Abstract
The present study aimed to assess the function of muscular and nervous systems in high-maneuvering jet fighters with the chosen method of clinical neurophysiology, which methodologically has not yet been presented in detail. Fifteen pilots with the experience of more than 1700 flying hours at 7G overloading on average and fifteen healthy subjects for the comparison of health status declared to participate in this study. The dermatomal perception from C4 to S1 was examined using von Frey’s filaments tactile method. Surface electromyography (sEMG) recordings examined the activity of proximal and distal muscles in the upper and lower extremities, the transmission of motor nerve impulses peripherally was diagnosed by electroneurography (ENG), the efferent transmission from C5–C7 and L4–L5 spinal centers to muscles was entirely verified with recordings of motor-evoked potentials induced oververtebrally with the magnetic field (MEP). The pilots estimated more lumbosacral than cervical pain at about 2 on the 10-point visual analog scale (VAS). Sensory perception studies did not reveal abnormal symptoms in the C2–S1 dermatomes innervation. Clinical neurophysiology studies indicated, in general, the lack of pathology during sEMG tests in comparison to healthy subjects or even better muscle motor unit contractile properties in pilots, both in the upper and lower extremities. In pilots, the parameters of ENG and MEP examinations show a statistically significant sensitivity for detecting the slight changes and their consequences in the transmission of neural impulses within L4–L5 ventral root fibers. The research results enable specifying the algorithm of future preventing rehabilitative treatment in high-maneuvering jet fighters with an average flight experience of 2000 h and working conditions at 7G on average. This study, for the first time, describes the application of a set of diagnostic neurophysiological methods with the particular importance of MEPs in the clinical evaluation of the jet fighters’ health status.
1. Introduction
It can be assumed that the overload accompanying the work of a high-maneuver jet fighter pilot may lead to changes in the biomechanics of the spine and cause changes in the transmission of nerve impulses in the fibers of the spinal roots. Moreover, it can be a source of pathological, generalized changes in peripheral nerve fiber conduction, and changes in the function of muscle motor units by neurogenic and/or overload origins. Additional expected clinical symptoms of the pathology may be a pain in the cervical and lumbosacral spine, sensory disturbances, or decreased muscle strength. Only a few papers describe the effectiveness of treatment of the mentioned pathologies in military aviators, with only a few kinesiotherapeutic methods that minimize the risk of abnormalities in the nervous and muscular systems [1]. Considering the G overloading influence, it can be supposed that there may be a maximum value beyond which the severity of pain does not increase. However, the intensity of exposure to overload is responsible for the stronger pain in the neck [2]. The literature describing the pathologies in high-maneuvering jet fighters focuses mainly on the cervical spine level. Rintala and co-workers point to persistent high overload forces and changes in head position resulting in musculoskeletal pathologies [3]. The combination of a tight cockpit where the pilot is sitting and works while wearing a helmet increases the loading and may cause acute or chronic pain in the spine and upper extremities [4]. The fact of loading by the auxiliary pilot’s equipment becomes essential; there are night-vision goggles or the helmet signaling system. Such devices are the most commonly reported cause of neck pain [5]. Drew considers that the exposure of the jet military fighter to overloading can cause pain in the cervical spine, reduces the pilot’s efficiency, and cause acute or chronic diseases [6]. Oksa and co-workers demonstrated the phenomenon of muscle fatigue, mainly innervated from the cervical spine, and increasing the risk of spine injury, thus reducing the effectiveness of air missions [7]. In their research, the muscle’s strength and the muscle’s motor unit activity decreased with the number of air maneuvers performed by the pilot. It has not been entirely explained the real cause of muscle pathology, the phenomenon of overload, or the secondary effects of damage to the nerve structures [8]. Most of the studies conducted so far used clinical assessment methods of pain intensity increase or muscle strength decline, based mainly on subjective questionnaire assessment [9]. These studies show that high-maneuvering jet fighters report severe pain after the flight; therefore, there is a growing concern about their operational ability [10].
The possibility of a correlation between age or the time of flying hours and the intensity of lumbosacral back pain was also underlined. Activities such as sitting and standing are also reported to be the most painful, relieved after stretching kinesiotherapy exercises [11]. Few studies describe the use of clinical neurophysiology techniques, including electromyography (EMG) to assess the activity of the muscles of the neck and upper or lower extremities, associated with increased activity during aerobatics or flight control [12]. Diagnostic tests of clinical neurophysiology used in the presented study are performed to confirm or deny the clinical diagnosis of the presence of pathological symptoms in the muscular system and abnormal transmission of nerve impulses in the central and peripheral nervous systems. Electroneurography (ENG) or motor-evoked potential induced with the magnetic field (MEP) studies were never or casuistically utilized in the evaluation of the health status of the high-maneuvering jet fighters. In cases where there are no obvious structural pathologies in the ultrasound image or high-resolution neuroimaging (computed tomography or magnetic resonance imaging), these tests may highlight the functional nature of the abnormality. No changes in MRI were observed in jet fighters after 13 years of flying fighter planes. The exposure to large overloads did not cause significant radiological changes in the spine [13].
