Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment
Abstract
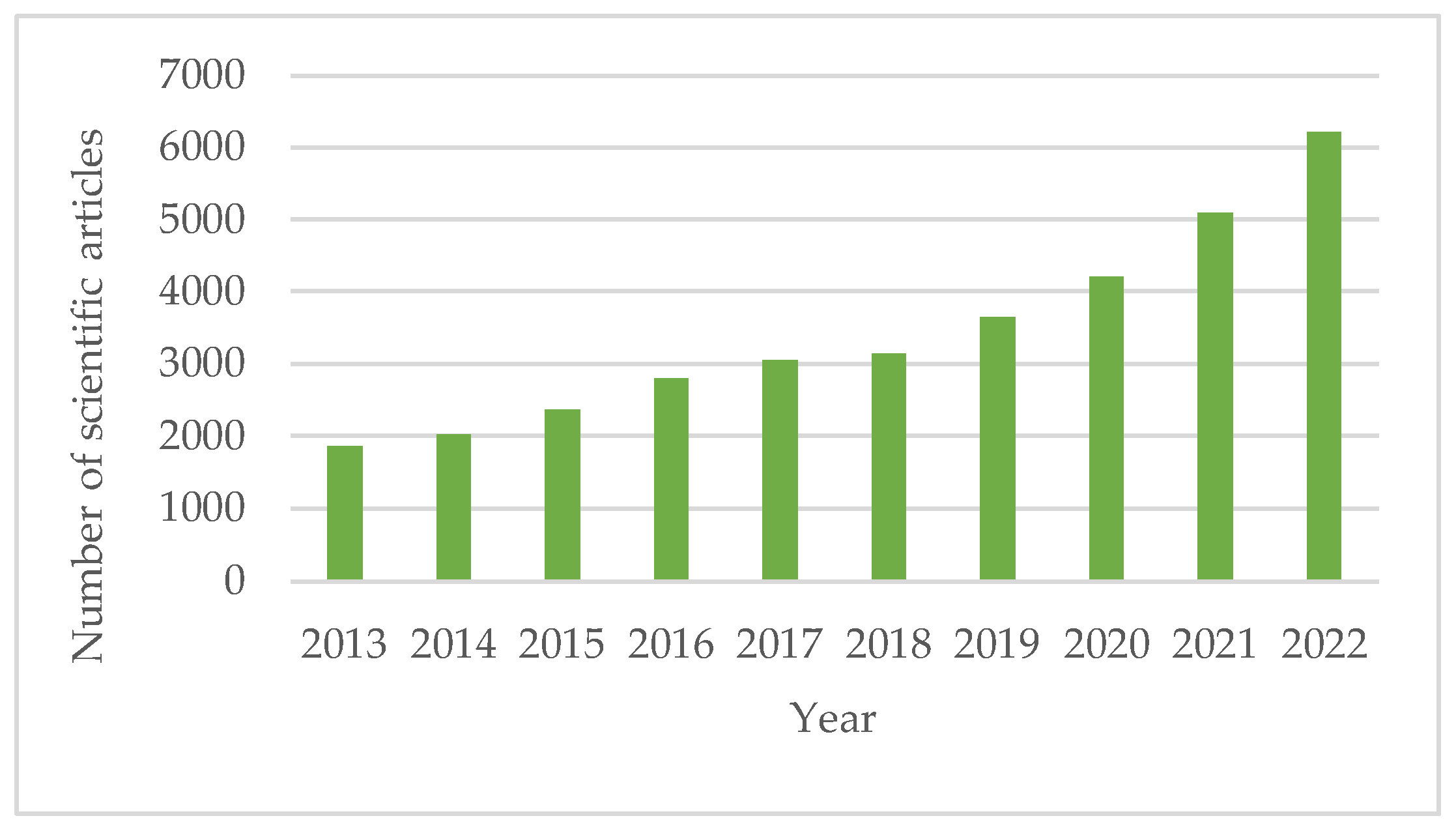
1. Introduction
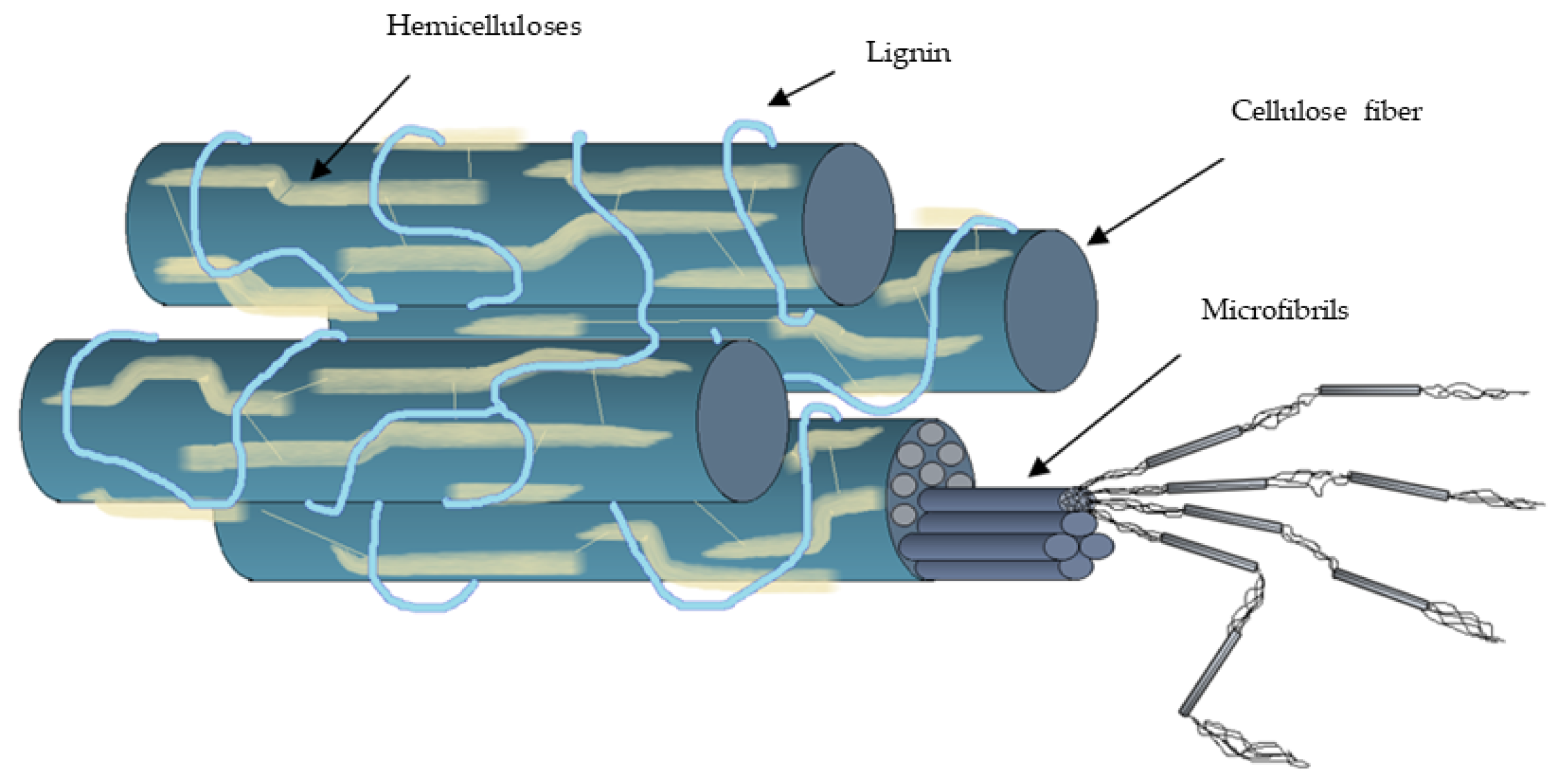
2. General Characteristics of Lignocellulosic Biomass
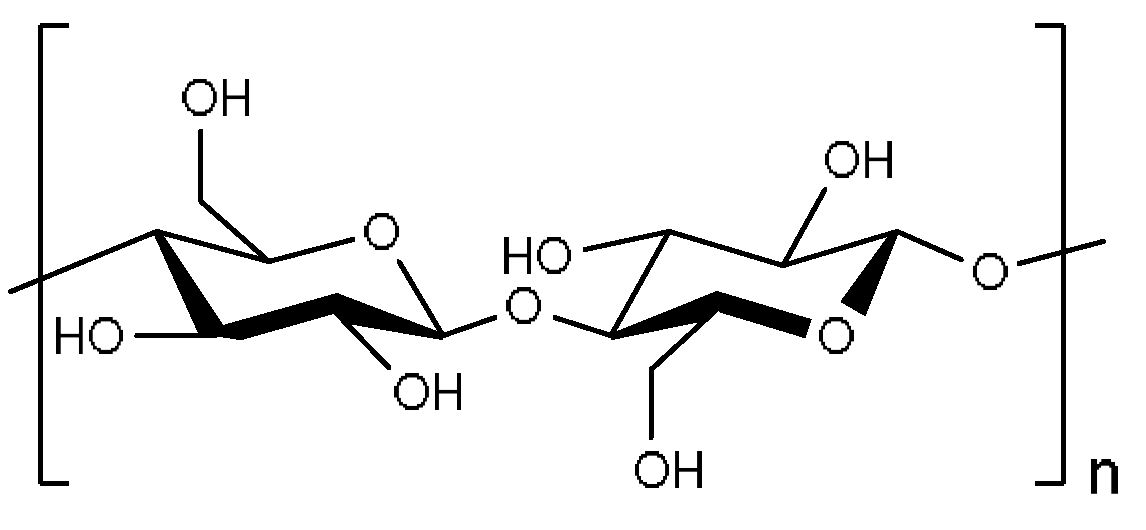
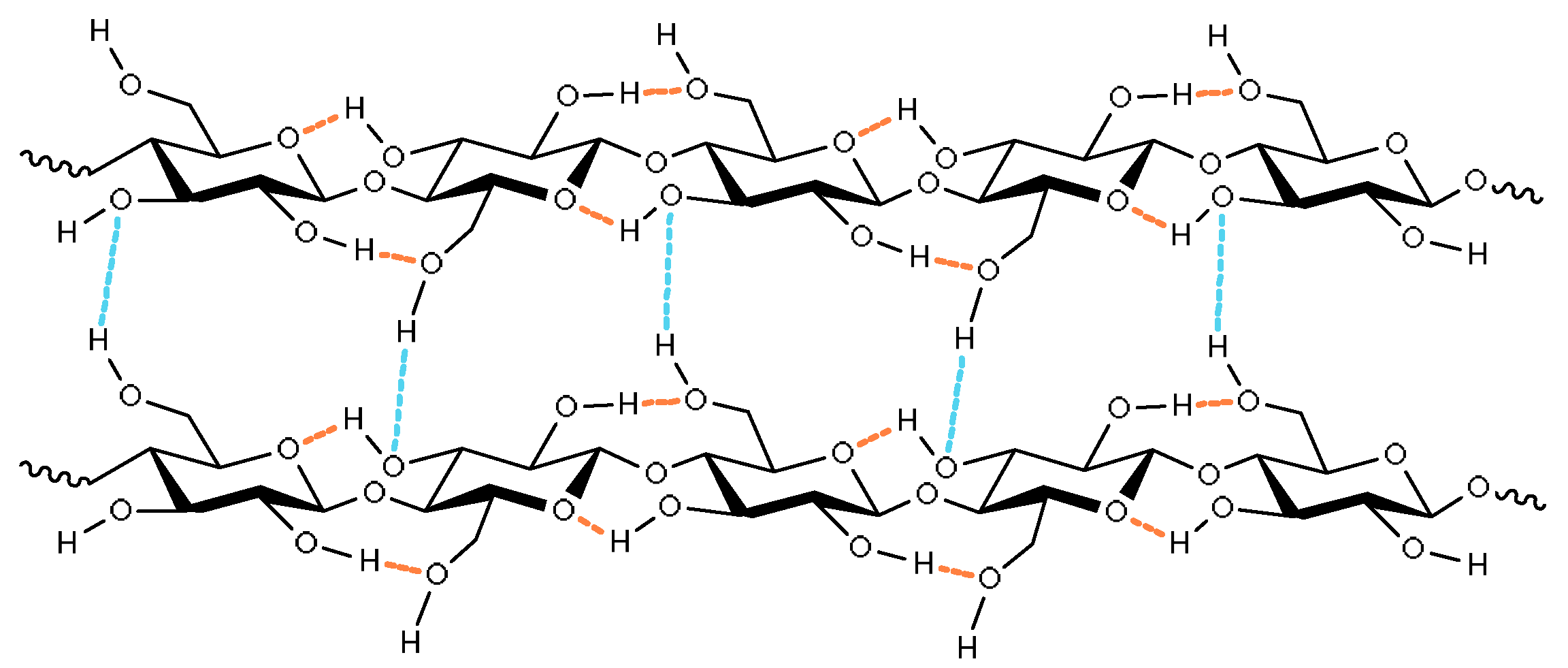
2.1. Cellulose
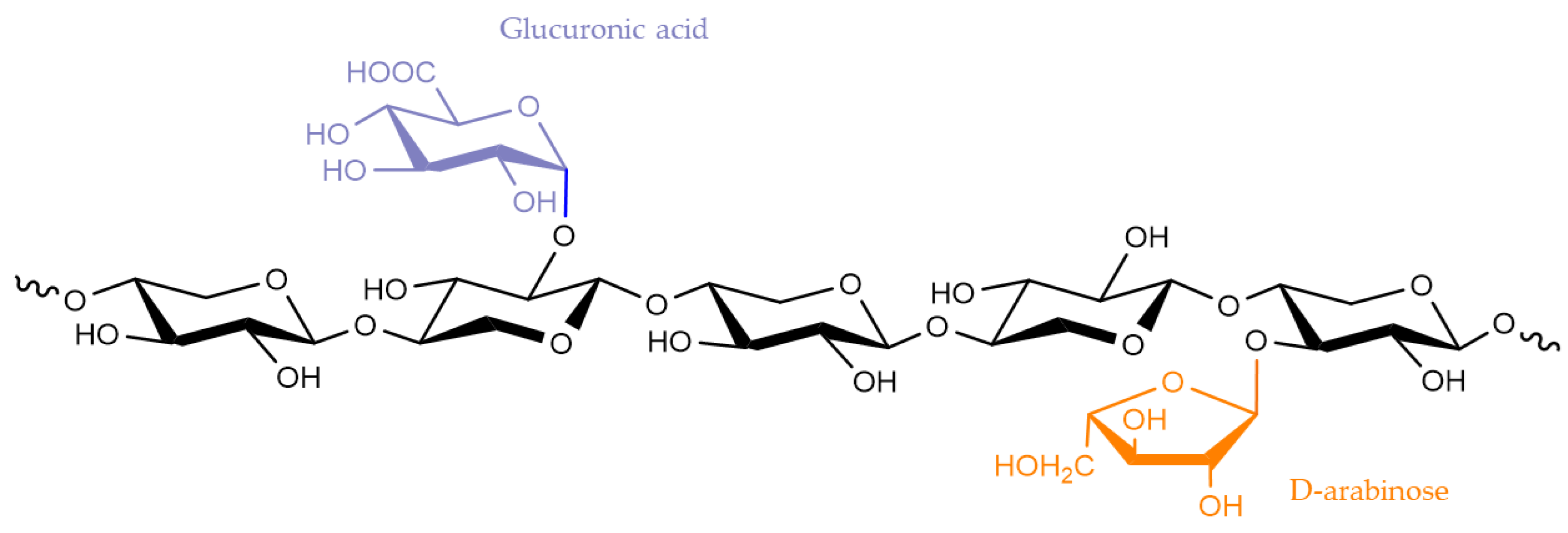
2.2. Hemicelluloses
2.3. Lignin
3. Dissolution of Biomass and Its Components
3.1. Alkaline Solutions
3.2. Acid Solutions
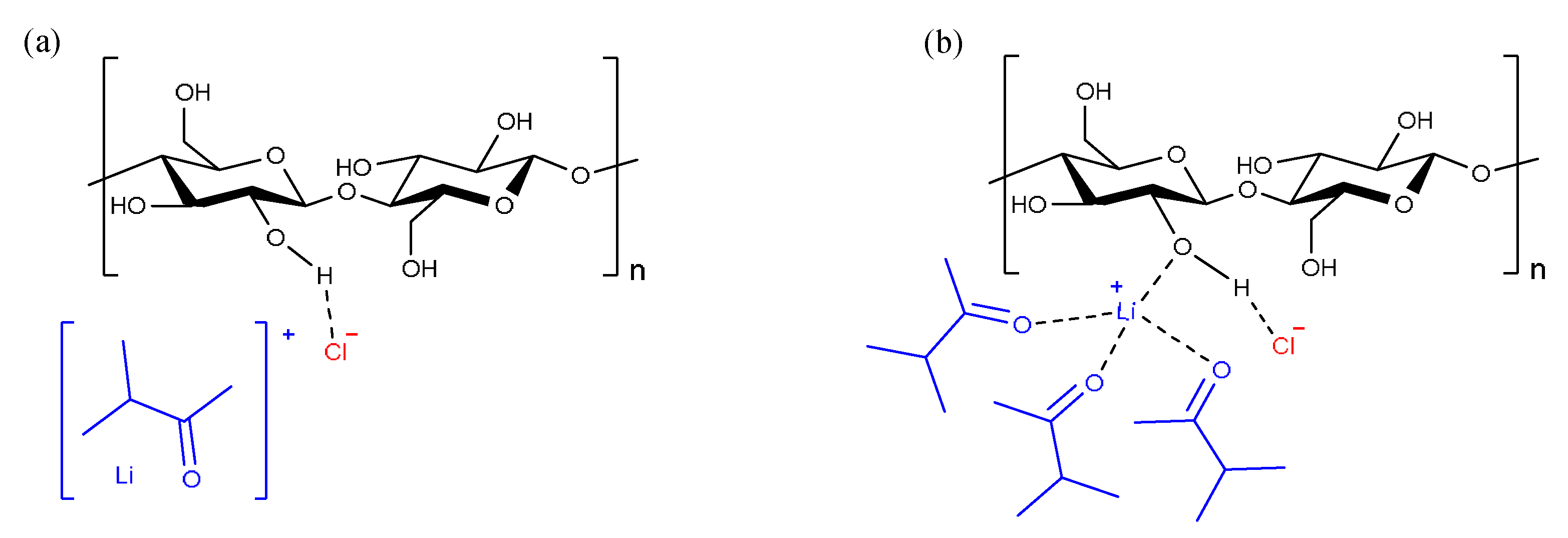
3.3. Inorganic Molten Salt Hydrates
3.4. Metal Complexes
3.5. Inorganic Salts in Organic Solvents
3.6. Ionic Liquids
3.6.1. Ionic Liquids in Biomass Dissolution
3.6.2. Cellulose Dissolution in Ionic Liquids
3.6.3. The Impact of Anion in Ionic Liquid on Cellulose Dissolution Effectiveness
3.6.4. The Impact of Ionic Liquid Cation on Cellulose Dissolution Effectiveness
3.6.5. The Impact of the Addition of Other Chemicals and Process Conditions on Cellulose Dissolution Effectiveness in Ionic Liquids
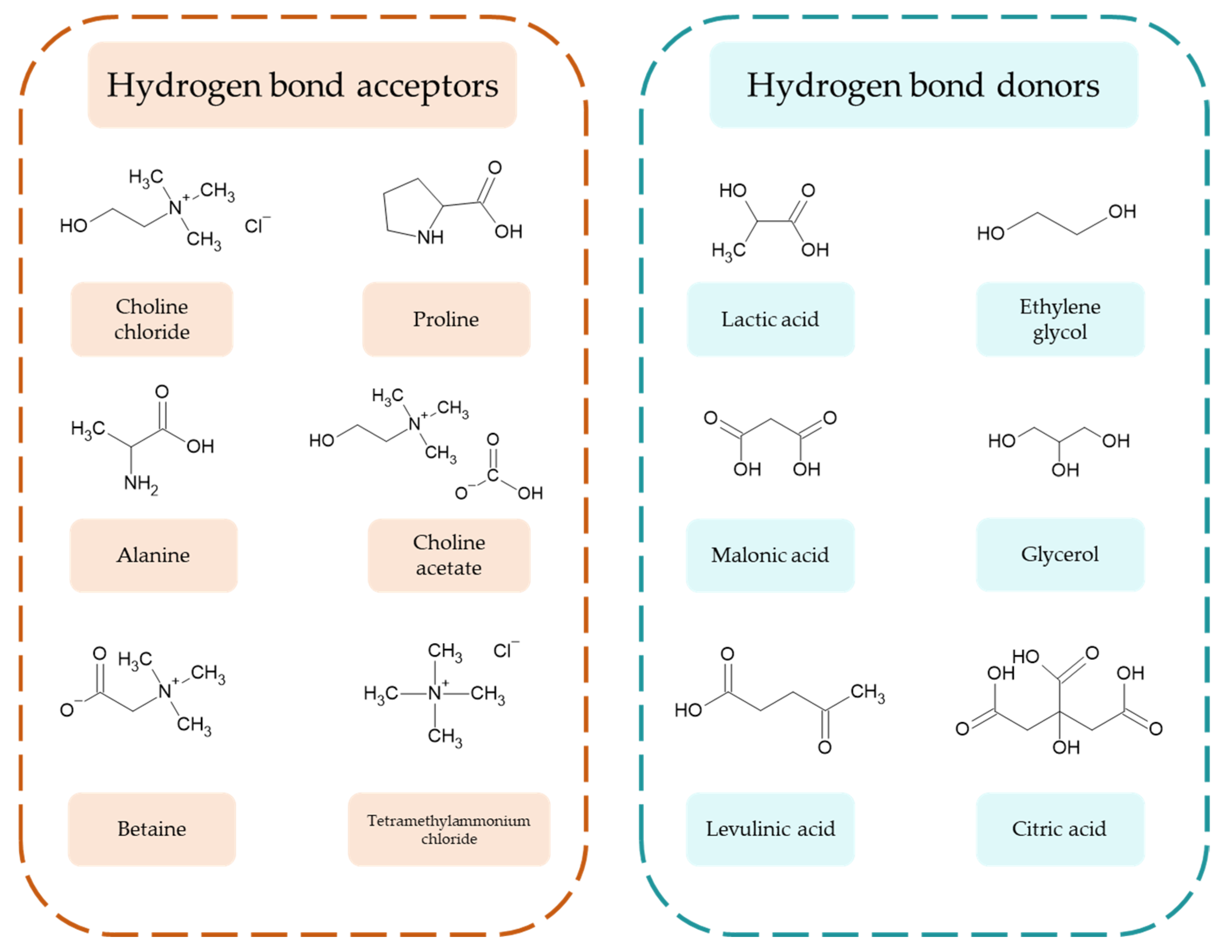
3.7. Deep Eutectic Solvents (DESs) in Biomass Processing
3.7.1. Delignification of Biomass with DESs
3.7.2. Hemicelluloses Dissolution in DESs
3.7.3. Cellulose Dissolution in DESs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quintana-Rojo, C.; Callejas-Albiñana, F.E.; Tarancón, M.Á.; Martínez-Rodríguez, I. Econometric studies on the development of renewable energy sources to support the European Union 2020–2030 climate and energy framework: A critical appraisal. Sustainability 2020, 12, 4828. [Google Scholar] [CrossRef]
- Camia, A. Biomass Production, Supply, Uses and Flows in the European Union; JRC: Brussels, Belgium, 2018. [Google Scholar]
- Ramos, A.; Monteiro, E.; Rouboa, A. Biomass pre-treatment techniques for the production of biofuels using thermal conversion methods—A review. Energy Convers. Manag. 2022, 270, 116271. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A.; Le Coeur, C. Commodity fuels from biomass through pretreatment and torrefaction: Effects of mineral content on torrefied fuel characteristics and quality. Energy Fuels 2012, 26, 6466–6474. [Google Scholar] [CrossRef]
- Prabha, S.; Durgalakshmi, D.; Rajendran, S.; Lichtfouse, E. Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: A review. Environ. Chem. Lett. 2021, 19, 1667–1691. [Google Scholar] [CrossRef]
- Schmitt, V.E.M.; Kaltschmitt, M. Effect of straw proportion and Ca- and Al-containing additives on ash composition and sintering of wood-straw pellets. Fuel 2013, 109, 551–558. [Google Scholar] [CrossRef]
- Juneja, A.; Kumar, D.; Williams, J.D.; Wysocki, D.J.; Murthy, G.S. Potential for ethanol production from conservation reserve program lands in Oregon. J. Renew. Sustain. Energy 2011, 3, 063102. [Google Scholar] [CrossRef]
- Surendra, K.C.; Richard, O.; Zaleski, H.M.; Hashimoto, A.G.; Khanal, S.K. High yielding tropical energy crops for bioenergy production: Effects of plant components, harvest years and locations on biomass composition. Bioresour. Technol. 2018, 251, 218–229. [Google Scholar] [CrossRef]
- Ruan, R.; Zhang, Y.; Chen, P.; Liu, S.; Fan, L.; Zhou, N.; Ding, K.; Peng, P.; Addy, M.; Cheng, Y.; et al. Biofuels: Introduction. In Biomass, Biofuels, Biochemicals: Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–43. [Google Scholar]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefinery 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Demirbaş, A. Calculation of higher heating values of biomass fuels. Fuel 1997, 76, 431–434. [Google Scholar] [CrossRef]
- Španić, N.; Jambreković, V.; Klarić, M. Basic chemical composition of wood as a parameter in raw material selection for biocomposite production. Cellul. Chem. Technol. 2018, 52, 163–169. [Google Scholar]
- Garcia-Maraver, A.; Salvachúa, D.; Martínez, M.J.; Diaz, L.F.; Zamorano, M. Analysis of the relation between the cellulose, hemicellulose and lignin content and the thermal behavior of residual biomass from olive trees. Waste Manag. 2013, 33, 2245–2249. [Google Scholar] [CrossRef]
