Innovative Tool for Automatic Detection of Arterial Stenosis on Cone Beam Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
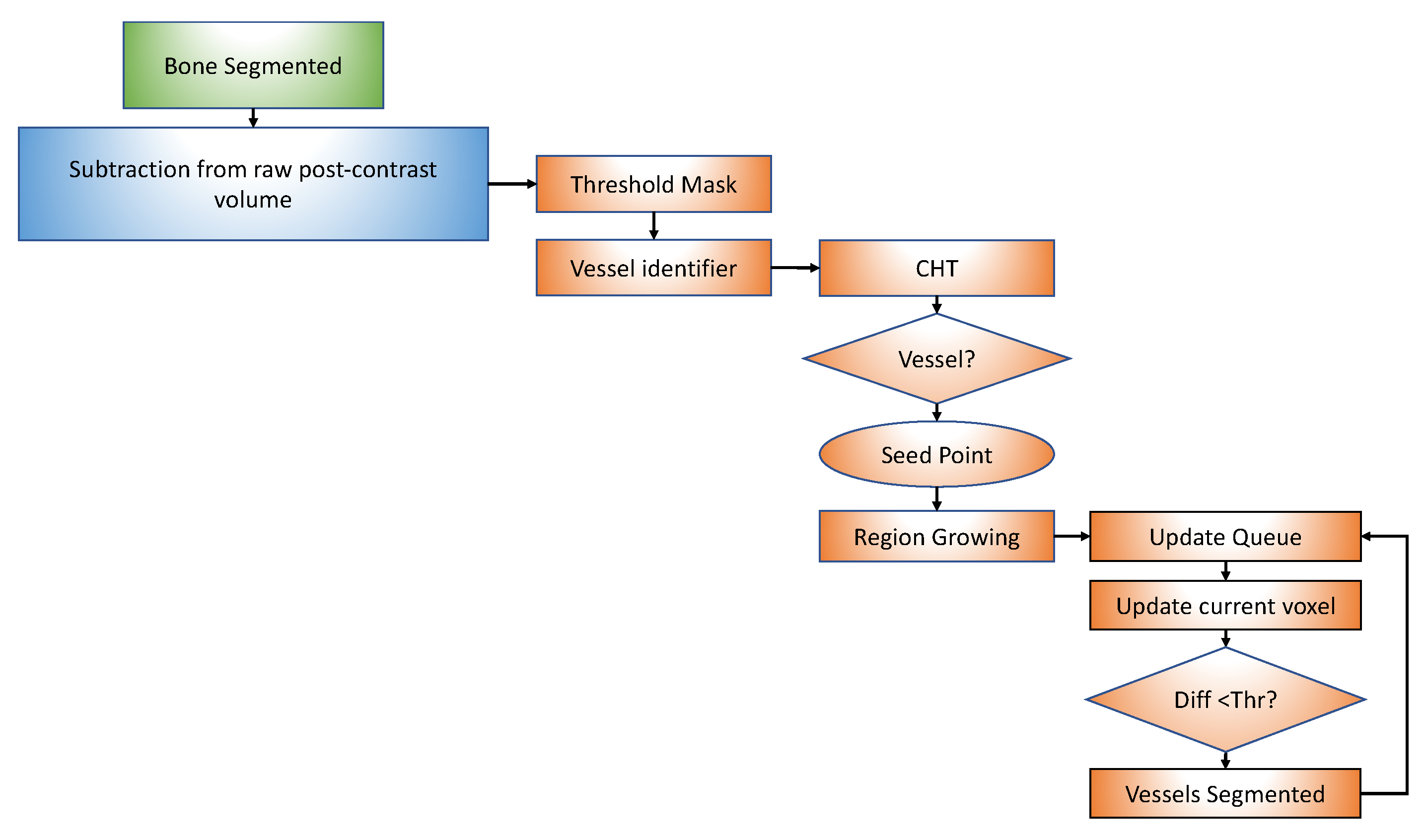
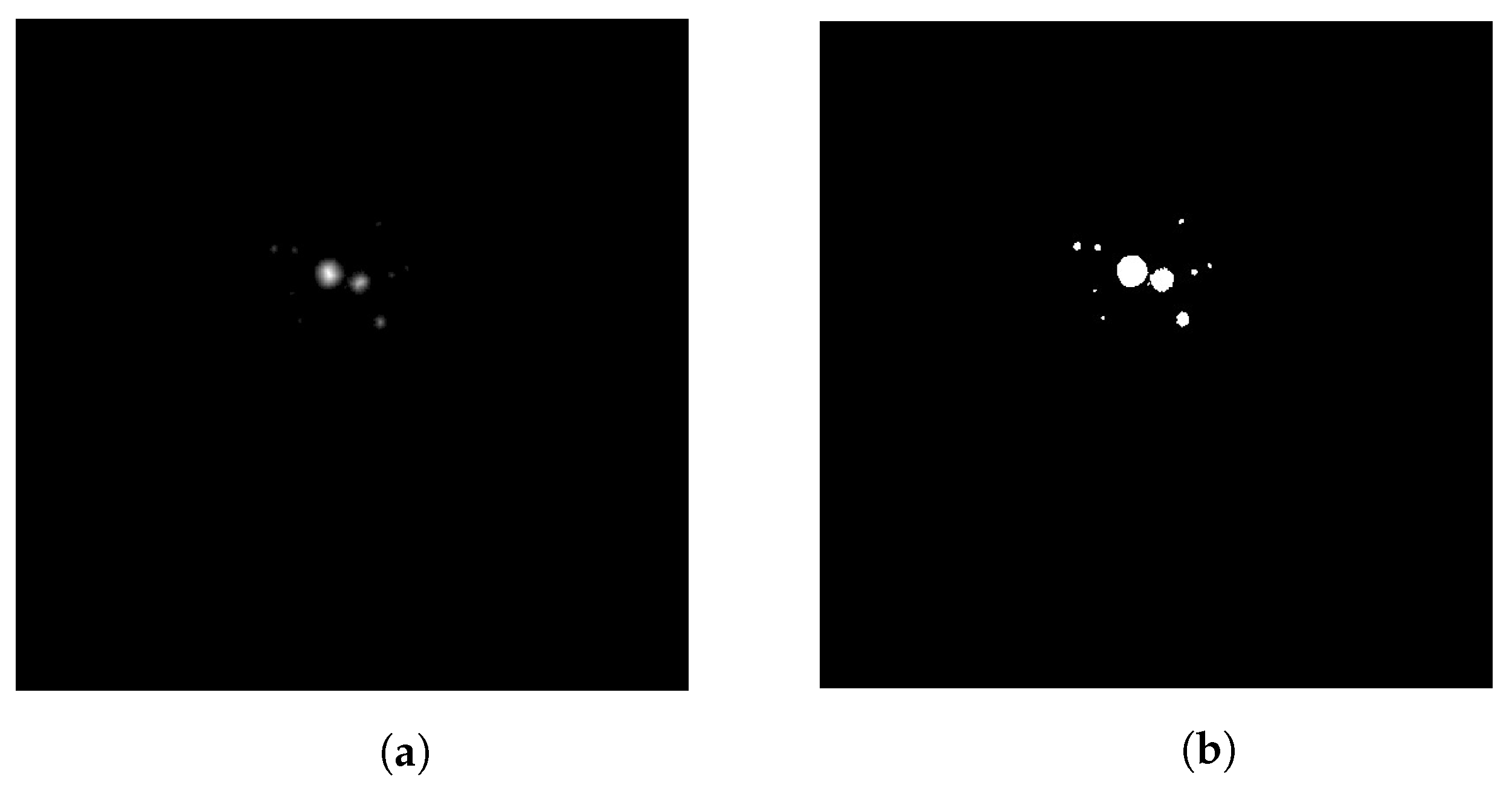
2.1. Segmentation
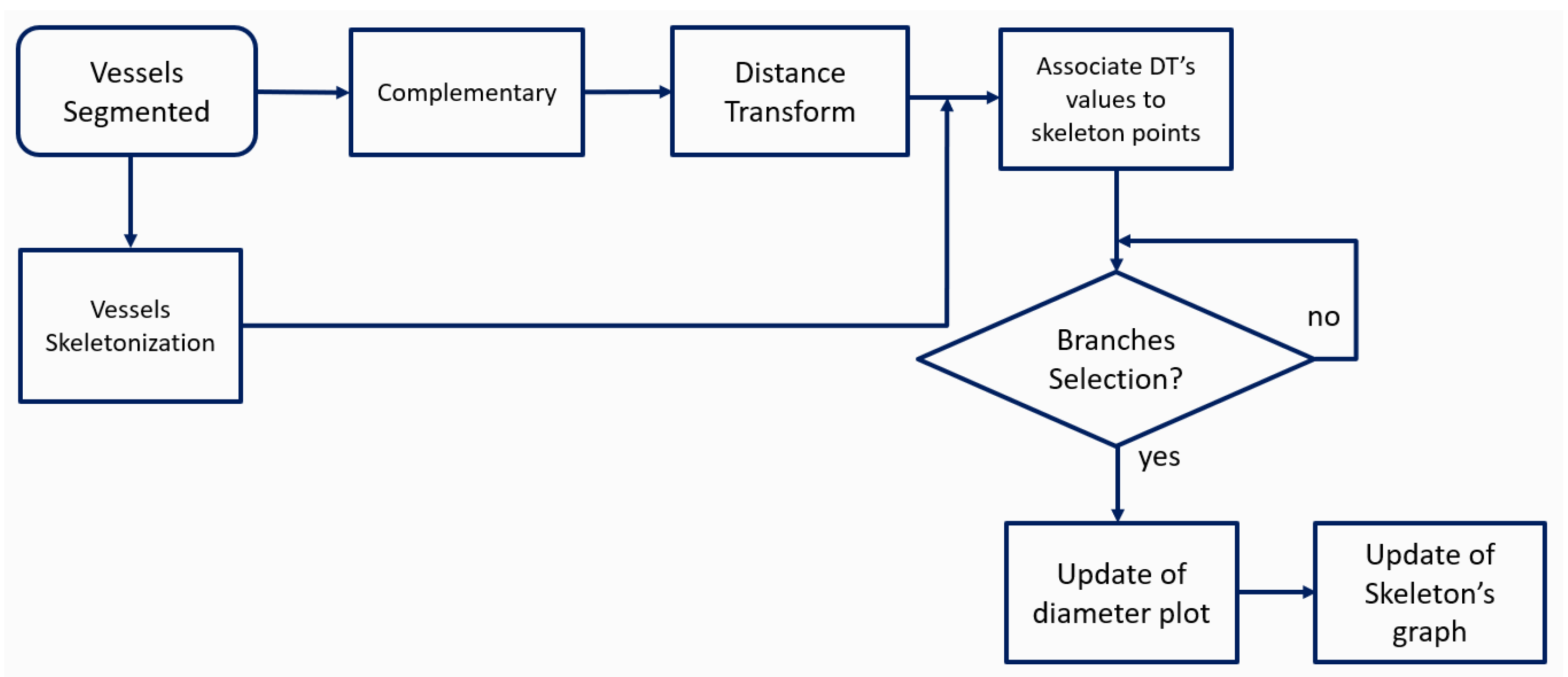
2.2. Skeletonization
2.3. Diameter Analysis
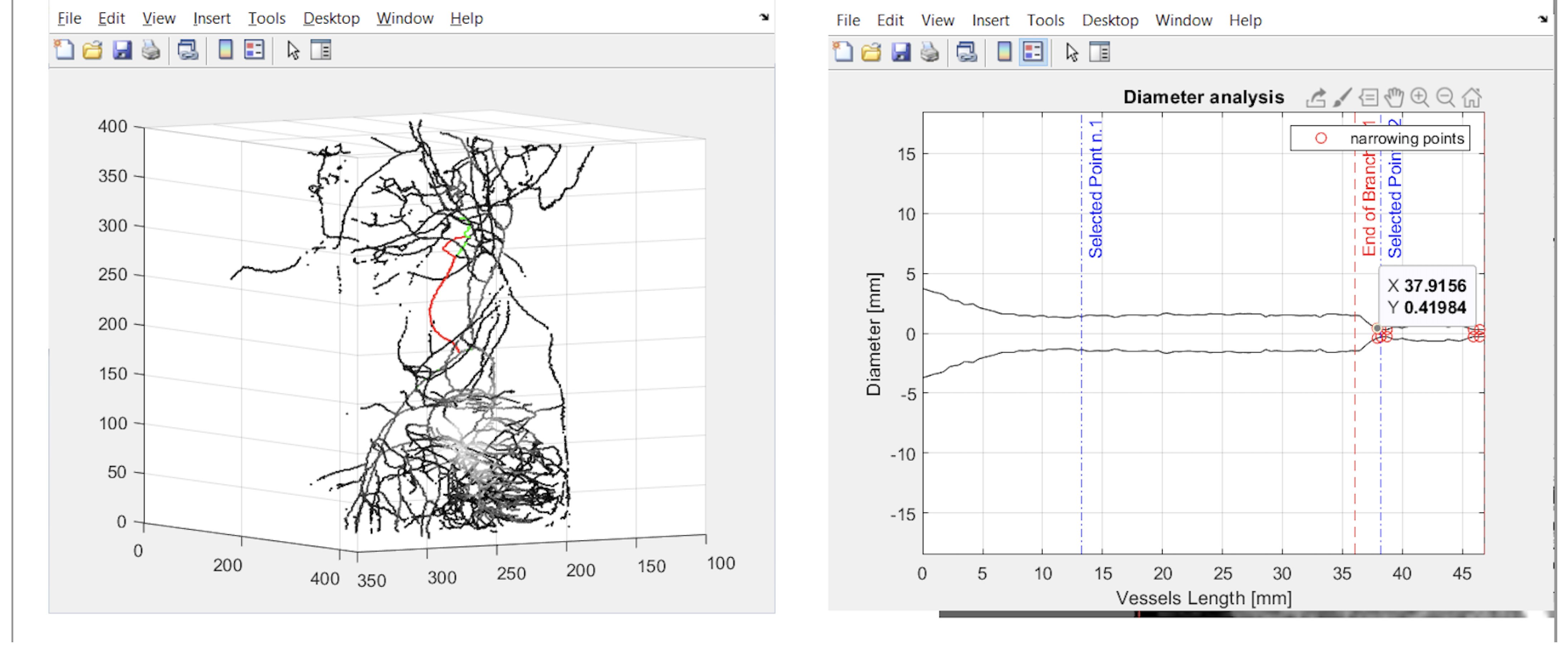
2.4. User Interface
2.5. Detection of Suspected Stenosis
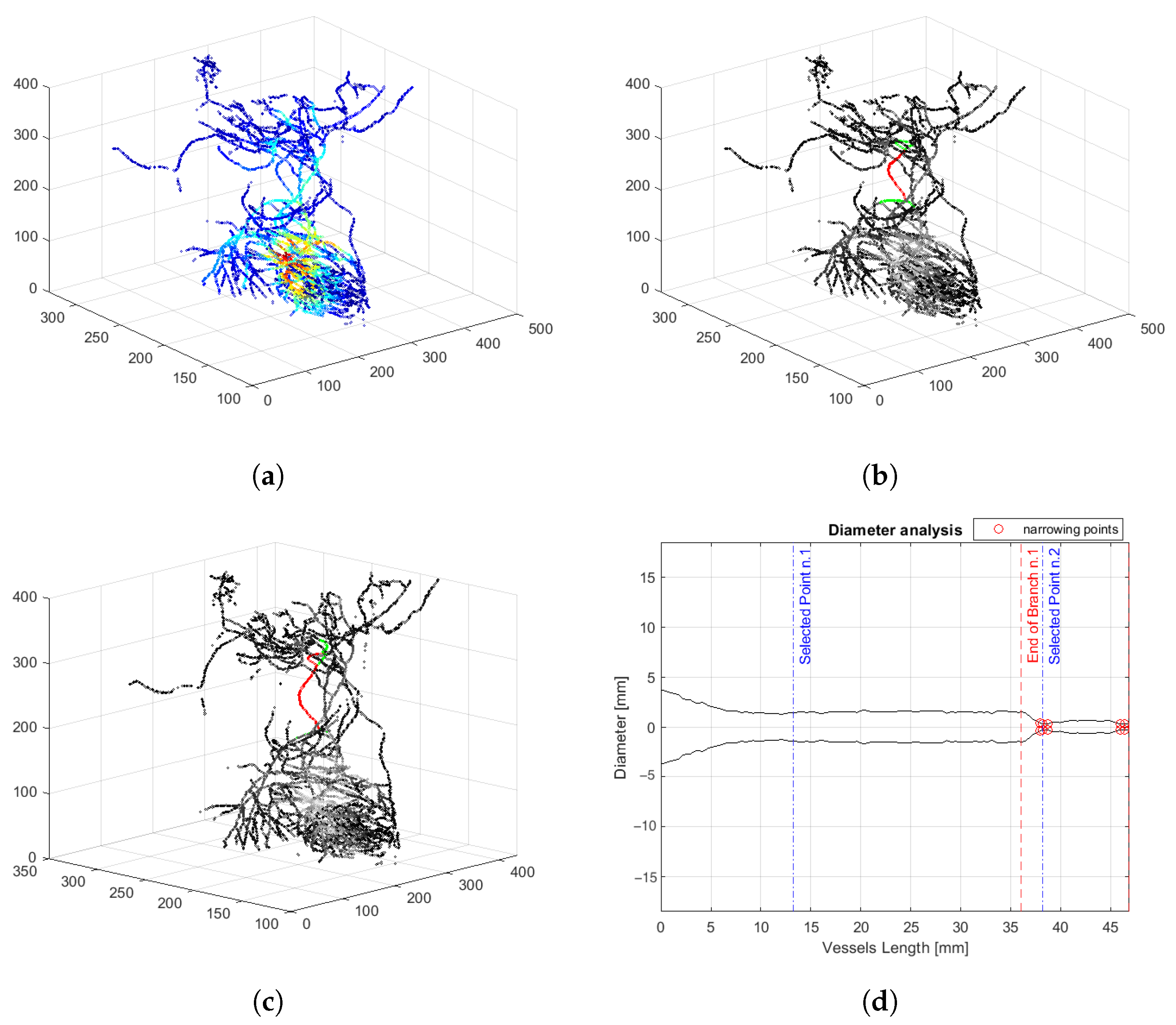
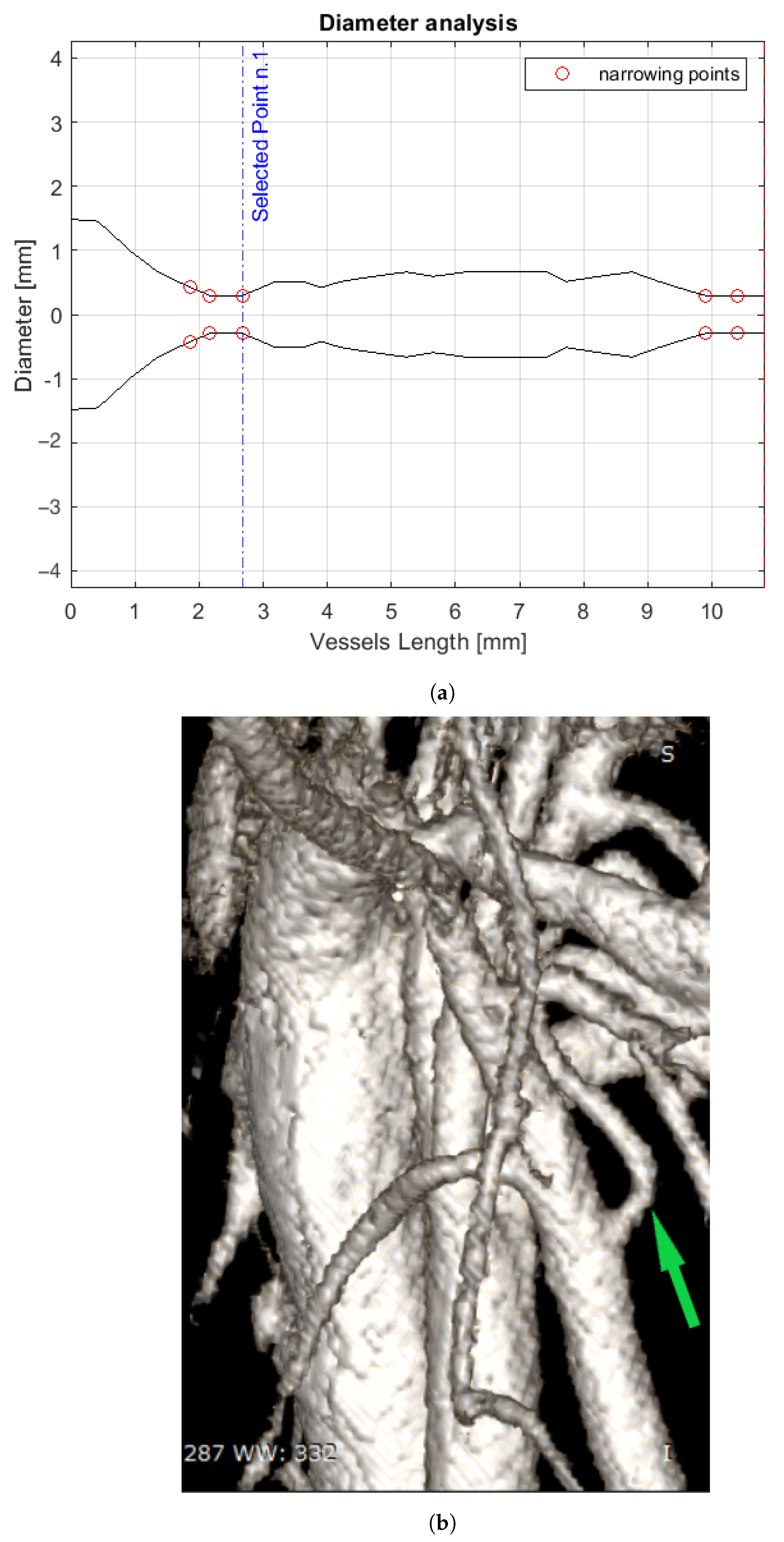
3. Results
4. Discussion
5. Conclusions and Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cleary, K.; Peters, T.M. Image-guided interventions: Technology review and clinical applications. Annu. Rev. Biomed. Eng. 2010, 12, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T. Roles of universal three-dimensional image analysis devices that assist surgical operations. