Abstract
Human zoonotic infection with Campylobacter is a major cause of gastroenteritis in the United States and worldwide. Listeria monocytogenes causes a potentially fatal infection in humans and is often attributed to contaminated food. Genetic typing has demonstrated that Campylobacter infection is often associated with the consumption of contaminated poultry products, with Campylobacter often colonizing the poultry gastrointestinal tract, while listeriosis is commonly associated with the consumption of contaminated ready-to-eat (RTE) foods. In this study, a strain of endospore-forming bacterium (strain NH) that is bactericidal towards the human food pathogens Campylobacter jejuni and Listeria monocytogenes was identified and characterized. Transwell experiments demonstrated that the bactericidal effect on both C. jejuni and L. monocytogenes is due to secretions from the spore former. These foodborne pathogens consistently exhibited 7 log reductions in growth when exposed to the NH cell-free culture filtrate. Sequencing of the 16s rRNA gene V4 variable region and analysis of the full-length 16s rRNA gene sequence from the WGS indicated that strain NH belongs to the species Aneurinibacillus aneurinilyticus. A microplate bioassay demonstrated that a bactericidal substance that is sensitive to protease could be collected from cell-free filtrates by salting out with ammonium sulfate. Gel filtration chromatography indicated a native molecular weight for the bactericidal protein of ca. 50 kDa, consistent with a class III bacteriocin. The active protein bound strongly to a cation-exchange resin and with an isoelectric point of ten, suggesting a positively charged protein. Both cation-exchange chromatography and isoelectric focusing indicated the enrichment of an 11 kDa protein on SDS-PAGE. This protein was identified through mass spectroscopy as the flgM protein, an anti-sigma factor. Analysis of whole genome sequencing (WGS) of the strain NH genome indicated the presence of a number of non-conservative amino acid substitutions in the flgM-gene-derived amino acid sequence of strain NH and A. aneurinilyticus compared to other members of the Aneurinibacillus genus. Further investigation is needed to determine whether these substitutions are correlated with the bactericidal activity. The identified strain may be useful as a feed additive for the pre-harvest control of Campylobacter jejuni in poultry.
1. Introduction
Campylobacter infection in humans (campylobacteriosis) is a major cause of foodborne illness and is the most frequently isolated foodborne bacterial pathogen from clinical samples in the US [1]. The number of campylobacteriosis cases in the US has been estimated at between 0.9 and 1.5 million per year, with greater than 8000 hospitalizations [2]. The monetary cost of Campylobacter infections in the US has been estimated at USD 1.75 billion per year [3,4]. Campylobacter infections commonly cause symptoms such as diarrhea and fever that resolve after about one week [5]. Although most cases of Campylobacter infection are not life-threatening, complications such as bacteremia, arthritis, and Guillain-Barré syndrome, a potentially life-threatening neurological condition, are known to occur, especially in patients with an immune deficiency or liver disease [5,6]. Enteritis caused by Campylobacter is a common risk factor for the development of irritable bowel syndrome (IBS). Infectious enteritis (IE) patients are four times more likely to develop IBS compared to those without IE [7]. Treatments for human campylobacteriosis include fluoroquinolone, macrolide, and aminoglycoside antibiotics, but resistance to all three has been reported [8]. Beta-lactam resistance in Campylobacter is common, with the addition of clavulanic acid and sulbactam as beta-lactamase inhibitors improving susceptibility but not completely eliminating it from foodborne isolates [9].
Campylobacter was previously thought to be strictly a commensal microbe of food animals but has more recently been implicated in animal disease as well as human disease. The experimental infection of broiler chickens with C. jejuni has been shown to elevate pro-inflammatory chemokine expression in the ceca and ileum and has been linked to intestinal villus damage [10]. In addition, Campylobacter jejuni and C. fetus subsp. fetus cause abortion in sheep [11], and C. hepaticus causes spotty liver disease in layer hens [12]. Treatments for Campylobacter in food animals include whole-cell attenuated vaccines in sheep, but these need to be species-specific to Campylobacter, and antibody titers are short-lived [11]. Other treatments in animals include chlortetracycline and tulathromycin [13], although Campylobacter resistance to chlortetracycline has occurred [14].
The consumption of poultry products is a major risk factor for campylobacteriosis, mainly in developed countries [15,16,17,18]. Campylobacter colonizes the poultry GI tract and is commonly present there in large numbers in broilers at slaughter age, ranging from log 6 to log 7 CFU/g of cecal content [10,19]. During evisceration, the contents of the ceca and other organs are released, contaminating equipment, working surfaces, water that is part of the process, and air, as well as contaminating the skin of the poultry carcass [20]. Although the subsequent washing and chilling of the carcass reduces bacteria counts, Campylobacter is often still present in a large proportion (85–91%) of carcasses after packaging [20]. A strong correlation exists between Campylobacter concentrations in the poultry intestinal tract and the concentration of Campylobacter on carcasses [21]. Thus, any interventions that reduce Campylobacter in the intestinal tract before the carcass is processed could reduce the spread of Campylobacter to carcass surfaces and parts and in turn reduce the incidence of campylobacteriosis.
There are three main pre-harvest intervention measures for the control of Campylobacter in poultry currently under development: vaccination, breeding for genetic resistance, and competitive exclusion [22]. Vaccination studies have demonstrated both mucosal and systemic humoral responses in Campylobacter-colonized poultry and anti-Campylobacter maternal antibodies [23], along with the development of subunit vaccines [24,25]. The vaccination of poultry with attenuated Salmonella-expressing Campylobacter outer-membrane proteins or epitopes resulted in multiple log reductions during a homologous Campylobacter challenge [24,26]. However, vaccines tend to be narrow in their efficacy, inhibiting only a few strains of a particular pathogen but not providing broader coverage for the entire spectrum of strains. In the case of Campylobacter, this may result from the known genetic heterogeneity of surface structures through the action of phase variation, and the consequent evasion of host immunity [27,28]. In addition, these vaccines are administered to poultry by oral gavage, which can be impractical for large-scale producers. Many of the experimental vaccines express a subunit of Campylobacter in live enteric bacteria through genetic modification, causing GMO regulatory difficulties. Breeding for genetic resistance to Campylobacter is confounded by the differences between individual poultry of the same breed, and essentially nothing is known at present about allelic variation that would be pertinent to Campylobacter resistance [22]. In competitive exclusion, defined or undefined mixtures of beneficial bacteria or prebiotics are orally administered to compete with Campylobacter for binding and/or resources in the poultry GI tract. The results following competitive exclusion have tended to be inconsistent, which may reflect the undefined nature of the agents or inadequate selective agents in the agar plate counting of Campylobacter [29]. Other potential treatments for Campylobacter are the application of short- and medium-chain fatty acids [30,31] or bacteriocins [32,33] to animal feeds.