- McKendry, P. Energy Production from Biomass (Part 1): Overview of Biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of hemicellulose, cellulose, and lignin content in different types of biomasses by thermogravimetric analysis and pseudocomponent kinetic model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Rusanen, A.; Lappalainen, K.; Kärkkäinen, J.; Tuuttila, T.; Mikola, M.; Lassi, U. Selective hemicellulose hydrolysis of scots pine sawdust. Biomass Convers. Biorefinery 2019, 9, 283–291. [Google Scholar] [CrossRef]
- Raitanen, J.E.; Järvenpää, E.; Korpinen, R.; Mäkinen, S.; Hellström, J.; Kilpeläinen, P.; Liimatainen, J.; Ora, A.; Tupasela, T.; Jyske, T. Tannins of conifer bark as Nordic piquancy—Sustainable preservative and aroma? Molecules 2020, 25, 567. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and opportunities in the characterization of cellulose—An important regulator of cell wall growth and mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.d.L.T.M.; Teixeira, J.A. Nanocellulose production: Exploring the enzymatic route and residues of pulp and paper industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef]
- Owonubi, S.J.; Agwuncha, S.C.; Malima, N.M.; Shombe, G.B.; Makhatha, E.M.; Revaprasadu, N. Non-woody biomass as sources of nanocellulose particles: A review of extraction procedures. Front. Energy Res. 2021, 9, 608825. [Google Scholar] [CrossRef]
- Lucenius, J.; Valle-Delgado, J.J.; Parikka, K.; Österberg, M. Understanding hemicellulose-cellulose interactions in cellulose nanofibril-based composites. J. Colloid Interface Sci. 2019, 555, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Hasanov, I.; Raud, M.; Kikas, T. The Role of ionic liquids in the lignin separation from lignocellulosic biomass. Energies 2020, 13, 4864. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Polo, C.C.; Pereira, L.; Mazzafera, P.; Flores-Borges, D.N.A.; Mayer, J.L.S.; Guizar-Sicairos, M.; Holler, M.; Barsi-Andreeta, M.; Westfahl, H.; Meneau, F. Correlations between lignin content and structural robustness in plants revealed by X-ray ptychography. Sci. Rep. 2020, 10, 6023. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A review on the partial and complete dissolution and fractionation of wood and lignocelluloses using imidazolium ionic liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent advances in organosolv fractionation: Towards biomass fractionation technology of the future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Credou, J.; Berthelot, T. Cellulose: From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef] [PubMed]
- Ioelovich, M. Preparation, characterization and application of amorphized cellulose—A review. Polymers 2021, 13, 4313. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Wood and fiber fundamentals. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 19–74. [Google Scholar]
- Jones, D.; Ormondroyd, G.O.; Curling, S.F.; Popescu, C.M.; Popescu, M.C. Chemical compositions of natural fibres. In Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels: Chemistry, Extractives, Lignins, Hemicelluloses and Cellulose; Elsevier: Amsterdam, The Netherlands, 2010; pp. 23–58. [Google Scholar]
- Kobetičová, K.; Nábělková, J. Effect of wood hemicellulose composition on binding interactions with caffeine. Buildings 2021, 11, 515. [Google Scholar] [CrossRef]
- Schädel, C.; Blöchl, A.; Richter, A.; Hoch, G. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Phys. Biochem. 2010, 48, 1–8. [Google Scholar] [CrossRef]
- Patel, J.P.; Parsania, P.H. Characterization, testing, and reinforcing materials of biodegradable composites. In Biodegradable and Biocompatible Polymer Composites: Processing, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 55–79. [Google Scholar]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Ajao, O.; Jeaidi, J.; Benali, M.; Restrepo, A.M.; El Mehdi, N.; Boumghar, Y. Quantification and variability analysis of lignin optical properties for colour-dependent industrial applications. Molecules 2018, 23, 377. [Google Scholar] [CrossRef]
- Melro, E.; Alves, L.; Antunes, F.E.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265, 578–584. [Google Scholar] [CrossRef]