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Stella, F.; Dolci, G.; Dell’Amore, A.; Badiali, G.; De Matteis, M.; Asadi, N.; Marchetti, C.; Bini, A. Three-dimensional surgical simulation-guided navigation in thoracic surgery: A new approach to improve results in chest wall resection and reconstruction for malignant diseases. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Tiribilli, E.; Iadanza, E.; Lorenzetto, C.; Manetti, L.; Bocchi, L. A Novel Implementation of Road Mapping from Digital Subtraction Angiography Images. In Proceedings of the CMBEBIH 2021, Mostar, Bosnia and Herzegovina, 21–24 April 2021. [Google Scholar]
- Porumb, M.; Iadanza, E.; Massaro, S.; Pecchia, L. A convolutional neural network approach to detect congestive heart failure. Biomed. Signal Process. Control. 2020, 55, 101597. [Google Scholar] [CrossRef]
- Neumuth, T. Surgical process modeling. Innov Surg Sci 2017, 2, 123–137. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Chan, H.H.; Frommlet, F.; Schweiger, T.; Keshavjee, S.; Waddell, T.K.; Klepetko, W.; Irish, J.C.; Yasufuku, K. 3D Models in the Diagnosis of Subglottic Airway Stenosis. Ann. Thorac. Surg. 2019, 107, 1860–1865. [Google Scholar] [CrossRef]
- Shi, C.; Sun, B.; Tang, G.; Xu, N.; He, H.; Ye, X.; Xu, G.; Gu, X. Clinical and radiological outcomes of endoscopic foraminoplasty and decompression assisted with preoperative planning software for lumbar foraminal stenosisSurgical process modeling. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 1829–1839. [Google Scholar] [CrossRef]
- Maragiannis, D.; MS, J.; Igo, S.; Schutt, R.; Connell, P.; Grande-Allen, J.; Barker, C.; Chang, S.; Reardon, M.; Zoghbi, W.; et al. Replicating Patient-Specific Severe Aortic Valve Stenosis With Functional 3D Modeling. Int. J. Comput. Assist. Radiol. Surg. 2015, 8, e00362. [Google Scholar] [CrossRef]
- Blankenhorn, D.H.; Kramsch, D.M. Reversal of atherosis and sclerosis. The two components of atherosclerosis. Circulation 1989, 79, 1–7. [Google Scholar] [CrossRef]
