Auditory Fine-Tuned Suppressor of TMS-Clicks (TMS-Click AFTS): A Novel, Perceptually Driven/Tuned Approach for the Reduction in AEP Artifacts in TMS-EEG Studies
Abstract
1. Introduction
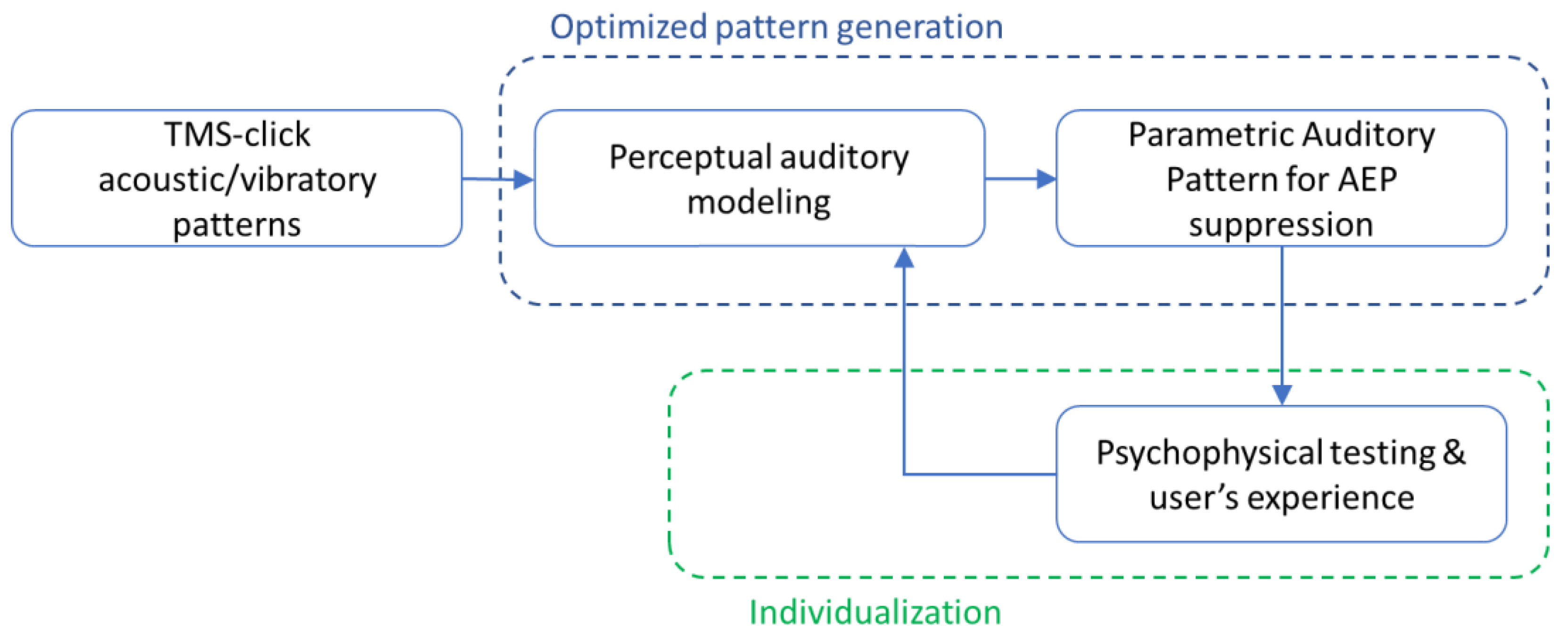
2. The Proposed Approach
- A module for generating a psychophysically determined auditory potentials suppression stimulus, which consists of properly shaped continuous wide-band noise (WBN), matched to the TMS clicks’ characteristics.
- Individualization by means of an adaptive procedure for optimal level determination, together with a GUI-based software for the automation and standardization of the procedure.
- Other benefits (facilitation of more precise control of pattern specification, increased suppression efficiency, hearing safety and comfort).
