The Impact of the Growing Substrate on Morphological and Biochemical Features of Salicornia europaea L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Biological Material
2.3. Sample Preparation for Optical Microscopy
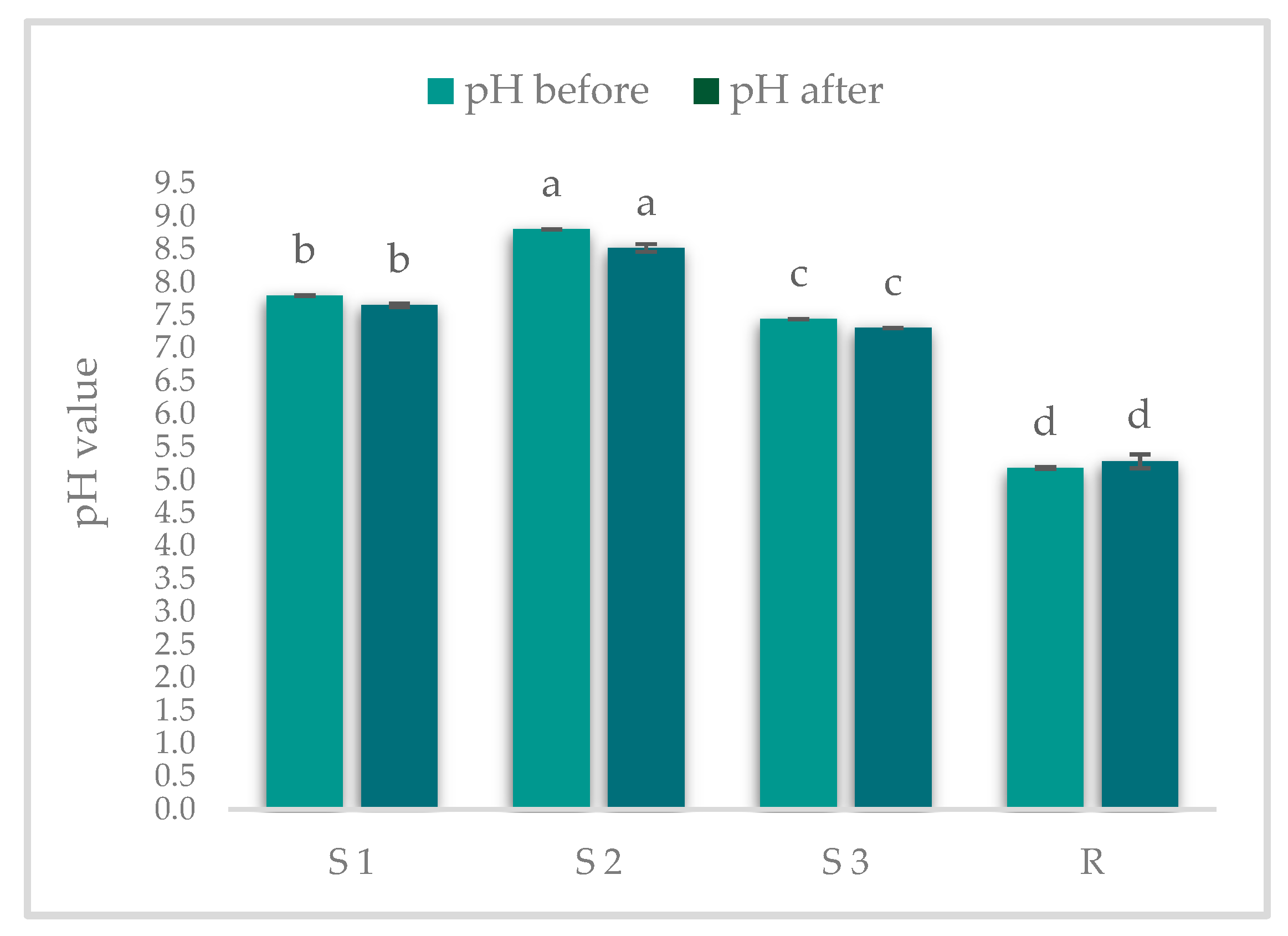
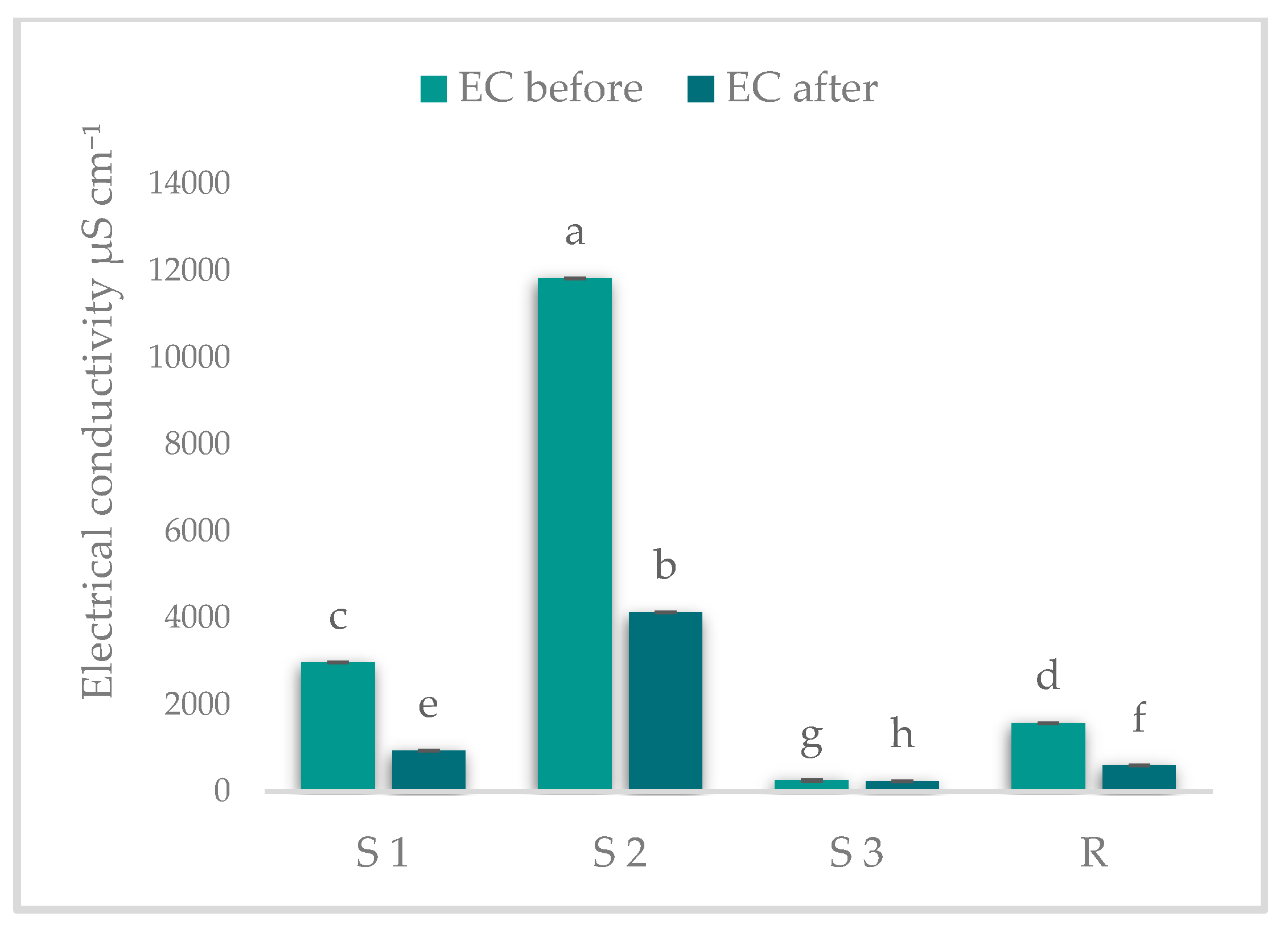
2.4. Determination of pH and Electrical Conductivity (EC)
2.5. Preparation of Plant Extracts for UV-VIS Analysis
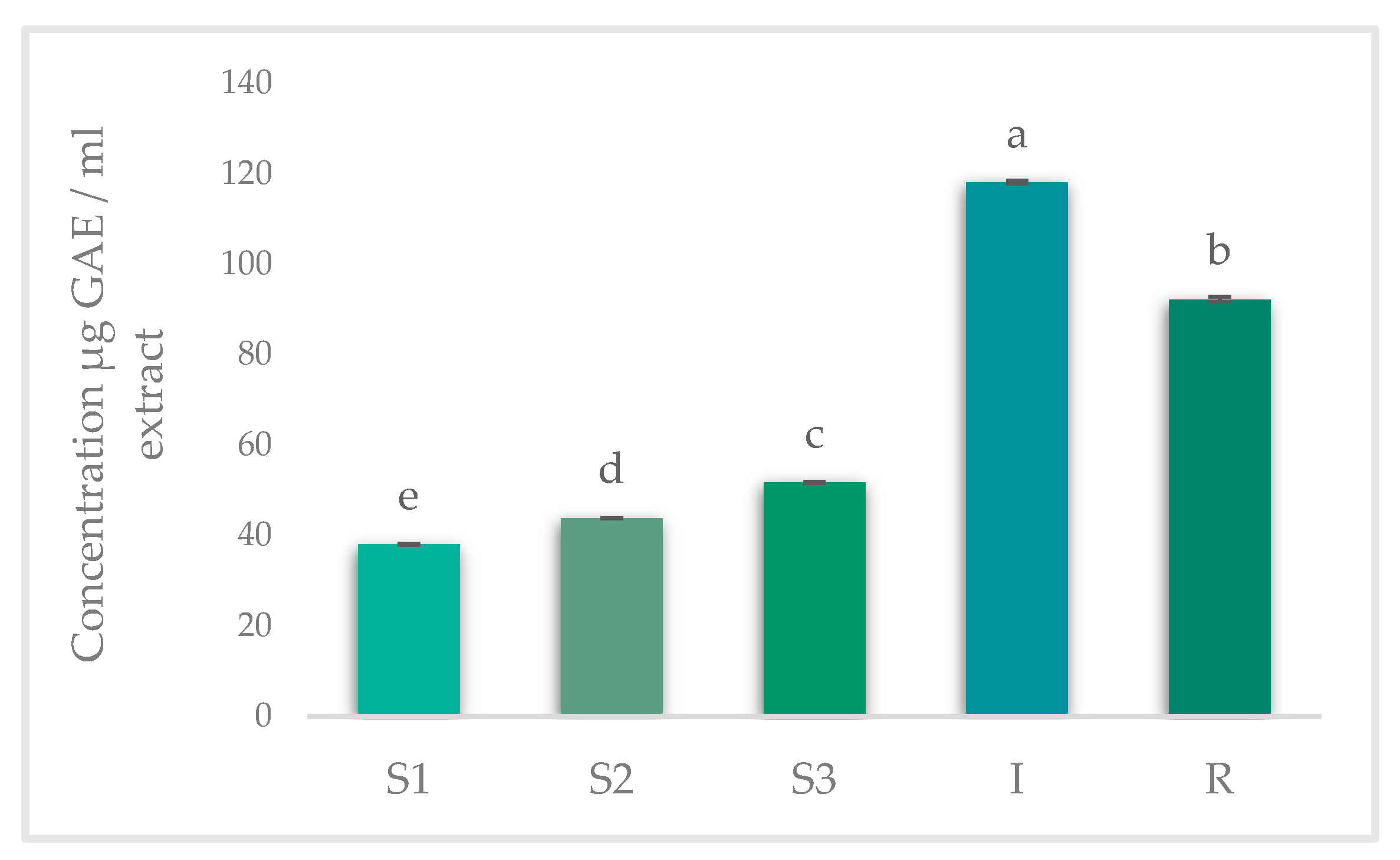
2.6. Total Phenolic Content (TPC)
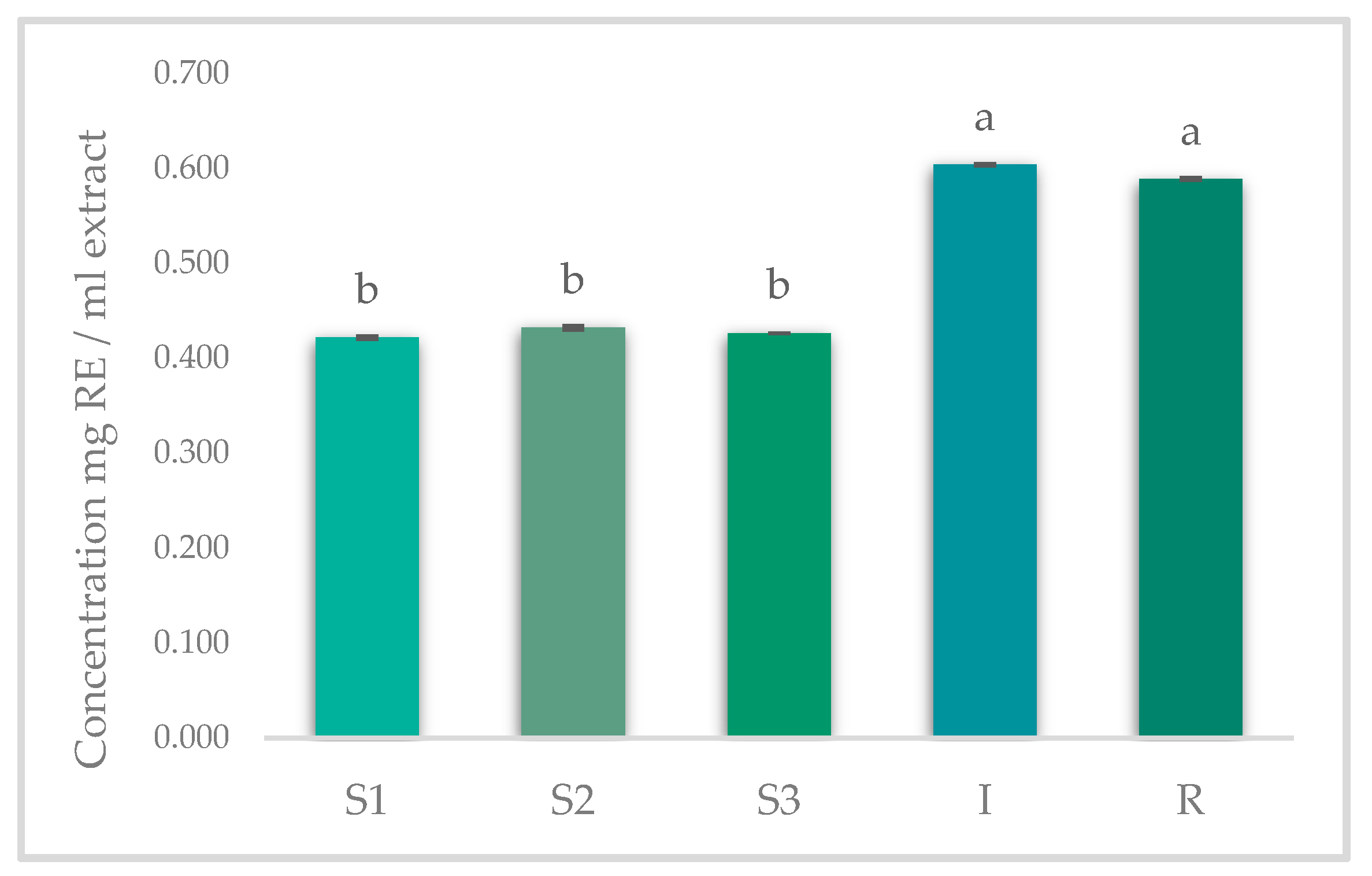
2.7. Total Flavonoids Content (TFC)
2.8. Microwaves-Assisted Extraction (MAE)
2.9. The Elemental Analysis of Plant Samples
2.10. Statistical Analysis
3. Results
3.1. Results on pH and EC Determinations
3.2. Results of Spectrophotometric Analysis
3.3. Results on Elemental Quantification Using ICP-MS
3.4. Correlation between Phenolic Compounds and Minerals
3.5. Results Related to Cell Morphology
3.6. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vermue, E.; Metselaar, K.; van der Zee, S.E.A.T.M. Modelling of Soil Salinity and Halophyte Crop Production. Environ. Exp. Bot. 2013, 92, 186–196. [Google Scholar] [CrossRef]
- Sarkar, D.; Kar, S.K.; Chattopadhyay, A.; Shikha; Rakshit, A.; Tripathi, V.K.; Dubey, P.K.; Abhilash, P.C. Low Input Sustainable Agriculture: A Viable Climate-Smart Option for Boosting Food Production in a Warming World. Ecol. Indic. 2020, 115, 106412. