Determination of Arsenic Species Distribution in Arsenide Tailings and Leakage Using Geochemical and Geophysical Methods
Abstract
1. Introduction
2. Study Object
3. Materials and Methods
3.1. Field Sampling
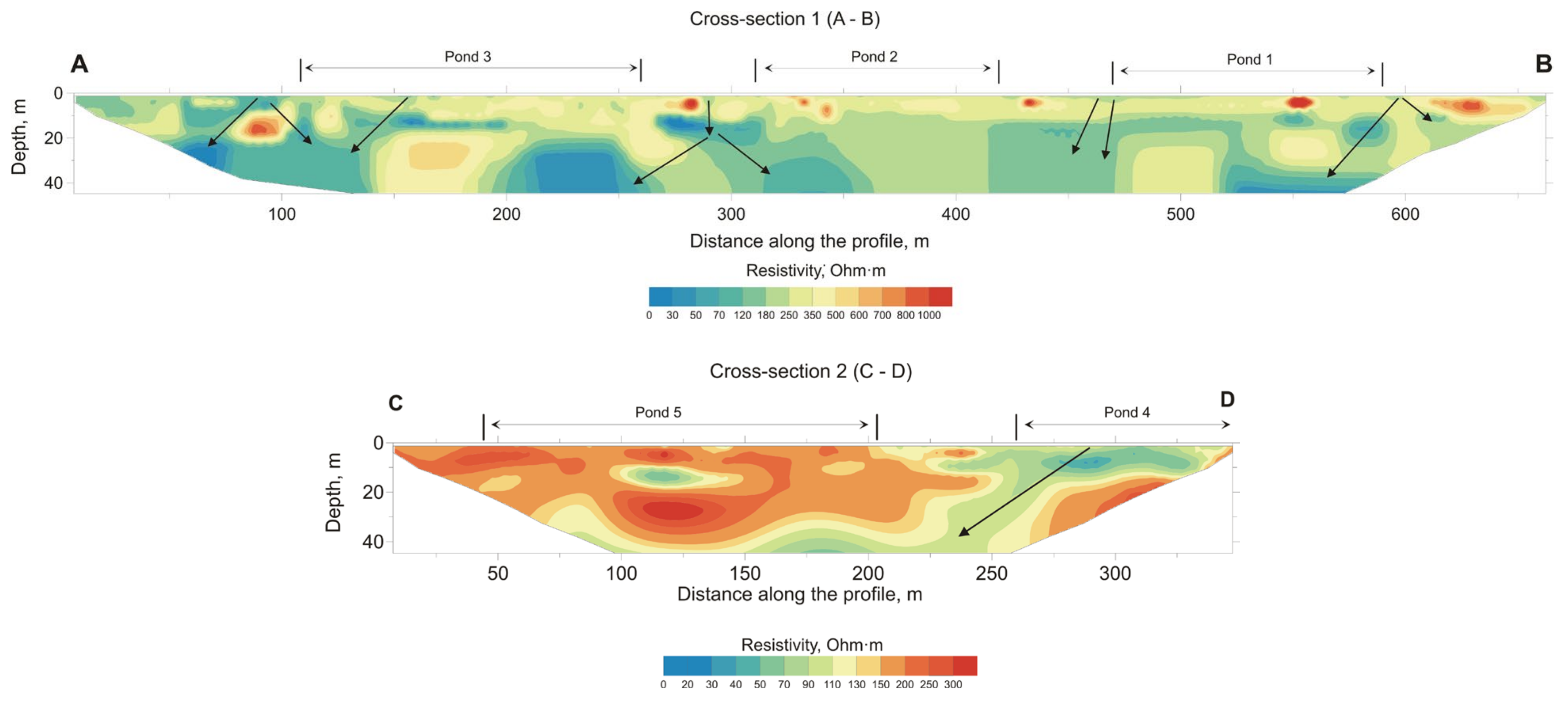
3.2. Geophysical Investigation
3.3. Laboratory Procedures and Analyses
3.3.1. Preparation of Solid
3.3.2. Water Extracts
3.3.3. Sequential Extraction Scheme
3.4. Electrotomography Processing
3.5. Laboratory Analyses
3.5.1. Solid
3.5.2. Solutions
3.5.3. As Species
3.5.4. Anions
4. Results of Geochemical and Geophysical Studies
4.1. Solid
4.2. Water Extract
4.3. Arsenic Species
- (F1)
- In total, 9.3% of the total arsenic is represented by water-soluble forms, specifically—3.7% of AsV and 5.6 % of AsIII (for example, in Mg, Ca, ammonia, arsenate, and arsenite according to its solubility product);
- (F2)
- in total, 30% is represented by bound/co-precipitated with magnesium, i.e., potentially water-soluble forms, 18% of AsV and 12% of AsIII (corresponding to Mg and Ca arsenates and arsenites remaining in the precipitate according to their solubility product);
- (F3)
- in total, 17% is represented by adsorbed on the surface of carbonates (calcite and dolomite) 9.8% of AsV and 7% of AsIII;
- (F4)
- in total, 17% is associated with oxides/hydroxides of iron/manganese, of which 9.3% is AsIII, 7.5% is AsV;
- (F5)
- in total, 19% is associated with easily oxidized minerals (residual arsenides and isomorphic admixture in sulfides) and organic material;
- (F6)
- and in total, only 7.7% is accounted for by non-oxidized arsenic minerals (for example, As-crystallohydrates, AsIII oxides).
4.4. Forms of Metals
- F1—water-soluble;
- F2 and F3—exchangeable;
- F4—sorbed on the surface of oxides/hydroxides of iron and manganese;
- F5—associated with easily oxidized minerals, as well as being associated with organic material;
- F6—associated with sparingly soluble minerals.
4.5. Composition of Surface Reservoirs
4.6. Inner Zonality of Pond Dumps
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahter, M. Health effects of early life exposure to arsenic. Basic Clin. Pharmacol. Toxicol. 2008, 102, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Adeloju, S.B.; Khan, S.; Patti, A.F. Arsenic contamination of groundwater and its implications for drinking water quality and human health in under-developed countries and remote communities—A review. Appl. Sci. 2011, 11, 1926. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Arsenic: A review on a great health issue worldwide. Appl. Sci. 2022, 12, 6184. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hirata, S. Arsenic speciation in environmental samples. Anal. Lett. 2003, 36, 2355–2366. [Google Scholar] [CrossRef]
