Abstract
Cement production is the primary source of global CO2 emissions in the construction industry. Blast furnace slag (BFS) has been examined as a potential substitute for cement to reduce CO2 emissions. In addition, this substitution increases the long-term strength and improves the chemical resistance of mortar. However, a glassy film is formed on the surface of BFS while it is generated as a byproduct, lowering the initial strength of mortar. Notably, this film is destroyed in an alkaline environment. Thus, several studies have used solutions with various alkali activators. However, alkali activators are unsafe, as they are strong alkaline materials, and have low economic efficiency. This study experimentally improved the initial hydration reactivity of a mortar containing BFS as a substitute for cement, thereby improving its initial strength. We observed an increase in carbonation resistance. In addition, this study focused on evaluating the compressive strength and carbonation resistance of mortar prepared using BFS and alkaline water obtained from the electrolysis of a K2CO3 electrolyte. Results show that alkali-activated mortar using an electrolyzed alkaline aqueous solution has higher strength and contains more hydration products than that using conventional mixing water.
1. Introduction
Many countries have introduced policies for carbon emission reduction to address global warming, which is a major challenge to modern society. It is common knowledge that global warming is caused by the emission of greenhouse gases (GHGs), such as carbon dioxide (CO2), methane, and nitrous oxide. The contribution of the construction industry to these emissions consists mainly of carbon dioxide, a significant portion of which is emitted by the cement manufacturing industry during the clinker production process owing to the use of calcium carbonate as a raw material. In Korea, greenhouse gas emissions from the mineral industry account for 62% (32,612 thousand tons CO2eq) of the total greenhouse emissions from the entire industrial process sector, 73% of which (23,990 thousand tons CO2eq) is from the cement manufacturing industry [1]. Furthermore, 52% of this CO2 emission originates from the pyro-processing of cement, and the remaining 48% originates from the transportation process.
Similarly, cement is used in the production of concrete, a material frequently used in modern construction, thus emitting considerable amounts of GHGs [2]. To reduce CO2 emissions from the construction sector, some studies have attempted to replace cement intended for producing concrete with different admixtures based on industrial byproducts. Two of the most frequently used cement substitutes to date are fly ash and blast furnace slag (BFS), which are industrial byproducts of coal-fired power plants and the steel industry, respectively, with the latter being more frequently utilized. Fly ash is obtained from quenching or precipitating byproducts from power plants that use coal as the main raw material.
BFS is an industrial byproduct produced in steel mills. Several studies have investigated the replacement of cement with BFS to reduce CO2 emissions from cement manufacturing. This substitution is also environmentally advantageous because it involves the reuse of an industrial byproduct. Notably, the components of BFS are similar to those of cement, namely, SiO2 (33%), CaO (42%), Al2O3 (14%), MgO (6% or less), and Fe2O3 (0.5%) [3]. Furthermore, substituting cement with BFS instead of using only ordinary Portland cement (OPC) improves the long-term strength and chemical resistance of concrete [4]. However, the initial strength of concrete decreases due to the latent hydraulic property of BFS, and the resistance of the matrix to neutralization erosion weakens because the lower unit weight of cement inhibits the generation of the calcium hydroxide (Ca(OH)2) responsible for creating an alkaline environment. The glassy film interferes with the hydration reaction between BFS and water. In other words, the hydration reaction does not occur because the glassy film prevents the elution of reactive materials inside BFS. When the film is destroyed by alkaline materials, the reactive materials are eluted, leading to a hydration reaction [5,6].
To overcome the latent hydraulic property of BFS and improve the initial strength of mortar, previous studies have investigated the acceleration of the hydration reaction using alkali activators [7,8,9]. When strong alkaline substances, such as NaOH, KOH, and Na2SiO3, exist in the BFS and fly ash, ions, such as Ca2+ and Si4+, are leached, and a hydration reaction occurs. Such substances that react with BFS and fly ash without the use of cement are called alkaline activators. However, alkali activators are expensive, and using them as construction materials may be dangerous, owing to their strong alkalinity [10]. In particular, previous studies did not use large portions of BFS in concrete.
In this study, an alkaline solution was generated through electrolysis and used as mixing water to improve the low initial strength of BFS and address challenges related to alkali activators. The initial strength of the mortar was experimentally verified by improving the initial hydration reactivity of BFS using alkaline water, which was electrolyzed using potassium carbonate (K2CO3) as an electrolyte. The experimental results show an increase in carbonation resistance.
2. Materials and Methods
2.1. Electrolysis for Alkaline Water Production
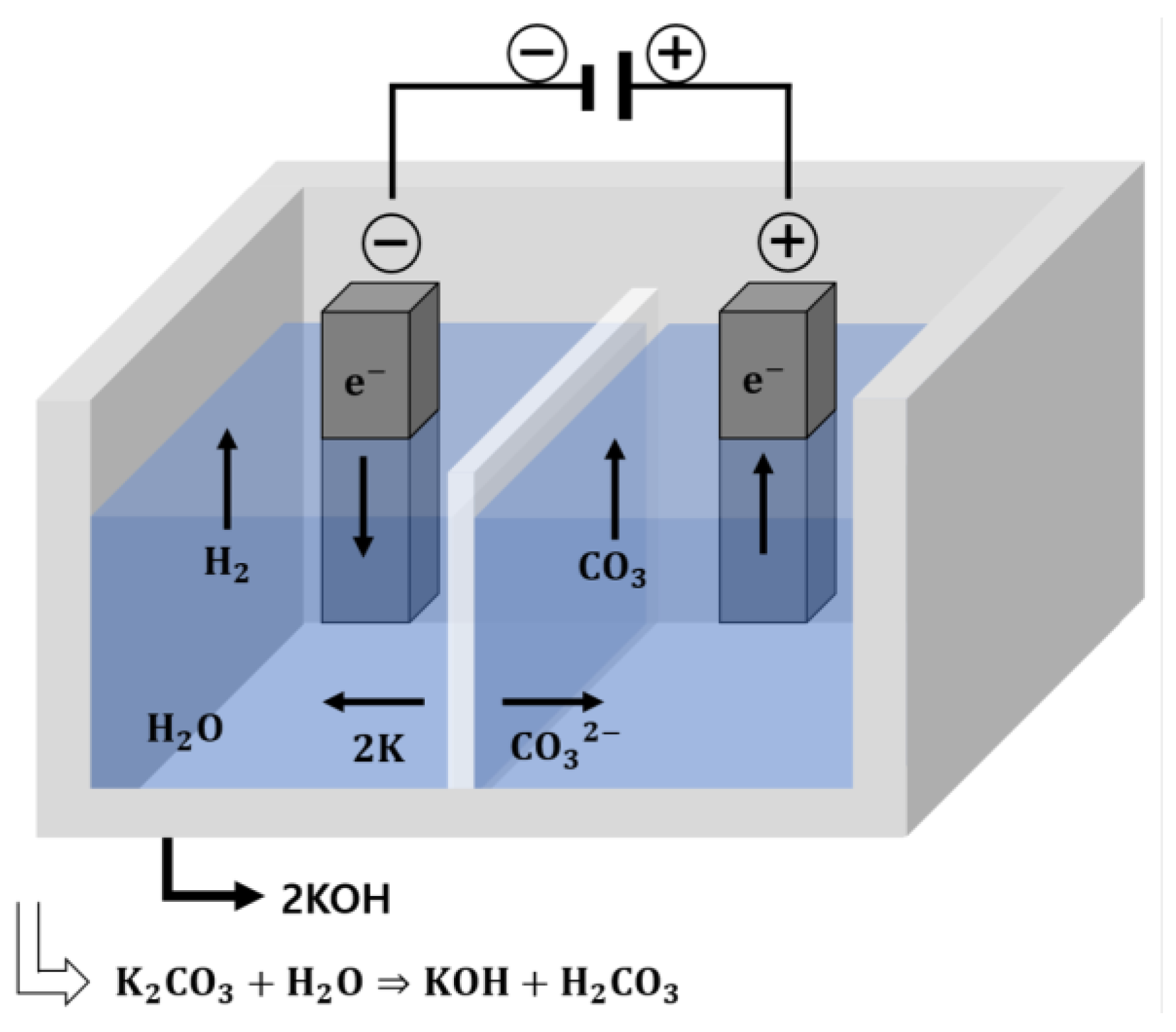
Electrolysis is an electrochemical process in which a voltage is applied to induce the reaction of a sample that would otherwise not undergo spontaneous oxidation (in which molecules or ions gain O2 or lose H2) or reduction (in which they lose O2 or gain H2) [11]. The electrolysis-generated alkaline water used in this study had high alkalinity; hence, it could act as an activator for the hydration reaction of BFS. In addition, in contrast to existing alkaline activators, electrolysis-generated alkaline water has low risks and may be a more economical choice. In this study, electrolysis-generated alkaline water was used as mixing water, thereby acting as a substitute for conventional alkali activators during mortar fabrication. Because pure water does not spontaneously undergo electrolysis, the potassium carbonate (K2CO3), which is highly alkaline, was used as an electrolyte to obtain a solution with a pH between 12.0 and 12.5; the electrolysis process is shown in Figure 1. The reaction equations for each electrode in the potassium carbonate (K2CO3) solution are expressed as follows:
Cathode (−): 2K(aq) + e− = 2K + 2H2O = 2KOH(aq) + H2(g)
Anode (+): CO32−(aq) + 2e− = CO3 = CO2(g) + ½ O2(g)
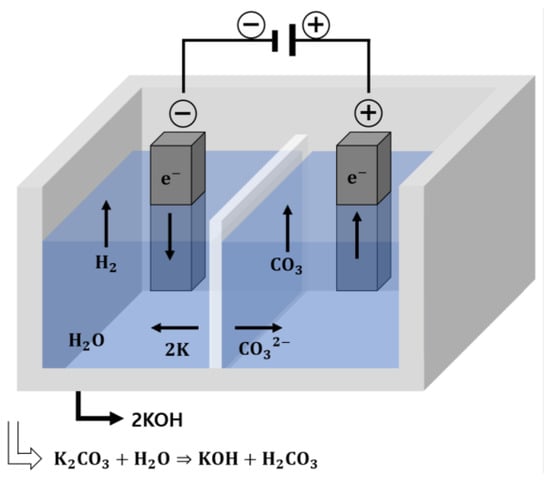
Figure 1.