- Sorelli, M.; Perrella, A.; Bocchi, L. Detecting vascular age using the analysis of peripheral pulse. IEEE Trans. Biomed. Eng. 2018, 65, 2742–2750. [Google Scholar] [CrossRef]
- Selzer, R.H.; Mack, W.J.; Lee, P.L.; Kwong-Fu, H.; Hodis, H.N. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis 2001, 154, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Dobbe, J.G.; Streekstra, G.J.; Atasever, B.; Van Zijderveld, R.; Ince, C. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med. Biol. Eng. Comput. 2008, 46, 659. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Hazim Alkawaz, M.; Rehman, A.; Saba, T. Retinal imaging analysis based on vessel detection. Microscopy research and technique 2017, 80, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Kovács, G.; Hajdu, A. A self-calibrating approach for the segmentation of retinal vessels by template matching and contour reconstruction. Med. Image Anal. 2016, 29, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Sorelli, M.; Perrella, A.; Bocchi, L. Cardiac pulse waves modeling and analysis in laser doppler perfusion signals of the skin microcirculation. In Proceedings of the IFMBE Proceedings, Sarajevo, Bosnia and Herzegovina, 16–18 March 2017; Volume 62, pp. 20–25. [Google Scholar]
- Rogai, F.; Manfredi, C.; Bocchi, L. Metaheuristics for specialization of a segmentation algorithm for ultrasound images. IEEE Trans. Evol. Comput. 2016, 20, 730–741. [Google Scholar] [CrossRef]
- Simoni, A.; Tiribilli, E.; Lorenzetto, C.; Manetti, L.; Iadanza, E.; Bocchi, L. 3D Vessel Segmentation in CT for Augmented and Virtual Reality. In Proceedings of the Mediterranean Forum—Data Science Conference. Springer Computer Science, Sarajevo, Bosnia and Herzegovina, 24 October 2020. [Google Scholar] [CrossRef]