- Gupta, D.K.; Tiwari, S.; Razafindrabe, B.H.N.; Chatterjee, S. Arsenic Contamination from Historical Aspects to the Present. In Arsenic Contamination in the Environment; Springer: Cham, Switzerland, 2017; pp. 1–12. [Google Scholar]
- Bortnikova, S.; Olenchenko, V.; Gaskova, O.; Yurkevicha, N.; Abrosimovaa, N.; Shevko, E.; Edelev, A.; Korneeva, T.; Provornaya, I.; Eder, L. Characterization of a gold extraction plant environment in assessing the hazardous nature of accumulated wastes (Kemerovo region, Russia). Appl. Geochem. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Akhavan, A.; Golchin, A. Environmental risk assessment of arsenic in Zn-Pb mine tailings. J. Water Soil Conserv. 2021, 27, 1–26. [Google Scholar]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Arsenic and iron speciation and mobilization during phytostabilization of pyritic mine tailings. Geochim. Cosmochim. Acta 2020, 286, 306–323. [Google Scholar] [CrossRef]
- Swęd, M.; Uzarowicz, Ł.; Duczmal-Czernikiewicz, A.; Kwasowski, W.; Pędziwiatr, A.; Siepak, M.; Niedzielski, P. Forms of metal (loid) s in soils derived from historical calamine mining waste and tailings of the Olkusz Zn–Pb ore district, southern Poland: A combined pedological, geochemical and mineralogical approach. Appl. Geochem. 2022, 139, 105218. [Google Scholar] [CrossRef]
- da Silva, F.F.; Quináglia, G.A.; Oliveira, P.V. Assessment of arsenic and lead mobility in the Ribeira do Iguape Valley, Southeastern Brazil. Environ. Earth Sci. 2021, 80, 1–12. [Google Scholar] [CrossRef]
- Fischer, C. Mineralogical and Hydrogeochemical Characterization of Legacy Mine Wastes near Cobalt. Master’s Thesis, University of Ottawa, Ottawa, ON, Canada, 2022. [Google Scholar]
- Sahira Joshi, S.; Sharma, M.; Kumari, A.; Surendra Shrestha, S.; Shrestha, B. Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Tuyen, T.T.; Shirzadi, A.; Pham, B.T.; Shahabi, H.; Omidvar, E.; Amini, A.; Entezami, H.; Prakash, I.; Phong, T.V. Development of a novel hybrid intelligence approach for landslide spatial prediction. Appl. Sci. 2019, 9, 2824. [Google Scholar] [CrossRef]
- Siddique, T.A.; Dutta, N.K.; Choudhury, N.R. Nanofiltration for arsenic removal: Challenges, recent developments, and perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Zouboulis, A.I. Application of biological processes for the removal of arsenic from groundwaters. Water Res. 2004, 38, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, D.; Viraraghavan, T. Arsenic removal from an aqueous solution by a modified fungal biomass. Water Res. 2006, 40, 549–552. [Google Scholar] [CrossRef]