3. Procedures and Material
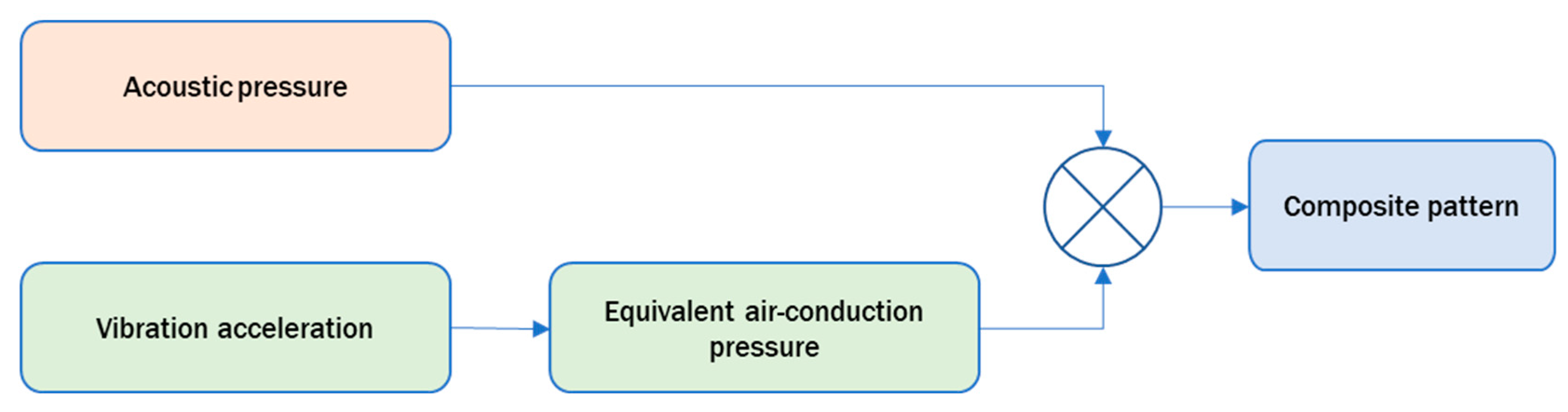
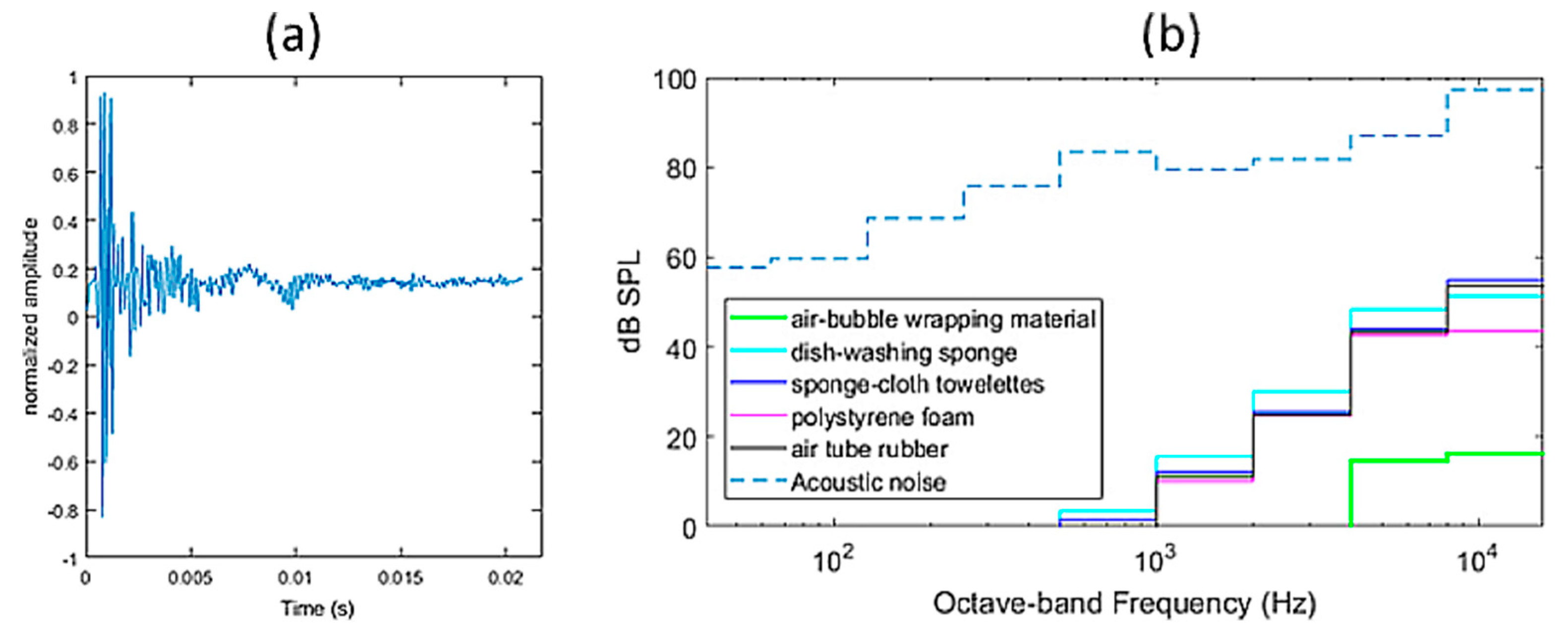
Suppressor’s Pattern Specification
- Intersubject variability of external ear’s acoustics and deviations from the measured rTMS-click pattern at the concha of the standard torso simulator’s pinna.