To sum up, most of the authors of the works related to the described problems point to the necessity to undertake further detailed research with the use of objective methods based on measurable functional tests on a larger population of pilots. Such criteria are met by electroneurographic studies of impulse conduction within the nerve fibers of the upper and lower extremities peripherally and within the spinal roots induced by electrical impulses (ENG) [14,15]. In turn, the determination of the correct or altered impulse transmission parameters from the level of the spinal motor center in the cervical or lumbosacral spinal cord directly to the muscles is possible in clinical neurophysiology tests using the method of recording motor potentials induced by magnetic field pulses (MEP) [16,17]. In the available world literature, the above-mentioned methods were not used to assess the efficiency of impulse conduction in the peripheral and central nervous systems in pilots of high-maneuvering fighters. The present study is intended to determine whether the above-mentioned functional abnormalities, even of a subclinical nature, can be diagnosed with complementary methods of clinical neurophysiology in high-maneuvering fighter pilots.
This study aimed to assess the function of muscular and nervous systems in high-maneuvering jet fighters with the chosen methods of clinical neurophysiology, surface electromyography (sEMG), electroneurography (ENG), and recordings of motor-evoked potentials induced oververtebrally with the magnetic field (MEP), in comparison to the healthy volunteers.
2. Materials and Methods
2.1. Participants
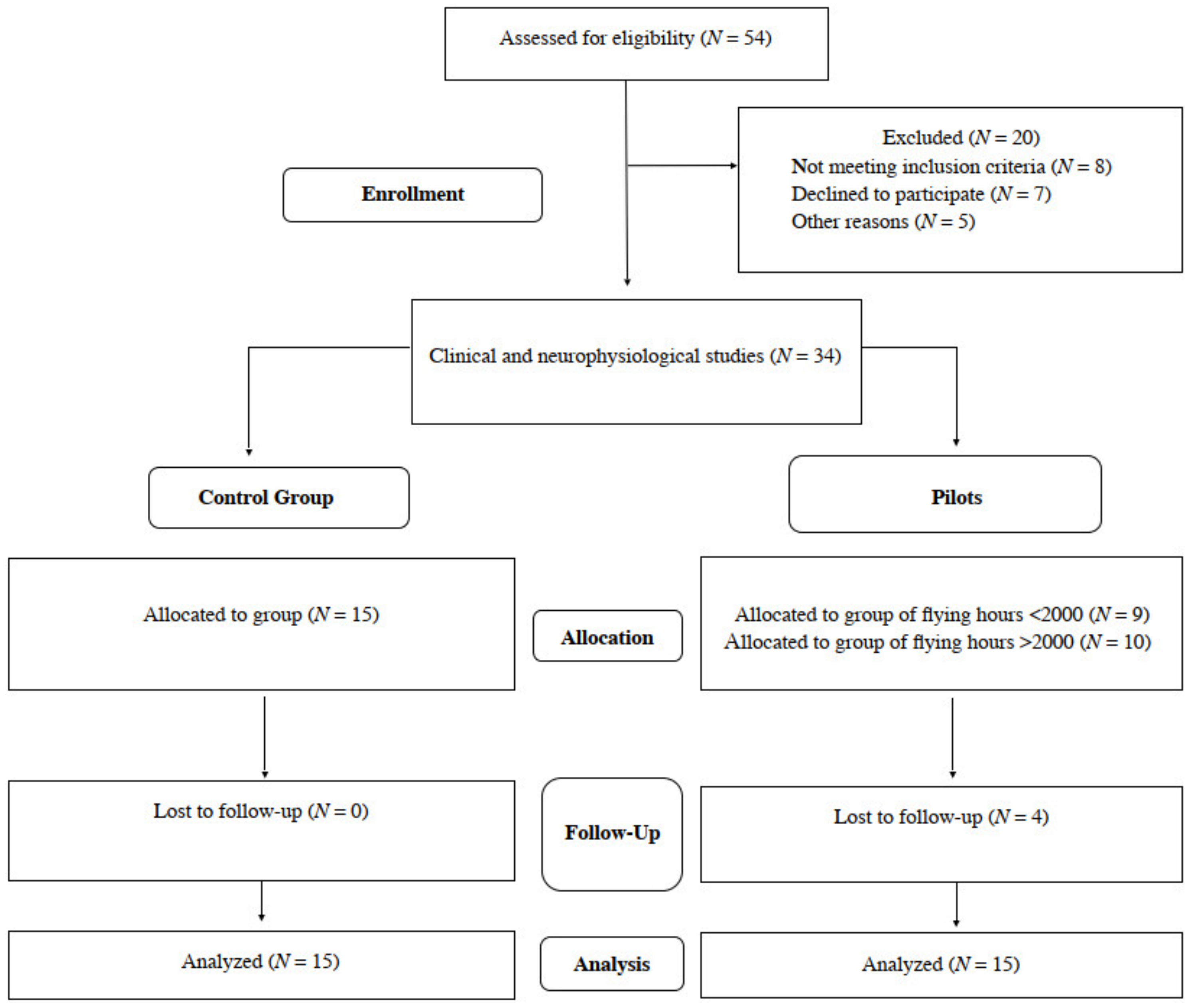
We preliminarily recruited a total number of 54 male subjects, both F-16 pilots and healthy volunteers. Information on certain steps of the study is provided in Figure 1. The main inclusion criteria were the general good health status, especially no episodes of epilepsy and other neurological syndromes, severe disorders of the cardiovascular system, electronic implants such as pacemakers and cochlear implants, inflammatory diseases and spine or head traumas, and COVID-19 related symptoms. Special attention was paid to the similar body constitution of all preliminary recruited subjects. Ethical considerations were in agreement with the Helsinki Declaration. Approval was also received from the Bioethical Committee of the University of Medical Sciences in Poznań, Poland (including studies on healthy people, decisions No 554/17 and 942/21). All subjects understood that there was no financial benefit from participation, and they signed a written consent form for voluntary participation in the study. Twenty out of fifty-four subjects were excluded from the project, they did not meet the inclusion criteria, declined to participate during this stage of enrollment or were ill with COVID-19. All of them were right-handed. Finally, the same clinical neurophysiology tests were conducted once on 19 pilots and 15 healthy subjects to obtain reference values.