Electrolysis scheme of potassium carbonate.
2.2. Experimental Procedure
The experimental parameters of this study are listed in Table 1. In the experiment, the water/binder (W/B) ratio was set to 65%, and the BFS replacement ratio was set to either 25 or 50% to examine the hydration reactivity as a function of the replacement ratio. The compressive strength of mortar was measured at 3, 7, and 28 d of age to examine the strength development according to the addition of alkaline water. For scanning electron microscope (SEM) analysis, measurements were performed after interrupting the hydration of the mortar at 3, 7, and 28 d of age to examine the difference in the amount of hydrates generated. In addition, the carbonation resistance of the mortar according to the use of the alkaline water was examined through an accelerated carbonation test. Mercury intrusion porosimetry (MIP) was performed on the mortar at 7 d of age to investigate the change in its internal porosity due to the use of alkaline water.

Table 1.
Experimental plan.
2.3. Materials
In this experiment, type I OPC from company “H” was used. BFS produced from steel mill “A” was used as a substitute for cement. The physical properties of cement and BFS are listed in Table 2, and their chemical compositions are listed in Table 3. River sand from company “E” was used as fine aggregate; its physical properties are listed in Table 4. Alkaline water with a pH between 12.0 and 12.5 generated through the electrolysis of potassium carbonate (K2CO3) was used as mixing water. The specifications of the electrolysis equipment used in this study to produce the alkaline water are listed in Table 5.

Table 2.
Physical properties of cement and blast furnace slag.

Table 3.
Chemical composition of cement and blast furnace slag.

Table 4.
Physical analysis of sand.

Table 5.
Specifications of the equipment used to produce the alkaline aqueous solution.
2.4. Experimental Method
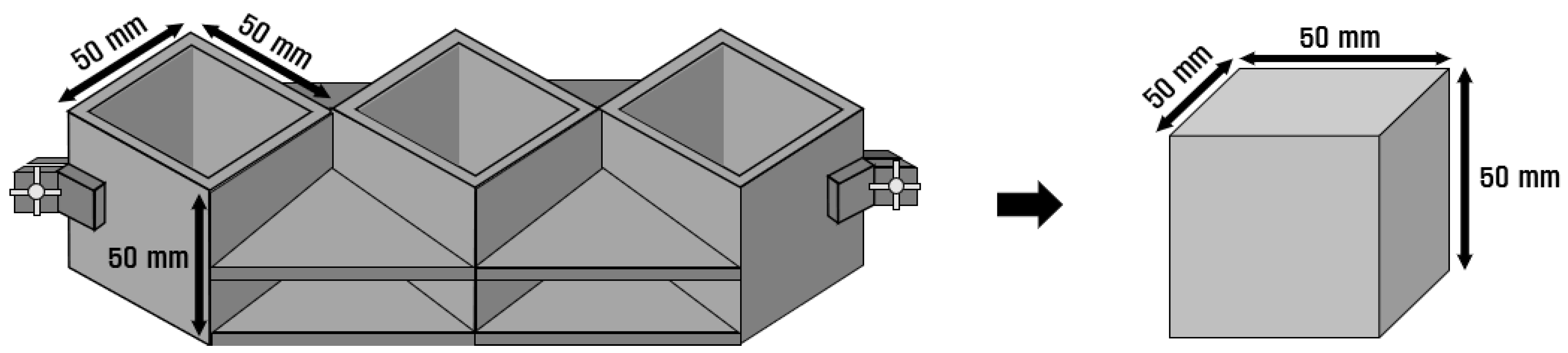
The mortar used in the experiment was mixed per ASTM C 305 [12]. For compressive strength specimens, a 50 mm × 50 mm × 50 mm cube-shaped mold was used, as shown in Figure 2, per ASTM C 109 [13]. The specimens were subjected to water-curing for 3, 7, and 28 d at a temperature of 20 ± 2 °C. The compressive strengths of the three specimens were measured and averaged. The samples used for the SEM analysis were prepared by pulverizing the specimens used in the compressive strength tests. The prepared samples were immersed in acetone, and measurements were performed after interrupting the hydration by evaporating moisture in a vacuum chamber for 24 h.
Figure 2.
Compressive strength specimen mold.
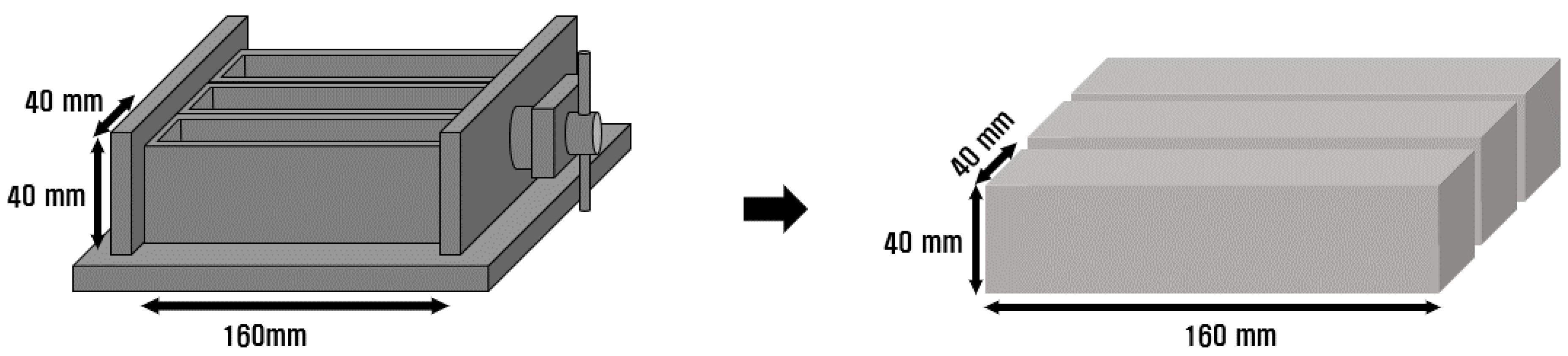
Accelerated carbonation test specimens were prepared using a 40 mm × 40 mm × 160 mm prismatic mold, as shown in Figure 3. Before the accelerated carbonation test, the specimens were subjected to water-curing at a temperature of 20 ± 2 °C for 28 d, followed by an additional 28-day curing in a humidity chamber at a constant temperature of 20 ± 2 °C and relative humidity of 60 ± 5%. The accelerated carbonation test was conducted in an environment with a temperature of 20 ± 2 °C, relative humidity of 60 ± 5%, and CO2 concentration of 5 ± 0.2%, as per the Korean Industrial Standard (KS) F 2584 [14]. The carbonation depth was measured weekly, as per KS F 2596 [15]. Samples 7 d in age were used for the MIP analysis to identify the pore structure of the accelerated carbonation test specimens. For the samples used in the MIP analysis, the hardened samples whose hydration had been interrupted were cut into hexahedral shapes in the same manner as the SEM analysis samples and dried in an oven at 100 °C for 24 h before the test.
Figure 3.
Accelerated carbonation test specimen mold.
3. Results and Discussion
3.1. Compressive Strength
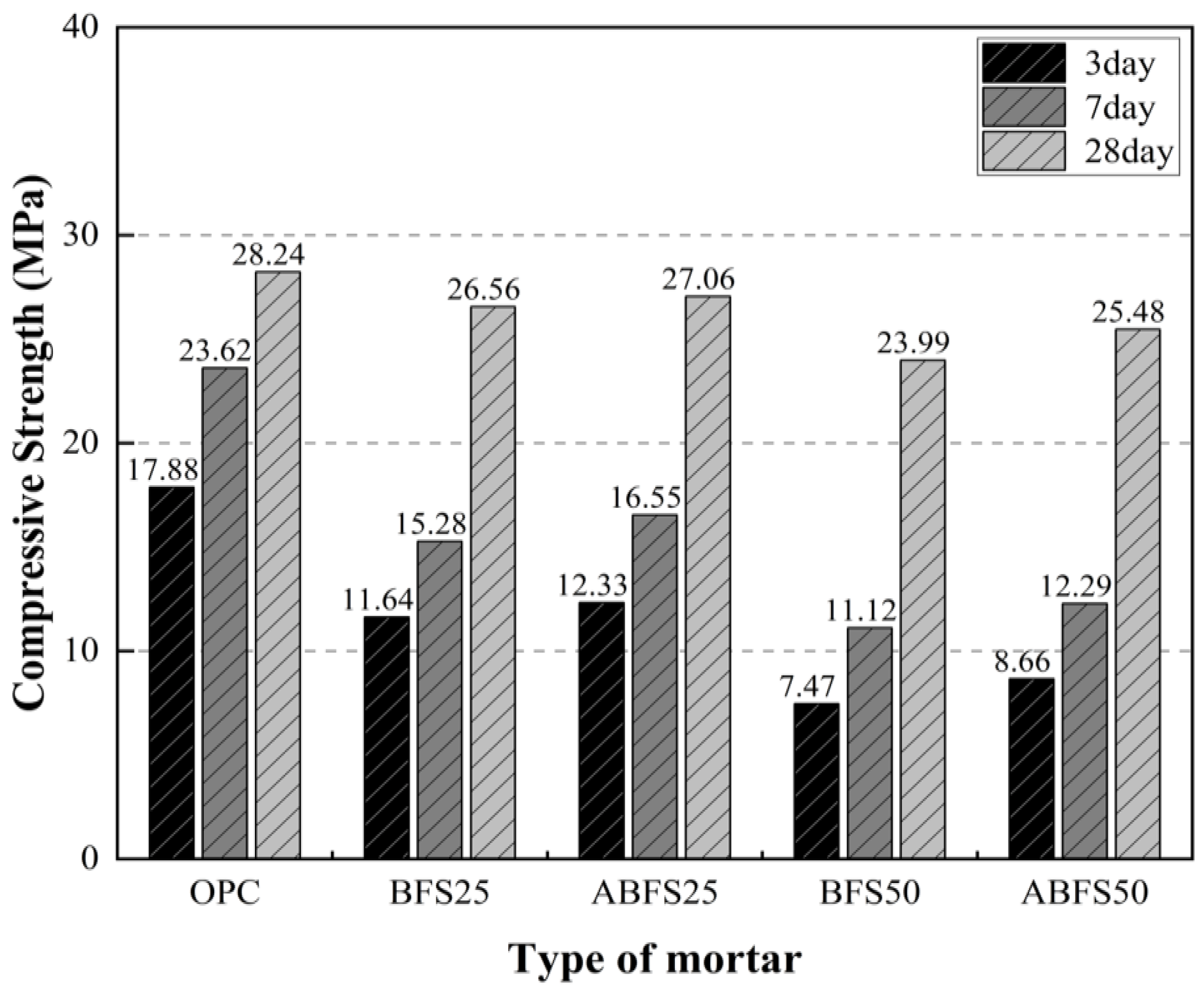
The compressive strengths at 3, 7, and 28 d of age are shown in Figure 4. The compressive strengths after 3, 7, and 28 d of age were 17.88, 23.62, and 28.24 MPa for samples with only OPC; 7.47, 11.12, and 23.99 MPa for BFS50 specimens; 12.33, 16.55, and 27.06 MPa for ABFS25 specimens; and 8.66, 12.29, and 25.48 MPa for ABFS50 specimens, respectively.