- Nguyen, K.M.; Nguyen, B.Q.; Nguyen, H.T.; Nguyen, H.T.H. Adsorption of arsenic and heavy metals from solution by unmodified iron-ore sludge. Appl. Sci. 2019, 9, 619. [Google Scholar] [CrossRef]
- Kaloop, M.R.; Kumar, D.; Samui, P.; Gabr, A.R.; Hu, J.W.; Jin, X.; Roy, B. particle swarm optimization algorithm-extreme learning machine (PSO-ELM) model for predicting resilient modulus of stabilized aggregate bases. Appl. Sci. 2019, 9, 3221. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, J.; Asteris, P.G.; Armaghani, D.J.; Tahir, M.M. Supervised machine learning techniques to the prediction of tunnel boring machine penetration rate. Appl. Sci. 2019, 9, 3715. [Google Scholar] [CrossRef]
- Le, L.T.; Nguyen, H.; Dou, J.; Zhou, J. A Comparative study of PSO-ANN, GA-ANN, ICA-ANN, and ABC-ANN in estimating the heating load of buildings’ energy efficiency for smart city planning. Appl. Sci. 2019, 9, 2630. [Google Scholar] [CrossRef]
- Al-Yaari, M.; Aldhyani, T.H.H.; Rushd, S. Prediction of arsenic removal from contaminated water using artificial neural network model. Appl. Sci. 2022, 12, 999. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Majzlan, J.; Konigsberger, E. Thermodynamic properties for arsenic minerals and aqueous species. Rev. Mineral. Geochem. 2014, 79, 217–255. [Google Scholar] [CrossRef]
- Campbell, K.M.; Nordstrom, D.K. Arsenic speciation and sorption in natural environments. Rev. Mineral. Geochem. 2014, 79, 185–216. [Google Scholar] [CrossRef]
- Chen, M.-L.; Ma, L.-Y.; Chen, X.-W. New procedures for arsenic speciation: A review. Talanta 2014, 125, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Száková, J.; Tlustoš, P.; Goessler, W.; Frková, Z.; Najmanová, J. Mobility of arsenic and its compounds in soil and soil solution: The effect of soil pretreatment and extraction methods. J. Hazard. Mater. 2009, 172, 1244–1251. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, S.-H.; Kim, J.-G. Assessment of fraction and mobility of arsenic in soil near the mine waste dam. Sustainability 2020, 12, 1480. [Google Scholar] [CrossRef]
- Ahn, J.S.; Park, Y.S.; Kim, J.-Y.; Kim, K.-W. Mineralogical and geochemical characterization of arsenic in an abandoned mine tailings of Korea. Environ. Geochem. Health 2005, 27, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Anawar, H.M.; Garcia-Sanchez, A.; Santa Regina, I. Evaluation of various chemical extraction methods to estimate plant-available arsenic in mine soils. Chemosphere 2008, 70, 1459–1467. [Google Scholar] [CrossRef]