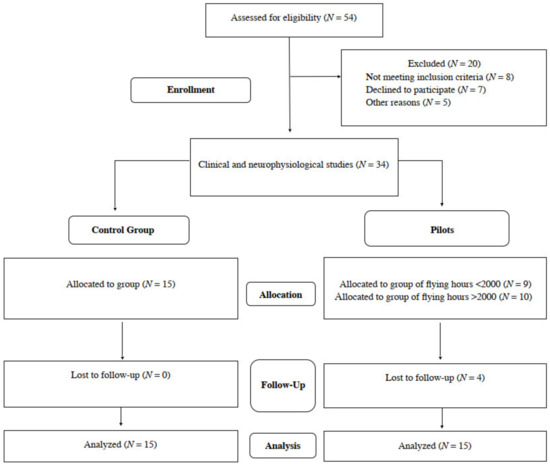
Figure 1.
Flow chart of the study.
In 19 jet fighters with an average flight experience of 1711.7 h, working in conditions of 7G in an average, with an average age of 38.2 years, with similar anthropometric characteristics, a confidential interview was conducted regarding seniority, physical activity and the occurrence of pain in the VAS scale. The group of 19 pilots was then divided according to the number of flying hours. The value of 2000 h has become the limit. Nine subjects were below this value, ten were above. Unfortunately, the continuity of research was lost in 4 pilots. Finally, 15 of them took part in the research utilizing the neurophysiological evaluation, also performed in the same fashion to the population of healthy people (control group with the similar demographic and anthropometric properties).
2.2. Clinical Neurophysiology Tests
Clinical neurophysiology studies were performed using the integrated KeyPoint system (Dantec, Skovlunde, Denmark) and MagPro (Medtronic A/S, Denmark), in an air-conditioned room with an average temperature of 22 °C, according to the guidelines of The International Federation of Clinical Neurophysiology, European Chapter.
Clinical evaluation of symptoms typical for the consequences of disc-root conflicts at cervical and lumbosacral spine included assessment of pain intensity in a ten-points VAS scale and the numbness in tips of fingers and toes.
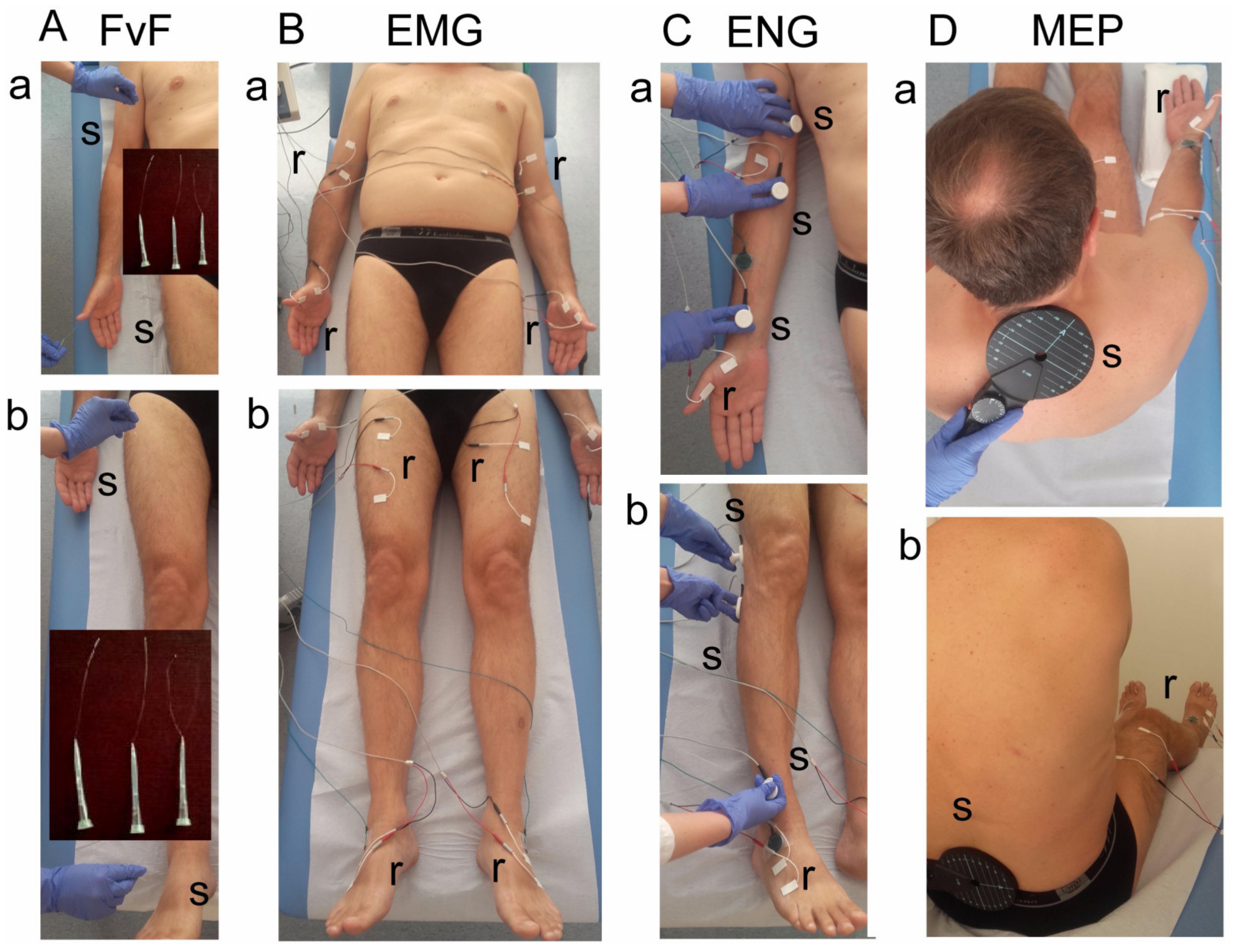
Using the method of examining the sensory perception with von Frey filaments (FvF, 0—decreased, 1—normal), the functional state of dermatomal innervation in the range C2–S1 was bilaterally examined (Figure 2A). It was performed during three-touch trials at areas of dermatomal innervations in upper (Figure 2A(a)) and lower (Figure 2A(b)) extremities. If the subject reports the touch sensation applied to skin twice, it is assumed as a positive test result. Perception evoked by pressure with filament of 0.30 mm in diameter corresponds to normal perception, with 0.12 mm to hyperesthesia and with 0.55 mm to analgesia.
