Abstract
The consequences of traumatic injuries are pulp necrosis, periapical inflammation, and possible external cervical resorption (ECR). The concomitant cessation of root maturation and dentinogenesis in premature teeth result in a fragile tooth. Revascularization of the pulp might be an alternative treatment option. A 12-year-old patient was referred for retreatment of chronic apical periodontitis with acute exacerbation after root canal treatment of an upper central incisor. During gutta-percha removal, an ECR was detected. First, it was closed internally with Biodentine, followed by an external composite closure after a cone beam computed tomography evaluation. In the first session, chemomechanical cleaning was aided with sodium hypochlorite and hyperpure chlorine dioxide, and the canal was dressed with Ca(OH)2 until the next session. In the next session, only the low-toxicity, but adequate, bactericide hyperpure chlorine dioxide was applied for irrigation, before the provocation of periapical bleeding into the canal. The blood clots were covered with Biodentine, and the tooth was restored with composite filling. During the 24-month follow-up, clinical signs/symptoms had disappeared, and the periapical lesion was resolved. Increased root thickness in the apical third and decreased size of the apical foramen were detected. In conclusion, regenerative endodontic procedures could potentially be used to retreat immature teeth with persistent apical periodontitis and external cervical resorption.
1. Introduction
The root canal treatment of immature teeth with apical periodontitis can be challenging due to the thin dentin walls and open apex. In such cases, apexification was considered the treatment of choice for necrotized immature teeth because of its success rate in periapical healing [1]. However, it led to the halting of dentin formation, resulting in a thin root wall. Consequently, the fragility of the root could compromise the long-term prognosis of the tooth [2].
A recent paradigm shift towards regenerative endodontic treatment (RET) may address this limitation. This biological treatment modality utilizes the principles of tissue engineering, such as the application of progenitor cells, growth factors, and scaffolds, to replace lost tissues with functional ones [3]. Several approaches have been described since the early 2000s when this field of endodontics began to emerge [4]. The methods varied according to the source of stem cells (allogenic or autologous technique) and the type of growth factors and scaffolds [5]. However, the most practical way to obtain autologous stem cells, growth factors, and scaffold simultaneously, is to induce bleeding into the root canal via laceration of the periapical area [6].
This novel treatment modality offers several benefits, including the resolution of periapical lesions, ongoing root development, improved crown–root ratio, thickened root canal walls, and, consequently, increased fracture resistance [7,8]. Furthermore, successful regeneration can restore the immune defense function and sensibility of the tooth once vital tissue reoccupies the root canal.
However, regeneration can only occur after disinfection and creation of a microenvironment adequate for stem cell survival and differentiation [9]. Complete root canal disinfection is always complex during primary treatment [10] but even more difficult during retreatment. Therefore, revascularizing the tooth after previous endodontic therapy is challenging, and thus, only a few cases of using revascularization for re-treatment have been reported [8]. Hyperpure ClO2 does not inhibit the viability of periodontal ligamental stem cells [11] at the toxic concentration for microbes due to its size-selective properties [12]. Therefore, it could be an excellent candidate for disinfecting the canal before revascularization.
External cervical resorption (ECR) could further complicate a traumatic case. This progressive resorptive condition can adversely affect the success of regenerative treatment if it establishes a connection between the oral cavity and the root canal. To the best of our knowledge, no reported case studies documenting regeneration in the presence of ECR exist. In such a case, cleaning the resorptive defect and hermetically sealing the perforation are essential to establish a bacteria-tight barrier before the regenerative procedure [13].
This report aimed to showcase the positive outcomes of the regenerative treatment approach performed on an immature upper central incisor with external cervical resorption. The tooth had a poorly obturated root canal and exhibited radiographic and clinical signs of apical periodontitis with acute exacerbation.