- Basu, A.; Schreiber, M.E. Arsenic release from arsenopyrite weathering: Insights from sequential extraction and microscopic studies. J. Hazard. Mater. 2013, 262, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Fazle Bari, A.S.M.; Lamb, D.; Choppala, G.; Bolan, N.; Seshadri, B.; Rahman, M.A.; Rahman, M.M. Geochemical fractionation and mineralogy of metal (loid)s in abandoned mine soils: Insights into arsenic behaviour and implications to remediation. J. Hazard. Mater. 2020, 399, 123029. [Google Scholar] [CrossRef]
- Courchesne, B.; Schindler, M.; Lussier, A.J.; Mykytczuk, N. Macro-to nanoscale mineral relationships in surficial cobalt-arsenic-bearing mine tailings of the Cobalt Mining Camp, Northeastern Ontario, Canada. Can. Miner. 2022, 60, 309–329. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, R.; Dong, K.; Zhang, S.; Zhao, R.; Jiang, Z.; Lan, X. An experimental comparison: Horizontal evaluation of valuable metal extraction and arsenic emission characteristics of tailings from different copper smelting slag recovery processes. J. Hazard. Mater. 2022, 430, 128493. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tang, Z.; Jin, Z.; Chi, Q.; He, B.; Jiang, G. Determination of As (III) and As (V) in soils using sequential extraction combined with flow injection hydride generation atomic fluorescence detection. Anal. Chim. Acta 2003, 477, 139–147. [Google Scholar] [CrossRef]
- Welna, M.; Szymczycha-Madeja, A.; Pohl, P. Non-chromatographic speciation of As by HG technique—Analysis of samples with different matrices. Molecules 2020, 25, 4944. [Google Scholar] [CrossRef] [PubMed]
- Bortnikova, S.; Bessonova, E.; Gaskova, O. Geochemistry of arsenic and metals in stored tailings of a Co-Ni arsenide-ore, Khovu-Aksy area, Russia. Appl. Geochem. 2012, 27, 2238–2250. [Google Scholar] [CrossRef]
- Lebedev, V.I. The khovu-aksy cobalt-arsenide deposit, Republic of Tuva, Russia: New perspectives on the problems of production and renewal of processing. Geol Ore Depos. 2021, 63, 212–238. [Google Scholar] [CrossRef]
- Borisenko, A.S.; Lebedev, V.I.; Tul’kin, V.G. Conditions of Formation of Hydrothermal Cobalt Deposits; Nauka: Novosibirsk, Russia, 1984; p. 172. (In Russian) [Google Scholar]
- Gaskova, O.L.; Bessonova, E.P.; Bortnikova, S.B. Leaching Geol Ore Depos. experiments on trace element release from the arsenic-bearing tailings of Khovu–Aksy (Tuva Republic, Russia). Appl. Geochem. 2003, 18, 1361–1371. [Google Scholar] [CrossRef]
