Effect of Time of Girdling on Leaf Photosynthetic Performance and Kiwifruit Quality Characteristics at Harvest and Post-Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Site Location—Plant Material—Treatments—Experimental Design
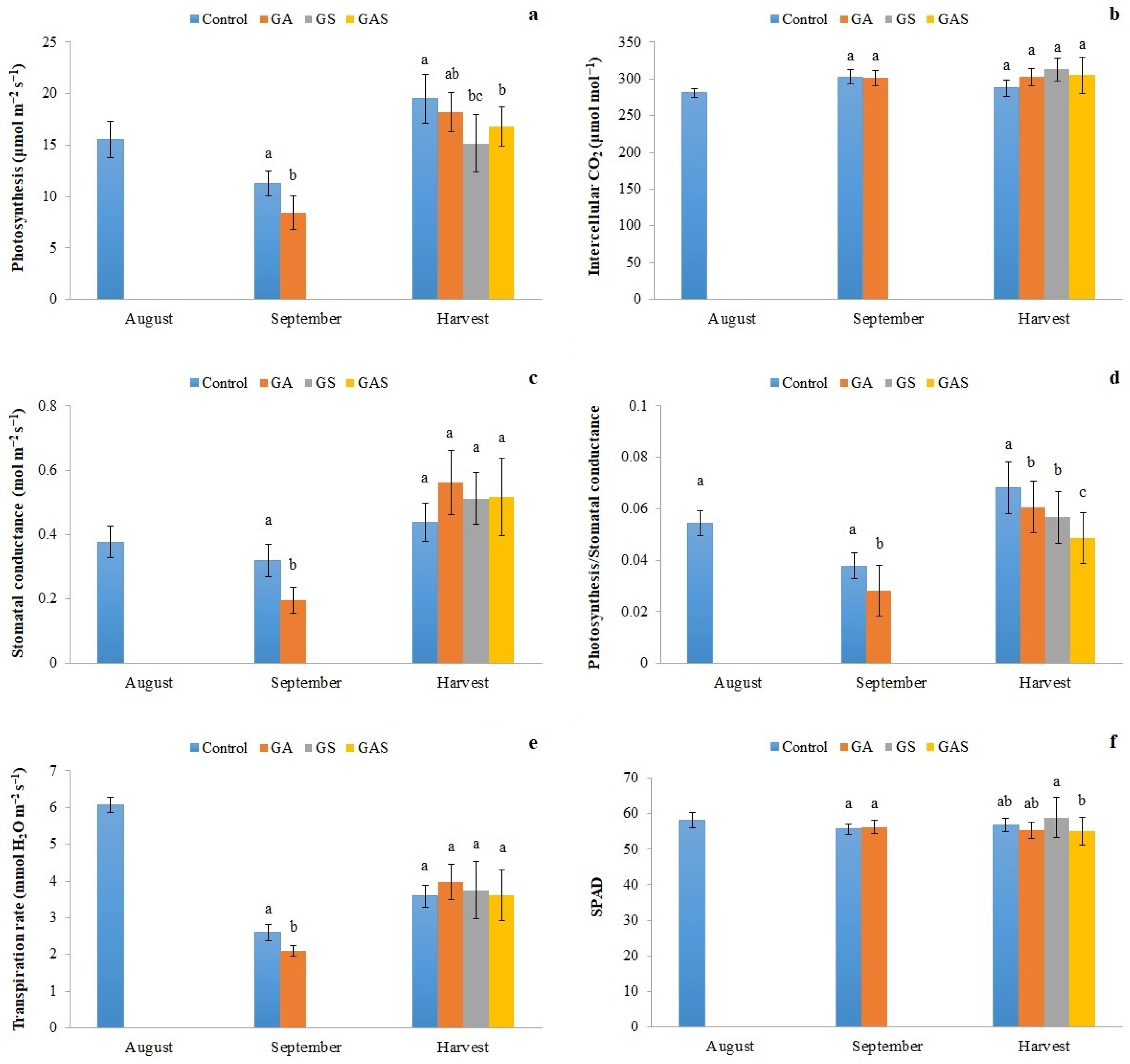
2.2. Photosynthesis and Photosynthetic Parameters
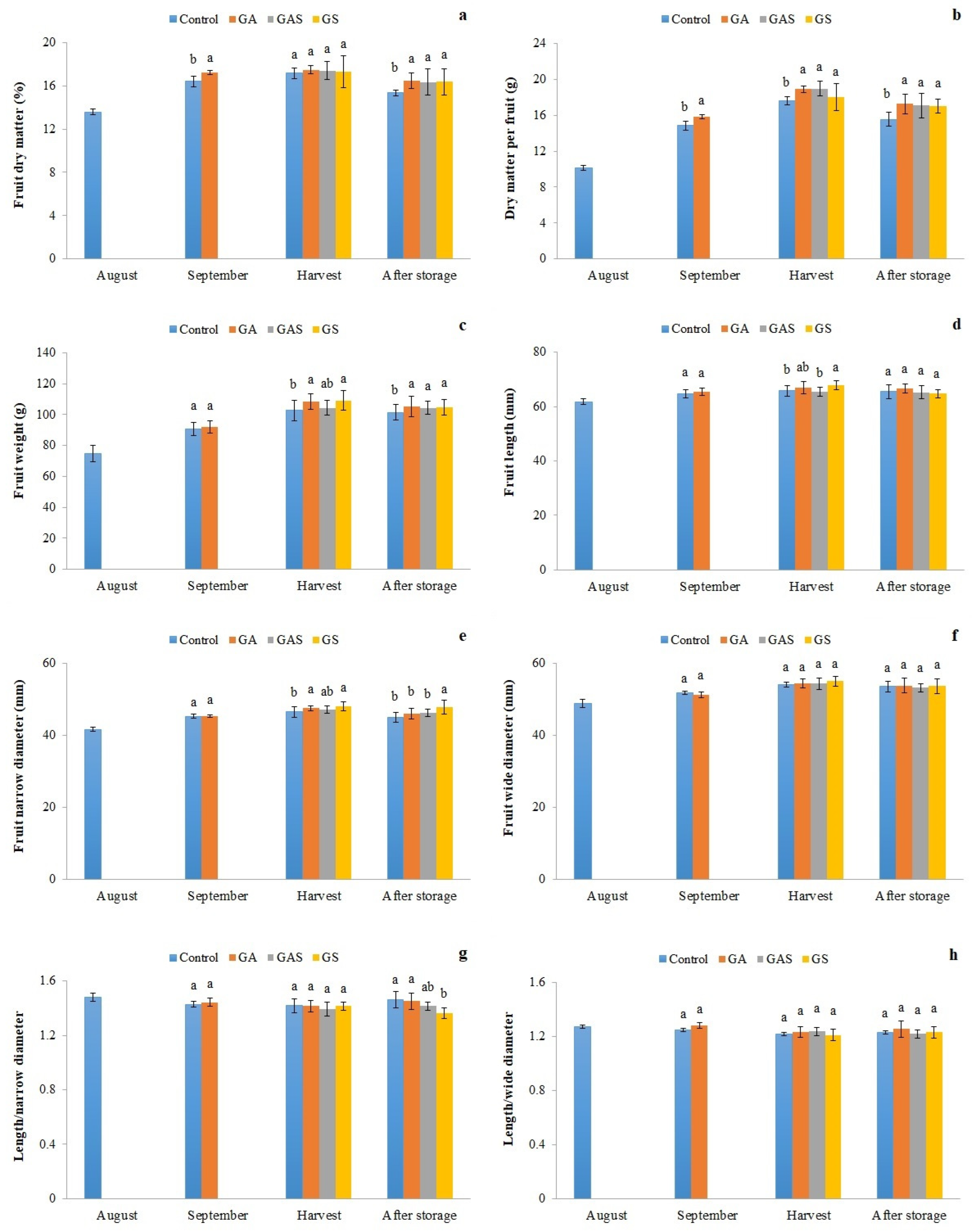
2.3. Sampling and Physiological Properties Determination
2.4. Determination of Organoleptic Characteristics
2.5. Chlorophyll and Carotenoids Determination
2.6. Soluble Sugars Determination
2.7. Organic Acids Determination
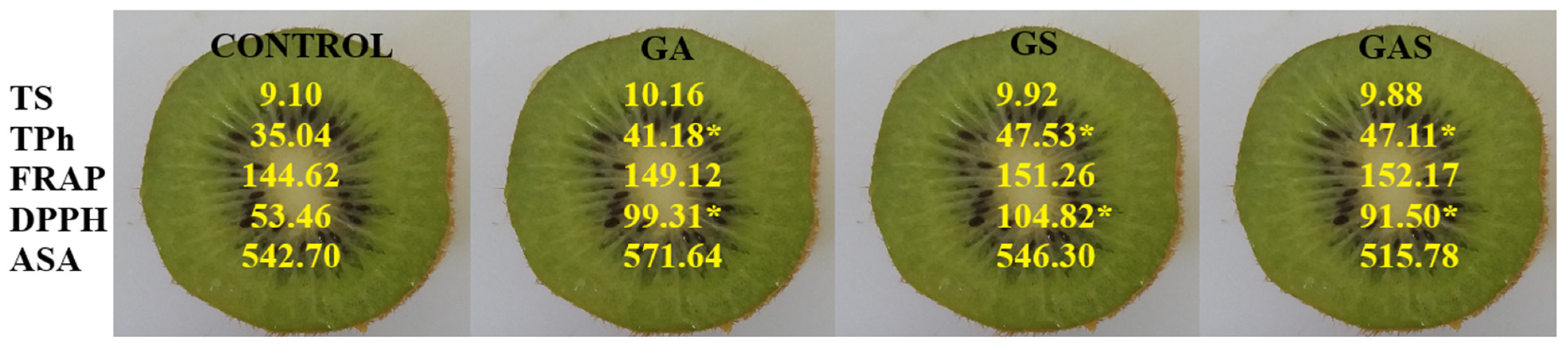
2.8. Phenolic Compounds Concentration and Antioxidant Capacity Determination
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.-Y.; Wang, W.-H.; Zhan, J.-Y.; Huang, Y.-L.; Cheng, W.-Y. Beneficial effects of golden kiwifruit consumption in overweight and obese young adults. J. Nutr. Sci. Vitaminol. 2020, 66, S356–S360. [Google Scholar] [CrossRef] [PubMed]
- Snelgar, W.P.; Hall, A.J.; Ferguson, A.R.; Blattmann, P. Temperature influences growth and maturation of fruit on ‘Hayward’ kiwifruit vines. Funct. Plant Biol. 2005, 32, 631–642. [Google Scholar] [CrossRef]
- Basile, B.; Giaccone, M.; Cirillo, C.; Ritieni, A.; Graziani, G.; Shahak, Y.; Forlani, M. Photo-selective hail nets affect fruit size and quality in Hayward kiwifruit. Sci. Hortic. 2012, 141, 91–97. [Google Scholar] [CrossRef]
- Roussos, P.A.; Tassis, A. Effects of girdling, nitrogen, zinc and auxin foliar spray applications on mandarin fruit ’Nova’ quality characteristics. Emir. J. Food Agric. 2011, 23, 431–439. [Google Scholar]
- Roussos, P.A.; Tsafouros, A.; Ntanos, E.; Denaxa, N.-K.; Kosta, A.; Bouchagier, P. Could black anti-hail net have an extra role as an amelioration agent against heat stress in kiwifruit? J. Berry Res. 2022, 12, 131–147. [Google Scholar] [CrossRef]
- Currie, M.; Barnett, A.; Boyd, L.; Max, S. Trunk girdling: Risks and opportunities. N. Z. Kiwifruit J. 2005, 167, 12–17. [Google Scholar]
- Ramming, D.W.; Tarailo, R. ‘Black Emerald’: An early-maturing, black seedless grape for the fresh market. HortScience 1998, 33, 353–354. [Google Scholar]
- Ülker, T.; Kamiloğlu, M.U. Influences of girdling and potassium treatments on fruit quality and some physiological characters of ‘Fremont’ mandarin variety. Folia Hortic. 2021, 33, 195–202. [Google Scholar] [CrossRef]
- Arakawa, O.; Kanno, K.; Kanetsuka, A.; Shiozaki, Y. Effects of girdling and bark inversion on tree growth and fruit quality of apple. Acta Hortic. 1996, 451, 579–586. [Google Scholar] [CrossRef]