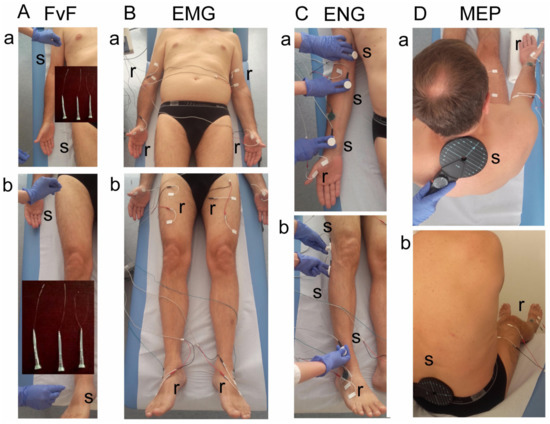
Figure 2.
Photograph illustrating the methodological details of clinical neurophysiology diagnostic tests. (A)—Method of tactile testing the sensory perception with von Frey’s filaments (FvF) within the dermatomes of (a) upper and (b) lower extremities. (B)—Electromyographic (sEMG) testing of motor units’ activity using the surface electrodes with the locations over muscles of (a) upper and (b) lower extremities. (C)—Electroneurographic (ENG) recordings of the evoked potential from muscles of (a) upper and (b) lower extremities with the surface electrodes following the stimulation of median and peroneal nerves along their anatomical passage, respectively. (D)—The method of motor evoked potentials (MEP) recordings induced by the single magnetic stimuli applied oververtebrally to the spinal cord centers at the (a) cervical and (b) lumbar levels. Abbreviations: r—recording, s—stimulation.
The function of the motor units in the selected proximal and distal muscles of the upper and lower extremities in a relaxed state (rEMG) and with the attempt of maximal contraction for 5 s (mcEMG) was examined by electromyography (sEMG) (Figure 2B). A pair of standard disposable Ag/AgCl surface electrodes with an active area of recording of 5 mm2 were applied bilaterally over the skin of examined muscles (the biceps brachii—BIC, the abductor pollicis brevis—APB, the rectus femoris—RECT muscles). The ground electrodes were placed on the distal part of the hand and leg. The upper 10 kHz and lower 20 Hz filters of the recorder were used. During the first stage of the examination, the patient was asked to fully relax muscles, and then to perform a maximal contraction for 5 s during which the simultaneous recording took place. Participants were instructed to contract the tested muscle as hard and quickly as possible until the neurophysiologist requested them to stop the attempt. The test was performed in triplicate, with a one-minute interval between each muscle contraction. The recording with the highest amplitude (measured peak-to-peak with the reference to isoelectric line in µV) and frequency (in Hz) parameters was chosen for the final analysis. Modified frequency index (FI, 3-0)—frequency of motor units action potentials recruitment during maximal contraction in sEMG recording; 3—95–70 Hz—normal; 2—65–40 Hz—moderate abnormality; 1—35–10 Hz—severe abnormality; 0—no contraction) was calculated using the automatic analysis software included in the KeyPoint system, compared to the online readings of sEMG recordings [14,15,16,17,18,19]. The sEMG recordings were made at the base time of 80 ms/D and the amplification of 20–1000 µV/D.
The conduction of nerve impulses in the motor fibers of nerves in the upper and lower extremities was diagnosed bilaterally by electroneurography (ENG, recordings of M and F waves in evoked potentials) (Figure 2C). Nerves were stimulated transcutaneously along their anatomical passage with the single electrical pulses in a rectangular shape, duration of 0.2 ms, at 1 Hz, and the intensity from 10 to 80 mA. ENG recordings were collected at the amplification of 100–5000 μV and a time base of 8 ms with the surface electrodes from APB and EXT muscles. The outcome measures were the parameters of amplitudes (in µV) and latencies (in ms) of M-waves (CMAP) and F-waves frequencies (not less than 14, during evoking 20 positive, successive recordings of M-waves). They aimed to evaluate the function of motor nerve fibers peripherally and the transmission of neural impulses in the spinal ventral roots, respectively.