- Bortnikova, S.B.; Yurkevich, N.V.; Gaskova, O.L.; Volynkin, S.S.; Edelev, A.V.; Grakhova, S.P.; Kurovskaya, V.V. Arsenic and metal quantities in abandoned arsenide tailings in dissolved, soluble, and volatile forms during 20 years of storage. Chem. Geol. 2021, 586, 120623. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Test Methods for Evaluating Solid Waste. Phys. Chem. Methods 1998, SW-846, 1. [Google Scholar]
- An Assessment of Laboratory Leaching Tests for Predicting the Impacts of Fill Material on Ground Water and Surface Water Quality. A Report to the Legislature; Publication No. 03-09-107; Washington State Department of Ecology: Washington, DC, USA, 2003. [Google Scholar]
- Meloni, F.; Montegrossi, G.; Lazzaroni, M.; Rappuoli, D.; Nisi, B.; Vaselli, O. Total and leached arsenic, mercury and antimony in the mining waste dumping area of Abbadia San Salvatore (Mt. Amiata, Central Italy). Appl. Sci. 2021, 11, 7893. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Bajpai, S.; Dewangan, U.K.; Tamrakar, R.K. Suitability of leaching test methods for fly ash and slag: A review. J. Radiat. Res. Appl. Sci. 2015, 8, 523–537. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Manful, G. Occurrence and Ecochemical Behaviour of Arsenic in a Goldsmelter Impacted Area in Ghana. Ph.D. Thesis, Centrum voor Milieusaneringen aan de RUG, University of Gent, Gent, Belgium, 1992. [Google Scholar]
- Baig, J.A.; Kazi, T.G.; Arain, M.B.; Shah, A.Q.; Sarfraz, R.A.; Afridi, H.I.; Khan, S. Arsenic fractionation in sediments of different origins using BCR sequential and single extraction methods. J. Hazard. Mater. 2009, 167, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Larios, R.; Fernández-Martínez, R.; LeHecho, I.; Rucandio, I. A methodological approach to evaluate arsenic speciation and bioaccumulation in different plant species from two highly polluted mining areas. Sci. Total Environ. 2012, 414, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Keon, N.E.; Swartz, C.H.; Brabander, D.J.; Harvey, C.; Hemond, H.F. Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ. Sci. Technol. 2001, 35, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, N. Analysis of Hydride Forming Elements with the Thermo Scientific iCAP 7000 Plus Series ICP-OES and the Basic Hydride Generator Kit; Application Note; ThermoFisher Scientific: Waltham, MA, USA, 2016. [Google Scholar]

| Steps | Extractable Components/Forms | Extractant and Conditions | Possible Mechanism |
|---|---|---|---|
| F1 | Water-soluble, arsenic forms | H2O (V = 50 mL) t 24 h | Dissolution in water |
| F2 | Arsenic forms sorbed on the surface of magnesium minerals and deposited as magnesium arsenates/arsenites | 0.1 M NaH2PO4 (pH = 8, V = 40 mL) t 24 h | Anionic exchange of ion phosphate for arsenate and arsenite |