2. Detailed Case Description
A 12-year-old girl with pain related to biting was referred to the Department of Restorative Dentistry and Endodontics by a pediatric general practitioner, for treatment of the upper right central incisor. This clinical case report was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Prior to any study procedures, the patient received comprehensive written information regarding the study objectives, procedures, potential risks, and benefits of the treatment being reported. Informed consent was obtained from the patient, and it was made clear that participation was entirely voluntary, and the patient had the right to withdraw consent at any time without repercussions. No medicaments or systemic disease influencing dental treatments were found in her medical history. Her dental history revealed a bike accident when she was eight years old, which resulted in the fracture of the crown of tooth 11 (FDI notation). A year later, due to the experience of pain related to biting, the dentist performed a root canal treatment. At age 12, the pain recurred, and she was referred to our department. On clinical examination, the crown of tooth 11 had a multisurface composite filling with dark discoloration (Figure 1a). The tooth was sensitive to palpation and percussion. No swelling was observed in the vestibule. It did not react to the sensibility test compared with the reference tooth. On the preoperative periapical X-ray, an insufficient root canal filling was visible in the short, immature root with a wide-open apex, and there was a large periapical radiolucency around it (Figure 1b), at class five according to the periapical index (PAI). Based on these findings, the case was diagnosed as a tooth with failed root canal treatment, chronic apical periodontitis, and acute exacerbation.
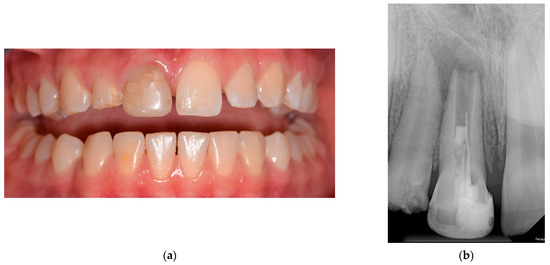
Figure 1.
The initial situation: (a) presenting discoloration of the right central incisor; (b) preoperative periapical X-ray highlighting inadequate obturation sealing with a short root canal filling.
RET was chosen for therapy because of its potential for strengthening the root by finishing the dentinogenesis.
At the first session, a silicone impression was taken of the front teeth, to make a chairside immediate provisional direct restoration at the end of the session, after the removal of the previous large composite filling from the tooth. After administering local anesthesia (Lidocaine-adrenaline 20 mg/0.01 mg/mL, Egis, Budapest, Hungary), tooth 11 was isolated with a rubber dam, and the canal was accessed. While removing the poorly-fitting gutta-percha with a size #30 Hedstrom file (Dentsply Maillefer, Ballaigues, Switzerland), a perforation filled with granulomatous tissue was found buccally at the cervical area involving the coronal third of the root (Figure 2a). The granulomatous tissue was removed with a small excavator, and the perforation was sealed internally with Biodentin (Figure 2b). After the Biodentin (Septodont Ltd., Saint Maur des Faussés, France) had set (~12 min), the canal was irrigated with 10 mL of 1.25% NaOCl (University Pharmacy, Semmelweis University, Budapest, Hungary) and 2 mL of 0.12% hyperpure ClO2 (Solumium Pental, Solumium Kft, Budapest, Hungary) to complete the irrigation. Next, the canal was dried and dressed with Ca(OH)2 (Calcipast; Cerkamed, Stalowa Wola, Poland) and sealed with polytetrafluoroethylene tape and glass–ionomer cement (GIC) (Ketac Molar; 3 M ESPE, Seefeld, Germany). Finally, an acrylate provisional restoration was fabricated and cemented.
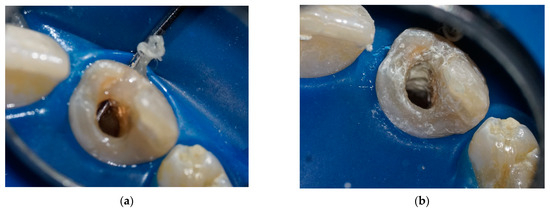
Figure 2.
Perforation management at the first session: (a) clinical photo illustrating the perforation across the buccal wall; (b) closure of the perforation with Biodentin.
A small field-of-view cone beam computed tomography (CBCT) image was taken to evaluate the extent and nature of the perforation and to create an appropriate treatment plan (Figure 2b,c and Figure 3a). The sections of the CBCT confirmed a type 2Ap (based on Patel classification) [14] perforating external cervical resorption.
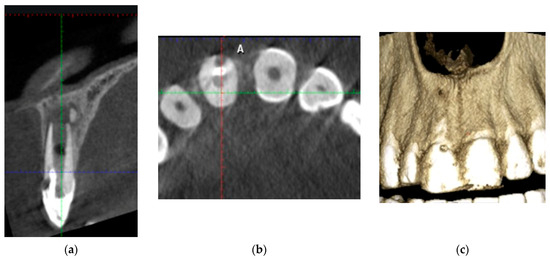
Figure 3.