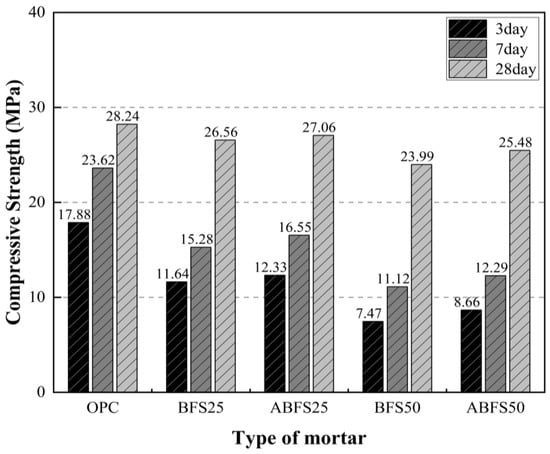
Figure 4.
Mortar compressive strength test results (MPa). (1) OPC: Ordinary Portland cement; OPC:BFS = 100:0 (normal water); (2) BFS25: Blast furnace slag; OPC:BFS = 75:25 (normal water); (3) ABFS25: Blast furnace slag; OPC:BFS = 75:25 (alkali water); (4) BFS50: Blast furnace slag; OPC:BFS = 50:50 (normal water); (5) ABFS50: Blast furnace slag; OPC:BFS = 50:50 (alkali water).
The mortar mixed with BFS showed a lower compressive strength than that with only OPC, regardless of the type of mixing water. The glassy film on the surface of the BFS particles was destroyed by the alkaline water, resulting in a hydration reaction between water and BFS. Thus, the mortar using alkaline water as mixing water had a higher strength than that using conventional mixing water regardless of age. Calcium silicate hydrates (C-S-H) and calcium aluminate hydrates (C-A-H) were generated owing to the reactive materials in BFS eluting through the glassy film and densely filling the pores inside the mortar.
3.2. Scanning Electron Microscopy (SEM)
Figure 5 and Figure 6 show the SEM results for the mortars with a BFS replacement ratio of 25%, mixed with conventional and alkaline water, respectively, at 3, 7, and 28 d of age. Similarly, Figure 7 and Figure 8 show the SEM results for the mortars with a BFS replacement ratio of 50%, mixed with conventional and alkaline water, respectively, at 3, 7, and 28 d of age.

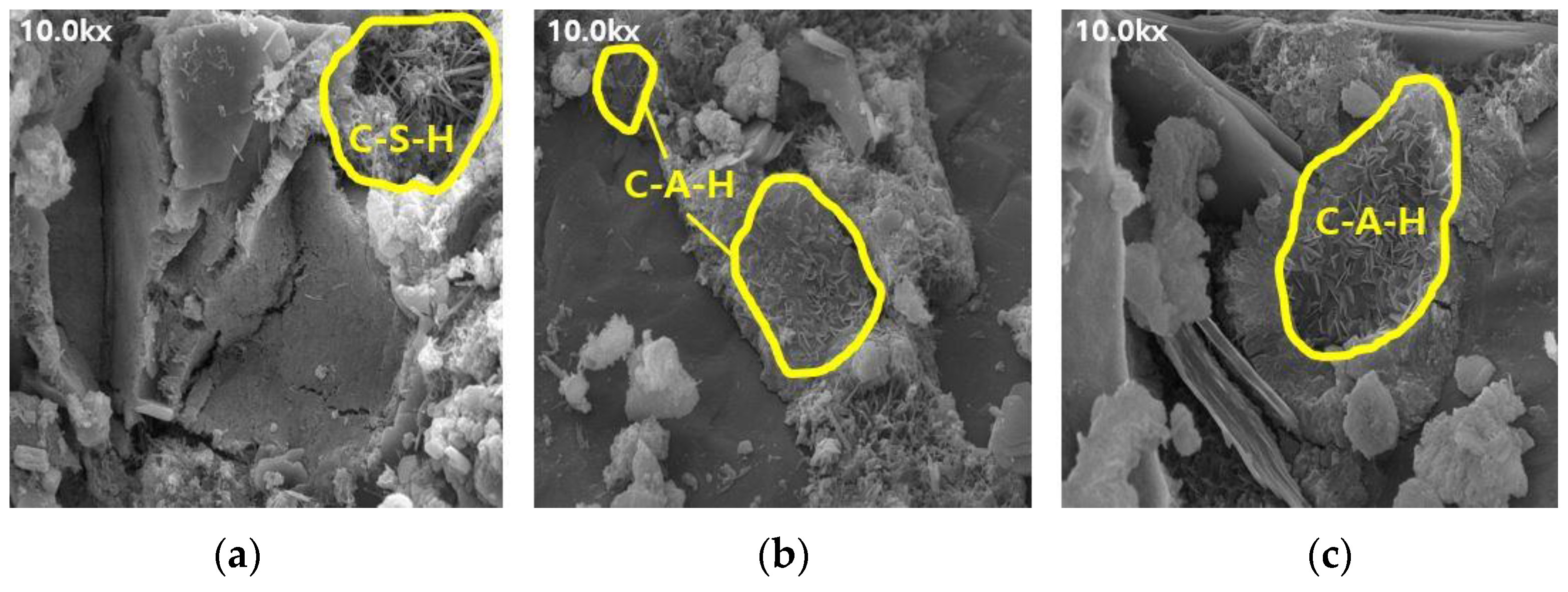
Figure 5.
SEM results of BFS25: (a) 3, (b) 7, and (c) 28 d.

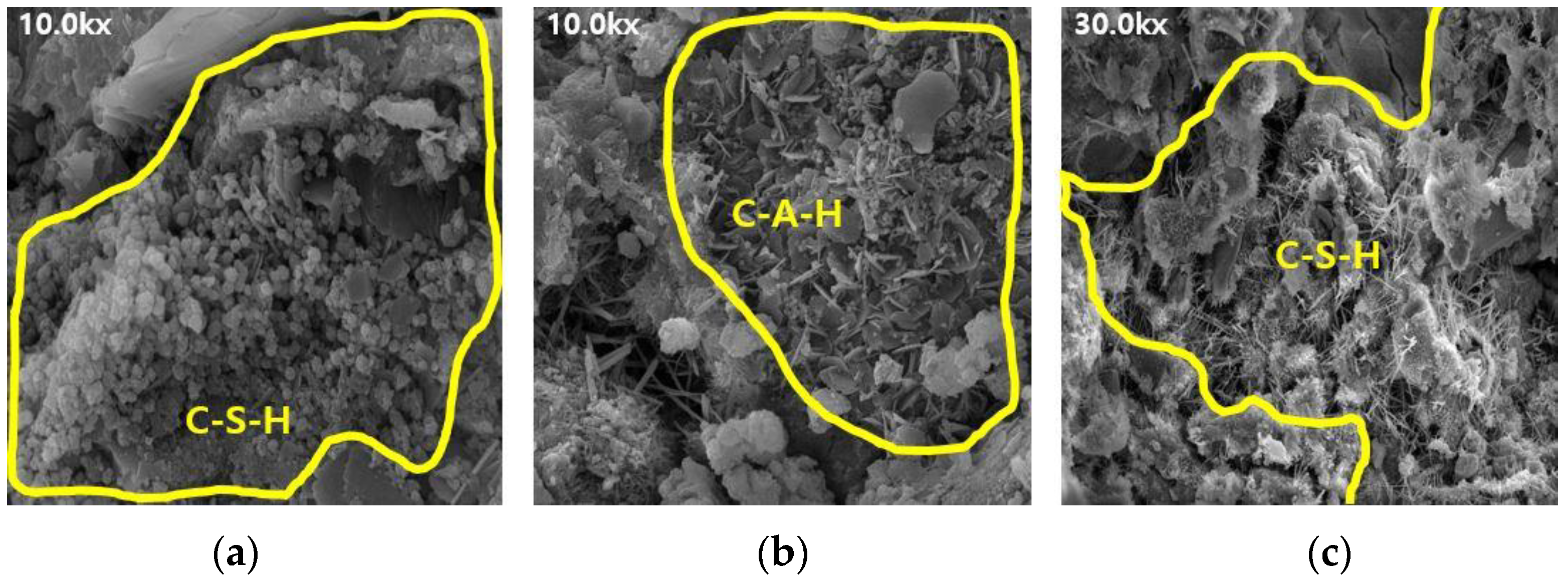
Figure 6.