- Intersubject variability in perceptual sensitivity of rTMS-click detection under the concurrent presentation of the suppressor.
- Intersubject variability in the perception of loudness of the suppressor and/or the rTMS-click due to binaural presentation.
- Variability due to earphones insertion and fit into the ear canal.
- parameter for frequency shifting of the whole pattern, namely
- spectral slope parameter around the frequency of maximum , namely , and
- overall gain which accounts for overall loudness adjustment.
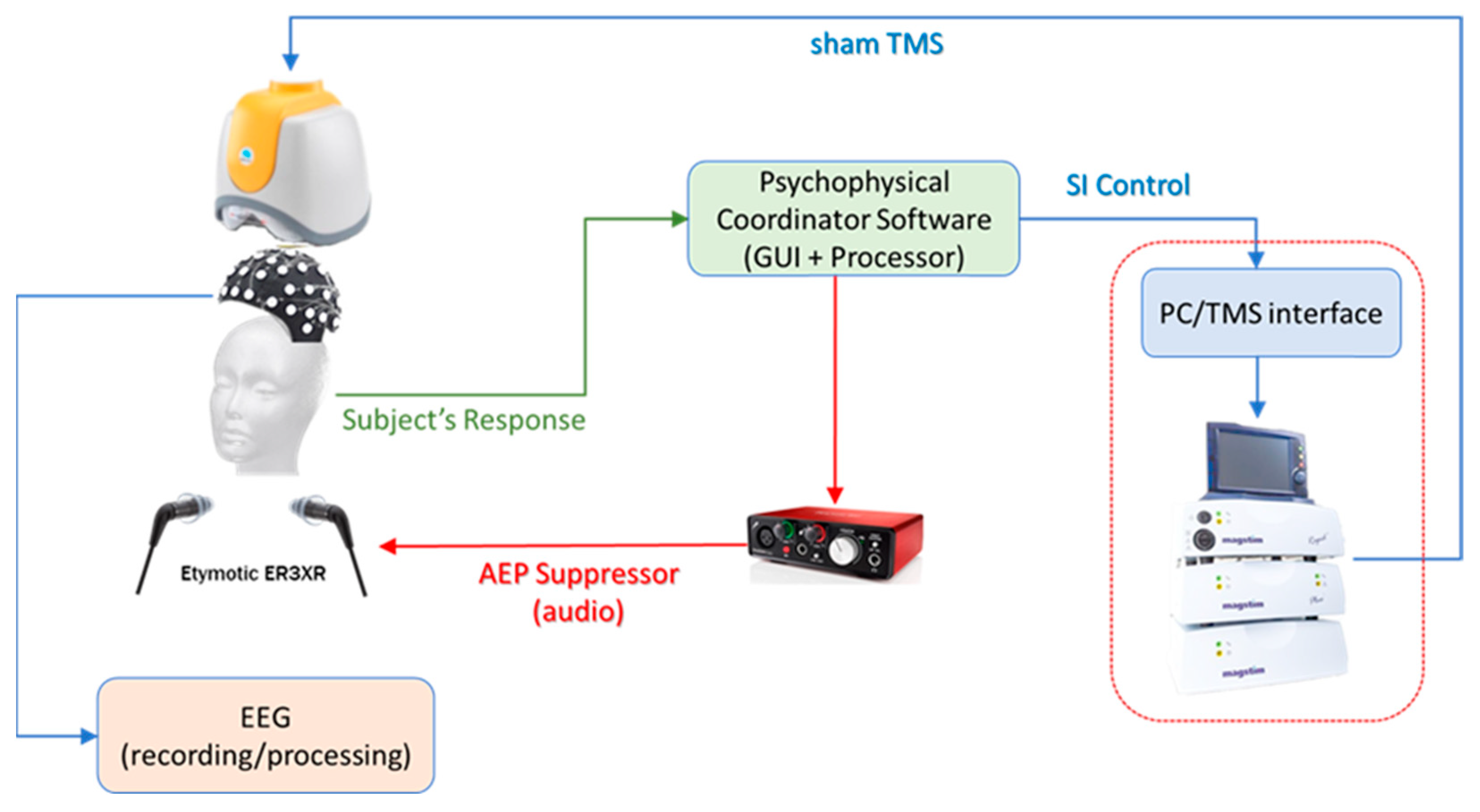
4. TMS Study Protocol and EEG Recording
4.1. EEG Data
4.2. EEG Signal Preprocessing and Analysis
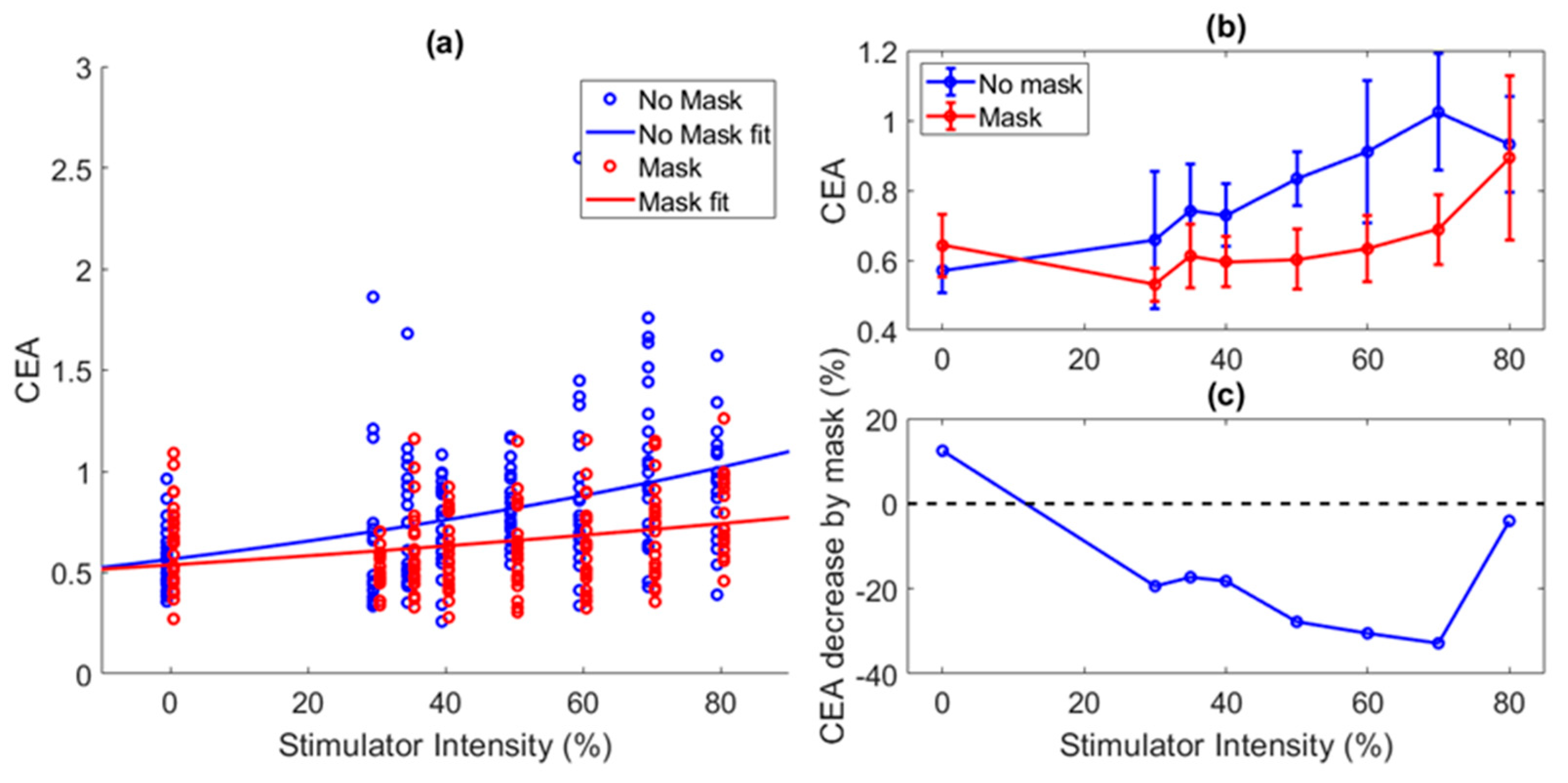
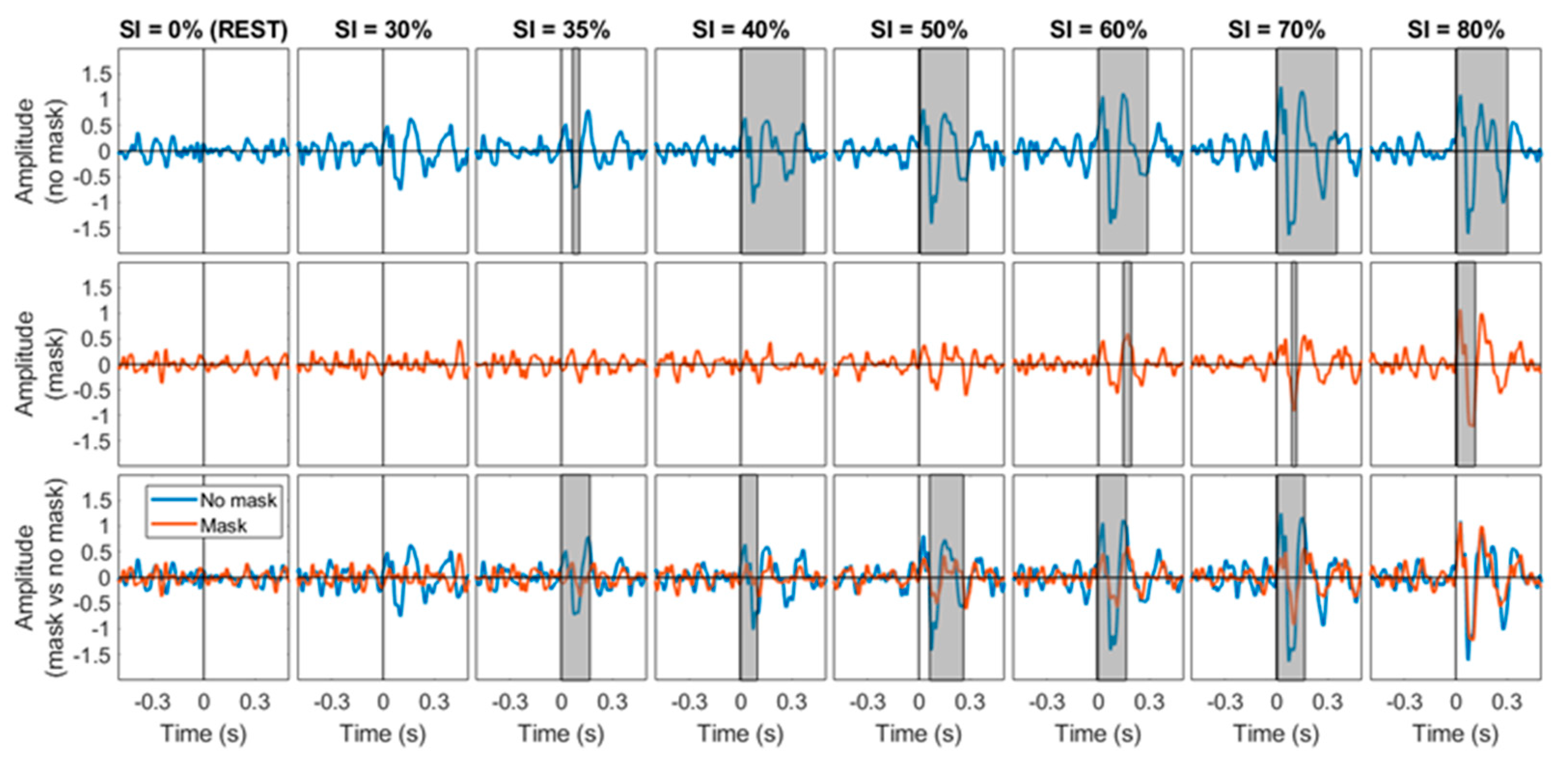
5. Results