- Dastpak, A.; Lourençon, T.V.; Balakshin, M.; Farhan Hashmi, S.; Lundström, M.; Wilson, B.P. Solubility study of lignin in industrial organic solvents and investigation of electrochemical properties of spray-coated solutions. Ind. Crops Prod. 2020, 148, 112310. [Google Scholar] [CrossRef]
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin—Recent routes reviewed. In European Polymer Journal; Elsevier: Amsterdam, The Netherlands, 2013; Volume 49, pp. 1151–1173. [Google Scholar]
- da Costa Lopes, A.M.; Gomes, J.R.B.; Coutinho, J.A.P.; Silvestre, A.J.D. Novel insights into biomass delignification with acidic deep eutectic solvents: A mechanistic study of β-O-4 ether bond cleavage and the role of the halide counterion in the catalytic performance. Green Chem. 2020, 22, 2474–2487. [Google Scholar] [CrossRef]
- Kang, X.; Kirui, A.; Dickwella Widanage, M.C.; Mentink-Vigier, F.; Cosgrove, D.J.; Wang, T. Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Mahdi, Y.S.; Mohammed, A.H.; Mohammed, A.K. Cellulose fibers dissolution in alkaline solution. Al-Khwarizmi Eng. J. 2019, 14, 107–115. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, P.; Liu, M.; Lu, A.; Zhang, L. Weak interactions and their impact on cellulose dissolution in an alkali/urea aqueous system. Phys. Chem. Chem. Phys. 2017, 19, 17909–17917. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Heiskanen, J.P. Room-temperature dissolution and chemical modification of cellulose in aqueous tetraethylammonium hydroxide–carbamide solutions. Cellulose 2020, 27, 1933–1950. [Google Scholar] [CrossRef]
- Yang, J.; Sun, M.; Jiao, L.; Dai, H. Molecular weight distribution and dissolution behavior of lignin in alkaline solutions. Polymers 2021, 13, 4166. [Google Scholar] [CrossRef]
- Lin, L.; Yan, R.; Jiang, W.; Shen, F.; Zhang, X.; Zhang, Y.; Deng, S.; Li, Z. Enhanced enzymatic hydrolysis of palm pressed fiber based on the three main components: Cellulose, hemicellulose, and lignin. Appl. Biochem. Biotechnol. 2014, 173, 409–420. [Google Scholar] [CrossRef]
- Mussatto, S.I. Biomass pretreatment with acids. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 169–185. [Google Scholar]
- Ioelovich, M. Study of cellulose interaction with concentrated solutions of sulfuric acid. ISRN Chem. Eng. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Fagerstedt, K.V.; Saranpää, P.; Tapanila, T.; Immanen, J.; Serra, J.A.A.; Nieminen, K. Determining the composition of lignins in different tissues of silver birch. Plants 2015, 4, 183–195. [Google Scholar] [CrossRef]
- Pfab, E.; Filiciotto, L.; Luque, R. The dark side of biomass valorization: A laboratory experiment to understand humin formation, catalysis, and green chemistry. J. Chem. Educ. 2019, 96, 3030–3037. [Google Scholar] [CrossRef]
- van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Olsson, C.; Westm, G. Direct dissolution of cellulose: Background, means and applications. In Cellulose—Fundamental Aspects; InTech: Singapore, 2013. [Google Scholar]
- Liebert, T. Cellulose solvents—Remarkable history, bright future. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; American Chemical Society: New York, NY, USA, 2010; pp. 3–54. [Google Scholar]
- Leipner, H.; Fischer, S.; Brendler, E.; Voigt, W. Structural changes of cellulose dissolved in molten salt hydrates. Macromol. Chem. Phys. 2000, 201, 2041–2049. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Recent advances in the application of inorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Deng, F.; Kommalapati, R.R.; Amarasekara, A.S. Iron based catalysts in biomass processing. Renew. Sustain. Energy Rev. 2020, 134, 110292. [Google Scholar]
- Zhang, Y.; Huang, M.; Su, J.; Hu, H.; Yang, M.; Huang, Z.; Chen, D.; Wu, J.; Feng, Z. Overcoming biomass recalcitrance by synergistic pretreatment of mechanical activation and metal salt for enhancing enzymatic conversion of lignocellulose. Biotechnol. Biofuels 2019, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Thümmler, K. Molten inorganic salts as reaction medium for cellulose. In ACS Symposium Series; American Chemical Society: New York, NY, USA, 2010; Volume 1033, pp. 91–101. [Google Scholar]