SEM results of ABFS25: (a) 3 d, (b) 7 d, and (c) 28 d.

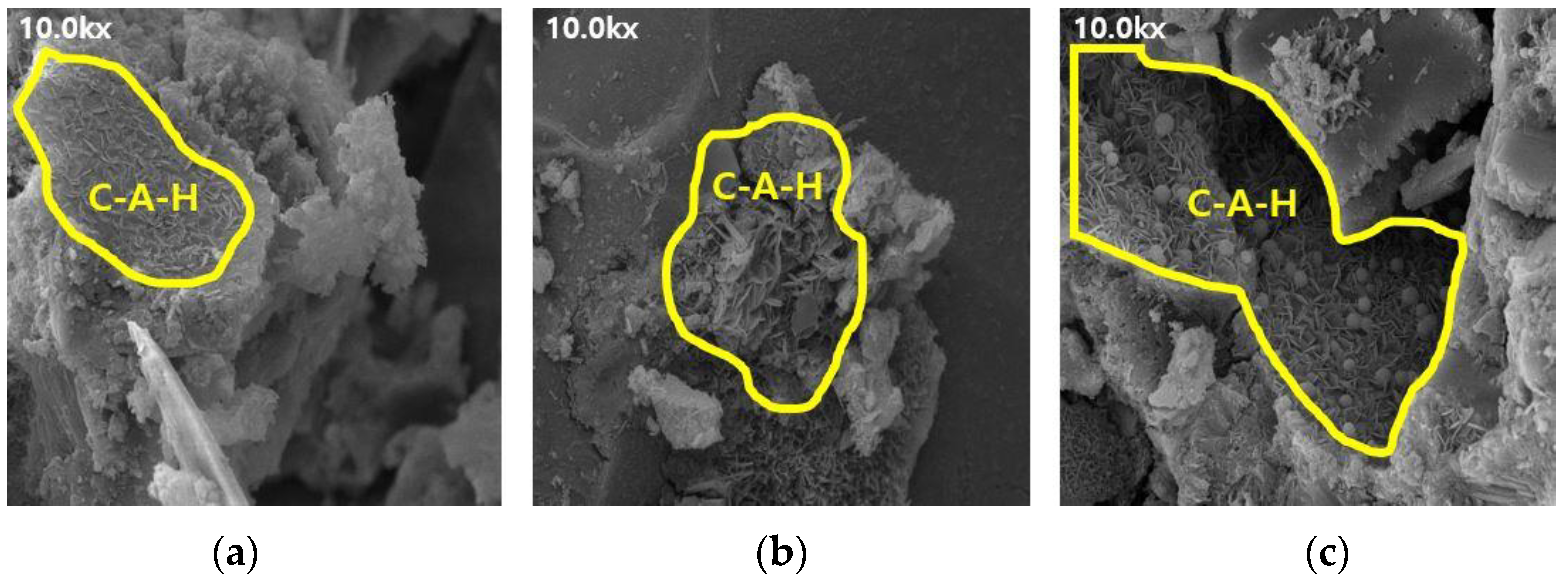
Figure 7.
SEM results of BFS50: (a) 3, (b) 7, and (c) 28 d.
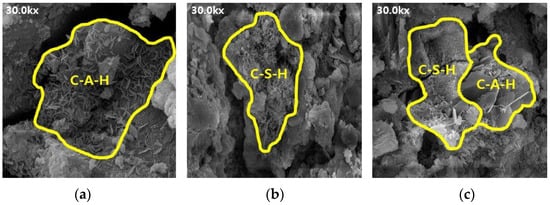
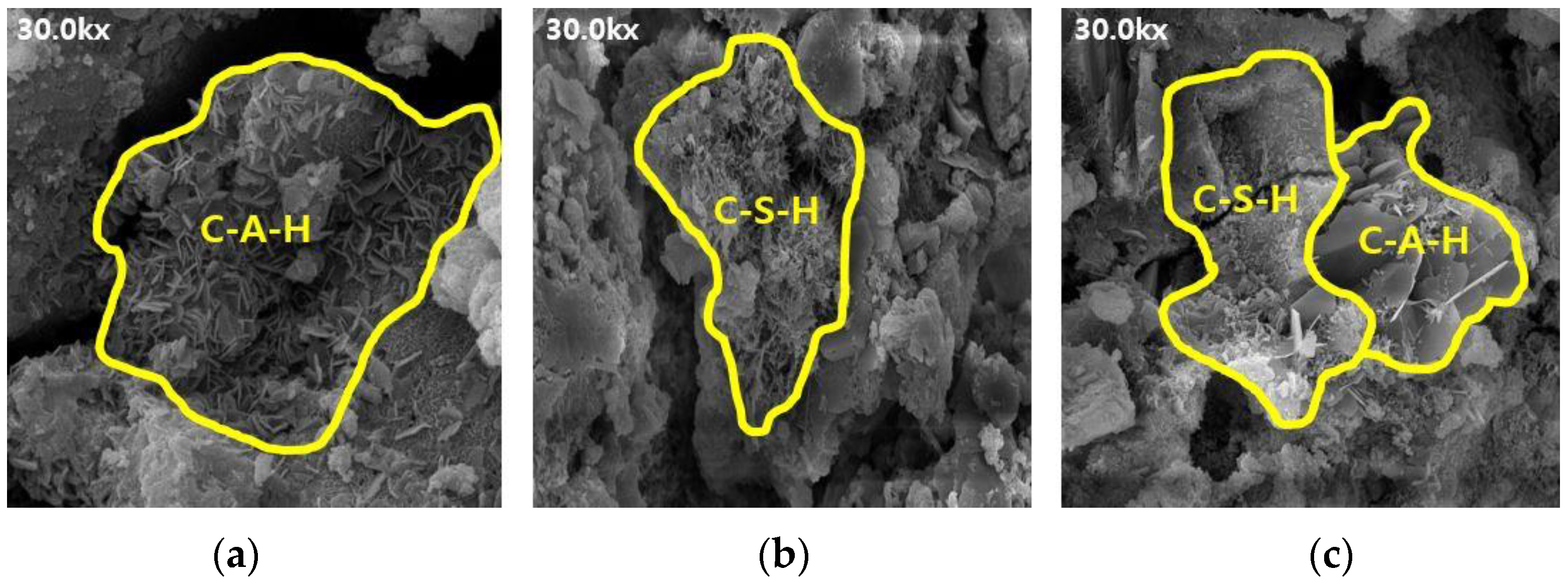
Figure 8.
SEM results of ABFS50: (a) 3, (b) 7, and (c) 28 d.
The SEM results indicate that the hydration products differed depending on the introduction of the alkaline water. The type of hydration product was confirmed to be the same form by referring to prior studies, and it was determined to be the same hydration product as well [16,17]. Owing to the generation of significantly more hydrates, such as C-S-H and C-A-H, the internal pores in the mortar using alkaline water (Figure 6 and Figure 8) were more densely filled than those in the mortar using conventional mixing water (Figure 5 and Figure 7), regardless of the BFS replacement ratio. This result indicates that using alkaline water affects the initial strength development of mortar, as confirmed by the compressive strength test.