Human Listeria monocytogenes infection (listeriosis) accounted for an estimated 14,169 illnesses and 3175 deaths globally in 2010 [34]. According to the FDA, there are about 1600 human cases of listeriosis and about 260 deaths from listeriosis each year in the US alone [35]. Although the global incidence of listeriosis is relatively low, it is nonetheless ranked as one of the most important foodborne illnesses due to its high fatality rate [36,37]. Human infection with Listeria monocytogenes can range from subclinical gastroenteritis to severe invasive disease, with three main clinical forms: (1) pregnancy-associated neonatal listeriosis; (2) septicemia; (3) central nervous system infection (meningitis). The majority of these clinical forms are of the septicemia type [38]. The treatment of human invasive L. monocytogenes is most effective with aminopenicillin or benzopenicillin. Treatment with meropenem has been associated with a higher mortality compared to treatment with these penicillins [39].
Listeriosis is frequently associated with refrigerated ready-to-eat (RTE) foods [15,16,17,18,40]. Common food associations for L. monocytogenes are RTE meats such as deli-sliced meats and those in prepacked sandwiches, cold-smoked fish, and prepared salads and fruits [38]. Recent US outbreaks include a 2020 outbreak across seventeen US states attributed to enoki mushrooms, and a 2023 outbreak across six states involving deli meat and cheese [41]. [Insert paragraph about Listeria monocytogenes, illness statistics, common food associations, etc.]
The US Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS) has ruled L. monocytogenes as an adulterant in ready-to-eat (RTE) products. The FSIS requires that producers of RTE products either test for Listeria species on food contact surfaces in the post-lethality processing environment or treat the product itself with a post-lethality antimicrobial treatment. Such a treatment must achieve at least a 1 log reduction in L. monocytogenes before the product leaves the plant; otherwise, the producer must establish the freezing of the product to suppress the growth of L. monocytogenes throughout the shelf life of the product [42]. Other important interventions include environmental monitoring programs within RTE food processing plants and physical barriers between the pre- and post-killing step at the processing plant [43]. A further Listeria intervention is the cautioning of vulnerable populations (women who are pregnant, those with immunocompromised conditions such as HIV, organ transplants, and cancer, and the elderly) to the high risks of Listeria in certain foods (such as unpasteurized milk, soft cheeses, and undercooked meats and fish) [44].
Aneurinibacillus aneurinilyticus is a relatively new species formed from the splitting of Bacillus brevis into two groups based on metabolic profiles, S-layer protein serology, hydrolysis of thiamine, and 16s rRNA gene sequence [45].
The aim of the present study was to test the hypothesis, based on preliminary observations, that an isolate from poultry feces, hereafter referred to as strain NH, controls the foodborne pathogen Campylobacter jejuni. Experiments were performed to characterize the antibacterial effect from this newly discovered strain, assigned via DNA sequencing to the species Aneurinibacillus aneurinilyticus. We also sought to assess its secretion of an antimicrobial protein into the culture medium, the chemical properties of this protein, and its bactericidal spectrum, which also impacts the effective control of the foodborne pathogen Listeria monocytogenes.
2. Materials and Methods
2.1. Bacterial Strains
The following strains were used in the competitive exclusion and bioassay experiments represented in this study. Campylobacter jejuni (NCTC 11168) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Listeria monocytogenes 1/2a (V7) was obtained from the US FDA and used in competitive exclusion and bioassay experiments. Salmonella enterica serovar Typhimurium 97 was received as a gift from Dr. B. Hargis, University of Arkansas, Fayetteville. Salmonella enterica serovars Heidelburg (ATCC 8326), Kentucky (1271-92), and Enteritidis (ATCC 13076) were provided by Dr. M. Slavik, University of Arkansas, Fayetteville. Escherichia coli O157:H7 (ATCC 43895) was obtained from Dr. S. Ricke, University of Wisconsin, Madison. Strain NH was originally isolated from poultry fecal material (fresh cecal dropping) obtained by the authors at the Milo Schult Agricultural Research station, University of Arkansas, Fayetteville. This strain is thus being described for the first time in the present publication. The cecal dropping was cultured as previously described [46]. An isolate from the culture was antagonistic to Campylobacter jejuni. Subsequent selection and isolation streaks of the culture on starch agar were performed as previously described [32]. The antibacterial spectrum of strain NH was tested against the above strains of Salmonella and E. coli, along with C. jejuni 81-176 (received as a gift from Dr. Young Min Kwon, University of Arkansas, Fayetteville).