The efferent transmission of neural impulses from the level of spinal center to the proximal and distal muscles of upper and lower extremities was bilaterally investigated using the motor-evoked potentials recording method (MEPs) (Figure 2D). They were induced by a single, sinusoidal, 5 ms duration magnetic stimulus released from the magnetic coil placed oververtebrally (C-100, 12 cm in diameter) at cervical and lumbosacral levels and recorded with surface electrodes from BIC, APB, RECT and EXT muscles. The magnetic field stream strength was 70–80% of the maximal stimulus output (2.4 T); the expected depth of stimulation was 3–5 cm deep. The parameters of amplitudes (in µV) and latencies (in ms) of MEPs were the main outcome measures. MEPs were usually recorded at the time base of 10 ms/D and amplification at 100–2000 µV; low-frequency 20 Hz and high-frequency 10 kHz filters were used in a KeyPoint recorder. A bandwidth of 10 Hz to 1000 Hz and digitalization at 2000 samples per second and channel were used during recordings.
Methodological details, analysis and interpretation of the results from VAS, von Frey’s filaments, sEMG, ENG and MEPs in the healthy people and the patients with symptoms of disc-root conflicts at the cervical and lumbosacral levels are described elsewhere [14,15,16,17,18,19,20,21].
2.3. Data Analysis and Statistics
Data were analyzed with Statistica software version 13.1 (StatSoft, Kraków, Poland). Descriptive statistics were reported as minimal and maximal values (range), mean or median and standard deviations (SD). The Shapiro–Wilk test was used to assess the normality of distributions in the test score. Wilcoxon’s signed-ranks test was conducted to compare the differences between results on the right and left side. The Friedman test was used to determine whether there were any differences between the measurements performed in pilots and healthy subjects. A post hoc analysis was used in the cases when there were statistically significant differences in the measurements. p-values ≤ 0.05 were considered statistically significant or at least at the level of significance. The statistical software was used to determine the required sample size using the primary outcome variable of sEMG amplitude recorded from APB and EXT muscles during maximal contraction with a power of 80% and a significance level of 0.05 (two-tailed). The mean and standard deviation (SD) were calculated using the data from the first seven patients and the sample size software estimated that more than ten pilots and ten healthy volunteers were needed for purposes of this study.
3. Results
The results of demographic and anthropometric data collection, the questionnaire and clinical studies are shown in Table 1. The pilots and the healthy volunteers did not differ significantly in demographic and anthropometric characteristics.

Table 1.
Demographic and anthropometric properties, results of reports and clinical studies in pilots and healthy subjects. Ranges, median or mean values with standard deviations are presented.
The pilots conducted moderate physical activity (training; moderate stretching exercises, bicycling, swimming) with a frequency of about two times a week, similar to the healthy subjects who were mainly the office workers (N = 7) than physical workers (N = 8). Only one pilot reported the incidence of a weak trauma, requiring short-lasting conservative treatment which could not permanently influence the general health status on the day of examination. Pilots experienced 1711 h of flying the high-maneuvering aircraft at 7G over-loading, all of them had four catapulting trainings, on average.
The intensity of pain with the occurrence onset at 1.7 years on average in the cervical and the lumbosacral region was rated as 1.4 on average on the 10-point VAS scale by pilots Table 1). The studies of sensory perception (FvF) did not reveal any symptoms of hyperesthesia or hypoaesthesia in the area of C2–S1 dermatomes on both sides. Neither the pilots nor the healthy volunteers complained of symptoms of pain or numbness in the distal areas of the upper and lower limbs on both sides.