3.3. Accelerated Carbonation Test
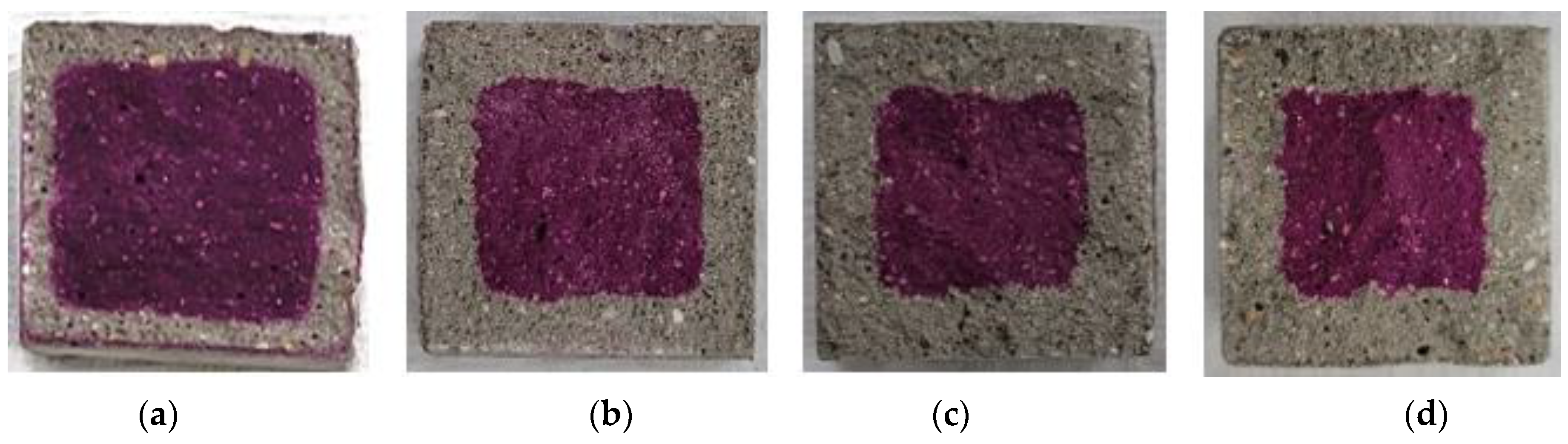
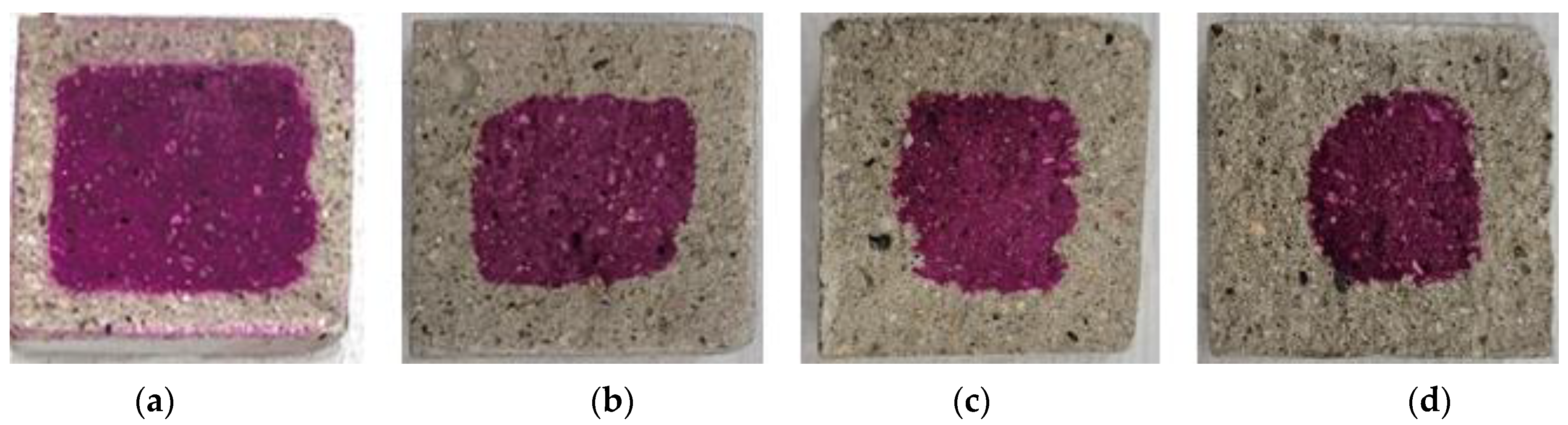
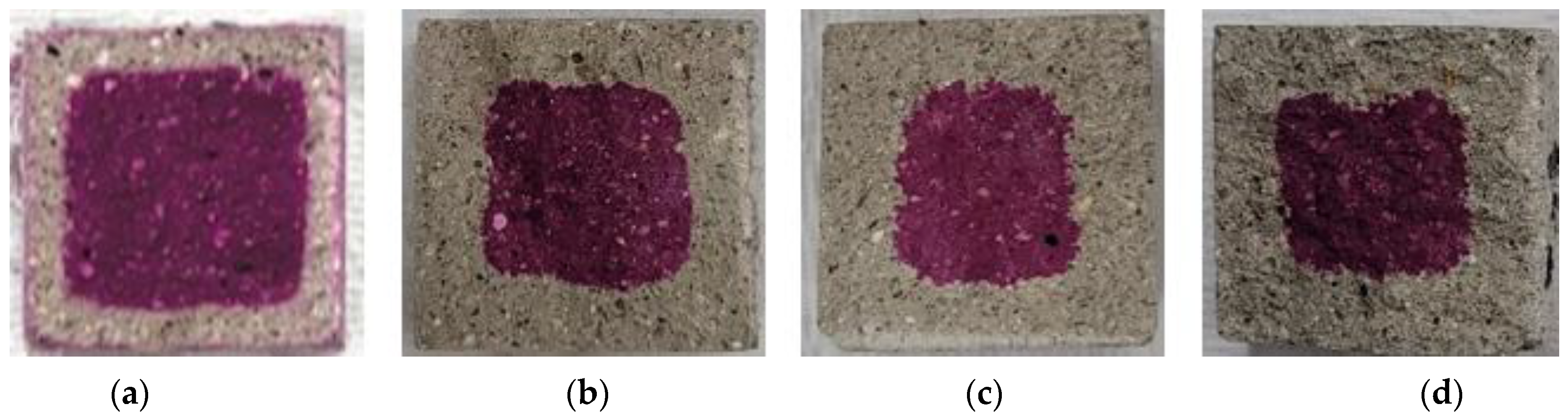
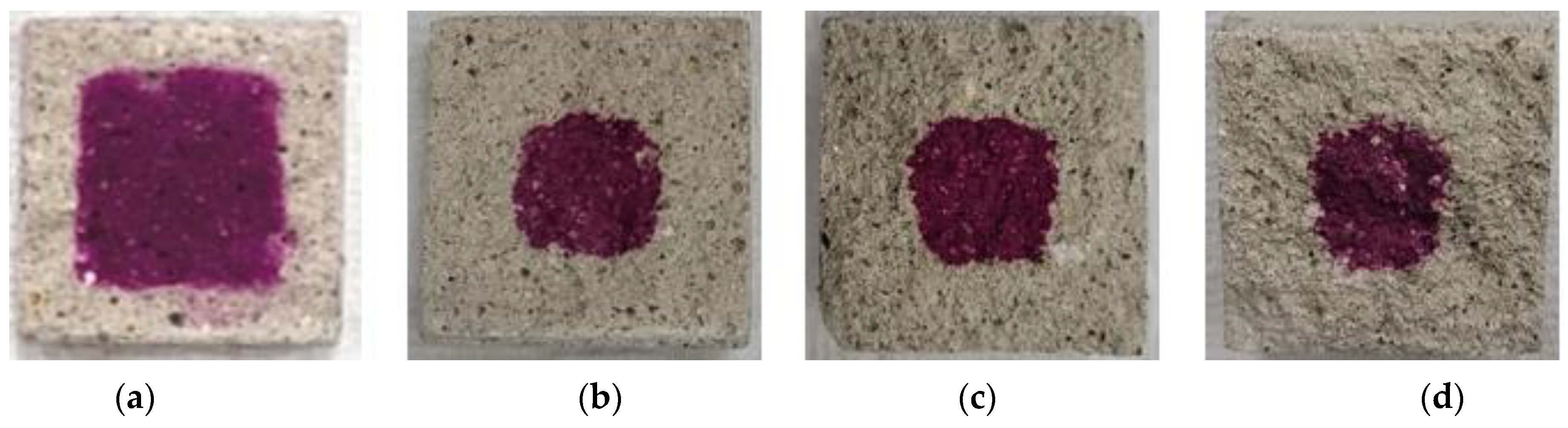
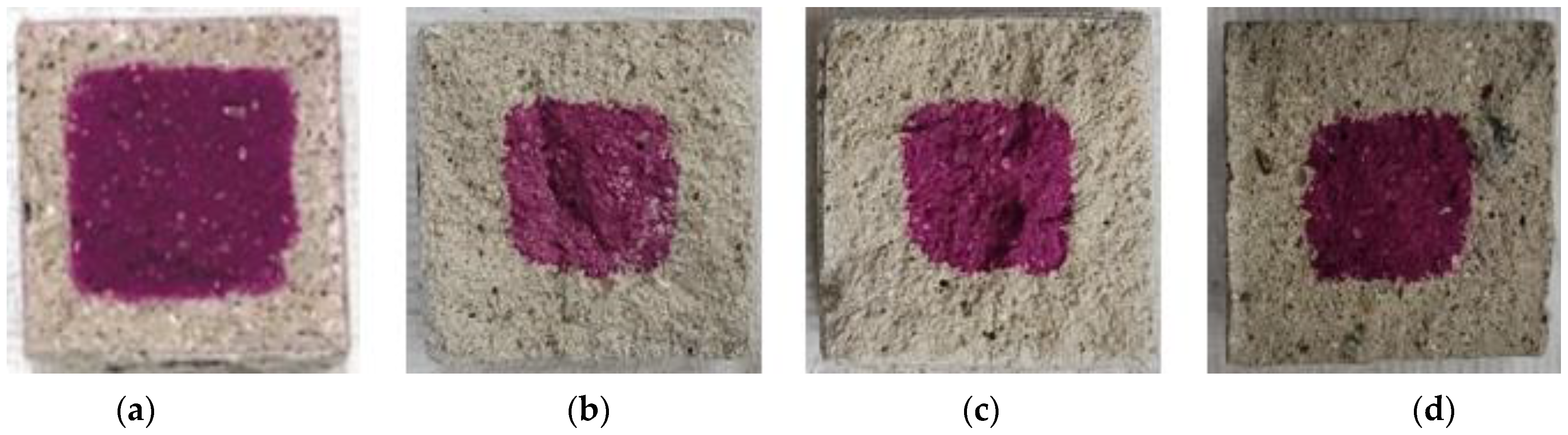
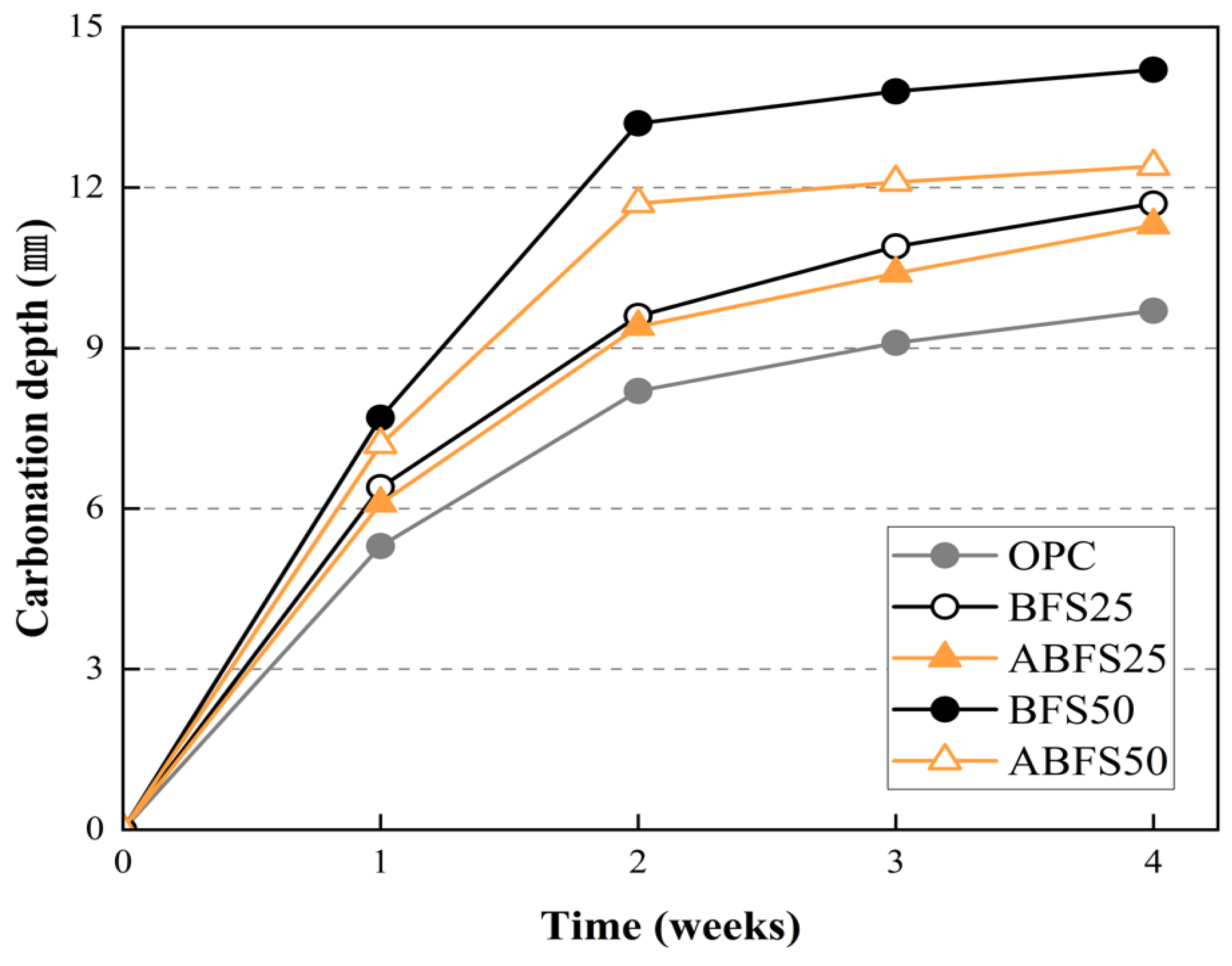
Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13 show the accelerated carbonation test results. For the mortar mixed with BFS using conventional mixing water, carbonation proceeded more rapidly compared to the mortar mixed with BFS using alkaline water. In addition, the carbonation depth measurement results in Figure 14 show that, for a given age, the carbonation depth increased as the BFS replacement ratio increased.