2.2. Transwell and Competitive Exclusion Experiments
Transwell experiments were conducted based on the method presented by Stempler et al. [47]. Strain NH and C. jejuni were cultured in separate serum bottles, each containing 20 mL Bolton broth (EMD Millipore, Billerica, MA, USA), and placed in a 7 L Anaeropack chamber (Mitsubishi Gas Chemical, Tokyo, Japan). This was followed by the addition of pressurized microaerobic atmosphere (10% CO2, 5% O2, 85% N2) to the box through a custom-designed valve, with subsequent growth occurring for 18 h at 42 °C with 120 RPM orbital shaking, as described previously [46]. Briefly, the cultures were washed twice in maximum recovery diluent (MRD, 0.1% peptone, 0.85% NaCl, pH 7.0), enumerated by dilution at 1:2 with 10% sterile buffered formalin (Sigma), and counted with a thin-body Petroff-Hauser counter and a phase-contrast microscope at 1000×. The washed cultures were then added at a density of 1 × 106 cells/mL in 2 mL Bolton broth per chamber of wells in a 6-well transwell plate (Corning, Kennebunk, ME, USA). The cultures were either added to the same side or opposite sides of the transwell membranes, as described in Section 3. Control wells contained either strain NH or C. jejuni alone. The inability of strain NH to pass through the membrane of the transwell inner chamber was confirmed in the control wells. Transwell plates were placed inside a sealable bag and the bag was inflated with a microaerobic atmosphere (10% CO2, 5% O2, 85% N2). The inflated bag was placed inside a glass dish and placed in a rotating incubator at 42 °C with 120 RPM shaking. Each chamber of each well was sampled by the removal of 0.23 mL at 0, 10, 24, and 30 h post-inoculation. Removed samples were serially diluted with MRD and plated on modified charcoal cefoperazone deoxycholate agar (mCCDA) consisting of Blood Free Campylobacter Agar Base (HiMedia, Mumbai, India), 1.5% agar, and 33 μg/mL cefoperazone sodium, and on tryptic soy agar (TSA). Preliminary experiments confirmed that strain NH failed to grow on mCCDA under a microaerobic atmosphere, while C. jejuni failed to grow aerobically on TSA. The mCCDA plates under an microaerobic atmosphere were thus used to quantify the growth of C. jejuni in the transwells, while aerobic TSA plates were used to quantify the growth of strain NH in the transwells. Identical experiments were conducted to investigate the competitive exclusion of L. monocytogenes by strain NH, except that the medium for the growth of L. monocytogenes in transwells was tryptic soy broth (TSB, EMD Millipore, Billerica, MA, USA). The transwell plates, for L. monocytogenes, were incubated in sealable bags inflated with ambient air from an aquarium air pump, and the enumeration of L. monocytogenes was performed on Oxford medium (Hardy Diagnostics, Santa Maria, CA, USA) with Listeria monocytogenes selective supplement (Oxoid, Basingstoke, UK).
2.3. Gram Stain and Photomicroscopy
The Listeria monocytogenes cultures from the control (Listeria alone) and experimental (Listeria + strain NH cell-free culture fluid) wells of the transwell experiment described above were spread on glass microscope slides, air dried in a sterile biosafety cabinet, and Gram stained using a commercial kit (Aldon Corp., Avon, NY, USA), as instructed by the manufacturer. Photomicrographs were taken with a cell phone camera (Apple iPhone SE, Cupertino, CA) through the left ocular lens of a Nikon Labophot-2 compound microscope (Tokyo, Japan). A built-in reticle in the left ocular lens was captured in the photos and used to draw a 10-micrometer scale bar onto the image. Subsequently, the reticle was cropped out of the images.
2.4. Preparation of Strain NH Cell-Free Culture Supernatant Ammonium Sulfate (CFCSAS) Filtrate Dialysate
The preparation of cell-free culture supernatant was performed as described previously [48]. Briefly, strain NH was inoculated from a glycerol stock into 5 mL Bolton broth (Neogen, Lansing, MI, USA) and grown at 37 °C for 18 h with 150 RPM shaking. A 1 mL aliquot of this culture was added to each of two batches of 1 L of room temperature Bolton broth in 2 L flasks. These were then incubated at 37 °C with 230 RPM shaking for 24 h. The cultures was subsequently centrifuged at 8000× g for 15 min, and the culture supernatants was transferred to two sterile bottles. The supernatants were subsequently filtered through 0.2-micron vacuum filtration units (Corning, Kennebunk, ME, USA) and combined in one sterile beaker. The combined filtrate was tested for sterility by plating on TSA and found to have no detectable colony forming units. The filtrate volume was measured with a sterilized cylinder and transferred with a sterile magnetic stir bar into a sterilized beaker on a magnetic stir plate at 4 °C. Solid ammonium sulfate was added slowly to a final concentration of 60% as described previously [49]. The proteins were allowed to salt out of the ammonium sulphate at 4 °C overnight. The salted-out proteins were subsequently harvested by centrifugation at 10,000× g for 15 min at 4 °C. The precipitate was resuspended in approximately 1× the pellet volume of 50 mM acetate buffer at pH 5.0. The resuspension was subsequently added to dialysis tubing with a 3500 Dalton molecular weight cutoff and dialyzed against 4 L of 50 mM acetate buffer at pH 5.0 at 4 °C for 18 h, followed by second and third dialyses, each against 3 L 50 mM acetate buffer at pH 5.0 at 4 °C. The resulting dialysate was centrifuged at 8000× g for 5 min to remove insoluble material and stored at 3 °C for subsequent testing.
2.5. Batch Adsorption of the Active Protein from Cell-Free Filtrate Dialysate to Ion-Exchange Resins
A batch adsorption experiment was conducted to optimize conditions for ion-exchange chromatography, as described previously [50]. Briefly, a 0.5 mL aliquot of strain NH CFCSAS precipitate dialysate was placed in each of 10 ultrafiltration devices (Microcon-10, Merck-Millipore, Cork, Ireland), with a molecular weight cutoff of 10 kDa to exchange buffers. The retentates were resuspended to 0.5 mL in 50 mM test buffers of different pH values (succinate pH 4.5, acetate pH 5.0, succinate pH 5.5, MES pH 6.0, MES pH 6.5, phosphate pH 7.0, imidazole pH 7.0, Tris pH 7.5, Tris pH 8.0, and Tris pH 8.5). Six 0.5 mL aliquots of pre-charged SP-Sepharose cation-exchange resin (Cytivia, Uppsala, Sweden) were each equilibrated with 10 volumes of the above pH 4.5 through pH 7.0 buffers up to and including the phosphate pH 7.0 buffer. Four 0.5 mL aliquots of pre-charged Q-Sepharose (Cytivia), an anion exchanger, were similarly equilibrated in 10 volumes of the imidazole pH 7.0 through Tris pH 8.5 buffers. After buffer removal by centrifugation, the NH CFCSAS retentates were added to the equilibrated resins and mixed for 20 min on a rocking platform. The resins were then centrifuged briefly at 12,000× g, and supernatants were collected for analysis. The pellets were washed twice with the corresponding test buffers and subsequently resuspended in either 0.4 mL of 50 mM phosphate pH 7.0/1M NaCl (SP-Sepharose-bound samples) or 0.4 mL 50 mM Tris pH 7.5/1M NaCl (Q-Sepharose-bound samples) to elute the protein. They were then mixed for 10 min on a rocking platform. The supernatants were collected from the resins by centrifugation and desalted by ultrafiltration, which was followed by resuspension in 0.3 mL 50 mM Tris pH 7.5 buffer. The bioassay for bactericidal activity against C. jejuni was subsequently performed on all unbound and bound fractions.