Sections of the CBCT: (a) bucco-lingual cross-section; (b) 3D reconstruction of the buccal bone plate; (c) horizontal cross-section indicating the localization of the cervical resorption.
In the second session, the resorptive cavity was explored externally to finalize the restoration of the cervical defect under visual control. In absolute isolation, restoration of the coronal part of the defect was undertaken and filled with composite (Filtek Z250; 3M ESPE, St. Paul, MN, USA). The working length was established at 1 mm short of the apex. It was determined on CBCT and verified using an electronic apex locator (Woodpex III, Guilin Woodpecker Medical Instrument Co., Ltd., Guilin, China).
The canal was mechanically prepared with gentle brushing motions with a #60 K-file (VDW, Munich, Germany). During chemical irrigation, 15 mL of 1.25% NaOCl was used and activated with ultrasound. Flushing with sterile saline was followed by irrigation with 20 mL of 17% EDTA. A side-vented needle, 1 mm short of the working length, was used to avoid extrusion of the solutions into the periapical area. The canal was dried and dressed with Ca(OH)2 [15]. The access cavity was closed with GIC and an acrylic temporary restoration.
Four weeks later, there were no clinical signs or symptoms of inflammation. Local anesthesia without vasoconstrictors (Scandonest 3% plain; Septodont Ltd., Saint Maur des Faussés, France) was given to avoid insufficient bleeding before absolute isolation. After accessing, the canal was irrigated with 10 mL of 0.12% hyperpure ClO2 to remove the Ca(OH)2 and disinfect the canal again. Ultrasonically activated EDTA (17%) was used to liberate fossilized growth factors in the dentin [16,17]. The apical tissues were lacerated with a #35 Hedstrom file to initiate bleeding into the canal space, which provides a blood clot as a scaffold. At the same time, the influx of mesenchymal stem cells and platelet-derived growth factors make pulp tissue regeneration possible. The level of the blood clot was established at 3 mm below the cementoenamel junction (Figure 4a). A resorbable collagen sponge (Collaplug; Zimmer Biomet Dental, Palm Beach Garden, FL, USA) was placed on the top of the blood clot to function as a matrix to facilitate the placement of the Biodentin. A 3 mm thick coronal plug of Biodentin (Figure 4b) was created to provide a bacteria-tight seal.
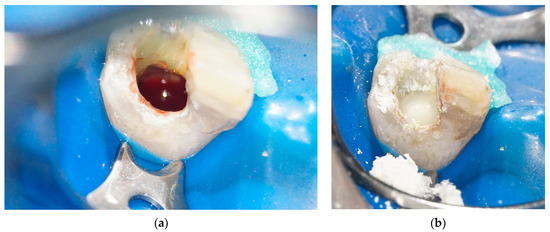
Figure 4.
Blood clot formation and Biodentin placement: (a) blood clot formed at the level of cementoenamel junction; (b) blood clot sealed with Biodentin.
After confirming the barrier, the tooth was restored with an extended composite direct veneer using the layering technique (Micerium Hri; MICERIUM S.p.A., Avegno GE Italy) (Figure 5).
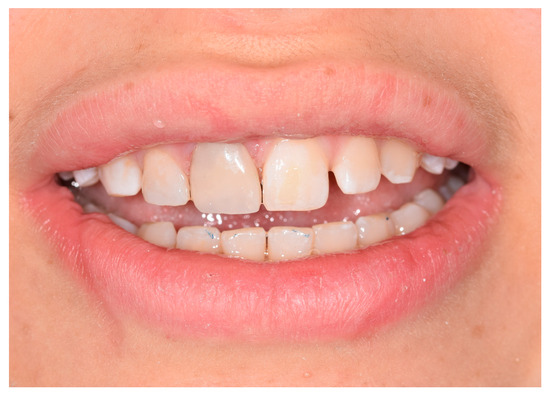
Figure 5.
Composite restoration.