- Saalwächter, K.; Burchard, W. Cellulose in new metal-complexing solvents. 2. Semidilute behavior in Cd-tren. Macromolecules 2001, 34, 5587–5598. [Google Scholar] [CrossRef]
- Sen, S.; Martin, J.D.; Argyropoulos, D.S. Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates. ACS Sustain. Chem. Eng. 2013, 1, 858–870. [Google Scholar] [CrossRef]
- McCormick, C.L.; Callais, P.A.; Hutchinson, B.H. Solution studies of cellulose in lithium chloride and N,N-dimethylacetamide. Macromolecules 1985, 18, 2394–2401. [Google Scholar] [CrossRef]
- Ali, N.; Hamouda, H.I.; Su, H.; Li, F.L.; Lu, M. Combinations of alkaline hydrogen peroxide and lithium chloride/N,N-dimethylacetamide pretreatments of corn stalk for improved biomethanation. Environ. Res. 2020, 186, 109563. [Google Scholar] [CrossRef]
- Li, H.; Xiong, L.; Chen, X.; Luo, M.; Chen, X.; Wang, C.; Huang, C.; Chen, X. Enhanced enzymatic hydrolysis of wheat straw via a combination of alkaline hydrogen peroxide and lithium chloride/N,N-dimethylacetamide pretreatment. Ind. Crops Prod. 2019, 137, 332–338. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution mechanism of cellulose in N,N-dimethylacetamide/lithium chloride: Revisiting through molecular interactions. J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Dicke, R.; Koschella, A.; Kull, A.H.; Klohr, E.A.; Koch, W. Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol. Chem. Phys. 2000, 201, 627–631. [Google Scholar] [CrossRef]
- Östlund, Å.; Lundberg, D.; Nordstierna, L.; Holmberg, K.; Nydén, M. Dissolution and gelation of cellulose in TBAF/DMSO solutions: The roles of fluoride ions and water. Biomacromolecules 2009, 10, 2401–2407. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Kragl, U.; Michalik, D.; Ludwig, R.; Hollmann, D. The effect of additives on the viscosity and dissolution of cellulose in tetrabutylphosphonium hydroxide. ChemSusChem 2019, 12, 3458–3462. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El Seoud, O.A. Recent advances in solvents for the dissolution, shaping and derivatization of cellulose: Quaternary ammonium electrolytes and their solutions in water and molecular solvents. Molecules 2018, 23, 511. [Google Scholar] [CrossRef] [PubMed]
- Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Critical review on sustainable homogeneous cellulose modification: Why renewability is not enough. ACS Sustain. Chem. Eng. 2019, 7, 1826–1840. [Google Scholar] [CrossRef]
- Ghandi, K. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Morais, E.S.; da Costa Lopes, A.M.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Setiawan, A.; Atkin, R.; Parthasarthy, R.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Progress on the pre-treatment of lignocellulosic biomass employing ionic liquids. Renew. Sustain. Energy Rev. 2019, 105, 268–292. [Google Scholar] [CrossRef]
- Fort, D.A.; Remsing, R.C.; Swatloski, R.P.; Moyna, P.; Moyna, G.; Rogers, R.D. Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem. 2007, 9, 63–69. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Factors governing dissolution process of lignocellulosic biomass in ionic liquid: Current status, overview and challenges. Bioresour. Technol. 2015, 178, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Rahim, A.H.A.; Yunus, N.M.; Hamzah, W.S.W.; Sarwono, A.; Muhammad, N. Low-viscosity ether-functionalized ionic liquids as solvents for the enhancement of lignocellulosic biomass dissolution. Processes 2021, 9, 261. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and valorization of cellulose, lignin and lignocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Moyer, P.; Smith, M.D.; Abdoulmoumine, N.; Chmely, S.C.; Smith, J.C.; Petridis, L.; Labbé, N. Relationship between lignocellulosic biomass dissolution and physicochemical properties of ionic liquids composed of 3-methylimidazolium cations and carboxylate anions. Phys. Chem. Chem. Phys. 2018, 20, 2508–2516. [Google Scholar] [CrossRef]