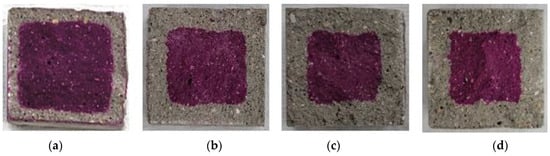
Figure 9.
Accelerated carbonation test result of OPC: (a) 1, (b) 2, (c) 3, and (d) 4 weeks.

Figure 10.
Accelerated carbonation test result of BFS25: (a) 1, (b) 2, (c) 3, and (d) 4 weeks.

Figure 11.
Accelerated carbonation test result of ABFS25: (a) 1, (b) 2, (c) 3, and (d) 4 weeks.
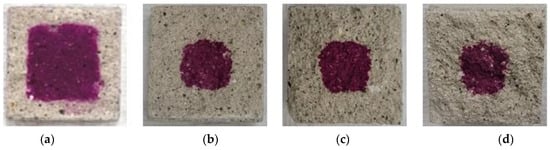
Figure 12.
Accelerated carbonation test result of BFS50: (a) 1, (b) 2, (c) 3, and (d) 4 weeks.

Figure 13.
Accelerated carbonation test result of ABFS50: (a) 1, (b) 2, (c) 3, and (d) 4 weeks.
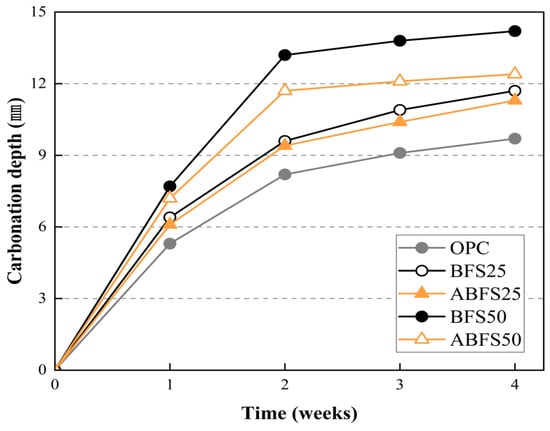
Figure 14.
Carbonation depth measurement results.