2.6. Bioassay for Campylobacter or Listeria Bactericidal Activity
The dialysate and desalted batch adsorption fractions (as well as all subsequent column chromatography and isoelectric focusing fractions) were tested for their ability to interfere with the normal growth of Campylobacter jejuni or Listeria monocytogenes in a microplate assay, based on the method of O’Bryan et al. [51]. Briefly, a 0.1 mL aliquot of each fraction or dialysate was added to an equal volume of Bolton broth (Campylobacter) or TSB (Listeria) in the first row (Row A) of a sterile microtiter plate (Greiner, Frickenhausen, Germany). The wells in the remaining rows received 0.1 mL sterile Bolton broth or TSB. The Row A mixture was mixed by pipetting up and down 6 times, and 0.1 mL was subsequently transferred to Row B. The process was repeated to obtain a serial 1:2 dilution of the dialysate or fraction down the plate. After serial dilution, each well received 20 μL of a washed C. jejuni NCTC 11168 or L. monocytogenes V7 culture. Positive control wells contained C. jejuni or L. monocytogenes, 0.1 mL medium, and 0.1 mL 50 mM Tris pH 7.5 buffer, but no dialysate or fraction. Negative control wells contained 0.1 mL medium and 0.1 mL 50 mM Tris pH 7.5 buffer, but no C. jejuni or L. monocytogenes. The microtiter plate was placed in a zip-lock bag and the bag was inflated with a microaerobic atmosphere (gas mix containing 10% CO2, 5% O2, and 85% N2). The bag with the plate was placed inside a glass tray and the tray was placed on a rotating incubator (New Brunswick Scientific, Edison, NJ, USA) at 42 °C with 120 RPM shaking for 18 h. The plate was subsequently removed from the bag, and the 600 nm optical absorbance of each well was recorded with a microplate reader (Tecan Infinite-200, Tecan, Salzburg, Austria). The levels of optical absorbance of the negative and positive control wells were compared to experimental wells to determine the end point dilutions for dialysate and the fractions. Identical experiments were performed with L. monocytogenes, except that the plate was incubated at 37 °C and an aquarium air pump (Topfin-4000, Topfin, Vietnam), pumping ambient air, was used in place of the microaerobic gas mix to inflate the bag.
2.7. Cation-Exchange Chromatography of Cell-Free Filtrate Dialysate
Cation-exchange chromatography of the strain NH CFCSAS filtrate dialysate was performed as described previously [50] and based on the results of the aforementioned batch adsorption of active protein (Section 2.5). Briefly, a 30 mL SP-sepharose column was equilibrated with acetate buffer at pH 5.0. A 50 mL aliquot of cell-free filtrate dialysate was applied to the top of the column and pumped at a flow rate of 0.44 mL/minute using a peristaltic pump (Dynamax RP-1, Rainin, San Diego, CA, USA), with additional acetate buffer at pH 5.0. After washing the column with 2 column volumes of acetate buffer, proteins were eluted from the column with a 0–500 mM NaCl isocratic salt gradient using a gradient former (Amersham, Piscataway, NJ, USA) in 50 mM acetate pH 5.0 buffer. Forty-four-drop fractions were collected using a fraction collector (BioRad, Hercules, CA, USA) from the column throughout the washing and elution steps and stored at 4 °C.
2.8. Isoelectric Focusing
The active protein peak from cation-exchange chromatography, identified via the bioassay (Section 2.6) of desalted protein peaks (Microcon-10, Merck-Millipore, Cork, Ireland), was further purified by isoelectric focusing on a MicroRotofor device (BioRad, Hercules, CA, USA), as described by the manufacturer. Briefly, broad-range ampholytes (BioRad, Hercules, CA, USA) were mixed with the desalted active protein peak to a final concentration of 1.5% and applied to the MicroRotofor. After 1 h of focusing, all ten fractions from the MicroRotofor were collected and desalted (Microcon-10, Merck-Millipore). Each fraction was tested for bactericidal activity against C. jejuni or L. monocytogenes, as described above (Section 2.6).
2.9. Species Determination of Strain NH
Genomic DNA was extracted from strain NH using the PureLink Genomic DNA Extraction Mini kit (Invitrogen, Carlsbad, CA, USA) as instructed by the manufacturer. Index primers A701 and A501 [52] and the low-error-rate Phusion polymerase (New England Biolabs, Ipswich, MA, USA) were used to amplify the V4 variable region of the 16s ribosomal RNA gene. The resulting PCR product was purified with the DNA Clean and Concentrator Kit, as instructed by the manufacturer (Zymo Research, Irvine, CA, USA), and sequenced (Eurofins, Louisville, KY, USA) with the same primers, A701 and A501 [52]. The resulting DNA sequence was compared to the nonredundant DNA database of the National Center for Biotechnology Information (NCBI) using the BLASTN algorithm. The sequence was also used as a query sequence to search the RDP classifier database of Michigan State University. The full-length 16s rRNA gene sequence of strain NH was obtained through whole-genome sequencing (University of Illinois Genomics Institute, Urbana, IL, USA) and also queried against the NCBI nonredundant DNA database with the BLASTN algorithm. A MAFFT multiple-sequence alignment and a phylogenetic tree were constructed with MegAlign Pro software, v 17.3.1 (11) (DNAStar, Madison, WI, USA, 2022).