The patient was recalled at six months, one year, and two years. Clinical follow-up was performed according to ESE quality guidelines [18]. The patient remained symptom-free. After six months, an X-ray showed no periapical radiolucency. After one year, an increase in root thickness in the apical third and a decrease in size of the apical foramen were visible (Figure 6). At the 24-month control, the tooth was clinically and radiologically asymptomatic; no sensitivity to percussion or palpation was detected. However, there was no reaction to cold, probably due to the technical difficulty of stimulation, as the revascularized pulp tissue was 3 mm below the bone level covered with Biodentin. A small field-of-view CBCT image (Figure 7) indicates that the resorption did not progress further. A calcified bridge formation was observed at the apex.
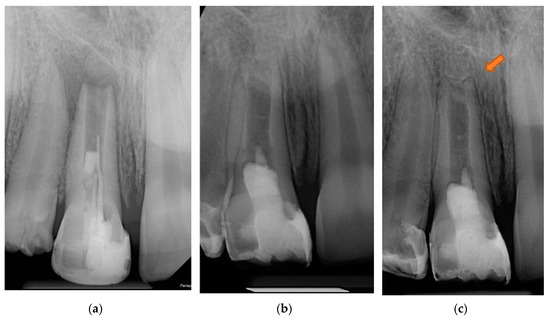
Figure 6.
Radiographic follow-up: (a) preoperative stage; (b) 6-month control; (c) 1-year control. The arrow indicates the thickening of dentinal walls and the lengthened root.
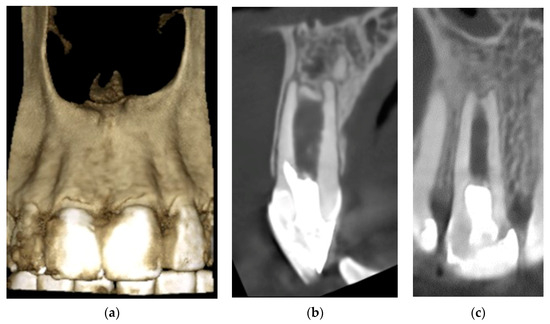
Figure 7.
24-month control CBCT: (a) 3D reconstruction of the healed buccal bone plate; (b) bucco-lingual cross-section; (c) mesio-distal cross-section.
3. Discussion
In the present case report, regenerative treatment was successful in a previously obturated, periodontally involved tooth with ECR. A case series study [19] indicates that successful treatment of necrotized immature teeth combined with external root resorption with RET is plausible. However, other case studies indicate that RET can be applied in other challenged situations as well. Lorono et al. [20] illustrate a case where RET salvaged a tooth with internal resorption. Kaval et al. [21] report the successful management of a case, with perforation due to internal resorption, using RET. Another case demonstrates successful regenerative treatment of a previous root canal treatment [8].
Traumatic tooth injury can result in neurovascular disruption, pulp necrosis, and damage to the periodontal ligament and cementum, which might cause ECR. The exact pathomechanism of ECR is still unknown, but trauma seems to be one of the most common etiologic factors of ECR [22]. Additionally, injury of the periapical tissues can seriously negatively affect normal root development, leaving the root short and the dentin wall thin. Recent publications [23,24] have shown that removing the protective pericanal resorption-resistant sheet during root canal treatment also predisposes the tooth to a more aggressive form of ECR. Perforation of the pulp happens only in the advanced stages of ECR [25]. However, penetration of the resorption to the pulp chamber cannot be prevented after root canal treatment [23]. As a result, the continuous influx of bacteria through the perforation into the pulp chamber results in a more severe root canal infection and hinders satisfactory disinfection during regenerative procedures. It is necessary to excavate and restore the resorptive defect before disinfection of the canal, in order to provide an optimal bacteria-free environment [26]. It is challenging to avoid excessive marginal bone and sound tooth structure removal and to completely eradicate the resorptive tissue simultaneously. If the size of the portal of entry is wide and the lesion penetrates the coronal part of the root, it is advisable to use external and internal methods as therapy [24]. Therefore, it was performed using two steps. First, the defect was cleaned and sealed internally. Second, the coronal part was finalized externally and restored with composite afterwards.
According to Cvek’s classification of root development [27], the tooth belonged to class 3 (2/3 root development with open apex). In class 3 teeth, RET is recommended because of the potential to facilitate dentinogenesis [10], contrarily to apexification. Although the success rate of periapical healing is similar, animal studies prove that teeth treated with RET can withstand more stress-induced fractures than untreated ones [7]. However, there is a lack of long-term clinical evidence.