The SEM analysis results indicate that more hydration products were generated inside the hardened body using alkaline water because an alkaline environment was created at the beginning. The carbonation proceeded slowly because the inner densification affected the permeation of CO2.
3.4. Mercury Intrusion Porosimetry (MIP)
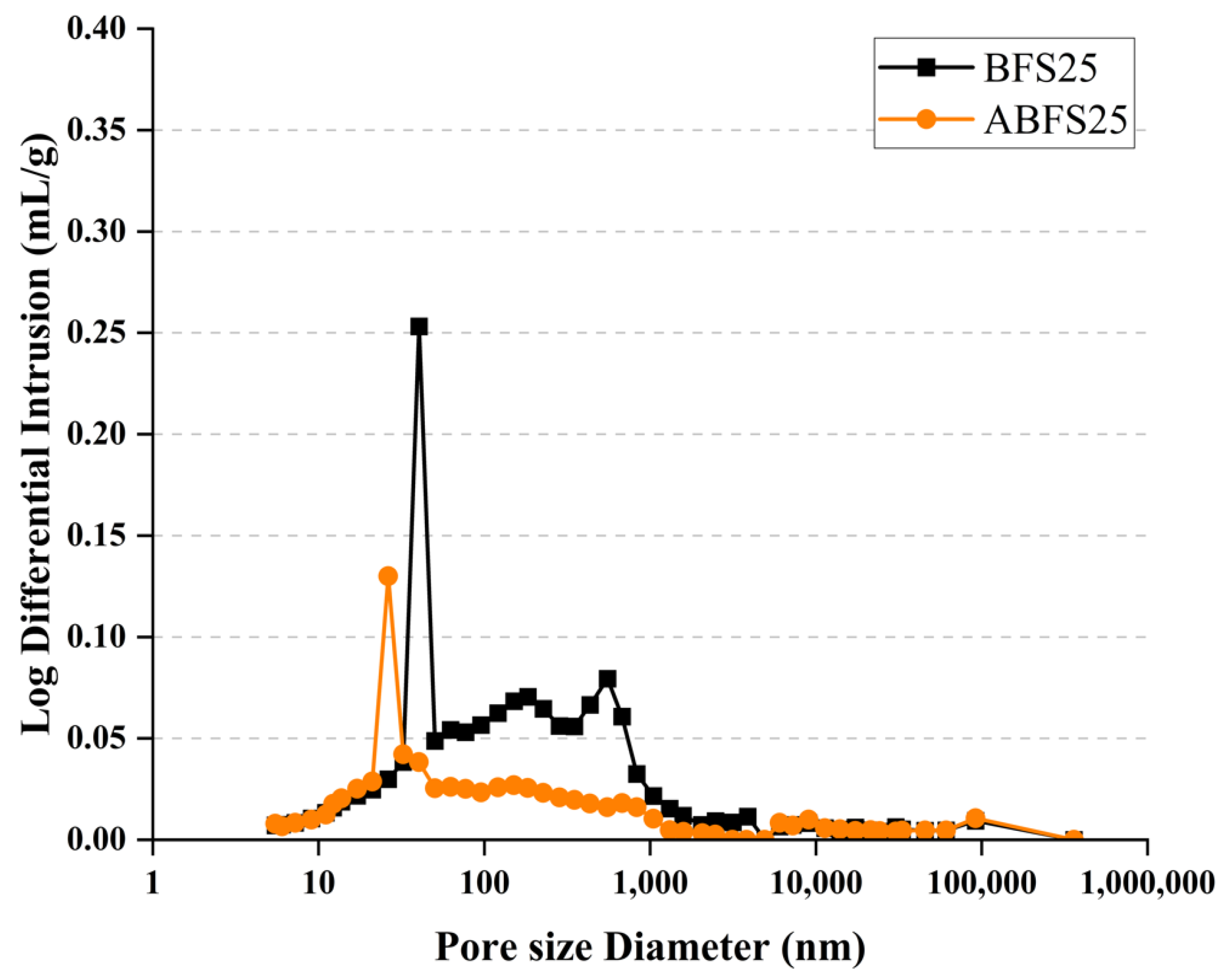
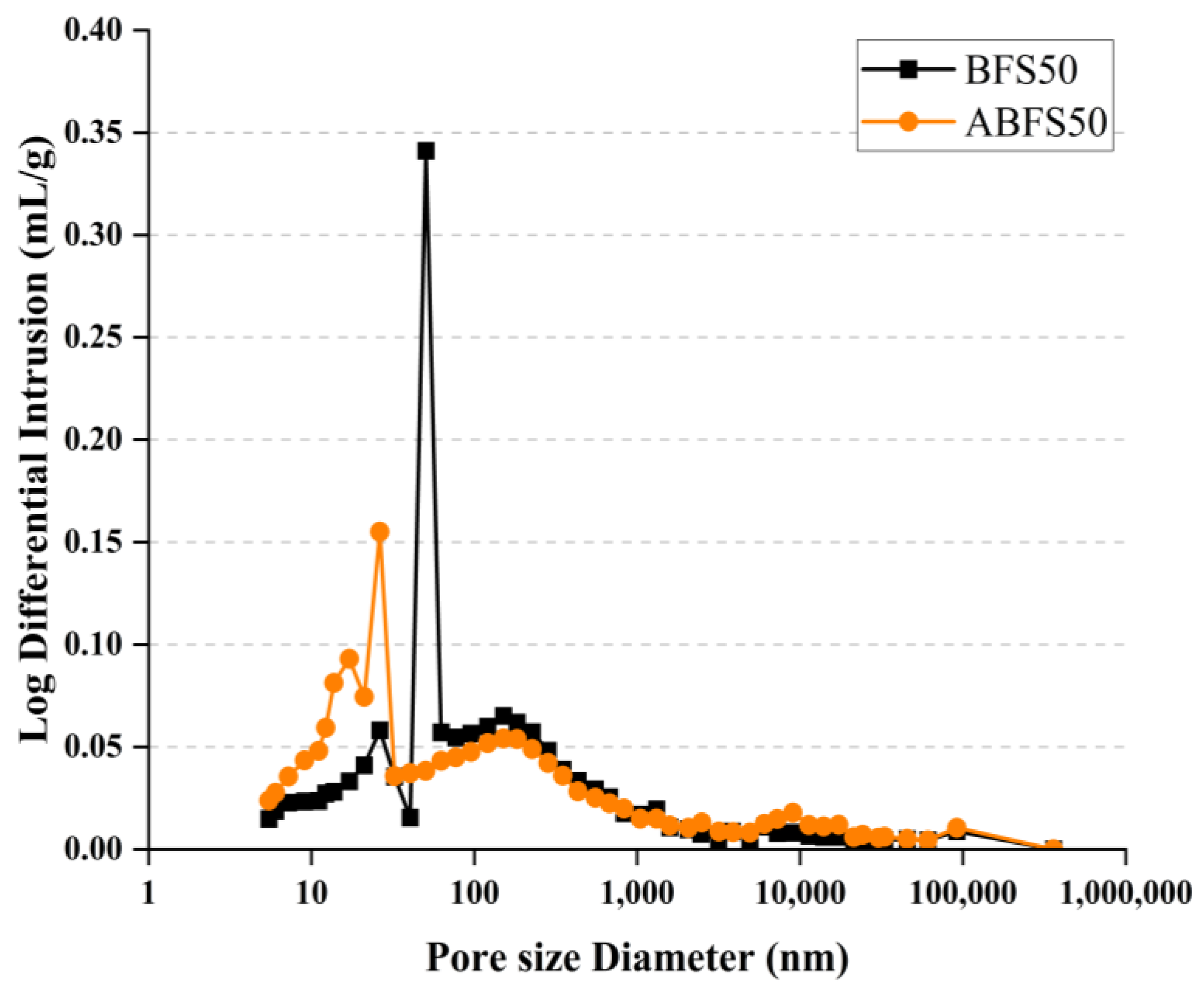
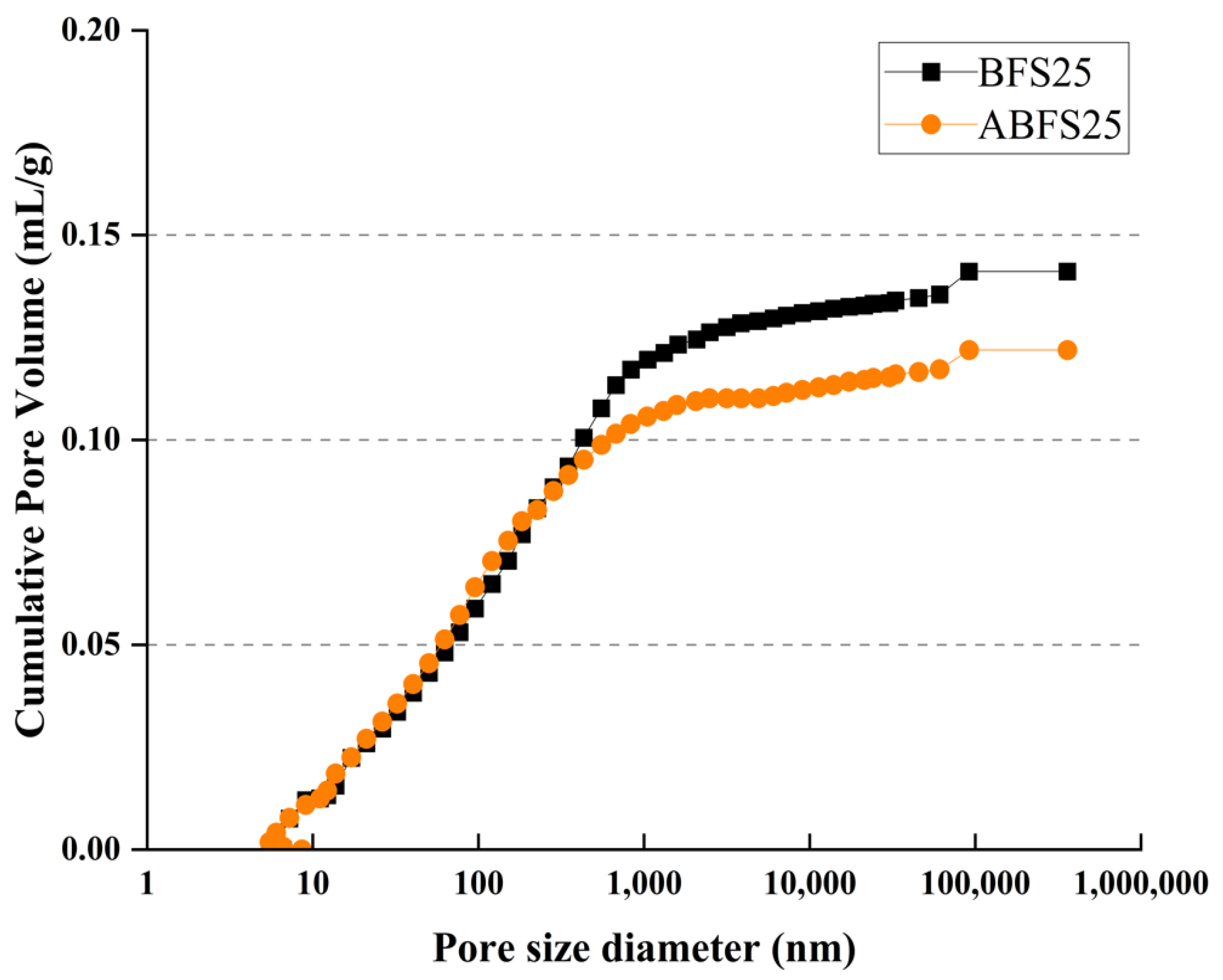
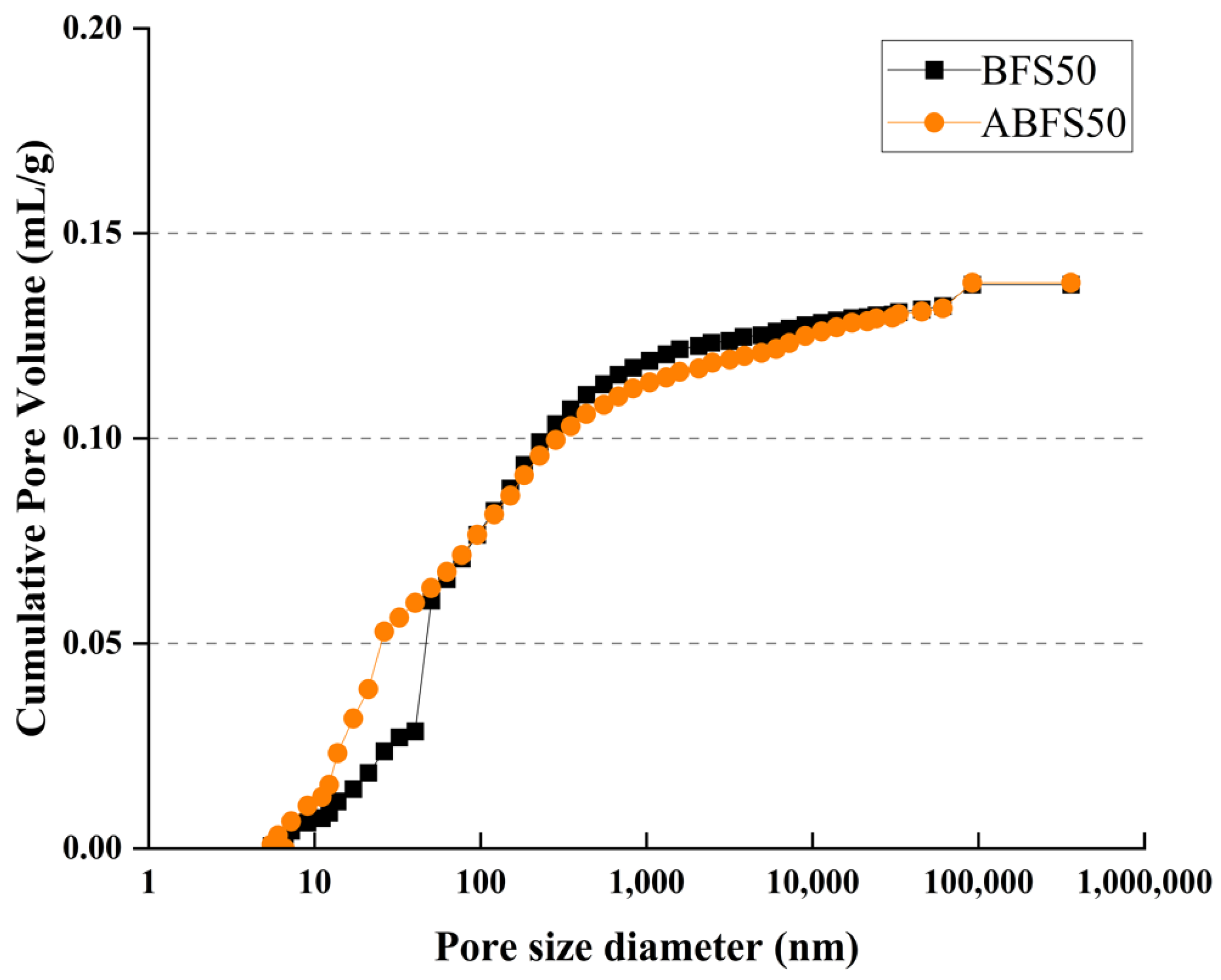
Figure 15 and Figure 16 show the pore size distributions obtained using MIP for conventional and alkali mortars with BFS replacement ratios of 25% and 50%, respectively, at 7 d of age. The pores inside the specimens are divided into micro- or macro-pores, depending on whether they are smaller or larger than 50, which positively and adversely affect the strength, respectively [18].