2.10. Mass Spectroscopy of SDS-PAGE Protein Band
2.10.1. Trypsin Digestion for Protein Identification
The gel bands were cut into small pieces (1 mm2) and washed with 1 mL of 25 mM ammonium bicarbonate for 30 min. Gel segments were destained with 25 mM ammonium bicarbonate, prepared in 50:50 H2O:acetonitrile for 60 min, followed by dehydration with 100% acetonitrile for 15 min. Excess acetonitrile was discarded, and the gel pieces were fully dried in a centrivap concentrator/evaporator (Labconco, Kansas City, MO, USA). The gel pieces were treated to reduce protein disulfide bonds with 10 mM dithiothreitol (DTT) and 25 mM ammonium bicarbonate for 60 min at 55 °C. The reducing solution was discarded, and 55 mM iodoacetamide in 25 mM ammonium bicarbonate was added. Then, the mixture was incubated for 60 min in the dark at room temperature to carbamidomethylate the cysteines. The gel pieces were washed with 25 mM ammonium bicarbonate before being dehydrated with acetonitrile for 15 min. The gel pieces were then dried again using the centrivap concentrator/evaporator. A sufficient 10 ng/µL of MS-grade trypsin in 25 mM ammonium bicarbonate was added to just cover the gel piece, then this was incubated at 4 °C for 30 min to allow the trypsin to penetrate into the gel pieces. An additional 200 uL of 25 mM ammonium bicarbonate was added, which was followed by incubation overnight at 37 ℃. The supernatant was transferred into a new microfuge tube. The gel pieces were resuspended in 5% formic acid with 60% acetonitrile and vortexed, and the supernatant was combined with the previous supernatant. This solution was dried using the centrivap system, reconstituted in 50 µL of 0.1% formic acid, and then subjected to proteomic analysis using liquid chromatography with tandem mass spectrometry (LC-MS/MS).
2.10.2. Mass Spectrometry Analysis for Proteomics
Tryptic peptides were analyzed using an Agilent 1200 series micro-flow high-performance liquid chromatography (HPLC) system (Agilent Technologies, Santa Clara, CA, USA) attached to a Bruker Amazon SL quadrupole ion trap mass spectrometer to perform LC-MS/MS (Bruker Daltoniks Inc., Billerica, MA, USA) with a captive spray ionization source, as has been previously described [53]. Peptides were separated by reverse-phase high-performance liquid chromatography (RP-HPLC) using a Zorbax SB C18 column (150 × 0.3 mm, 3.5 μm particle size, 300 Å pore size (Agilent, Santa Clara, CA, USA). The solvents were 5%–40% gradient of 0.1% formic acid (Solvent A) and acetonitrile in 0.1% formic acid (solvent B), with a flow rate of 4 μL/min over a duration of 300 min.
The default peak picking method for complex protein digest in the Bruker Compass DataAnalysis v 4.2 software (Build 383.1) (Bruker Daltonic GmbH, Billerica, MA, USA, 2013) was used to generate peak lists from LC-MS/MS chromatogram from the Mascot database search using the MASCOT v 2.2 search engine (Matrix Science, London, UK, 2021). Parent ion and fragment ion mass tolerances were set at 0.6 Da with cysteine carbamidomethyl fixed modification and methionine oxidation as variable modifications. The Mascot search was performed against the Aneurinibacillus aneurinilyticus proteins downloaded from uniport.org. Proteins discovered with a 95% confidence limit were further filtered by considering the false discovery rate (FDR), as calculated during the search by searching the reverse sequence database. Only proteins with an FDR of less than 5% were used.
2.11. Statistical Analysis
Means were compared using single-factor ANOVA and post-hoc two-tailed Student’s t-tests in Microsoft Excel v. 2308 (Microsoft, Redmond, WA, USA, 2023).
3. Results
3.1. Competitive Exclusion in Transwell Culture Plates
Early preliminary experiments indicated that the supernatant from the strain NH culture was effective at inhibiting the growth of Campylobacter jejuni and Listeria monocytogenes by up to 9 log (CFU/mL), while Salmonella enterica serovars Typhimurium, Enteritidis, Kentucky, Heidelburg, and Escherichia coli STEC serotype O157:H7 exhibited minimal inhibition by strain NH (ca. 1 log CFU/mL). A method was needed to determine whether the inhibition of C. jejuni and L. monocytogenes was actively caused by an antagonistic substance secreted from NH cells or merely a depletion of nutrients. Transwells are six-well culture plates that contain 0.4-micrometer pore size filters at the bottom of the inserts that fit inside each well. These filters were employed to indicate whether the inhibitory activity was due to a substance that was secreted from NH cells and whether it could cross a 0.4-micrometer membrane. Both L. monocytogenes and C. jejuni growth were inhibited when strain NH was inoculated in the same well, regardless of whether or not NH was inoculated into the same side of the membrane as the pathogens or was inoculated on the side of the membrane separated from the pathogens by the membrane. In control wells, strain NH cells were inoculated and grown alone in the inner chamber and were unable to cross the 0.4 µm membrane to the outer chamber (Figure 1C). These results indicated that strain NH secretes an inhibitory substance that can cross a 0.4-micrometer membrane and significantly reduce populations of C. jejuni and L. monocytogenes. A bactericidal effect of this substance on L. monocytogenes cells was further evident from the very marked lysis of L. monocytogenes cells co-cultured either on the same side of the membrane as strain NH or separated from NH by the membrane (Figure 2).
