eng. Chem. Res. 2020, 59, 20539–20550. [Google Scholar] [CrossRef]
- Müller, K.; Skeledzic, T.; Wasserscheid, P. Strategies for Low-Temperature Liquid Organic Hydrogen Carrier Dehydrogenation. Energy Fuels 2021, 35, 10929–10936. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Samarov, A.A.; Vostrikov, S.V.; Wasserscheid, P.; Müller, K. Comprehensive thermodynamic study of alkyl-cyclohexanes as Liquid Organic Hydrogen Carriers motifs. Hydrogen 2022. submitted. [Google Scholar] [CrossRef]
- Luyben, W.L. Design and Control of the Cumene Process. Ind. Eng. Chem. Res. 2010, 49, 719–734. [Google Scholar] [CrossRef]
- Kostrab, G.; Mravec, D.; Bajus, M.; Janotka, I.; Sugi, Y.; Cho, S.J.; Kim, J.H. tert-Butylation of toluene over mordenite and cerium-modified mordenite catalysts. Appl. Catal. A Gen. 2006, 299, 122–130. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemical properties of iso-propylbenzenes. Thermochim. Acta 1998, 316, 131–136. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemical properties of branched alkylsubstituted benzenes. J. Chem. Thermodyn. 1998, 30, 1029–1040. [Google Scholar] [CrossRef]
- Verevkin, S.P. Vapour pressures and enthalpies of vaporization of a series of the linear n-alkyl-benzenes. J. Chem. Thermodyn. 2006, 38, 1111–1123. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Kozlova, S.A.; Emel’yanenko, V.N.; Goodrich, P.; Hardacre, C. Thermochemistry of Ionic Liquid-Catalyzed Reactions. Experimental and Theoretical Study of Chemical Equilibria of Isomerization and Transalkylation of tert-Butylbenzenes. J. Phys. Chem. A 2008, 112, 11273–11282. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Schick, C.; Heym, F. Development of Direct and Indirect Methods for the Determination of Vaporization Enthalpies of Extremely Low-Volatile Compounds. In Handbook of Thermal Analysis and Calorimetry; Elsevier Science B.V: Amsterdam, The Netherlands, 2018; Volume 6, pp. 1–46. [Google Scholar]
- Verevkin, S.P. Gibbs Energy and Helmholtz Energy: Liquids, Solutions and Vapours; Wilhelm, E., Letcher, T.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2021; ISBN 978-1-83916-201-5. [Google Scholar]
- Kulikov, D.; Verevkin, S.P.; Heintz, A. Determination of Vapor Pressures and Vaporization Enthalpies of the Aliphatic Branched C 5 and C 6 Alcohols. J. Chem. Eng. Data 2001, 46, 1593–1600. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N. Transpiration method: Vapor pressures and enthalpies of vaporization of some low-boiling esters. Fluid Phase Equilib. 2008, 266, 64–75. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K.; Rassolov, V.; Pople, J.A. Gaussian-3 theory using reduced Mo/ller-Plesset order. J. Chem. Phys. 1999, 110, 4703–4709. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 theory. J. Chem. Phys. 2007, 126, 084108. [Google Scholar] [CrossRef]
- Majer, V.; Svoboda, V. Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation; Blackwell Scientific Publications: Oxford, UK, 1985. [Google Scholar]
- McDonald, R.A.; Shrader, S.A.; Stull, D.R. Vapor Pressures and Freezing Points of Thirty Pure Organic Compounds. J. Chem. Eng. Data 1959, 4, 311–313. [Google Scholar] [CrossRef]
- Reid, R.C. Handbook on vapor pressure and heats of vaporization of hydrocarbons and related compounds. AIChE J. 1972, 18, 1278. [Google Scholar] [CrossRef]
- Růẑička, V.; Zábranský, M.; Růẑička, K.; Majer, V. Vapor pressures for a group of high-boiling alkylbenzenes under environmental conditions. Thermochim. Acta 1994, 245, 121–144. [Google Scholar] [CrossRef]
- Hoskovec, M.; Grygarová, D.; Cvačka, J.; Streinz, L.; Zima, J.; Verevkin, S.P.; Koutek, B. Determining the vapour pressures of plant volatiles from gas chromatographic retention data. J. Chromatogr. A 2005, 1083, 161–172. [Google Scholar] [CrossRef]