Based on the data from the questionnaire (Table 2), the pilots with a number of flying hours <2000 reported the symptom of spine pain with a 0.88 intensity on average, while the pilots with a number of flying hours >2000 scored 1.67 on average. The twice as high pain level in pilots with a number of flying hours >2000 was not related to the frequency of weekly activity; similarly, about 2 h in all studied pilots.

Table 2.
Data on the number of flying hours, the physical activity and the symptoms of the spine pain evaluated in VAS among 19 studied pilots.
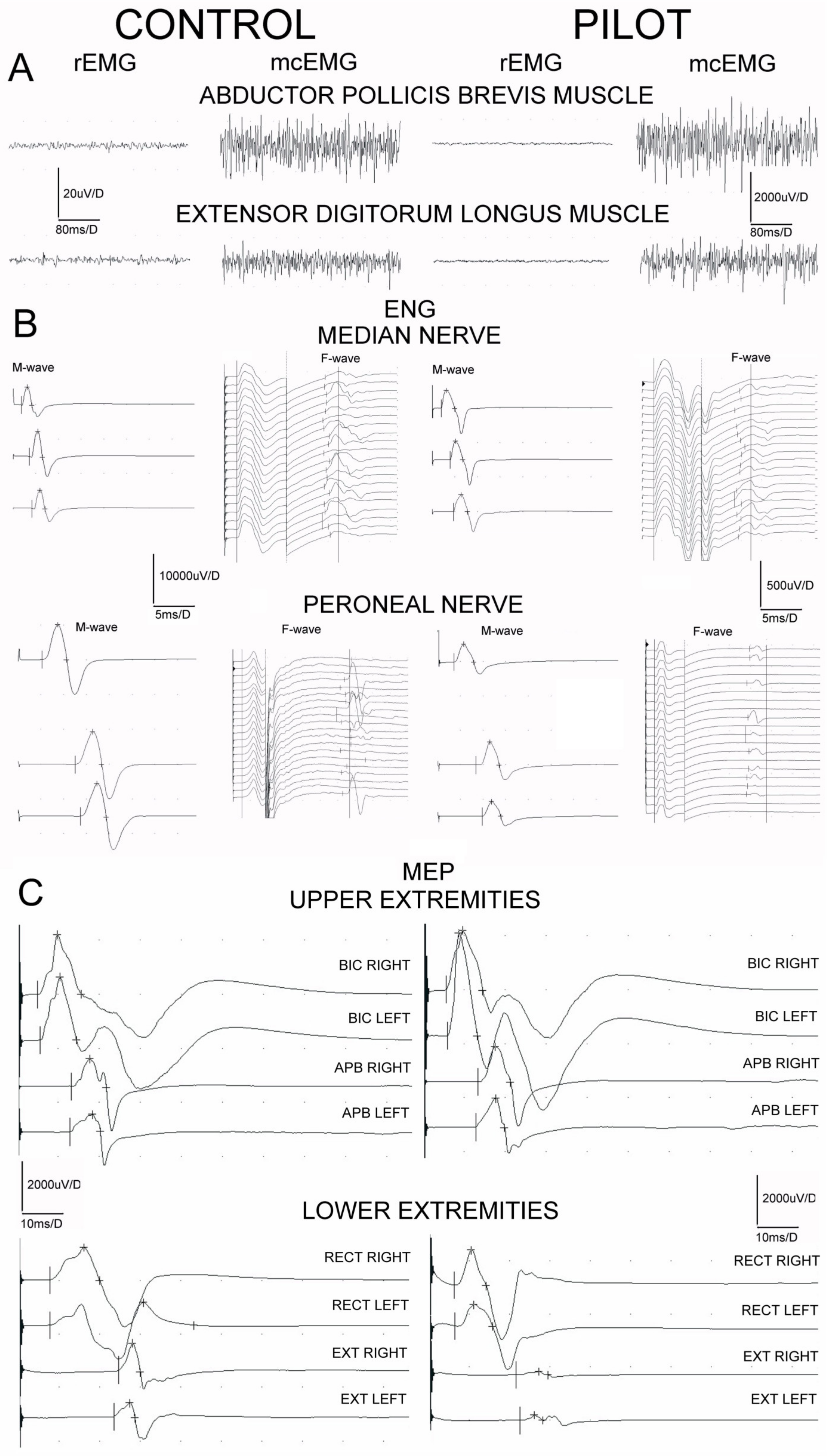
Examples of the recordings from neurophysiological studies performed in one of the pilots in comparison to one of the control subjects are presented in Figure 3. Table 3 summarizes the most significant differences found in sEMG, ENG, and MEPs recordings in two studied groups of subjects. The mean amplitudes of sEMG recordings performed at rest (rEMG) in all participants never reached values ≥25 μV, which proves no pathological symptoms of the increased muscle tension. The values of amplitudes of sEMG recordings during the attempt of maximal contraction (mcEMG) have been found to be significantly higher in all examined muscle groups in pilots at p = 0.02–0.04, pointing to the better ability of the muscle motor units for the contractile properties than in controls. No differences in FI indexes recorded in the two groups of examined subjects were found, indicating no pathological changes in the recruitment of the motor units of neurogenic origin.
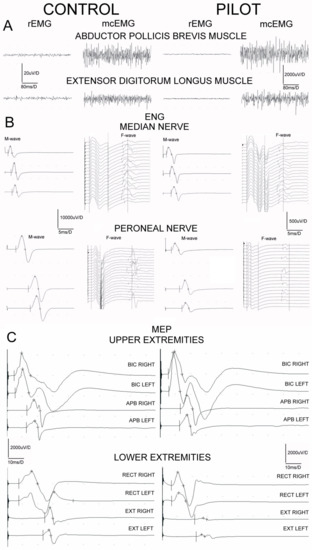
Figure 3.
Examples of electromyographic recordings ((A), sEMG) at rest (rEMG) and during the attempt of 5 s lasting maximal contraction (mcEMG), electroneurographical examinations ((B), ENG) and motor evoked potential recordings ((C), MEP) obtained in one of the healthy volunteers and in one pilot from the studied group for comparison. Calibration bars of amplitudes and time bases of recordings are the same for all sEMG, ENG and MEP recordings, subsequently.

Table 3.
Comparison of the results of neurophysiological tests in healthy people (N = 15) and pilots (N = 15). The mean values with standard deviations are presented. P—significant differences are marked bold. Arrow heads indicate increase (↑) or decrease (↓) the parameters recorded in pilots in comparison to the controls.
Results of the ENG studies in both groups of participants regarding the peripheral nerve impulses transmission in the motor fibers of median nerves and within C6 ventral roots bilaterally did not present clear-cut abnormalities in the parameters of M potentials (CMAP) amplitudes or recorded F wave frequencies. On the other hand, we have found significant (p = 0.05–0.04) decreases in the parameters of amplitudes and increases in the latencies parameters of recorded M potentials following peroneal nerves stimulations, which may suggest the moderate changes of axonal and demyelinating nature. They were related to the decreases in frequencies of the recorded F waves (at p = 0.05) suggesting the onset of abnormalities from the level of L4–L5 ventral roots.
Data presented at the bottom of Table 3 regarding parameters of MEPs recordings following the stimulation of the spinal motor centers at C6, C7 neuromeres do not indicate significant differences between the pilots and the healthy subjects. Differences in parameters of more amplitudes than latencies between pilots and controls have been found at p = 0.05–0.03, when MEPs induced oververtebrally excited L4 more than L5 spinal motoneurones and ventral roots and recordings were performed from the distal lower extremities muscles (see Figure 3C).