- Allan, P.; George, A.; Nissen, R.; Rasmussen, T. Effects of girdling time on growth, yield, and fruit maturity of the low chill peach cultivar Flordaprince. Aust. J. Exp. Agric. 1993, 33, 781–785. [Google Scholar] [CrossRef]
- Currie, M.B.; Patterson, K.J.; Snelgar, W.P.; Blattmann, P. Girdling kiwifruit vines for commercial advantage: Opportunities and risks. Acta Hortic. 2018, 1218, 405–412. [Google Scholar] [CrossRef]
- Rana, V.S.; Zarea, S.E.; Sharma, S.; Rana, N.; Kumar, V.; Sharma, U. Differential response of the leaf fruit ratio and girdling on the leaf nutrient concentrations, yield, and quality of nectarine. J. Plant Growth Regul. 2023, 42, 2360–2373. [Google Scholar] [CrossRef]
- Pereira, G.E.; Padhi, E.M.; Girardello, R.C.; Medina-Plaza, C.; Tseng, D.; Bruce, R.C.; Erdmann, J.N.; Kurtural, S.K.; Slupsky, C.M.; Oberholster, A. Trunk girdling increased stomatal conductance in Cabernet Sauvignon grapevines, reduced glutamine, and increased malvidin-3-glucoside and quercetin-3-glucoside concentrations in skins and pulp at harvest. Front. Plant Sci. 2020, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.; Léchaudel, M.; Lu, P. Effect of fruit load and girdling on leaf photosynthesis in Mangifera indica L. J. Exp. Bot. 2004, 55, 2075–2085. [Google Scholar] [CrossRef]
- Tóth, A.M.; Zsófi, Z.; Veres, S. Cane girdling influence on the berry texture properties of three table grape varieties. Horticulturae 2022, 8, 1101. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Araniti, F.; Landi, M.; Massai, R.; Guidi, L.; Abenavoli, M.R.; Remorini, D. Girdling stimulates anthocyanin accumulation and promotes sugar, organic acid, amino acid level and antioxidant activity in red plum: An overview of skin and pulp metabolomics. Sci. Hortic. 2021, 280, 109907. [Google Scholar] [CrossRef]
- Hackney, C.; Boshoff, M.; Slabbert, M. Increasing yield of young Hass avocado trees using the cincturing. S. Afr. Avocado Grow. Assoc. Yearb. 1993, 18, 54–55. [Google Scholar]
- Boyd, L.M.; Barnett, A.M. Manipulation of whole-vine carbon allocation using girdling, pruning, and fruit thinning affects fruit numbers and quality in kiwifruit. HortScience 2011, 46, 590–595. [Google Scholar] [CrossRef]
- Agusti, M.; Andreu, I.; Juan, M.; Almela, V.; Zacarias, L. Effects of ringing branches on fruit size and maturity of peach and nectarine cultivars. J. Hortic. Sci. Biotechnol. 1998, 73, 537–540. [Google Scholar] [CrossRef]
- Hoying, S.; Robinson, T. Effects of chain saw girdling and rootpruning of apple trees. Acta Hortic. 1992, 322, 167–172. [Google Scholar] [CrossRef]