- Dash, M.; Mohanty, K. Effect of different ionic liquids and anti-solvents on dissolution and regeneration of miscanthus towards bioethanol. Biomass Bioenergy 2019, 124, 33–42. [Google Scholar] [CrossRef]
- Carneiro, A.P.; Rodríguez, O.; Macedo, E.A. Dissolution and fractionation of nut shells in ionic liquids. Bioresour. Technol. 2017, 227, 188–196. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Kim, M.; Blanch, H.W.; Prausnitz, J.M. Solubility and rate of dissolution for miscanthus in hydrophilic ionic liquids. Fluid Phase Equilibria 2011, 309, 89–96. [Google Scholar] [CrossRef]
- Zhou, L.; Pan, F.; Liu, Y.; Kang, Z.; Zeng, S.; Nie, Y. Study on the regularity of cellulose degradation in ionic liquids. J. Mol. Liq. 2020, 308, 113153. [Google Scholar] [CrossRef]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3- methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Comm. 2006, 12, 1271–1273. [Google Scholar] [CrossRef]
- Uto, T.; Yamamoto, K.; Kadokawa, J.I. Cellulose crystal dissolution in imidazolium-based ionic liquids: A theoretical study. J. Phys. Chem. B 2018, 122, 258–266. [Google Scholar] [CrossRef]
- Gogoi, G.; Hazarika, S. Dissolution of lignocellulosic biomass in ionic liquid-water media: Interpretation from solubility parameter concept. Korean J. Chem. Eng. 2019, 36, 1626–1636. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Kim, K.; Bowland, C.C.; Keum, J.K.; Kearney, L.T.; André, N.; Labbé, N.; Naskar, A.K. A fundamental understanding of whole biomass dissolution in ionic liquid for regeneration of fiber by solution-spinning. Green Chem. 2019, 21, 4354–4367. [Google Scholar] [CrossRef]
- Ge, M.; Fang, T.; Zhou, G.; Li, C.; Li, Y.; Liu, X. Insight into the dual effect of water on lignin dissolution in ionic liquids. Int. J. Biol. Macromol. 2022, 205, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Manna, B.; Datta, S.; Ghosh, A. Understanding the dissolution of softwood lignin in ionic liquid and water mixed solvents. Int. J. Biol. Macromol. 2021, 182, 402–412. [Google Scholar] [CrossRef]
- Bahadur, I.; Phadagi, R. Ionic liquids as environmental benign solvents for cellulose chemistry: A review. In Solvents, Ionic Liquids and Solvent Effects; IntechOpen: London, UK, 2020. [Google Scholar]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Yukasik, E.; Bogel-Yukasik, R. Solubility of carbohydrates in ionic liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, Y.; Lu, W.; Yao, K.; Xu, H. Effect of alkyl chain length in anion on dissolution of cellulose in 1-butyl-3-methylimidazolium carboxylate ionic liquids. J. Mol. Liq. 2014, 197, 211–214. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Thomsen, K.; Nie, Y.; Zhang, S.-J.; Meyer, A.S. Predictive screening of ionic liquids for dissolving cellulose and experimental verification. Green Chem. 2016, 18, 6246–6254. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ling, Z.; Xu, D.; You, T.; Wu, Y.Y.; Xu, F. Room-temperature superbase-derived ionic liquids with facile synthesis and low viscosity: Powerful solvents for cellulose dissolution by destroying the cellulose aggregate structure. Macromolecules 2020, 53, 3284–3295. [Google Scholar] [CrossRef]
- Meng, X.; Devemy, J.; Verney, V.; Gautier, A.; Husson, P.; Andanson, J.M. Improving cellulose dissolution in ionic liquids by tuning the size of the ions: Impact of the length of the alkyl chains in tetraalkylammonium carboxylate. ChemSusChem 2017, 10, 1749–1760. [Google Scholar] [CrossRef]
- Kuzmina, O.; Bhardwaj, J.; Vincent, S.R.; Wanasekara, N.D.; Kalossaka, L.M.; Griffith, J.; Potthast, A.; Rahatekar, S.; Eichhorn, S.J.; Welton, T. Superbase ionic liquids for effective cellulose processing from dissolution to carbonisation. Green Chem. 2017, 19, 5949–5957. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, A.; Lu, B.; Li, Z.; Wang, J. Dissolution of cellulose in 1-allyl-3-methylimizodalium carboxylates at room temperature: A structure-property relationship study. Carbohydr. Polym. 2015, 117, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Becherini, S.; Mezzetta, A.; Chiappe, C.; Guazzelli, L. Levulinate amidinium protic ionic liquids (PILs) as suitable media for the dissolution and levulination of cellulose. New J. Chem. 2019, 43, 4554–4561. [Google Scholar] [CrossRef]