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Waiho, K.; Fazhan, H.; Necibi, M.C.; Hafsa, J.; Mrid, R.B.; Fal, S.; El Arroussi, H.; Peng, W.; Tabatabaei, M.; et al. Progress in Valorisation of Agriculture, Aquaculture and Shellfish Biomass into Biochemicals and Biomaterials towards Sustainable Bioeconomy. Chemosphere 2021, 291, 133036. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, M.; Tajbakhsh, A. Past, Present, and Prospective Themes of Sustainable Agricultural Supply Chains: A Content Analysis. J. Clean. Prod. 2020, 271, 122201. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural Waste Management Strategies for Environmental Sustainability. Environ. Res. 2021, 206, 112285. [Google Scholar] [CrossRef]
- Laurett, R.; Paço, A.; Mainardes, E.W. Antecedents and Consequences of Sustainable Development in Agriculture and the Moderator Role of the Barriers: Proposal and Test of a Structural Model. J. Rural Stud. 2021, 86, 270–281. [Google Scholar] [CrossRef]
- Lombardi, G.V.; Parrini, S.; Atzori, R.; Stefani, G.; Romano, D.; Gastaldi, M.; Liu, G. Sustainable Agriculture, Food Security and Diet Diversity. The Case Study of Tuscany, Italy. Ecol. Model. 2021, 458, 109702. [Google Scholar] [CrossRef]
- Dubey, P.K.; Singh, A.; Chaurasia, R.; Pandey, K.K.; Bundela, A.K.; Dubey, R.K.; Abhilash, P.C. Planet friendly agriculture: Farming for people and the planet. Curr. Res. Environ. Sustain. 2021, 3, 100041. [Google Scholar] [CrossRef]