Performing RET on a tooth that has already been root-canal-treated and has persistent apical periodontitis means a significant challenge [28]. First, the materials used during the first root canal treatment are not known, and their remnants might have a deleterious effect on regeneration. Second, eliminating persistent bacteria after a failed endodontic treatment requires a more potent chemical protocol [8].
Finding the balance between optimal disinfection and maintaining the vitality of periapical stem cells is the key to successful revascularization. High concentrations of NaOCl, additional use of chlorhexidine, and antibiotics can help reduce the bacterial load in the canal [29]. However, the cytotoxic effect of these chemicals can have a detrimental effect on the survival of the stem cells [30], which play a significant role in RET. In order to overcome this issue, gentle but effective chemical irrigation is needed [9]. NaOCl forms chloramine molecules when it reacts with organic tissue [31]. In the case of extrusion into the periapical space, this can cause necrosis of the tissues [32,33].
Therefore, hyperpure chlorine dioxide was used just before blood clot formation in the current case, instead of NaOCl. Chlorine dioxide, a disinfectant used in the food industry and drinking water treatment, has previously been suggested for use in endodontics because of its low toxicity and excellent antimicrobial properties [34,35,36,37]. However, the ordinary solution contains impurities because it is produced by mixing sodium chlorite with an acid [38]. Some of the unknown by-products are suspected to be harmful and carcinogenic [39]. Recently, a novel disinfectant, hyperpure ClO2 solution (Solumium Pental), was produced and used in endodontics, with a new membrane technology that contains no additional chemicals such as acids. Hyperpure ClO2 has no significant inhibitory effect on the viability of periodontal ligamental stem cells [11] at the toxic concentration for microbes, due to its size-selective property [12]. Furthermore, ultrasonic activation was performed to increase the effectiveness of the irrigants without increasing their concentration [40]. EDTA was also used to promote stem cell survival and release the growth factors embedded in dentin [41].
The commonly used interappointment medication for RET is triple antibiotic paste because of its bactericidal effect [42]. However, its side effects, such as crown discoloration, allergic reactions, and the development of bacterial resistance, can limit its usage [15]. In this case, Ca(OH)2 was chosen as an intersession medication [15] due to its antimicrobial capacity and ability to stimulate the survival and proliferation of stem cells of the apical papilla [43].
While regenerative endodontic procedures have shown promising results, they also come with several limitations and challenges. Not all cases are suitable for regenerative endodontic procedures, and success depends on the patient’s age, root development stage, and inflammation grade [44]. While growing evidence supports the effectiveness of regenerative endodontic procedures [8], long-term clinical studies are still relatively limited [45].
In this study, the presence of new neurons could not be revealed during the control appointments. However, positive sensibility testing can only be achieved in 50% of cases after successful RET [46].
The limitations of the laceration of periapical tissues are the relatively high chance of intracanal calcification and the unpredictable nature of the newly formed tissue [47]. Histological studies have indicated that, in many cases, tissue repair often involves the formation of ectopic cementum and bone-like tissue, rather than achieving true tissue regeneration [7,48,49]. The mesenchymal stem cells and mature cells from all periapical tissues (bone marrow, cementum, periodontal ligament) also enter the root canal and might be responsible for forming the nonendodontic mineralized tissues.
Additionally, the amount, type, and potential of stem cells delivered into the root canal space are unknown, and growth factors embedded in dentin could potentially be broken down by irrigants used during root canal treatment [50]. Therefore, new investigations should aim at genuine pulp regeneration by applying components of the tissue engineering triad in an evidence-based way. Numerous preclinical approaches have been introduced, such as applying pure dental stem cells from external sources [5] and using PRF/PRP- or polymer-based synthetic hydrogels as alternative scaffolds [51,52]. A novel cell-free method for regenerative endodontic therapy involves using secretome-derived products from mesenchymal stem cells [4,53]. A recent in vitro study [54] demonstrated that ascorbic acid treatment of mesenchymal stem cells improved their pluripotent activity. Thus, ascorbic acid treatment might provide further perspective, but more clinical investigations are needed to evaluate the effect of biological treatments on the regeneration of the pulp–dentin complex [3].
4. Conclusions
The presented case demonstrates that RET is possible when root development is severely deficient and after failed root canal therapy with persistent apical periodontitis. The presence of perforated cervical resorption makes the process more challenging due to canal sealing difficulties, but after proper management, success can be achieved in regeneration.