4. Discussion
The results of the presented research with the use of a set the clinical neurophysiology methods applied for diagnostic purposes of health status in high-maneuvering jet fighters are original and have not been fully described in the available literature so far. Except for this study, the results of motor transmission of impulses in peripheral nerves using electroneurography (ENG) and overall efferent transmission from the level of the motor center in the spinal cord to the muscles using motor potentials induced by a magnetic field (MEP) have not been presented in detail. Most reports describe pathological symptoms in pilots in the cervical spine diagnosed by subjective clinical methods of pain assessment [2,4,5,6] or by means of questionnaires [9,11], rarely with the use of electromyography or the range of movement and strength measurements in the examination of the neck and trunk muscles [7,12], and casuistically with the reference of the muscles of the pelvic girdle and lower extremities [12].
Based on our research results, it can be assumed that high-maneuver fighter pilots develop moderate ailments in the lumbosacral rather than cervical spine, with symptoms corresponding to the consequences of disc–root conflicts of various etiopathogenesis diagnosed using clinical neurophysiology methods and described in other studies [14,15,16,17,18,19]. It is likely that the effective methods of rehabilitation treatment of the consequences of cervical and back pain, as well as preventive methods limiting their development, should be the same ones that are applied in patients with symptoms of myofascial pain syndrome, disc–root conflict, and degenerative spine disease [1,14,15,18,22,23,24]. Early neurophysiological diagnosis of these syndromes in high-maneuver fighter pilots is all the more important because, in the early stages of the development of the ailment, there are no clear changes seen in the neuroimaging of the spinal structures [13]. Comparison of results from non-invasive neurophysiological tests may support monitoring and prognosis of the effectiveness of the treatment processes. Neurophysiological non-invasive diagnostic tests detecting the subclinical nature of abnormalities in the biomechanics of the spine may also be a reason to take action in the field of work ergonomics of a high-maneuvering fighter pilot [11].
Results of our study confirm observations of other researchers on the relation between the development of spine pain symptoms and the increasing number of flying hours and the overloading effect at 7G experienced by the high-maneuvering jet fighter pilot [3,4,6,8]. One of the most interesting aspects resulting from the health assessment of pilots presented in the study was the high efficiency of the motor units of the assessed muscle groups, and the ability to conduct impulses in the peripheral and central nervous systems, exceeding in some tests the standards of healthy volunteers to whom the results were compared. The reasons for such a high functional efficacy of the muscle and nervous systems should be sought in the high criteria of the initial qualification of fighter pilots according to the military psychomotor requirements and the care of the study participants about their health through regular exercises in accordance with the data from the interview that was conducted with them.
Our study has two main limitations. First of all, we have studied fifteen pilots only, who were not especially interested in the preliminary neuroimaging diagnostics and the neurophysiological testing in the absence of significant pain symptoms and changes in sensory perception or significant motor deficits. It should be also emphasized that participation in the study was anonymous and voluntary, participants during the recruitment process were randomly selected from a preliminary group of pilots and might abandon the project at any time. Moreover, the results of this work do not explain whether the discrete disturbances in the motor neural transmission detected in ENG and MEP studies were caused by the high overload itself at 7G accompanying the work of a high-maneuver jet fighter pilot or can be the secondary neurogenic consequences of the developed disc–root conflicts. An explanation of the above issues, including the influence of flying hours, requires further supplementary studies.
5. Conclusions
The long-lasting work of jet fighter pilots who are exposed to significant overload, in general, does not affect the function of the motor units of muscles in the upper extremities diagnosed on both sides with the electromyographic method, contrary to what has been found in this study in lower extremities. These factors also do not cause abnormalities in the sensory perception of the studied C2–S1 dermatomes nor in the transmission of impulses in motor fibers of median nerves and C6, C7 spinal ventral roots. On the other hand, electroneurographic examinations performed in pilots show changes in neural transmission typical for the moderate consequences of disc–root conflicts at the lumbosacral levels, also confirmed in neurophysiological diagnostics with the use of motor-evoked potentials induced oververtebrally with the magnetic field. The results of the research make it possible to specify the therapeutic algorithm of rehabilitation preventing the development of disc–root conflicts in high-maneuver fighter pilots with direct application to improving the ergonomics of pilots’ work.
Author Contributions
Conceptualization, A.W., P.D. and J.H.; Investigations, A.W. and J.H.; Methodology, J.H.; Software, J.H.; Validation, A.W., P.D. and J.H.; Formal analysis, A.W., P.D. and J.H.; Resources, P.D. and J.H.; Data curation, A.W., P.D. and J.H.; Writing—Original draft preparation, A.W., P.D. and J.H.; Writing—Review and editing, A.W. and J.H.; Visualization, A.W. and J.H.; Supervision, J.H.; Project administration, P.D. and J.H.; Funding acquisition, P.D. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee from the University of Medical Sciences (decision No 554/2017).