- Franco, S. Assessing the environmental sustainability of local agricultural systems: How and why. Curr. Res. Environ. Sustain. 2021, 3, 100028. [Google Scholar] [CrossRef]
- Federal Office for Spatial Development. 1987: Brundtland Report; Federal Office for Spatial Development: Ittigen, Switzerland, 1987. [Google Scholar]
- Volkov, A.; Morkunas, M.; Balezentis, T.; Streimikiene, D. Are Agricultural Sustainability and Resilience Complementary Notions? Evidence from the North European Agriculture. Land Use Policy 2022, 112, 105791. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, G.; Vishwakarma, S.; Dalin, C.; Komarek, A.M.; Kanter, D.R.; Davis, K.F.; Pfeifer, K.; Zhao, J.; Zou, T.; et al. Quantitative Assessment of Agricultural Sustainability Reveals Divergent Priorities among Nations. One Earth 2021, 4, 1262–1277. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, K.; Chen, S.; Fu, X.; Feng, S.; Zhuang, Z. A Sustainable Agricultural Supply Chain Considering Substituting Organic Manure for Chemical Fertilizer. Sustain. Prod. Consum. 2022, 29, 432–446. [Google Scholar] [CrossRef]
- Nuriyeva, S.; Akparov, Z.; Hajiyev, E.; Abbasov, M.; Sharma, R. Evaluation of Wheat Genetic Resources of Azerbaijan on Normal and Saline Fields. Turk. J. Agric. For. 2016, 40, 186–193. [Google Scholar] [CrossRef]
- Arbelet-Bonnın, D.; Hamed-Laoutı, I.B.; Laurentı, P.; Abdelly, C.; Hamed, K.B.; Bouteau, F. Cellular mechanisms to survive salt in the halophyte Cakile maritima. Plant Sci. 2018, 272, 173–178. [Google Scholar] [CrossRef]