Author Contributions
Conceptualization, M.P. and P.K.; methodology, M.P. and P.K.; investigation, M.P.; writing—original draft preparation, M.P. and Z.M.L.; writing—review and editing, visualization, M.P.; supervision, E.V.S. and P.K.; project administration, J.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This noninterventional observational study was conducted according to the guidelines of the Declaration of Helsinki. The subject involved in the present report was referred by public assistance to the Department of Restorative Dentistry and Endodontics, Semmelweis University (Budapest), and treated in accordance with her clinical needs. Therefore, no institutional ethics committee approval was required.
Informed Consent Statement
We affirm that this clinical case report was conducted with the utmost integrity, adherence to ethical principles, and patient welfare as the paramount concern. Written informed consent was obtained from the patient prior to publishing the photos and radiographic images in this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. This case report was presented in 2022 at the Biennial Congress of the European Society of Endodontology, Budapest, in the case report section.
References
- Lin, J.C.; Lu, J.X.; Zeng, Q.; Zhao, W.; Li, W.Q.; Ling, J.Q. Comparison of mineral trioxide aggregate and calcium hydroxide for apexification of immature permanent teeth: A systematic review and meta-analysis. J. Formos. Med. Assoc. 2016, 115, 523–530. [Google Scholar] [CrossRef]
- Lin, J.; Zeng, Q.; Wei, X.; Zhao, W.; Cui, M.; Gu, J.; Lu, J.; Yang, M.; Ling, J. Regenerative Endodontics Versus Apexification in Immature Permanent Teeth with Apical Periodontitis: A Prospective Randomized Controlled Study. J. Endod. 2017, 43, 1821–1827. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Diogenes, A.; Teixeira, F.B. Treatment options: Biological basis of regenerative endodontic procedures. Pediatr. Dent. 2013, 35, 129–140. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Vaca, A.; Vizoso, F.J. Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramirez, V.; Angelopoulos, I.; Cadiz, M.I.; Tapia-Limonchi, R.; Khoury, M. Cell-Based Regenerative Endodontics for Treatment of Periapical Lesions: A Randomized, Controlled Phase I/II Clinical Trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Chen, Y.; Chen, S.; Lyu, H.; Cai, Z.; Huang, X. Radiographic, Histologic, and Biomechanical Evaluation of Combined Application of Platelet-rich Fibrin with Blood Clot in Regenerative Endodontics. J. Endod. 2017, 43, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Cymerman, J.J.; Nosrat, A. Regenerative Endodontic Treatment as a Biologically Based Approach for Non-Surgical Retreatment of Immature Teeth. J. Endod. 2020, 46, 44–50. [Google Scholar] [CrossRef]
- Martin, D.E.; De Almeida, J.F.; Henry, M.A.; Khaing, Z.Z.; Schmidt, C.E.; Teixeira, F.B.; Diogenes, A. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J. Endod. 2014, 40, 51–55. [Google Scholar] [CrossRef]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef]
- Lang, O.; Nagy, K.S.; Lang, J.; Perczel-Kovach, K.; Herczegh, A.; Lohinai, Z.; Varga, G.; Kohidai, L. Comparative study of hyperpure chlorine dioxide with two other irrigants regarding the viability of periodontal ligament stem cells. Clin. Oral. Investig. 2021, 25, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- Noszticzius, Z.; Wittmann, M.; Kaly-Kullai, K.; Beregvari, Z.; Kiss, I.; Rosivall, L.; Szegedi, J. Chlorine dioxide is a size-selective antimicrobial agent. PLoS ONE 2013, 8, e79157. [Google Scholar] [CrossRef]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef]
- Patel, S.; Mavridou, A.M.; Lambrechts, P.; Saberi, N. External cervical resorption-part 1: Histopathology, distribution and presentation. Int. Endod. J. 2018, 51, 1205–1223. [Google Scholar] [CrossRef]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Duncan, H.F.; Kobayashi, Y.; Shimizu, E. Growth Factors and Cell Homing in Dental Tissue Regeneration. Curr. Oral. Health Rep. 2018, 5, 276–285. [Google Scholar] [CrossRef]