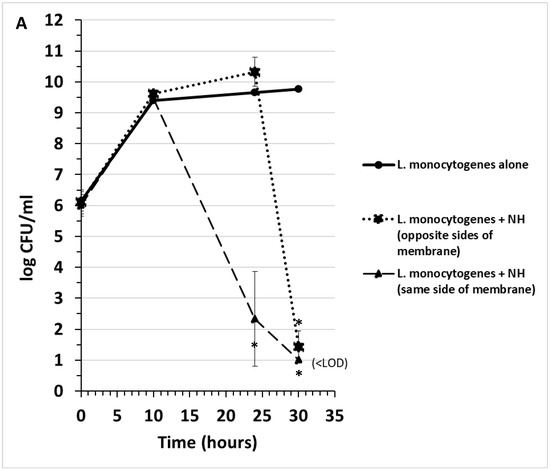
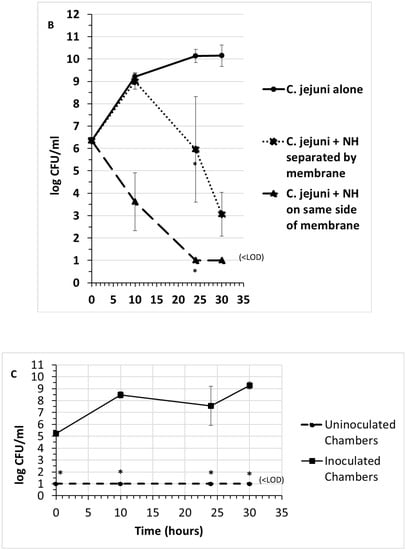
Figure 1.
Competitive exclusion in transwells of Listeria monocytogenes (A) and Campylobacter jejuni (B) by strain NH. When strain NH is grown in the transwell, it cannot cross the membrane of the transwell (C). “<LOD” = none detected, below limit of detection (10 CFU/mL). Data points and brackets represent the mean and standard deviation, respectively, of three independent experiments. Asterisks represent significantly different means at timepoints indicated compared to control cultures (pathogen alone or strain NH alone) (p < 0.05).
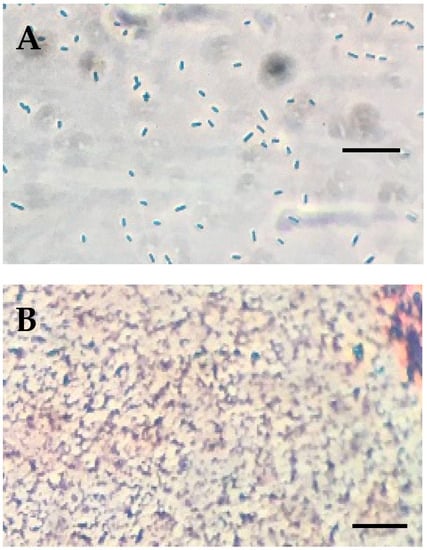
Figure 2.
Gram stain of Listeria monocytogenes cells from transwell not exposed to cell-free NH culture fluid (A), exhibiting normal appearance, and from transwell exposed to cell-free NH culture fluid (B), exhibiting the lysis of Listeria cells. Scale lines represent ten micrometers.
3.2. Characterization of the Size of the Antagonistic Substance and Correlation of Bactericidal Activity with Protein Concentration
Preliminary experiments indicated that the bactericidal substance was protease-sensitive, consistent with it being a protein. The inhibitory substance was therefore further characterized by collecting the cell-free culture supernatant by vacuum filtration through 0.2-micrometer vacuum filters, followed by ammonium sulfate precipitation. Preliminary experiments indicated that salting out by the addition of crystalline, proteomics-grade ammonium sulfate to a 60% final concentration precipitated essentially all of the bactericidal activity. This precipitate was dialyzed three times, which was followed by ultrafiltration. The flow-through and retentate fractions from differently sized ultrafiltration devices were then tested for bactericidal activity by measuring the inhibitory endpoint in microtiter plate dilutions of the fractions (Figure 3A) and by comparing the specific activity of the fractions (Figure 3B), calculated as the endpoint dilution factor divided by the mg of protein present. These experiments indicated that the bactericidal substance was present in the retentate from the 10 kDa cutoff ultrafiltration device but was not present in the filtrate from this same device (Figure 3A), suggesting that the substance exceeds 10 kDa in size. Further, the specific activity of the bactericidal substance was found to be directly proportional to the protein concentration in each fraction, which is consistent with the hypothesis that the substance is a protein (Figure 3B).
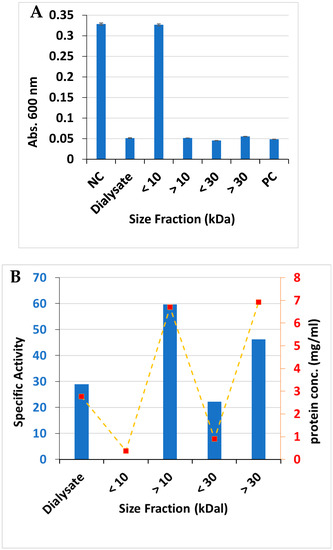
Figure 3.
(A) Campylobacter jejuni growth on culture filtrate dialysate and differently sized fractions obtained by ultrafiltration, n = 3. (B) Specific activity (endpoint dilution factor/mg protein) and protein concentration of ultrafiltration fractions. NC = C. jejuni growth in the absence of any protein fraction. PC = media + buffer alone (no C. jejuni).
The size of the bactericidal protein was further investigated via gel filtration chromatography. The strain NH cell-free culture supernatant ammonium sulfate (CFCSAS) precipitate dialysate was loaded on a Biogel P60 gel filtration column that had been calibrated with size standards the previous day. An active fraction consistently eluted from the P60 columns at a very similar cumulative elution volume in two independent experiments (Figure 4B). The molecular weight was calculated from the calibration curve and found to be approximately 49 ± 2 kDa.
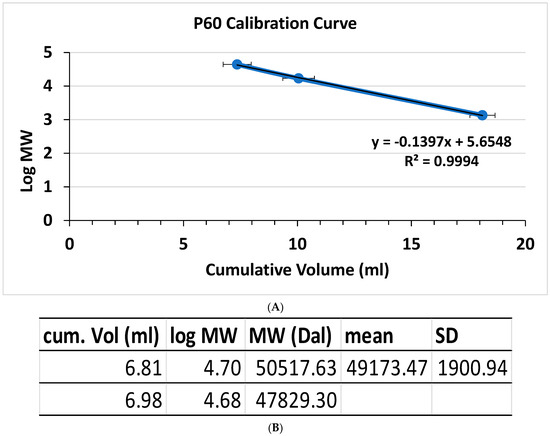
Figure 4.