- Melpolder, F.W.; Woodbridge, J.E.; Headington, C.E. The Isolation and Physical Properties of the Diisopropylbenzenes. J. Am. Chem. Soc. 1948, 70, 935–939. [Google Scholar] [CrossRef]
- Myers, H.S.; Fenske, M.R. Measurement and Correlation of Vapor Pressure Data for High Boiling Hydrocarbons. Ind. Eng. Chem. 1955, 47, 1652–1658. [Google Scholar] [CrossRef]
- Steele, W.V.; Chirico, R.D.; Cowell, A.B.; Knipmeyer, S.E.; Nguyen, A. Thermodynamic Properties and Ideal-Gas Enthalpies of Formation for 1,4-Diisopropylbenzene, 1,2,4,5-Tetraisopropylbenzene, Cyclohexanone Oxime, Dimethyl Malonate, Glutaric Acid, and Pimelic Acid. J. Chem. Eng. Data 2002, 47, 725–739. [Google Scholar] [CrossRef]
- VonNiederhausern, D.M.; Wilson, G.M.; Giles, N.F. Critical Point and Vapor Pressure Measurements for 17 Compounds by a Low Residence Time Flow Method. J. Chem. Eng. Data 2006, 51, 1990–1995. [Google Scholar] [CrossRef]
- Kasehgari, H.; Mokbel, I.; Viton, C.; Jose, J. Vapor pressure of 11 alkylbenzenes in the range 10-3—280 torr, correlation by equation of state. Fluid Phase Equilib. 1993, 87, 133–152. [Google Scholar] [CrossRef]
- SciFinder—Chemical Abstracts Service. Available online: http://scifinder.cas.org/ (accessed on 5 December 2022).
- Mokbel, I.; Rauzy, E.; Meille, J.P.; Jose, J. Low vapor pressures of 12 aromatic hydrocarbons. Experimental and calculated data using a group contribution method. Fluid Phase Equilib. 1998, 147, 271–284. [Google Scholar] [CrossRef]
- Stephenson, R.M.; Malanowski, S. Handbook of the Thermodynamics of Organic Compounds; Springer: Dordrecht, The Netherlands, 1987; ISBN 978-94-010-7923-5. [Google Scholar]
- Nazmutdinov, A.G.; Nesterov, I.A.; Nazmutdinov, T.A.; Nesterova, T.N. Investigation and prediction of vapor pressure of alkylbenzenes. Izv. Samar. Nauchnogo Tsentra RAN 2003, 8, 89–96. [Google Scholar]
- Steele, W.V.; Chirico, R.D.; Knipmeyer, S.E.; Nguyen, A. Vapor Pressure, Heat Capacity, and Density along the Saturation Line, Measurements for Cyclohexanol, 2-Cyclohexen-1-one, 1,2-Dichloropropane, 1,4-Di-tert-butylbenzene, (±)-2-Ethylhexanoic Acid, 2-(Methylamino)ethanol, Perfluoro-n-heptane, and Sulfolan. J. Chem. Eng. Data 1997, 42, 1021–1036. [Google Scholar] [CrossRef]
- Nesterov, I.A.; Nesterova, T.N.; Nazmutdinov, A.G.; Novozhenina, T.P. Study and prediction of alkylbenzenes’ vapour pressures. Fluid Phase Equilib. 2008, 269, 36–47. [Google Scholar] [CrossRef]
- Chirico, R.D.; Steele, W.V. Thermodynamic properties of tert-butylbenzene and 1,4-di-tert-butylbenzene. J. Chem. Thermodyn. 2009, 41, 392–401. [Google Scholar] [CrossRef]
- Hopke, E.R.; Sears, G.W. Vapor Pressures below 1 mm Hg of Several Aromatic Compounds. J. Chem. Phys. 1951, 19, 1345–1351. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Nagrimanov, R.N.; Varfolomeev, M.A.; Buzyurov, A.V.; Mukhametzyanov, T.A. Enthalpies of fusion and enthalpies of solvation of aromatic hydrocarbons derivatives: Estimation of sublimation enthalpies at 298.15 K. Thermochim. Acta 2016, 627–629, 77–82. [Google Scholar] [CrossRef]
- Davies, M.; Kybett, B. Sublimation and vaporization heats of long-chain alcohols. Trans. Faraday Soc. 1965, 61, 1608. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hosseini, S.; Hesse, D.G.; Liebman, J.F. Heat capacity corrections to a standard state: A comparison of new and some literature methods for organic liquids and solids. Struct. Chem. 1993, 4, 271–278. [Google Scholar] [CrossRef]
- Chickos, J.S.; Acree, W.E. Enthalpies of Sublimation of Organic and Organometallic Compounds. 1910–2001. J. Phys. Chem. Ref. Data 2002, 31, 537–698. [Google Scholar] [CrossRef]