- Dos Reis-Prado, A.H.; Abreu, L.G.; Fagundes, R.R.; Oliveira, S.C.; Bottino, M.C.; Ribeiro-Sobrinho, A.P.; Benetti, F. Influence of ethylenediaminetetraacetic acid on regenerative endodontics: A systematic review. Int. Endod. J. 2022, 55, 579–612. [Google Scholar] [CrossRef] [PubMed]
- European Society of Endodontology. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef]
- Yoshpe, M.; Einy, S.; Ruparel, N.; Lin, S.; Kaufman, A.Y. Regenerative Endodontics: A Potential Solution for External Root Resorption (Case Series). J. Endod. 2020, 46, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Lorono, G.; Jesus Conde, A.; Estevez, R.; Brizuela, C.; Cisneros, R.; Alfayate, R.P. Regenerative Endodontic Procedure in an Immature Permanent Incisor with Internal Root Resorption: A Case Report. J. Dent. 2022, 23, 155–160. [Google Scholar] [CrossRef]
- Kaval, M.E.; Guneri, P.; Caliskan, M.K. Regenerative endodontic treatment of perforated internal root resorption: A case report. Int. Endod. J. 2018, 51, 128–137. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Deng, X. External cervical resorption-a review of pathogenesis and potential predisposing factors. Int. J. Oral. Sci. 2021, 13, 19. [Google Scholar] [CrossRef]
- Mavridou, A.M.; Hauben, E.; Wevers, M.; Schepers, E.; Bergmans, L.; Lambrechts, P. Understanding external cervical resorption patterns in endodontically treated teeth. Int. Endod. J. 2017, 50, 1116–1133. [Google Scholar] [CrossRef]
- Mavridou, A.M.; Rubbers, E.; Schryvers, A.; Maes, A.; Linssen, M.; Barendregt, D.S.; Bergmans, L.; Lambrechts, P. A clinical approach strategy for the diagnosis, treatment and evaluation of external cervical resorption. Int. Endod. J. 2022, 55, 347–373. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Deng, X. A Review of External Cervical Resorption. J. Endod. 2021, 47, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Foschi, F.; Condon, R.; Pimentel, T.; Bhuva, B. External cervical resorption: Part 2—Management. Int. Endod. J. 2018, 51, 1224–1238. [Google Scholar] [CrossRef]
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod. Dent. Traumatol. 1992, 8, 45–55. [Google Scholar] [CrossRef]
- Saoud, T.M.; Huang, G.T.; Gibbs, J.L.; Sigurdsson, A.; Lin, L.M. Management of Teeth with Persistent Apical Periodontitis after Root Canal Treatment Using Regenerative Endodontic Therapy. J. Endod. 2015, 41, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Clegg, M.S.; Vertucci, F.J.; Walker, C.; Belanger, M.; Britto, L.R. The effect of exposure to irrigant solutions on apical dentin biofilms in vitro. J. Endod. 2006, 32, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Ruparel, N.B.; Teixeira, F.B.; Ferraz, C.C.; Diogenes, A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J. Endod. 2012, 38, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Sabala, C.L.; Powell, S.E. Sodium hypochlorite injection into periapical tissues. J. Endod. 1989, 15, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Becking, A.G. Complications in the use of sodium hypochlorite during endodontic treatment. Report of three cases. Oral Surg. Oral Med. Oral Pathol. 1991, 71, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Cobankara, F.K.; Ozkan, H.B.; Terlemez, A. Comparison of organic tissue dissolution capacities of sodium hypochlorite and chlorine dioxide. J. Endod. 2010, 36, 272–274. [Google Scholar] [CrossRef]
- Herczegh, A.; Gyurkovics, M.; Ghidan, A.; Megyesi, M.; Lohinai, Z. Effect of dentin powder on the antimicrobial properties of hyperpure chlorine-dioxide and its comparison to conventional endodontic disinfecting agents. Acta Microbiol. Immunol. Hung. 2014, 61, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Herczegh, A.; Gyurkovics, M.; Agababyan, H.; Ghidan, A.; Lohinai, Z. Comparing the efficacy of hyper-pure chlorine-dioxide with other oral antiseptics on oral pathogen microorganisms and biofilm in vitro. Acta Microbiol. Immunol. Hung. 2013, 60, 359–373. [Google Scholar] [CrossRef]