- Takemura, C.; Asakuma, H. Effect of shading and girdling on physiological fruit drop of Japanese persimmon ‘Akiou’. Acta Hortic. 2022, 1338, 185–190. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Johnson, R.S.; DeJong, T.; Day, K.R. Orchard factors affecting postharvest stone fruit quality. HortScience 1997, 32, 820–823. [Google Scholar] [CrossRef]
- Verreynne, J.; Rabe, E.; Theron, K. The effect of combined deficit irrigation and summer trunk girdling on the internal fruit quality of ‘Marisol’ Clementines. Sci. Hortic. 2001, 91, 25–37. [Google Scholar] [CrossRef]
- Black, M.Z.; Patterson, K.J.; Gould, K.S.; Clearwater, M.J. Physiological responses of kiwifruit vines (Actinidia chinensis Planch. var. chinensis) to trunk girdling and root pruning. N. Z. J. Crop Hortic. Sci. 2012, 40, 31–41. [Google Scholar] [CrossRef]
- Lallu, N.; Searle, A.N.; Macrae, E.A. An investigation of ripening and handling strategies for early season kiwifruit (Actinidia deliciosa cv Hayward). J. Sci. Food Agric. 1989, 47, 387–400. [Google Scholar] [CrossRef]
- Patterson, K.; Currie, M. Optimising kiwifruit vine performance for high productivity and superior fruit taste. Acta Hortic. 2011, 913, 257–268. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Aminifar, R. Effects of girdling treatment and forchlorfenuron spray on quality and storage life of Hayward kiwifruit (Actinidia deliciosa cv. ‘Hayward ‘). J. Plant Prod. Sci. 2021, 28, 89–102. [Google Scholar]
- Denaxa, N.-K.; Tsafouros, A.; Ntanos, E.; Kosta, A.; Roussos, P.A. Mitigation of high solar irradiance and heat stress in kiwifruit during summer via the use of alleviating products with different modes of action—Part 2 Effects on fruit quality, organoleptic, and phytochemical properties at harvest and after storage. Agriculture 2023, 13, 701. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophyll and carotenoids–pigments of photosynthetic biomembrances. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Roussos, P.A.; Denaxa, N.-K.; Damvakaris, T.; Stournaras, V.; Argyrokastritis, I. Effect of alleviating products with different mode of action on physiology and yield of olive under drought. Sci. Hortic. 2010, 125, 700–711. [Google Scholar] [CrossRef]
- Chai, L.; Li, Q.; Wang, H.; Wang, C.; Xu, J.; Yu, H.; Jiang, W. Girdling alters carbohydrate allocation to increase fruit size and advance harvest in tomato production. Sci. Hortic. 2021, 276, 109675. [Google Scholar] [CrossRef]