- Gobble, C.; Chickos, J.; Verevkin, S.P. Vapor Pressures and Vaporization Enthalpies of a Series of Dialkyl Phthalates by Correlation Gas Chromatography. J. Chem. Eng. Data 2014, 59, 1353–1365. [Google Scholar] [CrossRef]
- Walden, P. Über die Schmelzwärme, spezifische Kohäsion und Molekulargrösse bei der Schmelztemperatur. Z. Elektrotechnik Elektrochem. 1908, 14, 713–724. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Zaitsau, D.H.; Kuratieva, N.V.; Verevkin, S.P.; Schick, C. Melting of nucleobases. Getting the cutting edge of “Walden’s Rule”. Phys. Chem. Chem. Phys. 2019, 21, 12787–12797. [Google Scholar] [CrossRef]
- Kováts, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Gerasimenko, V.A.; Nabivach, V.M. Relationship between molecular structure and gas chromatographic retention of alkylbenzenes C8-C1 2 on polydimethylsiloxane. Zh. Anal. Khim. 1982, 37, 110–116. [Google Scholar]
- Engewald, W.; Wennrich, L. Molekülstruktur und Retentionsverhalten. VIII. Zum Retentionsverhalten höherer Alkylbenzole bei der Gas-Verteilungs-Chromatographie. Chromatographia 1976, 9, 540–547. [Google Scholar] [CrossRef]
- Poligné, I.; Collignan, A.; Trystram, G. Characterization of traditional processing of pork meat into boucané. Meat Sci. 2001, 59, 377–389. [Google Scholar] [CrossRef]
- Iraqi, R.; Vermeulen, C.; Benzekri, A.; Bouseta, A.; Collin, S. Screening for Key Odorants in Moroccan Green Olives by Gas Chromatography−Olfactometry/Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2005, 53, 1179–1184. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Heintz, A. Determination of Vaporization Enthalpies of the Branched Esters from Correlation Gas Chromatography and Transpiration Methods. J. Chem. Eng. Data 1999, 44, 1240–1244. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hosseini, S.; Hesse, D.G. Determination of vaporization enthalpies of simple organic molecules by correlations of changes in gas chromatographic net retention times. Thermochim. Acta 1995, 249, 41–62. [Google Scholar] [CrossRef]
- Nesterov, I.A.; Nesterova, T.N.; Pimerzin, A.A.; Tsvetkov, V.S. Thermodynamics of alkylbenzene sorption and evaporation. IV. Evaporation enthalpies and thermodynamic characteristics of sorption by stationary OV-101 and PEG-40M phases. Izv. Vyss. Uchebnykh Zaved. Khimiya i Khimicheskaya Tekhnologiya 2000, 43, 39–45. [Google Scholar]
- Connolly, M.L. Solvent-Accessible Surfaces of Proteins and Nucleic Acids. Science 1983, 221, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Rakus, K.; Verevkin, S.P.; Schätzer, J.; Beckhaus, H.; Rüchardt, C. Thermolabile Hydrocarbons, 33. Thermochemistry and Thermal Decomposition of 9,9′-Bifluorenyl and 9,9′-Dimethyl-9,9′-bifluorenyl—The Stabilization Energy of 9-Fluorenyl Radicals. Chem. Ber. 1994, 127, 1095–1103. [Google Scholar] [CrossRef]
- Connolly, M.L. QCPE Program No. 429, Quantum Chemistry Program Exchange; University of Indiana: Bloomington, IN, USA, 2013. [Google Scholar]
- Pedley, J.B.; Naylor, R.D.; Kirby, S.P. Thermochemical Data of Organic Compounds; Chapman and Hall: New York, NY, USA, 1986; pp. 1–792. [Google Scholar]
- Arnett, E.M.; Sanda, J.C.; Bollinger, J.M.; Barber, M. Crowded benzenes. VI. Strain energy in o-di-tert-butylbenzenes. J. Am. Chem. Soc. 1967, 89, 5389–5400. [Google Scholar] [CrossRef]
- Olofsson, G. Assignment of Uncertainties; International Union of Pure and Applied Chemistry: Zürich, Switzerland, 1979; Volume 9. [Google Scholar]