| F3 | Arsenic forms sorbed on the surface of carbonates | 0.1 M CH3COOH (V = 40 mL) pH 4 t 24 h | Dissolution of carbonate minerals with separation into solution As |
| F4 | Arsenic forms associated with iron hydroxides | 2 M NH2OH • HCl in 0.1 M CH3COOH solution (pH = 2, V = 40 mL), 12 h in a water bath (T = 80 °C) | Reduction of Fe oxyhydroxides |
| F5 | Forms of arsenic associated with oxidizable minerals and organic tailings | H2O2(conc) (V = 25 mL, T = 80 °C) | Oxidation of organic tailings and easily oxidizable minerals |
| F6 | Arsenic in the composition of hardly soluble minerals | Decomposition with a mixture of H2O2 and HNO3 by heating in a water bath (V = 25 mL) |
| Component | Content | Element | Content |
|---|---|---|---|
| SiO2 | 44 | Cr | 61 |
| TiO2 | 0.60 | Co | 420 |
| Al2O3 | 12 | Ni | 370 |
| Fe2O3 | 8.0 | Cu | 750 |
| MnO | 0.26 | Zn | 290 |
| MgO | 3.4 | As | 3900 |
| CaO | 15 | Ag | 8.4 |
| Na2O | 0.42 | Cd | 0.78 |
| K2O | 2.3 | Sn | 1.7 |
| P2O5 | 0.23 | Sb | 35 |
| BaO | 0.05 | Pb | 30 |
| SO3 | 3.5 | Bi | 19 |
| LOI | 10 | Sr | 100 |
| Component | Content | Element | Content |
| pH | 8.07 | Mn | 3.1 |
| Eh | 410 | Co | 16 |
| SO42− | 44 | Ni | 5.6 |
| Cl− | 0.4 | Cr | 0.15 |
| NO3− | 9.8 | Cu | 4.3 |
| HCO3− | 105 | Zn | 3.2 |
| Ca2+ | 11 | Pb | 0.053 |
| Mg2+ | 29 | Ag | 0.005 |
| Na+ | 0.75 | Cd | 0.018 |
| K+ | 1.5 | Sn | 0.065 |
| Fe | 0.022 | Hg | 0.024 |
| Al | 0.011 | Ba | 26 |
| As | 26 | Sr | 40 |
| Sb | 0.21 |
| Fraction | F1 | F2 | F3 | F4 | F5 | F6 | Total Content | |
|---|---|---|---|---|---|---|---|---|
| Solutions | AsIII | 3.1 ± 0.5 | 13 ± 1 | 7.6 ± 0.3 | 10 ± 1 | - | - | - |
| AsV | 5.0 ± 0.9 | 20 ± 5 | 10 ± 1 | 8 ± 3 | - | - | - | |
| Astotal | 8.1 ± 0.8 | 33 ± 5 | 18 ± 1 | 18 ± 3 | 33 ± 0.5 | 12 ± 1 | - | |
| Solid | AsIII | 160 ± 25 | 530 ± 60 | 300 ± 20 | 400 ± 40 | - | - | 1400 ± 140 (For F1-F4) |
| AsV | 240 ± 50 | 770 ± 160 | 420 ± 45 | 320 ± 130 | - | - | 1750 ± 220 (For F1-F4) | |
| Astotal | 400 ± 40 | 1300 ± 150 | 720 ± 40 | 720 ± 120 | 810 ± 20 | 330 ± 20 | 4300 ± 600 |
| Fraction | F1 | F2 | F3 | F4 | F5 | F6 | Share |
|---|---|---|---|---|---|---|---|
| AsIII | 3.7 | 12 | 7.0 | 9.3 | - | - | 32 (For F1-F4) |
| AsV | 5.6 | 18 | 9.8 | 7.5 | - | - | 41 (For F1-F4) |
| Astotal | 9.3 | 30 | 17 | 17 | 19 | 7.7 | 100 |
| Component | Water Extract | F1 Fraction | F2 Fraction |
|---|---|---|---|
| Solutions, mg/L | 26 ± 3 | 8.1 ± 0.8 | 33 ± 5 |
| Solid sample, mg/kg | 260 ± 30 | 400 ± 40 | 1300 ± 150 |
| Fraction | F1 | F2 | F3 | F4 | F5 | F6 | |