- Lievre, D.L.; Anderson, R.; Boldingh, H.; Cooney, J.; Seelye, R.; Gould, N.; Hunter, D.; Jensen, D.; Pereira, T.; Wohlers, M. Modifying carbohydrate supply to fruit during development changes the composition and flavour of Actinidia chinensis var. chinensis ‘Zesy002′ Kiwifruit. Plants 2021, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Snelgar, W.; Minchin, P.; Blatmann, P.; Hall, A. Sink priority on ‘Hayward’ kiwifruit vines. N. Z. J. Crop Hortic. Sci. 2012, 40, 253–263. [Google Scholar] [CrossRef]
- Morandi, B.; Losciale, P.; Manfrini, L.; Zibordi, M.; Studhalter, M.; Grappadelli, L.C. The growth of the kiwifruit in its final stages. Acta Hortic. 2006, 753, 369–374. [Google Scholar] [CrossRef]
- Clearwater, M.J.; Luo, Z.; Ong, S.E.C.; Blattmann, P.; Thorp, T.G. Vascular functioning and the water balance of ripening kiwifruit (Actinidia chinensis) berries. J. Exp. Bot. 2012, 63, 1835–1847. [Google Scholar] [CrossRef]
- Piller, G.; Greaves, A.; Meekings, J. Sensitivity of floral shoot growth, fruit set and early fruit size in Actinidia deliciosa to local carbon supply. Ann. Bot. 1998, 81, 723–728. [Google Scholar] [CrossRef]
- Di Vaio, C.; Petito, A.; Buccheri, M. Effect of girdling on gas exchanges and leaf mineral content in the ‘Independence’ nectarine. J. Plant Nutr. 2001, 24, 1047–1060. [Google Scholar] [CrossRef]
- Roper, T.R.; Williams, L.E. Net CO2 assimilation and carbohydrate partitioning of grapevine leaves in response to trunk girdling and gibberellic acid application. Plant Physiol. 1989, 89, 1136–1140. [Google Scholar] [CrossRef]
- Williams, L.; Ayars, J. Water use of Thompson Seedless grapevines as affected by the application of gibberellic acid (GA3) and trunk girdling–practices to increase berry size. Agric. For. Meteorol. 2005, 129, 85–94. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Famiani, F. Effect of different leaf-to-fruit ratios on photosynthesis and fruit growth in olive (Olea europaea L.). Photosynthetica 2006, 44, 275–285. [Google Scholar] [CrossRef]
- Moscatello, S.; Proietti, S.; Augusti, A.; Scartazza, A.; Walker, R.P.; Famiani, F.; Battistelli, A. Late summer photosynthesis and storage carbohydrates in walnut (Juglans regia L.): Feed-back and feed-forward effects. Plant Physiol. Biochem. 2017, 118, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Poirier-Pocovi, M.; Lothier, J.; Buck-Sorlin, G. Modelling temporal variation of parameters used in two photosynthesis models: Influence of fruit load and girdling on leaf photosynthesis in fruit-bearing branches of apple. Ann. Bot. 2018, 121, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, M.V.; Kafkaletou, M.; Karantzi, A.D.; Tsantili, E. Girdling effects on fruit maturity, kernel quality, and nutritional value of walnuts (Juglans regia L.) alongside the effects on leaf physiological characteristics. Agronomy 2021, 11, 200. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mohamed, A.O.; Mohamed, A.H.; Omar, A.A. Effect of some girdling treatments on fruiting behavior and physio-chemical properties of Washington navel orange trees. J. Agric. Vet. Sci. 2016, 9, 58–65. [Google Scholar]