6. Discussion and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilmoniemi, R.J.; Kičić, D. Methodology for combined TMS and EEG. Brain Topogr. 2010, 22, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical utility and prospective of TMS–EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef] [PubMed]
- Julkunen, P.; Kimiskidis, V.K.; Belardinelli, P. Bridging the gap: TMS-EEG from lab to clinic. J. Neurosci. Methods 2022, 369, 109482. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Sarasso, S.; Puglisi, G.; Palù, D.D.; Pigorini, A.; Casarotto, S.; D’Ambrosio, S.; Astolfi, A.; Massimini, M.; Rosanova, M.; et al. TAAC-TMS Adaptable Auditory Control: A universal tool to mask TMS clicks. J. Neurosci. Methods 2022, 370, 109491. [Google Scholar] [CrossRef]
- Eeg-Olofsson, M.; Stenfelt, S.; Taghavi, H.; Reinfeldt, S.; Håkansson, B.; Tengstrand, T.; Finizia, C. Transmission of bone conducted sound–Correlation between hearing perception and cochlear vibration. Hear. Res. 2013, 306, 11–20. [Google Scholar] [CrossRef]
- Reinfeldt, S.; Stenfelt, S.; Håkansson, B. Estimation of bone conduction skull transmission by hearing thresholds and ear-canal sound pressure. Hear. Res. 2013, 299, 19–28. [Google Scholar] [CrossRef]
- Ross, J.M.; Sarkar, M.; Keller, C.J. Experimental Suppression of TMS-EEG Sensory Potentials. Neuroscience 2022, 16, 1663. [Google Scholar] [CrossRef]
- Biabani, M.; Fornito, A.; Mutanen, T.P.; Morrow, J.; Rogasch, N.C. Characterizing and minimizing the contribution of sensory inputs to TMS-evoked potentials. Brain Stimul. 2019, 12, 1537–1552. [Google Scholar] [CrossRef]
- Conde, V.; Tomasevic, L.; Akopian, I.; Stanek, K.; Saturnino, G.B.; Thielscher, A.; Bergmann, T.O.; Siebner, H.R. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. Neuroimage 2019, 185, 300–312. [Google Scholar] [CrossRef]
- Harrison, R.; Bielefeld, E. Assessing Hidden Hearing Loss After Impulse Noise in a Mouse Model. Noise Health 2019, 21, 35. [Google Scholar] [CrossRef]
- Mantysalo, S.; Vuori, J. Effects of impulse noise and continuous steady state noise on hearing. Occup. Environ. Med. 1984, 41, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kardous, C.; Murphy, W. How Can We Measure Impulse Noise Properly? Available online: https://blogs.cdc.gov/niosh-science-blog/2018/07/18/impulse-noise/ (accessed on 16 May 2022).