|---|---|---|---|---|---|---|---|
| Elements | |||||||
| Mg | 6 ± 1.5 | 35 ± 7 | 20 ± 7 | 125 ± 25 | 330 ± 50 | 280 ± 20 | |
| Ca | 30 ± 15 | 60 ± 30 | 100 ± 50 | 1500 ± 130 | 290 ± 80 | 280 ± 60 | |
| Sr | 50 ± 3 | 220 ± 10 | 220 ± 90 | 1500 ± 130 | 620 ± 60 | 280 ± 10 | |
| Cd | <10 | <10 | <10 | 90 ± 15 | 60 ± 10 | 140 ± 20 | |
| Co | 20 ± 6 | 390 ± 80 | 1000 ± 400 | 7600 ± 740 | 5400 ± 680 | 1700 ± 300 | |
| Cu | 10 ± 6 | 160 ± 30 | 300 ± 140 | 10,000 ± 1000 | 11,000 ± 800 | 10,000 ± 1600 | |
| Ni | 8 ± 2 | 180 ± 50 | 370 ± 110 | 5000 ± 670 | 10,000 ± 1600 | 10,000 ± 1500 | |
| Zn | <10 | 440 ± 110 | 320 ± 100 | 5300 ± 660 | 6000 ± 1000 | 4200 ± 700 | |
| Ba | <15 | 20 ± 6 | 25 ± 15 | 1300 ± 400 | 1600 ± 200 | 960 ± 200 | |
| Cr | <25 | <75 | <75 | 250 ± 90 | 900 ± 130 | 1200 ± 100 | |
| Mn | <15 | 85 ± 20 | 490 ± 360 | 23,000 ± 3000 | 19,000 ± 4000 | 14,000 ± 1500 | |
| Fe | <300 | <300 | <300 | 1400 ± 500 | 590,000 ± 70,000 | 760,000 ± 70,000 | |
| Sb | 30 ± 3 | 70 ± 8 | 40 ± 4 | 170 ± 20 | 25 ± 5 | 290 ± 30 | |
| Pb | <10 | <20 | <20 | 400 ± 200 | 620 ± 180 | 250 ± 150 | |
| Fraction | F1 | F2 | F3 | F4 | F5 | F6 | Total Content | |
|---|---|---|---|---|---|---|---|---|
| Elements | ||||||||
| Mg | 0.30 ± 0.075 | 1.4 ± 0.28 | 0.79 ± 0.28 | 5.0 ± 1.0 | 8.1 ± 1.2 | 7.7 ± 0.55 | 23 ± 1.7 | |
| 1.3 | 6.1 | 3.4 | 22 | 35 | 33 | |||
| Ca | 1.5 ± 0.74 | 2.4 ± 1.2 | 3.9 ± 1.9 | 60 ± 5.2 | 7.1 ± 1.9 | 7.7 ± 1.5 | 83 ± 13 | |
| 1.8 | 2.9 | 4.7 | 72 | 8.6 | 9.3 | |||
| Sr | 2.5 ± 0.150 | 9.0 ± 0.4 | 8.7 ± 3.5 | 60 ± 5.2 | 15 ± 1.5 | 7.7 ± 0.27 | 100 ± 11 | |
| 2.5 | 9.0 | 8.7 | 60 | 15 | 7.7 | |||
| Cd | <0.50 | <0.40 | <0.40 | 3.6 ± 0.60 | 1.5 ± 0.25 | 3.5 ± 0.50 | 8.6 ± 1.4 | |
| 0 | 0 | 0 | 42 | 17 | 41 | |||
| Co | 0.99 ± 0.30 | 16 ± 3.3 | 40 ± 16 | 300 ± 30 | 130 ± 17 | 43 ± 7.5 | 540 ± 74 | |
| 0.18 | 3.0 | 7.4 | 56 | 24 | 7.9 | |||
| Cu | 0.49 ± 0.30 | 6.4 ± 1.2 | 12 ± 5.6 | 440 ± 40 | 270 ± 20 | 250 ± 4.4 | 1000 ± 100 | |
| 0.05 | 0.64 | 1.2 | 44 | 27 | 25 | |||
| Ni | 0.40 ± 0.099 | 7.3 ± 2.0 | 15.0 ± 4.3 | 200 ± 27 | 240 ± 39 | 250 ± 38 | 720 ± 110 | |
| 0.06 | 1.0 | 2.1 | 28 | 33 | 35 | |||
| Zn | <0.50 | 18 ± 4.5 | 13 ± 3.9 | 210 ± 26 | 150 ± 24 | 105 ± 18 | 500 ± 77 | |
| 0 | 3.6 | 2.6 | 42 | 30 | 21 | |||
| Ba | <0.75 | 0.81 ± 0.24 | 0.99 ± 0.59 | 52 ± 16 | 39 ± 4.9 | 24 ± 5.0 | 120 ± 27 | |
| 0 | 0.7 | 0.8 | 44 | 33 | 21 | |||
| Cr | <1.2 | <1.0 | <3.0 | 10 ± 3.6 | 23 ± 3.2 | 30 ± 2.5 | 62 ± 9.0 | |
| 0 | 0 | 0 | 16 | 37 | 48 | |||
| Mn | <0.75 | 3.5 ± 0.8 | 19 ± 14 | 920 ± 12 | 470 ± 98 | 350 ± 37.5 | 1800 ± 270 | |
| 0 | 0.2 | 1.1 | 52 | 27 | 20 | |||