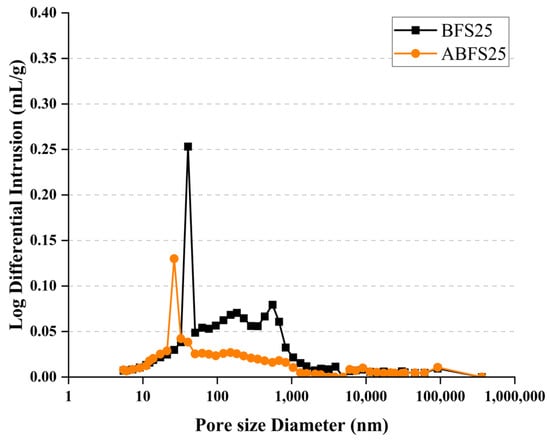
Figure 15.
MIP results for BFS25 and ABFS25.
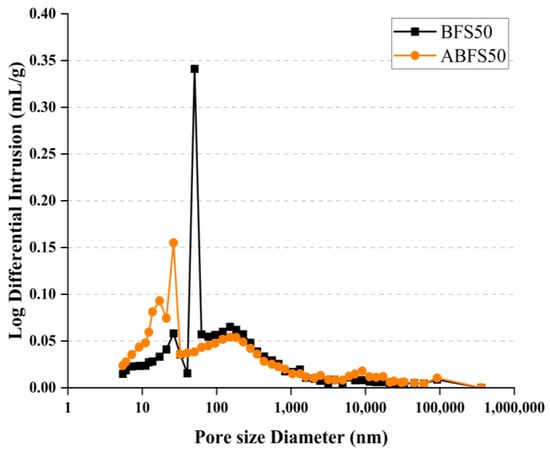
Figure 16.
MIP results for BFS50 and ABFS50.
These figures also show that the mortar mixed with BFS has numerous macropores, and the mortar mixed with BFS using alkaline water has numerous micropores, indicating a similar tendency to that observed in the accelerated carbonation test results for the specimens using alkaline water and BFS.
Figure 17 and Figure 18 show the cumulative pore volume according to the BFS replacement ratio. The mortar mixed with BFS using conventional mixing water exhibits a higher cumulative pore volume than that using alkaline water because pores in the latter are more densely filled due to the accelerated hydration reaction, which increases the formation of internal hydrates.
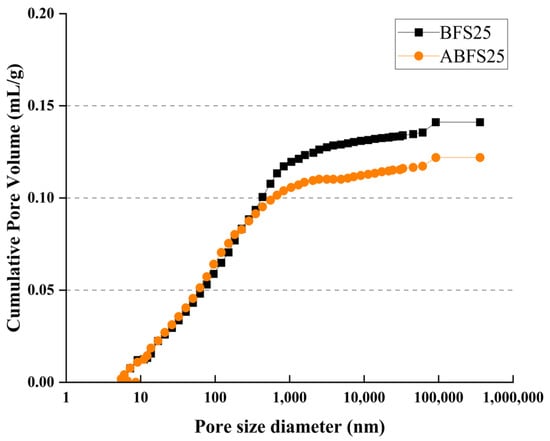
Figure 17.
Porosity results for BFS25 and ABFS25.
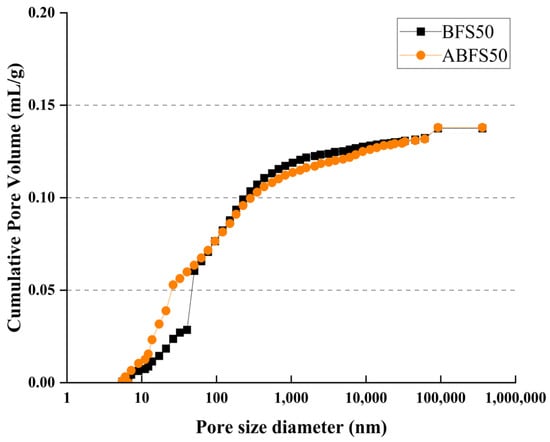
Figure 18.
Porosity results for BFS50 and ABFS50.
4. Conclusions
This study experimentally evaluated the improvement of the initial hydration reactivity and carbonation resistance of a mortar mixture produced by partially substituting cement with BFS and adding an alkaline aqueous solution obtained through an electrolytic process in which potassium carbonate (K2CO3) was used as an electrolyte. The conclusions drawn from this study are as follows:
- (1)
- The compressive strength at all ages decreased as the BFS replacement ratio increased. For mortar mixed with BFS, using the alkaline solution as mixing water led to a higher level of strength compared to that obtained when using pure water because the former created an alkaline environment inside the mortar; thus, the glassy film on the surface of the BFS particles was destroyed, improving the hydration reactivity.
- (2)
- From the SEM analysis results, it was confirmed that alkaline water destroyed the film on the surface of the BFS particles, thereby eluting the reactive materials in the BFS, resulting in more hydration products.
- (3)
- The accelerated carbonation test results confirmed that the mortar mixed using alkaline water had a higher level of carbonation resistance than the mortar mixed using conventional water because the carbonation proceeded slowly for the hardened body that used alkaline water. The internal pores became smaller and affected the permeation of the carbon gas, as shown in the MIP test results at 7 d of age.
This study utilized an electrolysis alkaline aqueous solution as mixing water to improve the initial strength of mortar produced by substituting a specific cement content with BFS, a byproduct of the steel industry. Because this study has limitations in experimental factors and levels, more experiments are required in the future.
Author Contributions
Conceptualization, S.J. and J.K.; formal analysis, S.J.; experiment, S.J. and J.K. and H.K.; writing—original draft preparation, S.J.; writing—review and editing, S.J.; visualization, S.J. and J.K.; supervision, S.P.; project administration, S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF), grant funded by the South Korean government (MSIT) (No. NRF-2020R1A2C1011957).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kim, R.H. Current state of carbon dioxide emission in cement industry and proposal for the environment load reducing cement used inorganic construction wastes. Mag. Korean Recycl. Constr. Resour. Inst. 2019, 14, 22–28. [Google Scholar]
- Park, S.G. Treatment of Ash from power plant by-product using plasma and arc discharge. Mag. Korean Recycl. Constr. Resour. Inst. 2020, 15, 43–47. [Google Scholar]
- Seo, H.; Kim, D.H. Development of reinforcement grout materials using reinforcing fiber and blast furnace slag powder. J. Korean Geosynth. Soc. 2019, 18, 101–112. [Google Scholar]
- Choi, S.W.; Kim, V.; Chang, W.S.; Kim, E.Y. The present situation of production and utilization of steel slag in Korea and other countries. Mag. Korea Concr. Inst. 2007, 19, 28–33. [Google Scholar]
- Park, S.K.; Kwon, S.J.; Kim, Y.M.; Lee, S.S. Reaction properties of non-cement mortar using ground granulated blast furnace slag. J. Korea Contents Assoc. 2013, 13, 392–399. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, W.K.; Kang, S.H. Hydration mechanism of ground granulated blast furnace slag. Mag. Korea Concr. Inst. 2012, 24, 31–34. [Google Scholar]
- Kim, H.J.; Yang, J.K. Shrinkage behavior of geopolymer mortar with expansive additive. J. Acad. Conf. Concr. Soc. 2017, 29, 597–598. [Google Scholar]
- Kim, R.H.; Kim, G.Y.; Kim, J.H.; Lee, B.K.; Cho, B.S. Effect of types and replacement ratio of alkali activator on compressive strength of ground granulated blast furnace slag mortar. J. Korean Recycl. Constr. Resour. Inst. 2014, 2, 360–366. [Google Scholar]
- Song, J.K.; Song, K.I.; Yang, K.H. Importance and characteristics of geopolymer concrete technology. Mag. RCR 2017, 12, 8–15. [Google Scholar]
- Kim, S.A.; Park, S.K. Hydration properties of high volume cement matrix using blast furnace slag and alkaline aqueous by electrolysis. J. Korean Recycl. Constr. Resour. Inst. 2017, 5, 8–13. [Google Scholar]
- Hwang, G.J.; Choi, H.S. Hydrogen production systems through water electrolysis. J. Membr. 2017, 27, 477–486. [Google Scholar] [CrossRef]
- ASTM C-305; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- ASTM C-109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- KS F 2584; Standard Test Method for Accelerated Carbonation of Concrete. Korean Agency for Technology and Standards: Eumseong County, Republic of Korea, 2020.
- KS F 2596; Standard Test Method for Measuring Carbonation Depth of Concrete. Korean Agency for Technology and Standards: Eumseong County, Republic of Korea, 2019.
- Lee, S.H.; Kim, H.J.; Lim, Y.J.; Kim, D.H.; Park, B.S. Effect of Na2SO4 on autogenous healing in initial cracking of blast furnace slag cement paste. J. Korea Concr. Inst. 2019, 31, 261–267. [Google Scholar] [CrossRef]
- Hou, Y.; Ding, P.; Han, D.; Zhang, X.; Cao, S. Study on the preparation and hydration properties of a new cementitious material for tailings discharge. Processes 2019, 7, 47. [Google Scholar] [CrossRef]
- Choi, Y.W.; Hooton, R.D. A study on pore structure of high-fluidity concrete using lime stone powder and fly-ash. J. Korea Inst. Struct. Maint. Insp. 2011, 15, 118–125. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).