- Popov, V.E.; Rozhnov, A.M.; Safronov, V.S.; Volkova, A.G. Disproportionation Equilibrium for Isopropylbenzene. Neftekhimiya 1974, 14, 364–367. [Google Scholar]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Houk, K.N.; Schleyer, P.v.R.; Allen, W.D. A Hierarchy of Homodesmotic Reactions for Thermochemistry. J. Am. Chem. Soc. 2009, 131, 2547–2560. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Notario, R.; Roux, M.V.; Chickos, J.S.; Liebman, J.F. Rediscovering the Wheel. Thermochemical Analysis of Energetics of the Aromatic Diazines. J. Phys. Chem. Lett. 2012, 3, 3454–3459. [Google Scholar] [CrossRef]
- Krüerke, U.; Hoogzand, C.; Hübel, W. Über Organometall-Komplexe, VI. 1.2.4-Tri-tert.-butyl-benzol. Chem. Ber. 1961, 94, 2817–2820. [Google Scholar] [CrossRef]
- Hoogzand, C.; Hűbel, W. On -tertiarybutylbenzenes 1,2,4,5-tetra-tertiarybutylbenzene. Tetrahedron Lett. 1961, 2, 637–643. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Sazonova, A.Y.; Emel’yanenko, V.N.; Zaitsau, D.H.; Varfolomeev, M.A.; Solomonov, B.N.; Zherikova, K.V. Thermochemistry of Halogen-Substituted Methylbenzenes. J. Chem. Eng. Data 2015, 60, 89–103. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P. Benchmark Thermodynamic Properties of 1,3-Propanediol: Comprehensive Experimental and Theoretical Study. J. Chem. Thermodyn. 2015, 85, 111–119. [Google Scholar] [CrossRef]
- Kurbatov, V.Y. Specific Heat of Liquids. I. Specific Heat of Benzenoid Hydrocarbons. Zhur. Obs. Khim. 1947, 17, 1999–2003. [Google Scholar]
- Steele, W.V.; Chirico, R.D.; Knipmeyer, S.E.; Nguyen, A. Vapor Pressure, Heat Capacity, and Density along the Saturation Line: Measurements for Benzenamine, Butylbenzene, Sec -Butylbenzene, Tert -Butylbenzene, 2,2-Dimethylbutanoic Acid, Tridecafluoroheptanoic Acid, 2-Butyl-2-Ethyl-1,3-Propanediol, 2,2,4-Trimeth. J. Chem. Eng. Data 2002, 47, 648–666. [Google Scholar] [CrossRef]
- Acree, W.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. Sublimation, Vaporization and Fusion Enthalpies from 1880 to 2015. Part 1. C 1−C 10. J. Phys. Chem. Ref. Data 2016, 45, 033101. [Google Scholar] [CrossRef]
- Clarke, E.C.W.; Glew, D.N. Evaluation of Thermodynamic Functions from Equilibrium Constants. Trans. Faraday Soc. 1966, 62, 539. [Google Scholar] [CrossRef]
- Olofsson, G.; Sunner, S. Combustion Calorimetry; Pergamon: New York, NY, USA, 1979. [Google Scholar]
- Hubbard, W.N.; Scott, D.W.; Waddington, G. Standard States and Corrections for Combustions in a Bomb at Constant Volume, Chap. 5. In Experimental Thermochemistry: Measurements of Heats of Reactions; Interscience; Rossini, F.D., Ed.; Interscience Publishers: New York, NY, USA, 1956; pp. 75–128. [Google Scholar]
- Roux, M.V.; Temprado, M.; Chickos, J.S.; Nagano, Y. Critically Evaluated Thermochemical Properties of Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. Ref. Data 2008, 37, 1855–1996. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hesse, D.G.; Liebman, J.F. Estimating Enthalpies of Sublimation of Hydrocarbons. In Energetics of Organometallic Species; Springer: Dordrecht, The Netherlands, 1992; pp. 159–169. [Google Scholar]
- Nagrimanov, R.N.; Ziganshin, M.A.; Solomonov, B.N.; Verevkin, S.P. Thermochemistry of Drugs: Experimental and Theoretical Study of Analgesics. Struct. Chem. 2019, 30, 247–261. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Nagrimanov, R.N. Nearest-Neighbor and Non-Nearest-Neighbor Interactions between Substituents in the Benzene Ring. Experimental and Theoretical Study of Functionally Substituted Benzamides. J. Phys. Chem. A 2016, 120, 9867–9877. [Google Scholar] [CrossRef]
- Colomina, M.; Jiménez, P.; Roux, M.; Turrión, C. Thermochemical Properties of 1,2,4,5-Tetramethylbenzene, Pentamethylbenzene, and Hexamethylbenzene. J. Chem. Thermodyn. 1989, 21, 275–281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).