- Nebauer, S.G.; Renau-Morata, B.; Guardiola, J.L.; Molina, R.-V. Photosynthesis down-regulation precedes carbohydrate accumulation under sink limitation in Citrus. Tree Physiol. 2011, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Li, W.D.; Duan, W.; Fan, P.G.; Yan, S.T.; Li, S.H. Photosynthesis in response to sink—Source activity and in relation to end products and activities of metabolic enzymes in peach trees. Tree Physiol. 2007, 27, 1307–1318. [Google Scholar] [CrossRef]
- Vemmos, S.; Papagiannopoulou, A.; Coward, S. Effects of shoot girdling on photosynthetic capacity, leaf carbohydrate, and bud abscission in pistachio (Pistacia vera L.). Photosynthetica 2012, 50, 35–48. [Google Scholar] [CrossRef]
- Choi, S.-T.; Song, W.-D.; Park, D.-S.; Kang, S.-M. Effect of different girdling dates on tree growth, fruit characteristics and reserve accumulation in a late-maturing persimmon. Sci. Hortic. 2010, 126, 152–155. [Google Scholar] [CrossRef]
- Liu, P.; Huang, J.-J.; Cai, Z.-Y.; Chen, H.-T.; Huang, X.; Yang, S.-N.; Su, Z.-X.; Azam, M.; Chen, H.-B.; Shen, J.-Y. Influence of girdling on growth of litchi (Litchi chinensis) roots during cold-dependent floral induction. Sci. Hortic. 2022, 297, 110928. [Google Scholar] [CrossRef]
- Dai, J.; Dong, H. Stem girdling influences concentrations of endogenous cytokinins and abscisic acid in relation to leaf senescence in cotton. Acta Physiol. Plantarum 2011, 33, 1697–1705. [Google Scholar] [CrossRef]
- Day, K.; DeJong, T. Girdling of early season ‘Mayfire’ nectarine trees. J. Hortic. Sci. 1990, 65, 529–534. [Google Scholar] [CrossRef]
- Dann, I.; Wildes, R.; Chalmers, D. Effects of limb girdling on growth and development of competing fruit and vegetative tissues of peach trees. Funct. Plant Biol. 1984, 11, 49–58. [Google Scholar] [CrossRef]
- Wang, S.; Pan, C.; Zhang, C.; Zhao, S. Key enzymes of sucrose metabolism play an important role in source–sink regulation of walnut fruit growth and development. N. Z. J. Crop Hortic. Sci. 2023, 51, 400–419. [Google Scholar] [CrossRef]
- Tyagi, K.; Maoz, I.; Lewinsohn, E.; Lerno, L.; Ebeler, S.E.; Lichter, A. Girdling of table grapes at fruit set can divert the phenylpropanoid pathway towards accumulation of proanthocyanidins and change the volatile composition. Plant Sci. 2020, 296, 110495. [Google Scholar] [PubMed]