| Fe | <1.5 | <1.2 | <1.2 | 56 ± 20 | 15,000 ± 1700 | 19,000 ± 1700 | 34,000 ± 3500 | |
| 0 | 0 | 0 | 0.16 | 44 | 56 | |||
| Sb | 1.5 ± 0.15 | 2.9 ± 0.33 | 1.6 ± 0.16 | 6.8 ± 0.8 | 0.61 ± 0.12 | 7.3 ± 0.75 | 20 ± 2.3 | |
| 7.1 | 14 | 7.6 | 32 | 2.9 | 35 | |||
| Pb | <0.50 | <0.80 | <0.80 | 16.0 ± 8.0 | 15 ± 4.4 | 6.2 ± 3.7 | 38 ± 17 | |
| 0 | 0 | 0 | 42 | 39 | 16 | |||
| Samples | P1–1 | P1–2 | P3–1 | P3–2 | P4–1 | P4–2 | P5 | |
|---|---|---|---|---|---|---|---|---|
| Components | ||||||||
| pH | 8.47 | 8.24 | 7.63 | 8.88 | 8.49 | 7.78 | 8.55 | |
| Eh, mV | 420 | 385 | 370 | 460 | 472 | 340 | 496 | |
| SO42− | 140 | 56 | 114 | 115 | 23 | 81 | 74 | |
| NO3− | 27 | 34 | 70 | 70 | 23 | 52 | 160 | |
| HCO3− | 70 | 160 | 140 | 140 | 110 | 120 | 87 | |
| NH4+ | 1.1 | 0.8 | 1.3 | 1.4 | 0.5 | 0.5 | 1.0 | |
| Ca2+ | 38 | 17 | 12 | 12 | 7.7 | 23 | 9.3 | |
| Mg2+ | 23 | 21 | 52 | 54 | 20 | 23 | 58 | |
| Na+ | 0.50 | 0.46 | 3.6 | 3.8 | 0.91 | 0.83 | 1.4 | |
| K+ | 2.2 | 2.0 | 2.9 | 3.1 | 0.68 | 0.78 | 1.4 | |
| As | 12 | 9.3 | 4.6 | 4.8 | 13 | 12 | 7.2 | |
| Fe | 52 | 23 | 15 | 20 | 11 | 39 | 46 | |
| Mn | 0.67 | 2.6 | 12 | 12 | 2.5 | 12 | 14 | |
| Co | 6.7 | 6.0 | 7.0 | 6.1 | 4.7 | 16 | 9.9 | |
| Ni | 4.8 | 3.0 | 3.9 | 2.9 | 4.9 | 12 | 7.2 | |
| Cu | 6.1 | 2.6 | 0.82 | 0.76 | 0.85 | 1.0 | 1.3 | |
| Zn | 2.0 | 0.42 | 1.4 | 0.61 | 0.91 | 0.96 | 1.2 | |
| Sb | 62 | 60 | 41 | 40 | 45 | 37 | 54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volynkin, S.S.; Bortnikova, S.B.; Yurkevich, N.V.; Shuvaeva, O.V.; Kohanova, S.P. Determination of Arsenic Species Distribution in Arsenide Tailings and Leakage Using Geochemical and Geophysical Methods. Appl. Sci. 2023, 13, 1067. https://doi.org/10.3390/app13021067
Volynkin SS, Bortnikova SB, Yurkevich NV, Shuvaeva OV, Kohanova SP. Determination of Arsenic Species Distribution in Arsenide Tailings and Leakage Using Geochemical and Geophysical Methods. Applied Sciences. 2023; 13(2):1067. https://doi.org/10.3390/app13021067
Chicago/Turabian StyleVolynkin, Sergey S., Svetlana B. Bortnikova, Nataliya V. Yurkevich, Olga V. Shuvaeva, and Sofia P. Kohanova. 2023. "Determination of Arsenic Species Distribution in Arsenide Tailings and Leakage Using Geochemical and Geophysical Methods" Applied Sciences 13, no. 2: 1067. https://doi.org/10.3390/app13021067
APA StyleVolynkin, S. S., Bortnikova, S. B., Yurkevich, N. V., Shuvaeva, O. V., & Kohanova, S. P. (2023). Determination of Arsenic Species Distribution in Arsenide Tailings and Leakage Using Geochemical and Geophysical Methods. Applied Sciences, 13(2), 1067. https://doi.org/10.3390/app13021067