- Dadaşoğlu, E. Ameliorative effects of nitric oxide on growth, physiology and biochemistry of chickpeaplants under salinity stress. Turk. J. Agric. For. 2022, 46, 224–233. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte Agriculture: Success Stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Ekinci, M.; Turan, M.; Yildirim, E. Biochar mitigates salt stress by regulating nutrient uptake and antioxidant activity, alleviating the oxidative stress and abscisic acid content in cabbage seedlings. Turk. J. Agric. For. 2022, 46, 28–37. [Google Scholar]
- Komaresofla, R.B.; Alikhani, H.A.; Etesami, H.; Khoshkholgh-Sima, N.A. Improved Growth and Salinity Tolerance of the Halophyte Salicornia Sp. By Co–Inoculation with Endophytic and Rhizosphere Bacteria. Appl. Soil Ecol. 2019, 138, 160–170. [Google Scholar] [CrossRef]
- Rozema, J.; Muscolo, A.; Flowers, T. Sustainable Cultivation and Exploitation of Halophyte Crops in a Salinising World. Environ. Exp. Bot. 2013, 92, 1–196. [Google Scholar] [CrossRef]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination Strategies of Halophyte Seeds under Salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Maggio, A. Functional Biology of Halophytes in the Phytoremediation of Heavy Metal Contaminated Soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Karakaş, S.; Çullu, M.A.; Dikilitaş, M. Comparison of Two Halophyte Species (Salsola Soda