- Khalkho, N.; Horo, P.; Jha, K. Effect of girdling and defoliation on physical properties of litchi fruit. J. Crop Weed 2015, 11, 200–203. [Google Scholar]
- Fallahi, E.; Kiester, M.J.; Fallahi, B.; Mahdavi, S. Rootstock, canopy architecture, bark girdling, and scoring influence on growth, productivity, and fruit quality at harvest in ‘Aztec Fuji’apple. HortScience 2018, 53, 1629–1633. [Google Scholar] [CrossRef]
- Goren, R.; Huberman, M.; Goldschmidt, E.E. Girdling: Physiological and horticultural aspects. Hortic. Rev. 2004, 30, 1–36. [Google Scholar]
- Soltekin, O.; Candemir, A.; Altindisli, A. Effects of cane girdling on yield, fruit quality and maturation of (Vitis vinifera L.) cv. Flame Seedless. BIO Web Conf. 2016, 7, 01032. [Google Scholar] [CrossRef]
- Gawankar, M.; Haldankar, P.; Salvi, B.; Parulekar, Y.; Dalvi, N.; Kulkarni, M.; Saitwal, Y.; Nalage, N. Effect of girdling on induction of flowering and quality of fruits in horticultural crops-a review. Adv. Agric. Res. Technol. J. 2019, 3, 201–215. [Google Scholar]
- Wargo, J.M.; Merwin, I.A.; Watkins, C.B. Nitrogen fertilization, midsummer trunk girdling, and avg treatments affect maturity and quality of ‘Jonagold’ apples. HortScience 2004, 39, 493–500. [Google Scholar] [CrossRef]
- Avendaño-Arrazate, C.H.; Moreno-Pérez, E.d.C.; Martínez-Damián, M.T.; Cruz-Alvarez, O.; Vargas-Madríz, H. Postharvest quality and behavior of rambutan (Nephelium lappaceum L.) fruits due to the effects of agronomic practices. Rev. Chapingo Ser. Hortic. 2018, 24, 13–26. [Google Scholar] [CrossRef]
- Ahmad, M.; Kumari Kaul, R.; Kaul, B.L. Effect of girdling, thinning and GA3 on fruit growth, yield, quality and shelf life of grapes (Vitis vinifera L.) cv. Perlette. Acta Hortic. 2005, 696, 309–313. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Samiotaki, M.; Sarrou, E.; Karamanoli, K.; Lazaridou, A.; Martens, S.; Molassiotis, A. Proteomic and metabolic analysis reveals novel sweet cherry fruit development regulatory points influenced by girdling. Plant Physiol. Biochem. 2020, 149, 233–244. [Google Scholar] [CrossRef]
- Mafrica, R.; De Bruno, A.; Lanza, D.; Poiana, M. Effects of altering carbohydrate supply to fruit during development on the carpometric and qualitative characteristics of ‘Feminello Zagara Bianca’ lemon. Horticulturae 2023, 9, 71. [Google Scholar] [CrossRef]

| Control | GA | GS | GAS | |
|---|---|---|---|---|
| Harvest | ||||
| pH | 2.87 ± 0.09 a | 2.86 ± 0.05 a | 2.89 ± 0.05 a | 2.89 ± 0.05 a |