Gel filtration chromatography of the strain NH CFCSAS precipitate dialysate. (A) Size standards: 1 = chicken ovalbumin (44 kDa); 2 = horse myoglobin (17 kDa); 3 = vitamin B12 (1.35 kDa). n = 3. (B) Bactericidal fraction cumulative volumes and corresponding mean and SD for the molecular weight, n = 2.
3.3. Batch Adsorption of the Active Protein from Cell-Free Filtrate Dialysate to Ion-Exchange Resins
The strain NH CFCSAS precipitate dialysate was tested for binding to ion-exchange resins at 10 different pH values, as described in the materials and methods. These experiments indicated that the bactericidal protein bound strongly to a cation-exchange resin at an acidic pH, but relatively poorly to an anion-exchange resin at all pH values (Figure 5). This information was subsequently used to partially purify the bactericidal protein via cation-exchange chromatography.
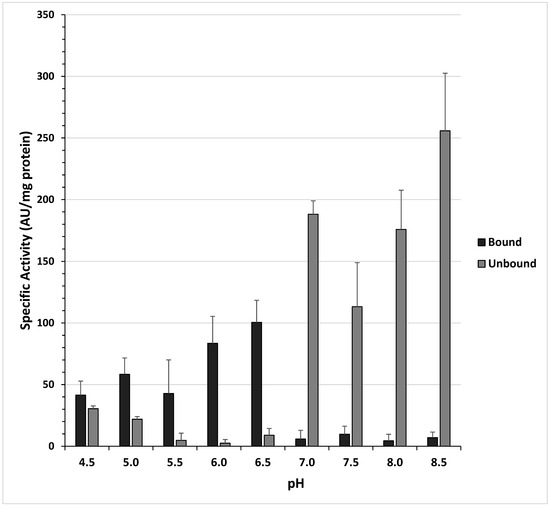
Figure 5.
Batch adsorption of anti-Campylobacter jejuni bactericidal activity to ion-exchange resins at various pH values. Activity units (AU) = reciprocal of the endpoint dilution (greatest dilution inhibitory to C. jejuni) per mL of fraction tested. Bars and brackets represent the mean and standard error of three independent experiments, respectively.
3.4. Partial Purification of the Bactericidal Protein via Cation-Exchange Chromatography and Isoelectric Focusing
The strain NH CFCSAS precipitate dialysate, equilibrated in 50 mM acetate buffer at pH 5.0, was loaded onto an equilibrated 30 mL SP-Sepharose column. Following washing of nonspecific material off of the column, bound proteins were eluted from the column with an isocratic 0–500 mM NaCl gradient in 50 mM acetate at pH 5.0. Fractions from the column elution were quantified by BCA protein assay. Protein peak fractions (Figure 6A) were pooled and concentrated by ultrafiltration (with a cutoff of 10 kDa). Each protein peak was then tested for bactericidal activity against C. jejuni (Figure 6B). Inactive and active peaks were then compared by SDS-PAGE to see if any protein bands correlated with the bactericidal activity (Figure 6C). An 11 kDa band on SDS-PAGE appeared to be enriched in the active peak and absent or very diminished in protein peaks that were found to be inactive in the bactericidal activity assay. Separation of active peak proteins by isoelectric focusing (Figure 7 and Figure 8) and bactericidal activity assay of focused fractions confirmed the association between the presence of the 11 kDa band and bactericidal activity against both C. jejuni and L. monocytogenes.
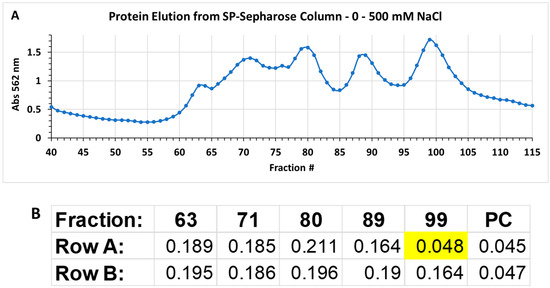
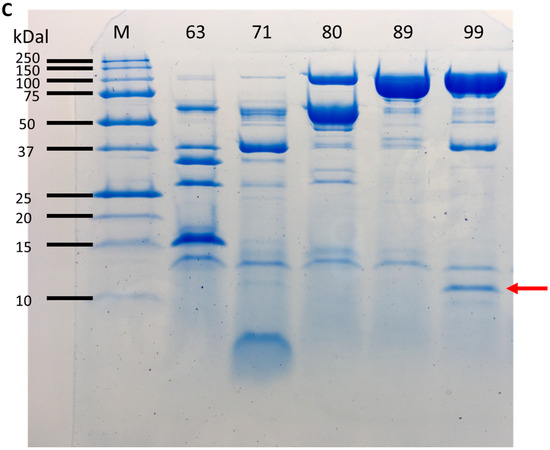
Figure 6.
(A) Protein elution from the cation-exchange column; (B) Bioassay on peak protein fractions (63, 71, 80, 89, and 99) from elution of cation exchange column. Numbers in cells of rows A and B are absorbance at 600 nm of C. jejuni cells, and proportional to C. jejuni growth. Inhibition of growth by active peak 99 is indicated by yellow highlighting. PC: positive control well lacking Campylobacter jejuni; (C) SDS–PAGE with 10 µg/lane of protein peaks from the cation exchange column. Arrow points to enriched protein band in the active peak, fraction 99.
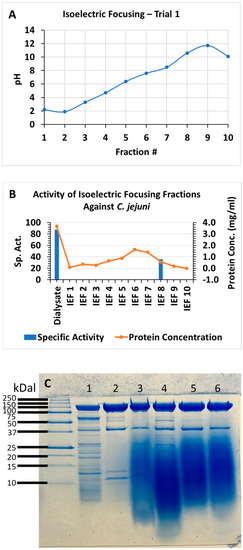
Figure 7.