and Portulaca Oleracea) for Salt Removal Potential under Different Soil Salinity Conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, J.; Wang, W.; Zhang, Y.; Yuan, H.; Huang, S. Transcriptome Analysis Reveals Complex Response of the Medicinal/Ornamental Halophyte Iris Halophila Pall. To High Environmental Salinity Ecotoxicol. Environ. Saf. 2018, 165, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Ameıxa, O.M.C.C.; Marques, B.; Fernandes, V.S.; Soares, A.M.V.M.; Calado, R.; Lıllebø, A.I. Dimorphic seeds of Salicornia ramosissima display contrasting germination responses under different salinities. Ecol. Eng. 2016, 87, 120–123. [Google Scholar] [CrossRef]
- Akınshına, N.; Azızov, A.; Karasyova, T.; Klose, E. On the issue of halophytes as energy plants in saline environment. Biomass Bioenergy 2016, 91, 306–311. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte Crop Cultivation: The Case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Ferronato, C.; Speranza, M.; Ferronı, L.; Buscarolı, A.; Vıanello, G.; Antısarı, L.V. Vegetation response to soil salinity and waterlogging in three saltmarsh hydrosequences through macronutrients distribution. Estuar. Coast. Shelf Sci. 2018, 200, 131–140. [Google Scholar] [CrossRef]
- Norman, H.C.; Masters, D.G.; Barrett-Lennard, E.G. Halophytes as Forages in Saline Landscapes: Interactions between Plant Genotype and Environment Change Their Feeding Value to Ruminants. Environ. Exp. Bot. 2013, 92, 96–109. [Google Scholar] [CrossRef]