| TA | 2.59 ± 0.28 a | 2.57 ± 0.12 a | 2.58 ± 0.29 a | 2.58 ± 0.33 a |
| TSS | 6.33 ± 0.49 a | 6.15 ± 0.42 a | 5.73 ± 0.57 a | 6.12 ± 0.27 a |
| TSS:TA | 2.66 ± 0.82 a | 2.43 ± 0.57 a | 2.26 ± 0.40 a | 2.36 ± 0.68 a |
| Firmness (N) | 38.03 ± 1.28 a | 35.75 ± 4.76 a | 36.41 ± 2.30 a | 37.73 ± 3.12 a |
| Storage | ||||
| pH | 3.09 ± 0.14 a | 3.01 ± 0.04 a | 3.01 ± 0.07 a | 3.03 ± 0.03 a |
| TA | 2.23 ± 0.24 a | 2.25 ± 0.14 a | 2.26 ± 0.21 a | 2.21 ± 0.14 a |
| TSS | 12.24 ± 0.55 b | 13.74 ± 0.39 a | 13.68 ± 0.89 a | 13.85 ± 0.72 a |
| TSS:TA | 5.54 ± 0.65 b | 6.27 ±0.42 a | 6.29 ± 0.57 a | 6.09 ± 0.49 a |
| Firmness (N) | 7.66 ± 1.26 a | 4.16 ± 0.79 b | 5.00 ± 1.66 b | 3.84 ± 0.78 b |
| Control | GA | GS | GAS | |
|---|---|---|---|---|
| Harvest | ||||
| Chla | 0.51 ± 0.07 a | 0.54 ± 0.09 a | 0.56 ± 0.04 a | 0.59 ± 0.16 a |
| Chlb | 0.38 ± 0.02 a | 0.43 ± 0.08 a | 0.46 ± 0.04 a | 0.47 ± 0.19 a |
| Total Chls | 0.89 ± 0.09 a | 0.97 ± 0.016 a | 1.02 ± 0.07 a | 1.07 ± 0.35 a |
| Carotenoids | 0.16 ± 0.02 a | 0.19 ± 0.03 a | 0.16 ± 0.05 a | 0.20 ± 0.05 a |
| Storage | ||||
| Chla | 0.35 ± 0.02 a | 0.35 ± 0.05 a | 0.38 ± 0.09 a | 0.37 ± 0.06 a |
| Chlb | 0.19 ± 0.03 a | 0.18 ± 0.06 a | 0.25 ± 0.12 a | 0.20 ± 0.06 a |
| Total Chls | 0.54 ± 0.04 a | 0.53 ± 0.10 a | 0.63 ± 0.18 a | 0.56 ± 0.12 a |
| Carotenoids | 0.15 ± 0.02 a | 0.13 ± 0.03 a | 0.16 ± 0.05 a | 0.16 ± 0.02 a |
| Control | GA | GS | GAS | |
|---|---|---|---|---|
| Harvest | ||||
| Fructose | 11.22 ± 3.19 a | 13.54 ± 4.76 a | 16.06 ± 5.10 a | 14.60 ± 4.62 a |
| Glucose | 6.13 ± 1.59 a | 7.16 ± 1.65 a | 7.74 ± 1.82 a | 7.51 ± 1.66 a |
| Sucrose | 0.74 ± 0.72 a | 1.18 ± 0.93 a | 0.70 ± 0.75 a | 1.40 ± 0.54 a |
| Inositol | 2.31 ± 0.65 a | 1.94 ± 0.63 a | 2.44 ± 0.75 a | 2.23 ± 0.54 a |
| Total sugars | 20.40 ± 5.16 a | 23.82 ± 5.18 a | 26.94 ± 6.64 a | 25.75 ± 7.54 a |
| Storage | ||||
| Fructose | 53.08 ± 9.87 a | 57.52 ± 3.04 a | 56.35 ± 5.80 a | 55.97 ± 6.94 a |
| Glucose | 27.19 ± 4.62 a | 28.93 ± 1.61 a | 28.19 ± 2.80 a | 28.04 ± 3.58 a |
| Sucrose | 8.49 ± 1.25 a | 9.13 ± 0.59 a | 9.07 ± 2.01 a | 9.57 ± 1.53 a |
| Inositol | 0.93 ± 1.03 a | 1.15 ± 0.22 a | 1.16 ± 0.22 a | 1.19 ± 0.22 a |
| Total sugars | 89.69 ± 13.25 a | 96.73 ± 5.13 a | 94.77 ± 9.91 a | 94.77 ± 11.89 a |
| Control | GA | GS | GAS | |
|---|---|---|---|---|
| Harvest | ||||
| Malic acid | 0.77 ± 0.26 a | 0.97 ± 0.64 a | 0.82 ± 0.19 a | 0.93 ± 0.29 a |
| Ascorbic acid | 6.14 ± 2.20 a | 5.79 ± 1.78 a | 5.94 ± 1.74 a | 5.53 ± 2.15 a |
| Citric acid | 3.85 ± 0.30 a | 3.91 ± 0.30 a | 3.69 ± 0.28 a | 3.99 ± 0.27 a |