- Brehm, M.; Pulst, M.; Kressler, J.; Sebastiani, D. Triazolium-based ionic liquids: A novel class of cellulose solvents. J. Phys. Chem. B 2019, 123, 3994–4003. [Google Scholar] [CrossRef]
- Xu, K.; Xiao, Y.; Cao, Y.; Peng, S.; Fan, M.; Wang, K. Dissolution of cellulose in 1-allyl-3-methylimidazolium methyl phosphonate ionic liquid and its composite system with Na2PHO3. Carbohydr. Polym. 2019, 209, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Meenatchi, B.; Renuga, V.; Manikandan, A. Cellulose dissolution and regeneration using various imidazolium based protic ionic liquids. J. Mol. Liq. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- Raut, D.G.; Sundman, O.; Su, W.; Virtanen, P.; Sugano, Y.; Kordas, K.; Mikkola, J.P. A Morpholinium ionic liquid for cellulose dissolution. Carbohydr. Polym. 2015, 130, 18–25. [Google Scholar] [CrossRef]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: Innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Islam, T.; Islam Sarker, M.Z.; Uddin, A.H.; Bin Yunus, K.; Prasad, R.; Mia, M.A.R.; Ferdosh, S. Kamlet Taft parameters: A tool to alternate the usage of hazardous solvent in pharmaceutical and chemical manufacturing/synthesis—A gateway towards green technology. Anal. Chem. Lett. 2020, 10, 550–561. [Google Scholar] [CrossRef]
- Weiß, N.; Schmidt, C.H.; Thielemann, G.; Heid, E.; Schröder, C.; Spange, S. The physical significance of the Kamlet-Taft: π ∗ parameter of ionic liquids. Phys. Chem. Chem. Phys. 2021, 23, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–442. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Kasprzak, D.; Krystkowiak, E.; Stępniak, I.; Galiński, M. Dissolution of cellulose in novel carboxylate-based ionic liquids and dimethyl sulfoxide mixed solvents. Eur. Polym. J. 2019, 113, 89–97. [Google Scholar] [CrossRef]
- Phadagi, R.; Singh, S.; Hashemi, H.; Kaya, S.; Venkatesu, P.; Ramjugernath, D.; Ebenso, E.E.; Bahadur, I. Understanding the role of dimethylformamide as co-solvents in the dissolution of cellulose in ionic liquids: Experimental and theoretical approach. J. Mol. Liq. 2021, 328, 115392. [Google Scholar] [CrossRef]
- Fendt, S.; Padmanabhan, S.; Blanch, H.W.; Prausnitz, J.M. Viscosities of acetate or chloride-based ionic liquids and some of their mixtures with water or other common solvents. J. Chem. Eng. Data 2011, 56, 31–34. [Google Scholar] [CrossRef]
- Xu, A.; Wang, J.; Wang, H. Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem. 2010, 12, 268–275. [Google Scholar] [CrossRef]
- Ding, Z.D.; Chi, Z.; Gu, W.X.; Gu, S.M.; Liu, J.H.; Wang, H.J. Theoretical and experimental investigation on dissolution and regeneration of cellulose in ionic liquid. Carbohydr. Polym. 2012, 89, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Asikkala, J.; Filpponen, I.; Argyropoulos, D.S. Factors affecting wood dissolution and regeneration of ionic liquids. Ind. Eng. Chem. Res. 2010, 49, 2477–2484. [Google Scholar] [CrossRef]
- Manna, B.; Ghosh, A. Dissolution of cellulose in ionic liquid and water mixtures as revealed by molecular dynamics simulations. J. Biomol. Struct. Dyn. 2019, 37, 3987–4005. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ju, Z.; Yu, M.; Luo, H.; Zhang, C.; Zhang, X.; Cheng, H.; Zheng, M.; Jin, L.; Ge, C. Cellulose regeneration in imidazolium-based ionic liquids and antisolvent mixtures: A density functional theory study. ACS Omega 2022, 7, 42170–42180. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Z.L. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- el Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Gomes, G.R.; Mattioli, R.R.; Pastre, J.C. Amino acid-based deep eutectic solvents in biomass processing—Recent advances. J. Braz. Chem. Soc. 2022, 33, 815–823. [Google Scholar] [CrossRef]
- Ji, H.; Lv, P. Mechanistic insights into the lignin dissolution behaviors of a recyclable acid hydrotrope, deep eutectic solvent (DES), and ionic liquid (IL). Green Chem. 2020, 22, 1378–1387. [Google Scholar] [CrossRef]