- Herczegh, A.; Ghidan, A.; Friedreich, D.; Gyurkovics, M.; Bendo, Z.; Lohinai, Z. Effectiveness of a high purity chlorine dioxide solution in eliminating intracanal Enterococcus faecalis biofilm. Acta Microbiol. Immunol. Hung. 2013, 60, 63–75. [Google Scholar] [CrossRef]
- Ma, J.W.; Huang, B.S.; Hsu, C.W.; Peng, C.W.; Cheng, M.L.; Kao, J.Y.; Way, T.D.; Yin, H.C.; Wang, S.S. Efficacy and Safety Evaluation of a Chlorine Dioxide Solution. Int. J. Environ. Res. Public Health 2017, 14, 329. [Google Scholar] [CrossRef]
- Couri, D.; Abdel-Rahman, M.S.; Bull, R.J. Toxicological effects of chlorine dioxide, chlorite and chlorate. Environ. Health Perspect. 1982, 46, 13–17. [Google Scholar] [CrossRef]
- Nakamura, V.C.; Pinheiro, E.T.; Prado, L.C.; Silveira, A.C.; Carvalho, A.P.L.; Mayer, M.P.A.; Gavini, G. Effect of ultrasonic activation on the reduction of bacteria and endotoxins in root canals: A randomized clinical trial. Int. Endod. J. 2018, 51 (Suppl. S1), e12–e22. [Google Scholar] [CrossRef]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef]
- Windley, W., 3rd; Teixeira, F.; Levin, L.; Sigurdsson, A.; Trope, M. Disinfection of immature teeth with a triple antibiotic paste. J. Endod. 2005, 31, 439–443. [Google Scholar] [CrossRef]
- Althumairy, R.I.; Teixeira, F.B.; Diogenes, A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J. Endod. 2014, 40, 521–525. [Google Scholar] [CrossRef]
- Estefan, B.S.; El Batouty, K.M.; Nagy, M.M.; Diogenes, A. Influence of Age and Apical Diameter on the Success of Endodontic Regeneration Procedures. J. Endod. 2016, 42, 1620–1625. [Google Scholar] [CrossRef]
- Wikström, A.A.-O.; Brundin, M.A.-O.; Lopes, M.F.; El Sayed, M.; Tsilingaridis, G.A.-O. What is the best long-term treatment modality for immature permanent teeth with pulp necrosis and apical periodontitis? Eur. Arch. Paediatr. Dent. 2021, 22, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A.; Ruparel, N.B. Regenerative Endodontic Procedures: Clinical Outcomes. Dent. Clin. N. Am. 2017, 61, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cao, Y.; Shin, S.J.; Shon, W.J.; Chugal, N.; Kim, R.H.; Kim, E.; Kang, M.K. Revascularization-associated Intracanal Calcification: Assessment of Prevalence and Contributing Factors. J. Endod. 2017, 43, 2025–2033. [Google Scholar] [CrossRef]
- Becerra, P.; Ricucci, D.; Loghin, S.; Gibbs, J.L.; Lin, L.M. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J. Endod. 2014, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Faras, H.; Corr, R.; Wright, K.R.; Shabahang, S. Histologic examinations of teeth treated with 2 scaffolds: A pilot animal investigation. J. Endod. 2014, 40, 515–520. [Google Scholar] [CrossRef]
- Galler, K.M.; Buchalla, W.; Hiller, K.A.; Federlin, M.; Eidt, A.; Schiefersteiner, M.; Schmalz, G. Influence of root canal disinfectants on growth factor release from dentin. J. Endod. 2015, 41, 363–368. [Google Scholar] [CrossRef]
- Fukushima, K.A.; Marques, M.M.; Tedesco, T.K.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.; Morimoto, S.; Moreira, M.S. Screening of hydrogel-based scaffolds for dental pulp regeneration-A systematic review. Arch. Oral Biol. 2019, 98, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.M.; Tawfik, H.E.; Hashem, A.A.; Abu-Seida, A.M. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J. Endod. 2014, 40, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal stem cells secretome: Current trends and future challenges. Neural Regen. Res. 2020, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Bhandi, S.; Alkahtani, A.; Mashyakhy, M.; Abumelha, A.S.; Albar, N.H.M.; Renugalakshmi, A.; Alkahtany, M.F.; Robaian, A.; Almeslet, A.S.; Patil, V.R.; et al. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J. Pers. Med. 2021, 11, 589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).