- Smillie, C. Salicornia spp. as a biomonitor of Cu and Zn in salt marsh sediments. Ecol. Indic. 2015, 56, 70–78. [Google Scholar] [CrossRef]
- Sánchez-Gavilán, I.; Ramírez, E.; de la Fuente, V. Bioactive Compounds in Salicornia patula Duval-Jouve: A Mediterranean Edible Euhalophyte. Foods 2021, 10, 410. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Pıernık, A.; Chanona-Pérez, J.J.; Grıgore, M.N.; Perea-Flores, M.J. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Rathore, A.P.; Chaudhary, D.R.; Jha, B. Biomass Production, Nutrient Cycling, and Carbon Fixation by Salicornia brachiate Roxb.: A Promising Halophyte for Coastal Saline Soil Rehabilitation. Int. J. Phytoremediation 2016, 18, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.-N.; Oprica, L. Halophytes as Possible Source of Antioxidant Compounds, in a Scenario Based on Threatened Agriculture and Food Crisis. Iran. J. Public Health 2015, 44, 1153–1155. [Google Scholar]
- Poenaru, V.; Badea, A.; Cimpeanu, S.M.; Irimescu, A. Multi-Temporal Multi-Spectral and Radar Remote Sensing for Agricultural Monitoring in the Braila Plain. Agric. Agric. Sci. Procedia 2015, 6, 506–516. [Google Scholar] [CrossRef]
- Toma, F.; Georgescu, M.I.; Petra, S.; Dobrescu, E. Some Aspects Concerning the Rest Period of Tuberose Bulbs. Agric. Agric. Sci. Procedia 2015, 6, 179–183. [Google Scholar] [CrossRef]
- SR ISO ISO 11265 + A1: 1998 Soil quality.