| Total organic acids | 10.18 ± 3.0 a | 10.31 ± 3.33 a | 10.07 ± 2.26 a | 10.03 ± 3.01 a |
| Storage | ||||
| Malic acid | 0.75 ± 0.39 a | 1.06 ± 0.53 a | 0.86 ± 0.19 a | 1.02 ± 0.44 a |
| Ascorbic acid | 5.35 ± 0.50 a | 5.44 ± 0.97 a | 4.93 ± 0.41 a | 5.24 ± 0.26 a |
| Citric acid | 3.28 ± 0.99 a | 3.55 ± 1.47 a | 3.30 ± 0.65 a | 3.54 ± 1.24 a |
| Total organic acids | 9.38 ± 1.80 a | 10.05 ± 1.87 a | 9.09 ± 0.77 a | 9.80 ± 0.98 a |
| Control | GA | GS | GAS | |
|---|---|---|---|---|
| Harvest | ||||
| Total phenols | 0.42 ± 0.05 a | 0.39 ± 0.07 a | 0.44 ± 0.05 a | 0.43 ± 0.04 a |
| Total o-diphenols | 10.0 ± 0.01 a | 8.9 ± 0.01 a | 9.6 ± 0.03 a | 9.8 ± 0.01 a |
| Total flavanols | 3.3 ± 0.01 a | 2.8 ± 0.01 a | 2.7 ± 0.01 a | 2.6 ± 0.01 a |
| Total flavonoids | 17.0 ± 0.01 a | 12.4 ± 0.01 b | 15.3 ± 0.01 a | 16.0 ± 0.01 a |
| FRAP | 1.35 ± 0.11 b | 1.41 ± 0.10 ab | 1.45 ± 0.09 a | 1.40 ± 0.10 ab |
| DPPH | 0.88 ± 0.17 b | 0.97 ± 0.23 ab | 1.17 ± 0.36 a | 1.08 ± 0.14 ab |
| Storage | ||||
| Total phenols | 0.35 ± 0.06 b | 0.39 ± 0.05 ab | 0.45 ± 0.10 a | 0.45 ± 0.06 a |
| Total o-diphenols | 15.6 ± 1.9 a | 13.3 ± 1.6 ab | 13.1 ± 1.6 b | 15.1 ± 2.5 ab |
| Total flavanols | 1.3 ± 0.09 b | 2.2 ± 0.1 ab | 2.6 ± 0.1 a | 2.5 ± 0.2 a |
| Total flavonoids | 15.3 ± 0.10 ab | 6.5 ± 0.21 b | 16.7 ± 0.17 a | 6.6 ± 0.25 b |
| FRAP | 1.43 ± 0.10 a | 1.41 ± 0.07 a | 1.44 ± 0.12 a | 1.46 ± 0.06 a |
| DPPH | 0.53 ± 0.09 b | 0.95 ± 0.12 a | 1.00 ± 0.20 a | 0.88 ± 0.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roussos, P.A.; Denaxa, N.-K.; Tsafouros, A.; Ntanos, E. Effect of Time of Girdling on Leaf Photosynthetic Performance and Kiwifruit Quality Characteristics at Harvest and Post-Storage. Appl. Sci. 2023, 13, 11087. https://doi.org/10.3390/app131911087
Roussos PA, Denaxa N-K, Tsafouros A, Ntanos E. Effect of Time of Girdling on Leaf Photosynthetic Performance and Kiwifruit Quality Characteristics at Harvest and Post-Storage. Applied Sciences. 2023; 13(19):11087. https://doi.org/10.3390/app131911087
Chicago/Turabian StyleRoussos, Peter A., Nikoleta-Kleio Denaxa, Athanassios Tsafouros, and Efstathios Ntanos. 2023. "Effect of Time of Girdling on Leaf Photosynthetic Performance and Kiwifruit Quality Characteristics at Harvest and Post-Storage" Applied Sciences 13, no. 19: 11087. https://doi.org/10.3390/app131911087
APA StyleRoussos, P. A., Denaxa, N.-K., Tsafouros, A., & Ntanos, E. (2023). Effect of Time of Girdling on Leaf Photosynthetic Performance and Kiwifruit Quality Characteristics at Harvest and Post-Storage. Applied Sciences, 13(19), 11087. https://doi.org/10.3390/app131911087