- Margolis, R.H.; Stiepan, S.M. Acoustic method for calibration of audiometric bone vibrators. J. Acoust. Soc. Am. 2012, 131, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Katz, J. Handbook of Clinical Audiology, 7th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Voran, S. Observations on auditory excitation and masking patterns. In Proceedings of the 1995 Workshop on Applications of Signal Processing to Audio and Acoustics, New Paltz, NY, USA, 15–18 October 1995; pp. 206–209. [Google Scholar] [CrossRef]
- Moore, B.C.J.; Glasberg, B.R.; Baer, T. A model for the prediction of thresholds, loudness, and partial loudness. J. Audio Eng. Soc. 1997, 45, 224–240. [Google Scholar]
- Necciari, T.; Balazs, P.; Kronland-Martinet, R.; Ystad, S.; Laback, B.; Savel, S.; Meunier, S. Auditory Time-Frequency Masking: Psychoacoustical Data and Application to Audio Representations. In Speech, Sound and Music Processing: Embracing Research in India; Ystad, S., Aramaki, M., Kronland-Martinet, R., Jensen, K., Mohanty, S., Eds.; CMMR FRSM 2011; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7172. [Google Scholar] [CrossRef]
- Heijden, M.V.; Kohlrausch, A. Using an excitation-pattern model to predict auditory masking. Hear. Res. 2012, 80, 38–52. [Google Scholar] [CrossRef]
- Glasberg, B.R.; Moore, B.C.J. A model of loudness applicable to time-varying sounds. J. Audio Eng. Soc. 2002, 50, 331–342. [Google Scholar]
- Leek, M.R. Adaptive procedures in psychophysical research. Percept. Psychophys. 2001, 63, 1279–1292. [Google Scholar] [CrossRef]
- Daly, E.J., 3rd; Wells, N.J.; Swanger-Gagné, M.S.; Carr, J.E.; Kunz, G.M.; Taylor, A.M. Evaluation of the multiple-stimulus without replacement preference assessment method using activities as stimuli. J. Appl. Behav. Anal. 2009, 42, 563–574. [Google Scholar] [CrossRef]
- Chowdhury, N.S.; Rogasch, N.C.; Chiang, A.K.I.; Millard, S.K.; Skippen, P.; Chang, W.J.; Bilska, K.; Si, E.; Seminowicz, D.A.; Schabrun, S.M. The influence of sensory potentials on transcranial magnetic stimulation-Electroencephalography recordings. Clin. Neurophysiol. 2022, 140, 98–109. [Google Scholar] [CrossRef]
- Carmi, L.; Tendler, A.; Bystritsky, A.; Hollander, E.; Blumberger, D.M.; Daskalakis, J.; Ward, H.; Lapidus, K.; Goodman, W.; Casuto, L.; et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 931–993. [Google Scholar] [CrossRef]
- Carmi, L.; Alyagon, U.; Barnea-Ygael, N.; Zohar, J.; Dar, R.; Zangen, A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018, 11, 158–165. [Google Scholar] [CrossRef]
- Virtanen, J.; Ruohonen, J.; Näätänen, R.; Ilmoniemi, R.J. Instrumentation for the Measurement of Electric Brain Responses to Transcranial Magnetic Stimulation. Med. Biol. Eng. Comput. 1999, 37, 322–326. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wilson, G.; Russell, C. Removal of Ocular Artifacts from Electro-Encephalogram by Adaptive Filtering. Med Biol. Eng. Comput. 2004, 24, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Bokil, H. Observed Brain Dynamics; Oxford University Press: Oxford, UK, 2007; ISBN 9780199864829. [Google Scholar]
- Kothe, C.A.; Makeig, S. BCILAB: A Platform for Brain-Computer Interface Development. J. Neural Eng. 2013, 10, 056014. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.R.; Kothe, C.A.E.; Chi, Y.M.; Ojeda, A.; Kerth, T.; Makeig, S.; Jung, T.P.; Cauwenberghs, G. Real-Time Neuroimaging and Cognitive Monitoring Using Wearable Dry EEG. IEEE Trans. Biomed. Eng. 2015, 62, 2553–2567. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Sullivan, C.; Thomson, R.H.; Rose, N.S.; Bailey, N.W.; Fitzgerald, P.B.; Farzan, F.; Hernandez-Pavon, J.C. Analysing Concurrent Transcranial Magnetic Stimulation and Electroencephalographic Data: A Review and Introduction to the Open-Source TESA Software. Neuroimage 2017, 147, 934–951. [Google Scholar] [CrossRef]