- Cassoni, A.C.; Mota, I.; Costa, P.; Vasconcelos, M.W.; Pintado, M. Effect of alkaline and deep eutectic solvents pretreatments on the recovery of lignin with antioxidant activity from grape stalks. Int. J. Biol. Macromol. 2022, 220, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, K.; He, Z.; Wu, T.; Huang, C.; Liang, L.; Fang, G. Deep eutectic solvent recycling to prepare high purity dissolving pulp. Cellulose 2021, 28, 11503–11517. [Google Scholar] [CrossRef]
- Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K. Effective delignification of lignocellulosic biomass by microwave assisted deep eutectic solvents. Bioresour. Technol. 2020, 303, 122897. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.; da Costa Lopes, A.M.; Silvestre, A.J.D.; Rodrigues Pinto, P.C.; Freire, C.S.R.; Coutinho, J.A.P. Wood delignification with aqueous solutions of deep eutectic solvents. Ind. Crops Prod. 2021, 160, 113128. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Wang, W.; Li, B. Mechanism of deep eutectic solvent delignification: Insights from molecular dynamics simulations. ACS Sustain. Chem. Eng. 2021, 9, 7101–7111. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Nair, A.; Jayavel, A.; Sathiasivan, K.; Rajesh, M.; Ramaswamy, S.; Tamilarasan, K. Choline chloride-based deep eutectic solvents for efficient delignification of Bambusa bambos in bio-refinery applications. Chem. Pap. 2020, 74, 4533–4545. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Ricci, P.; Karvonen, V.; Ottolina, G.; Liimatainen, H. Acidic and alkaline deep eutectic solvents in delignification and nanofibrillation of corn stalk, wheat straw, and rapeseed stem residues. Ind. Crops Prod. 2020, 145, 111956. [Google Scholar] [CrossRef]
- Wu, M.; Gong, L.; Ma, C.; He, Y.C. Enhanced enzymatic saccharification of sorghum straw by effective delignification via combined pretreatment with alkali extraction and deep eutectic solvent soaking. Bioresour. Technol. 2021, 340, 125695. [Google Scholar] [CrossRef]
- Lin, W.; Xing, S.; Jin, Y.; Lu, X.; Huang, C.; Yong, Q. Insight into understanding the performance of deep eutectic solvent pretreatment on improving enzymatic digestibility of bamboo residues. Bioresour. Technol. 2020, 306, 123163. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, T.; Huang, C.; Yao, J. Using deep eutectic solvent pretreatment for enhanced enzymatic saccharification and lignin utilization of Masson pine. Renew. Energy 2022, 195, 681–687. [Google Scholar] [CrossRef]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.J. Effect of choline-based deep eutectic solvent pretreatment on the structure of cellulose and lignin in bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.J.; Yang, J.Y.; Li, M.F.; Peng, F.; Bian, J. Efficient fractionation of woody biomass hemicelluloses using cholinium amino acids-based deep eutectic solvents and their aqueous mixtures. Bioresour. Technol. 2022, 354, 127139. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Zhang, W.; Li, M.; Peng, F.; Bian, J. Alkaline deep eutectic solvents as novel and effective pretreatment media for hemicellulose dissociation and enzymatic hydrolysis enhancement. Int. J. Biol. Macromol. 2021, 193, 1610–1616. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, J.; Lan, P.; Yang, H.; Lu, J.; Wang, Z. Study on the dissolution mechanism of cellulose by ChCl-based deep eutectic solvents. Materials 2020, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Ma, Z.; Yan, L. Dissolution of highly molecular weight cellulose isolated from wheat straw in deep eutectic solvent of choline/L-lysine hydrochloride. Green Energy Environ. 2020, 5, 232–239. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]



| Type of Biomass | Content [%] | References | ||
|---|---|---|---|---|
| Cellulose | Hemicelluloses | Lignin | ||
| Grass | 28.8–38.0 | 18.4–30.0 | 4.0–17.5 | [10,11] |
| Sawmill wood chips | 35.0–50.0 | 15.0–39.0 | 20.0–34.0 | [6,12] |
| Walnut shells | 25.6 | 22.7 | 29.9–52.3 | [6,13] |
| Hazelnut shells | 25.9 | 29.9 | 42.5 | [14] |
| Walnut tree wood | 40.8–49.8 | 33.4 | 21.8–29.1 | [15] |
| Olive tree wood | 31.5–31.9 | 11.3–15.5 | 32.5 | [16] |