- Babin, D.; Pižurica, A.; Velicki, L.; Matić, V.; Galić, I.; Leventić, H.; Zlokolica, V.; Philips, W. Skeletonization method for vessel delineation of arteriovenous malformation. Comput. Biol. Med. 2018, 93, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Kollmannsberger, P.; Kerschnitzki, M.; Repp, F.; Wagermaier, W.; Weinkamer, R.; Fratzl, P. The small world of osteocytes: Connectomics of the lacuno-canalicular network in bone. New J. Phys. 2017, 19, 073019. [Google Scholar] [CrossRef]
- Felzenszwalb, P.F.; Huttenlocher, D.P. Distance transforms of sampled functions. Theory Comput. 2012, 8, 415–428. [Google Scholar] [CrossRef]
- Barcali, E.; Iadanza, E.; Manetti, L.; Francia, P.; Nardi, C.; Bocchi, L. Augmented Reality in Surgery: A Scoping Review. Appl. Sci. 2022, 12, 6890. [Google Scholar] [CrossRef]
- Vilser, W. Retinal vessel analyzer (RVA)-a new measuring system for examination of local and temporal vessel behaviour. Investig. Ophthalmol. Vis. Sci. 1997, 38, 678. [Google Scholar] [CrossRef]
- Heneghan, C.; Flynn, J.; O’Keefe, M.; Cahill, M. Characterization of changes in blood vessel width and tortuosity in retinopathy of prematurity using image analysis. Med. Image Anal. 2002, 6, 407–429. [Google Scholar] [CrossRef] [PubMed]
- Boskamp, T.; Rinck, D.; Link, F.; Kummerlen, B.; Stamm, G.; Mildenberger, P. New vessel analysis tool for morphometric quantification and visualization of vessels in CT and MR imaging data sets. Radiographics 2004, 24, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.; Salerno, S.; Molteni, R.; Occhipinti, M.; Grazzini, G.; Norberti, N.; Cordopatri, C.; Colagrande, S. Radiation dose in non-dental cone beam CT applications: A systematic review. Radiol. Medica 2018, 123, 765–777. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simoni, A.; Barcali, E.; Lorenzetto, C.; Tiribilli, E.; Rastrelli, V.; Manetti, L.; Nardi, C.; Iadanza, E.; Bocchi, L. Innovative Tool for Automatic Detection of Arterial Stenosis on Cone Beam Computed Tomography. Appl. Sci. 2023, 13, 805. https://doi.org/10.3390/app13020805
Simoni A, Barcali E, Lorenzetto C, Tiribilli E, Rastrelli V, Manetti L, Nardi C, Iadanza E, Bocchi L. Innovative Tool for Automatic Detection of Arterial Stenosis on Cone Beam Computed Tomography. Applied Sciences. 2023; 13(2):805. https://doi.org/10.3390/app13020805
Chicago/Turabian StyleSimoni, Agnese, Eleonora Barcali, Cosimo Lorenzetto, Eleonora Tiribilli, Vieri Rastrelli, Leonardo Manetti, Cosimo Nardi, Ernesto Iadanza, and Leonardo Bocchi. 2023. "Innovative Tool for Automatic Detection of Arterial Stenosis on Cone Beam Computed Tomography" Applied Sciences 13, no. 2: 805. https://doi.org/10.3390/app13020805
APA StyleSimoni, A., Barcali, E., Lorenzetto, C., Tiribilli, E., Rastrelli, V., Manetti, L., Nardi, C., Iadanza, E., & Bocchi, L. (2023). Innovative Tool for Automatic Detection of Arterial Stenosis on Cone Beam Computed Tomography. Applied Sciences, 13(2), 805. https://doi.org/10.3390/app13020805