- Kayser, J.; Tenke, C.E. Principal Components Analysis of Laplacian Waveforms as a Generic Method for Identifying ERP Generator Patterns: II. Adequacy of Low-Density Estimates. Clin. Neurophysiol. 2006, 117, 369–380. [Google Scholar] [CrossRef]
- Kayser, J.; Tenke, C.E. Principal Components Analysis of Laplacian Waveforms as a Generic Method for Identifying ERP Generator Patterns: I. Evaluation with Auditory Oddball Tasks. Clin. Neurophysiol. 2006, 117, 348–368. [Google Scholar] [CrossRef]
- Rosanova, M.; Casali, A.; Bellina, V.; Resta, F.; Mariotti, M.; Massimini, M.J. Natural frequencies of human corticothalamic circuits. Neuroscience 2009, 29, 7679–7685. [Google Scholar] [CrossRef]
- Fecchio, M.; Pigorini, A.; Comanducci, A.; Sarasso, S.; Casarotto, S.; Premoli, I.; Derchi, C.-C.; Mazza, A.; Russo, S.; Resta, F.; et al. The spectral features of EEG responses to transcranial magnetic stimulation of the primary motor cortex depend on the amplitude of the motor evoked potentials. PLoS ONE 2017, 12, e0184910. [Google Scholar] [CrossRef]
- Ross, J.M.; Ozdemir, R.A.; Lian, S.J.; Fried, P.J.; Schmitt, E.M.; Inouye, S.K.; Pascual-Leone, A.; Shafi, M.M. A structured ICA-based process for removing auditory evoked potentials. Sci. Rep. 2022, 12, 1–19. [Google Scholar]
- McCulloch, C.E.; Searle, S.R. Generalized, Linear, and Mixed Models; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open-source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]

| Group | N | Age: Mean (Std) | Sex: M/F | # of Stimulations: Median (Range) |
|---|---|---|---|---|
| Healthy | 11 | 34.6(9.5) | 5/6 | 31 *, ([11, 97]) |
| Epileptic | 13 | 28.4(7.9) | 5/8 | 31 *, ([13, 81]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastiadis, K.; Vlachos, I.; Chatzikyriakou, E.; Roth, Y.; Zibman, S.; Zangen, A.; Kugiumtzis, D.; Kimiskidis, V.K. Auditory Fine-Tuned Suppressor of TMS-Clicks (TMS-Click AFTS): A Novel, Perceptually Driven/Tuned Approach for the Reduction in AEP Artifacts in TMS-EEG Studies. Appl. Sci. 2023, 13, 1047. https://doi.org/10.3390/app13021047
Pastiadis K, Vlachos I, Chatzikyriakou E, Roth Y, Zibman S, Zangen A, Kugiumtzis D, Kimiskidis VK. Auditory Fine-Tuned Suppressor of TMS-Clicks (TMS-Click AFTS): A Novel, Perceptually Driven/Tuned Approach for the Reduction in AEP Artifacts in TMS-EEG Studies. Applied Sciences. 2023; 13(2):1047. https://doi.org/10.3390/app13021047
Chicago/Turabian StylePastiadis, Konstantinos, Ioannis Vlachos, Evangelia Chatzikyriakou, Yiftach Roth, Samuel Zibman, Abraham Zangen, Dimitris Kugiumtzis, and Vasilios K. Kimiskidis. 2023. "Auditory Fine-Tuned Suppressor of TMS-Clicks (TMS-Click AFTS): A Novel, Perceptually Driven/Tuned Approach for the Reduction in AEP Artifacts in TMS-EEG Studies" Applied Sciences 13, no. 2: 1047. https://doi.org/10.3390/app13021047
APA StylePastiadis, K., Vlachos, I., Chatzikyriakou, E., Roth, Y., Zibman, S., Zangen, A., Kugiumtzis, D., & Kimiskidis, V. K. (2023). Auditory Fine-Tuned Suppressor of TMS-Clicks (TMS-Click AFTS): A Novel, Perceptually Driven/Tuned Approach for the Reduction in AEP Artifacts in TMS-EEG Studies. Applied Sciences, 13(2), 1047. https://doi.org/10.3390/app13021047