- Al Hassan, M.; Chaura, J.; Lopez-Gresa, M.P.; Borsai, O.; Daniso, E.; Donat-Torres, M.P.; Mayoral, O.; Vicente, O.; Boscaiu, M. Native-invasive plants vs. halophytes in Mediterranean salt marshes: Stress tolerance mechanisms in two related species. Front. Plant Sci. 2016, 7, 473. [Google Scholar] [CrossRef]
- Bendokas, V.; Šarkınas, A.; Jasınauskıenė, D.; Anısımovıenė, N.; Morkūnaıtė-Haımı, Š.; Stanys, V.; Šıkšnıanas, T. Antimicrobial activity of berries extracts of four Ribes species, their phenolic content and anthocyanin composition. Folia Hort. 2018, 30, 249–257. [Google Scholar] [CrossRef]
- Dimitriu, L.; Preda, D.; Constantinescu-Aruxandei, D.; Oancea, F.; Băbeanu, N. Optimization of ultrasound-assisted extraction of polyphenols from honeysuckle (Lonicera caprifolium). AgroLife Sci. J. 2021, 10, 47–55. [Google Scholar] [CrossRef]
- Fintineru, A.; Manole, C.G.; Smedescu, D.; Rodino, S.; Fintineru, S.C.; Butu, A. Bioaccumulation of Health Promoting Compounds in Five Ribes rubrum L. Varieties during Fruit Maturation. Rom. Biotechnol. Lett. 2015, 20, 10036–10046. [Google Scholar]
- Wu, S.; Feng, X.; Wittmeier, A. Microwave Digestion of Plant and Grain Reference Materials in Nitric Acid or a Mixture of Nitric Acid or a Mixture of Nitric Acid and Hydrogen Peroxide for the Determination of Multi-Elements by Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom. 1997, 12, 797–806. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil PH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Lee, A.T.; Lin, Z.; Pickering, I.J.; Terry, N. X-Ray Absorption Spectroscopy Study Shows That the Rapid Selenium Volatilizer, Pickleweed (Salicornia bigelovii Torr.), Reduces Selenate to Organic Forms without the Aid of Microbes. Planta 2001, 213, 977–980. [Google Scholar] [CrossRef]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.-T. Phytoremediation of Salt-Affected Soils: A Review of Processes, Applicability, and the Impact of Climate Change. Environ. Sci. Pollut. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile Maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional characterization and storage ability of Salicornia ramosissima and sarcocornia perennis for fresh vegetable salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.; Khatib, A.; Abdull Razis, A. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative Analysis of Bioactive Phenolic Compounds Composition from 26 Medicinal Plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Padalino, L.; Costa, C.; Del Nobile, M.A.; Conte, A. Extract of Salicornia Europaea in Fresh Pasta to Enhance Phenolic Compounds and Antioxidant Activity. Int. J. Food Sci. Technol. 2019, 54, 3051–3057. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Lin, X.; Gonzalez Neira, A.C.; Tabay Zambon, F.; Hu, H.; Wang, X.; Huang, J.-H.; Fan, G. Substrate PH Influences the Nutrient Absorption and Rhizosphere Microbiome of Huanglongbing-Affected Grapefruit Plants. Front. Plant Sci. 2022, 13, 856937. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Lopes, M.D.; Roque, M.J.; Cavaleıro, C.; Ramos, F. Nutrient value of Salicornia ramosissima—A green extraction process for mineral analysis. J. Food Compos. Anal. 2021, 104, 104135. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Ghimire, B.K.; Kim, S.H.; Yu, C.Y.; Oh, D.H.; Chelliah, R.; Kwon, C.; Kim, Y.J.; Chung, I.M. Assessment of Mineral and Phenolic Profiles and Their Association with the Antioxidant, Cytotoxic Effect, and Antimicrobial Potential of Lycium chinense Miller. Plants 2020, 13, 1023. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Bahamonde, H.A.; Javier Peguero-Pina, J.; Gil-Pelegrín, E.; Sancho-Knapik, D.; Gil, L.; Goldbach, H.E.; Eichert, T. Physico-Chemical Properties of Plant Cuticles and Their Functional and Ecological Significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.-N.; Vicente, O. Wild Halophytes: Tools for Understanding Salt Tolerance Mechanisms of Plants and for Adapting Agriculture to Climate Change. Plants 2023, 12, 221. [Google Scholar] [CrossRef]