| Softwood | 35.0–45.2 | 25.0–31.3 | 21.7–30.0 | [14,17] |
| Hardwood | 45.0–50.0 | 20.0–25.0 | 20.0–28.0 | [14,17] |
| Poplar | 42.7 | 21.7 | 26.9 | [18] |
| Willow | 44.3 | 22.6 | 25.1 | [18] |
| Pine sawmill chips | 44.0 | 26.0 | 26.0 | [19] |
| Pine bark | 19.0–21.9 | 18.3–25.0 | 38.0–40.7 | [18,20] |
| Spruce bark | 29.7 | 13.9 | 45.1 | [18] |
| Olive tree leaves | 5.7–8.5 | 3.8–5.4 | 39.6 | [16] |
| Wheat straw | 28.8–40.0 | 20.0–39.1 | 15.0–20.5 | [14,17,18] |
| Switchgrass millet | 30.0–50.0 | 10.0–40.0 | 5.0–20.0 | [17] |
| Biomass Type | Ionic Liquid | Temperature [°C] | Solubility [wt.%] | References |
|---|---|---|---|---|
| Hybrid poplar | [emim][CH3COO] | 80 | 5.3 | [84] |
| [emim][HCOO] | 5.3 | |||
| [amim][CH3COO] | 7.0 | |||
| [amim][HCOO] | 7.4 | |||
| Miscanthus | [emim][CH3COO] | 100 | 1.7 | [85] |
| [emim][CH3SO3] | 4.0 | |||
| [emim][HSO4] | 9.6 | |||
| Peanut shells | [bmim]Cl | 120 | 2.3 | [86] |
| [emim][CH3COO] | 5.8 | |||
| Chestnut shells | [bmim]Cl | 120 | 4.7 | |
| [emim][CH3COO] | 7.0 | |||
| Miscanthus | [bmim][CH3COO] | 130 | 4.0 | [87] |
| [bmim]Cl | 3.0 | |||
| [emim]Cl | 4.0 |
| Cellulose Type | Ionic liquid | Temperature [°C] | Solubility [wt. %] | References |
|---|---|---|---|---|
| MCC | [bmim][HCOO] | 70 | 12.5 | [99] |
| [bmim][CH3COO] | 15.5 | |||
| [bmim][CH3CH2COO] | 17.5 | |||
| [bmim][CH3(CH2)2COO] | 14.0 | |||
| MCC | [emim][CH3COO] | 90 | 27.0 | [100] |
| [emim]DEP | 15.0 | |||
| MCC | [DBUH][HCOO] | 90 | 18.9 | [101] |
| [DBUH][CH3COO] | 14.8 | |||
| [DBUH][CH3CH2COO] | 12.6 | |||
| MCC | [N2226][CH3COO] | 80 | 15.0 | [102] |
| 90 | 22.0 | |||
| Commercial Cellulose | [emim][CH3COO] | 80 | 35.2 | [103] |
| [emim][DEP] | 23.0 | |||
| [DBUH][CH3COO] | 22.5 | |||
| [DBUH]][Prop] | 4.3 | |||
| [DBNH][CH3COO] | 22.0 | |||
| [DBNH][Hex] | 16.1 | |||
| MCC | [amim][CH3CH2COO] | 30 | 19.0 | [104] |
| [amim][C6H5COO] | <5.0 | |||
| MCC | [DBUH][Lev] | 100 | 15.0 | [105] |
| [DBNH][Lev] | 20.0 | |||
| MCC | [emtr124][CH3COO] | 80 | 30.0 | [106] |
| [emtr123]Br | 4.0 | |||
| [emim][CH3COO] | 21.1 | |||
| MCC | [amim][MP] | 80 | 22.0 | [107] |
| Commercial cellulose | [hmim][CH3CH(OH)COO] | 80 | 5.0 | [108] |
| MCC | [ammorp][CH3COO] | 80 | 17.0 | [109] |
| Biomass Type | DES (HBA:HBD Molar Ratio) | Temperature [°C] | Dissolution Technique | Time [h] | Delignification Level [%] | References |
|---|---|---|---|---|---|---|
| Rice straw | ChCl:Lac (1:5) | 60 | Stirring and heating | 12 | 60.0 | [138] |
| Bet:Lac (1:5) | 53.0 | |||||
| Miscanthus | ChCl:Fa (1:2) | 60 | Microwave | 1 | 4.8 | [135] |
| 130 | 82.0 | |||||
| Birchwood | ChCl:Ox (1:1) | 60 | Microwave | 1 | 43.0 | |
| 130 | 85.0 | |||||
| Bamboo stem | ChCl:U (1:2) | 120 | Stirring and heating | 10 | 19.4 | [139] |
| ChCl:Ox (1:2) | 25.4 | |||||
| Wheat straw | ChCl:Lac (1:5) | 100 | Stirring and heating | 16 | 8.5 | [140] |
| Corn stalk | 9.5 | |||||
| Rapeseed stem | 11.8 | |||||
| Sorghum straw | ChCl:Lac | 150 | Stirring and heating | 0.5 | 49.0 | [141] |
| Bamboo residues | ChCl:Lac (1:4) | 110 | Stirring and heating | 1.5 | 42.3 | [142] |
| 130 | 83.6 | |||||
| ChCl:Lac (1:8) | 110 | 41.2 | ||||
| 130 | 84.1 | |||||
| Masson pine | ChCl:Lac (1:10) | 110 | Stirring and heating | 6 | 58.1 | [143] |
| 140 | 87.5 | |||||
| Bagasse | ChCl:Ox (1:1) | 100 | Stirring and heating | 4 | 47.9 | [144] |
| ChCl:Et (1:2) | 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przypis, M.; Wawoczny, A.; Gillner, D. Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment. Appl. Sci. 2023, 13, 1055. https://doi.org/10.3390/app13021055
Przypis M, Wawoczny A, Gillner D. Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment. Applied Sciences. 2023; 13(2):1055. https://doi.org/10.3390/app13021055
Chicago/Turabian StylePrzypis, Marta, Agata Wawoczny, and Danuta Gillner. 2023. "Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment" Applied Sciences 13, no. 2: 1055. https://doi.org/10.3390/app13021055
APA StylePrzypis, M., Wawoczny, A., & Gillner, D. (2023). Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment. Applied Sciences, 13(2), 1055. https://doi.org/10.3390/app13021055