Informed Consent Statement
Written informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Green, B.N.; Dunn, A.S.; Pearce, S.M.; Johnson, C.D. Conservative management of uncomplicated mechanical neck pain in a military aviator. J. Can. Chiropr. Assoc. 2010, 54, 92–99. [Google Scholar] [PubMed]
- Kang, S.; Hwang, S.; Lee, E.T.; Yang, S.; Park, J. Measuring the cumulative effect of G force on aviator neck pain. Aviat. Space Environ. Med. 2011, 82, 1042–1048. [Google Scholar] [CrossRef]
- Rintala, H.; Häkkinen, A.; Siitonen, S.; Kyröläinen, H. Relationships Between Physical Fitness, Demands of Flight Duty, and Musculoskeletal Symptoms Among Military Pilots. Mil. Med. 2015, 180, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.S.; Jahr, K.I.; Rodskier, S. +Gz-induced spinal symptoms in fighter pilots: Operational and individual associated factors. Aviat. Space Environ. Med. 2012, 83, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Thoolen, S.J.J.; van den Oord, M.H.A.H. Modern air combat developments and their influence on neck and back pain in F-16 pilots. Aerosp. Med. Hum. Perform. 2015, 86, 936–941. [Google Scholar] [CrossRef]
- Drew, W.E.S. Spinal symptoms in aviators and their relationship to G-exposure and aircraft seating angle. Aviat. Space Environ. Med. 2000, 71, 22–30. [Google Scholar] [PubMed]
- Oksa, J.; Hämäläinen, O.; Rissanen, S.; Salminen, M.; Kuronen, P. Muscle fatigue caused by repeated aerial combat maneuvering exercises. Aviat. Space Environ. Med. 1999, 70, 556–560. [Google Scholar] [PubMed]
- Coakwell, M.R.; Bloswick, D.S.; Moser, R., Jr. High-risk head and neck movements at high G and interventions to reduce associated neck injury. Aviat. Space Environ. Med. 2004, 75, 68–80. [Google Scholar]
- Truszczyńska, A.; Lewkowicz, R.; Truszczyński, O.; Wojtkowiak, M. Back pain and its consequences among Polish Air Force pilots flying high performance aircraft. Int. J. Occup. Med. Environ. Health 2014, 27, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Verde, P.; Trivelloni, P.; Angelino, G.; Morgagni, F.; Tomao, E. Neck Pain in F-16 vs. Typhoon Fighter Pilots. Aerosp. Med. Hum. Perform. 2015, 86, 402–406. [Google Scholar] [CrossRef]
- Kelley, A.M.; Macdonnell, J.; Grigley, D.; Campbell, J.; Gaydos, S.J. Reported back pain in army aircrew in relation to airframe, gender, age, and experience. Aerosp. Med. Hum. Perform. 2017, 88, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hewson, D.J.; McNair, P.J.; Marshall, R.N. Aircraft control forces and EMG activity: Comparison of novice and experienced pilots during simulated take-off and landing. Aviat. Space Environ. Med. 1999, 70, 745–751. [Google Scholar] [PubMed]
- Sovelius, R.; Salonen, O.; Lamminen, A.; Huhtala, H.; Hämäläinen, O. Spinal MRI in fighter pilots and controls: A 13-year longitudinal study. Aviat. Space Environ. Med. 2008, 79, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Lisiński, P.; Huber, J. Evolution of muscles dysfunction from myofascial pain syndrome through cervical disc-root conflict to degenerative spine disease. Spine 2017, 42, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Lisiński, P. Early results of supervised versus unsupervised rehabilitation of patients with cervical pain. Int. J. Artif. Organs 2019, 42, 695–703. [Google Scholar] [CrossRef]
- Bryndal, A.; Wojtysiak, M.; Moskal, J.; Lipiec-Kowalska, J.; Borowczyk, M.; Tańska, M.; Grochulska, A.; Huber, J.; Majchrzycki, M. Motor evoked potentials after supraspinal stimulation in pre- and postoperative evaluations of patients with cervical radiculopathy. Biomed Res. Int. 2019, 2019, 4576493. [Google Scholar] [CrossRef] [PubMed]
- Wojtysiak, M.; Huber, J.; Wiertel-Krawczuk, A.; Szymankiewicz-Szukała, A.; Moskal, J.; Janicki, J. Pre- and postoperative evaluation of patients with lumbosacral disc herniation by neurophysiological and clinical assessment. Spine 2014, 39, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Lisinski, P.; Samborski, W.; Wytrazek, M. The effect of early isometric exercises on clinical and neurophysiological parameters in patients with sciatica: An interventional randomized single-blinded study. Isokinet. Exerc. Sci. 2011, 19, 207–214. [Google Scholar] [CrossRef]
- Huber, J.; Lisiński, P.; Polowczyk, A. Reinvestigation of the dysfunction in neck and shoulder girdle muscles as the reason of cervicognic headache among office workers. Disabil. Rehabil. 2013, 35, 793–802. [Google Scholar] [CrossRef]
- Leszczyńska, K.; Wincek, A.; Fortuna, W.; Huber, J.; Łukaszek, J.; Okurowski, S.; Chmielak, K.; Tabakow, P. Treatment of patients with cervical and upper thoracic incomplete spinal cord injury using repetitive transcranial magnetic stimulation. Int. J. Artif. Organs 2019, 43, 323–331. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Huber, J.; Leszczyńska, K.; Wietrzak, P.; Kaczmarek, K. Relationships between the Clinical Test Results and Neurophysiological Findings in Patients with Thoracic Outlet Syndrome. Bioengineering 2022, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Alricsson, M.; Harms-Ringdahl, K.; Larsson, B.; Linder, J.; Werner, S. Neck muscle strength and endurance in fighter pilots: Effects of a supervised training program. Aviat. Space Environ. Med. 2004, 75, 23–28. [Google Scholar] [PubMed]
- Clark, J.B. Cervical dystonia following exposure to high-G forces. Aviat. Space Environ. Med. 1990, 61, 935–937. [Google Scholar]
- Chumbley, E.M.; O’Hai, N.; Stolfi, A.; Lienesch, C.; McEachen, J.C.; Wright, B.A. Home Cervical Traction to Reduce Neck Pain in Fighter Pilots. Aerosp. Med. Hum. Perform. 2016, 87, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).