- Grigore, M.N.; Ivanescu, L.; Toma, C.; Grigore, M.N.; Ivanescu, L.; Toma, C. General Morphological and Anatomical Adaptations in Halophytes. In Halophytes: An Integrative Anatomical Study; Springer: Berlin/Heidelberg, Germany, 2014; pp. 33–37. [Google Scholar]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, Anatomical and Metabolic Implications of Salt Tolerance in the Halophyte Salvadora Persica under Hydroponic Culture Condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Wolf, W.M. The Impact of Soil PH on Heavy Metals Uptake and Photosynthesis Efficiency in Melissa officinalis, Taraxacum officinalis, Ocimum basilicum. Molecules 2022, 27, 4671. [Google Scholar] [CrossRef]

| Cultivation Substrate | Initially pH Values | Na g/kg | Mg g/kg | K g/kg | Ca g/kg |
|---|---|---|---|---|---|
| S1 | 7.8 ± 0.017 | 72.166 ± 0.405 c | 6.874 ± 0.119 c | 19.038 ± 0.133 c | 36.359 ± 0.526 b |
| S2 | 8.81 ± 0.03 | 156 ± 0.479 b | 11.734 ± 0.016 b | 15.400 ± 0.061 d | 8.057 ± 0.307 d |
| S3 | 7.45 ± 0.03 | 54.487 ± 0.302 d | 7.388 ± 0.052 c | 23.389 ± 0.089 b | 46.207 ± 0.461 a |
| I | 8.81 ± 0.06 | 170.336 ± 1.566 a | 15.039 ± 0.133 a | 7.685 ± 0.022 e | 3.943 ± 0.438 e |
| Peat and perlite (R) | 5.18 ± 0.03 | 45.962 ± 0.292 e | 11.329 ± 0.124 b | 40.867 ± 0.229 a | 25.520 ± 0.346 c |
| TPC | TFC | Na | Mg | K | Ca | |
|---|---|---|---|---|---|---|
| TPC | 1 | |||||
| TFC | 0.969 | 1 | ||||
| Na | 0.298 | 0.194 | 1 | |||
| Mg | 0.802 | 0.774 | 0.737 | 1 | ||
| K | −0.019 | 0.162 | −0.816 | −0.306 | 1 | |
| Ca | −0.523 | −0.516 | −0.880 | −0.915 | 0.465 | 1 |
| Substrate | Cuticle (µm) | Epidermis (µm) | Parenchyma (µm) |
|---|---|---|---|
| I | 0.297 ± 0.014 b | 1.707 ± 0.090 c | 6.565 ± 0.566 c |
| S1 | 0.278 ± 0.016 b | 2.394 ± 0.331 a | 7.675 ± 0.423 b |
| S2 | 0.240 ± 0.008 b | 2.023 ± 0.121 b | 8.584 ± 0.599 a |
| S3 | 0.533 ± 0.058 a | 1.657 ± 0.099 c | 8.694 ± 0.677 a |
| Peat and perlite (R) | 0.151 ± 0.053 c | 1.783 ± 0.162 c | 5.226 ± 0.412 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, C.G.; Zugravu, M.M.; Georgescu, M.; Constantin, M.F.; Moț, A.; Paraschiv, M.; Dobrin, A. The Impact of the Growing Substrate on Morphological and Biochemical Features of Salicornia europaea L. Appl. Sci. 2023, 13, 10835. https://doi.org/10.3390/app131910835
Constantin CG, Zugravu MM, Georgescu M, Constantin MF, Moț A, Paraschiv M, Dobrin A. The Impact of the Growing Substrate on Morphological and Biochemical Features of Salicornia europaea L. Applied Sciences. 2023; 13(19):10835. https://doi.org/10.3390/app131910835
Chicago/Turabian StyleConstantin, Carmen Gabriela, Mihaela Maria Zugravu, Mihaela Georgescu, Mugurași Florin Constantin, Andrei Moț, Maria Paraschiv, and Aurora Dobrin. 2023. "The Impact of the Growing Substrate on Morphological and Biochemical Features of Salicornia europaea L." Applied Sciences 13, no. 19: 10835. https://doi.org/10.3390/app131910835
APA StyleConstantin, C. G., Zugravu, M. M., Georgescu, M., Constantin, M. F., Moț, A., Paraschiv, M., & Dobrin, A. (2023). The Impact of the Growing Substrate on Morphological and Biochemical Features of Salicornia europaea L. Applied Sciences, 13(19), 10835. https://doi.org/10.3390/app131910835