Isoelectric focusing of protein peak active against C. jejuni from cation exchange column, trial 1. (A) pH of fractions, trial 1; (B) bioassay, trial 1; (C) SDS–PAGE trial 1: dialysate (lane 1), active peak from cation-exchange column and isoelectric focusing fractions 8, 5, 6, and 7, with each lane loaded with 10 micrograms protein.
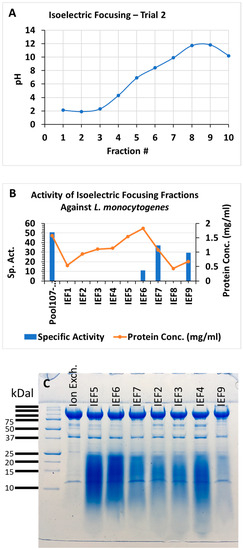
Figure 8.
Isoelectric focusing of protein peak active against L. monocytogenes from cation-exchange column, trial 2. (A) pH of fractions, trial 2; (B) bioassay trial 2; (C) SDS–PAGE trial 2: active peak from cation-exchange column and IEF fractions 5, 6, 7, 2, 3, 4, and 9, with each lane loaded with 10 micrograms protein.
3.5. Mass Spectrometric Identification of the SDS-PAGE Protein Band
The 11 kDa protein band correlating with bactericidal activity was cut out of the SDS-PAGE gel and analyzed via mass spectrometry. Out of the 28 total mass spectra, 54 of the 86 amino acids of a flagellin regulatory protein were covered by the spectra, totaling a 63% coverage of this protein by the mass spectra. This analysis indicated a probability of 100% for the flgM protein of Aneurinibacillus aneurinilyticus ATCC 12856, which has a predicted molecular weight of 10 kDa.
3.6. Identification of Strain NH as Aneurinibacillus aneurinilyticus
In order to assign a species identity to strain NH, we amplified the NH genomic DNA with PCR primers previously described in the literature [52] that bound to conserved sequences flanking the V4 region of the 16s rRNA gene. This PCR product was DNA-sequenced, and the resulting sequence was used as the query sequence to search the RDP classifier database of Michigan State University, and also in a Smith–Waterman local alignment to a strain NH whole-genome sequence. The RDP database result indicated that NH is a member of the genus Aneurinibacillus. The Smith–Waterman alignment identified the corresponding region in the NH whole-genome sequence. Comparison of the surrounding sequence to the published 16s rRNA gene [54] allowed us to obtain the entire 16s rRNA gene sequence of NH. This full-length 16s sequence was subsequently used as the query sequence to search for matching sequences using the BLAST algorithm search of all non-redundant nucleotide sequences in the NCBI database. The resulting BLAST search revealed a percentage nucleotide sequence identity between Aneurinibacillus aneurinilyticus strain Murayama (accession no. NR_036798) and NH of 99.9%. This value exceeds the 98.7% cutoff established by Stackebrandt [55] for bacterial species identification. Lastly, a MAFFT multiple-sequence alignment and a phylogenetic tree were constructed (Figure S1). The heat map and phylogenetic tree both indicated that NH is highly related to species Aneurinibacillus aneurinilyticus and diverges from species A. migulanus. We conclude that strain NH is a new strain of Aneurinibacillus aneurinilyticus. Thus, we propose that strain NH be called “Aneurinibacillus aneurinilyticus strain NH”. From these results, one would predict that other A. aneurinilyticus strains should exhibit the same bactericidal effect on C. jejuni and L. monocytogenes. This was indeed found to be the case for CFCSAS precipitate dialysate of A. aneurinilyticus strain ATCC 12856 when partially purified by cation-exchange chromatography.
3.7. Analysis of the flgM Gene of A. aneurinibacillus Strain NH
The flgM gene of A. aneurinibacillus strain NH was identified by conducting a Smith–Waterman local alignment of the NH whole-genome sequence with the flgM gene coding sequence of A. aneurinibacillus strain XH2 (GenBank accession no. CP014140). The resulting alignment (Figure S2) had no gaps, a reasonably high alignment score (193), and 74.2% nucleotide identity. Translation of the NH flgM gene was performed to obtain the derived amino acid sequence. This sequence was then compared to that of flgM proteins of several species from the Aneurinibacillus genus (Figure S3). Although the NH strain’s FlgM protein is highly related to that of species Aneurinibacillus. aneurinilyticus (98% amino acid identity), these two species exhibit several non-conservative substitutions from the consensus sequence compiled by the alignment algorithm (Figure S3). These substitutions are H8Q, T39N, E51T, Q64K, and I76D.
4. Discussion
In the present study, we have identified a strain of A. aneurinilyticus (strain NH) that is highly effective in controlling the human foodborne pathogens C. jejuni and L. monocytogenes through the secretion of one of more substances, including a relatively high molecular weight component of 50 kDa (Figure 3 and Figure 4). Bacteriocins (bactericidal peptides and proteins) have been extensively investigated in lactic acid bacteria and Bacillus species. Three classes of bacteriocins have been described [56]. Class I includes peptides that undergo post-translational modifications. This class includes lanthionine-containing antibiotic peptides (“lantibiotics”). Lanthionine is an amino acid that results from post-translational cross-linking of alanine residues via a thioether linkage. Other common post-translational modifications present in Class I bacteriocins are the unsaturated amino acids 2,3-didehydroalanine (Dha) and 2,3-didehydrobutyrine (Dhb), derived from the action of serine/threonine dehydratase and addition of thioether linkages via internal cysteine residues. These modifications introduce internal cyclic thioether structures to the peptide. Class I bacteriocins are <5 kDa in size, and examples include nisin, subtilin, and epidermin, produced by L. lactis, B. subtilis, and S. epidermis, respectively [57]. Class II bacteriocins (<10 kDa) are non-modified, linear peptides that are heat and pH stable. Three subclasses of class II have been identified based on conserved amino acid motifs near the N-terminus. Subclass II.1 includes pediocin-like proteins such as coagulin (B. coagulans) [58] and the Paenibacillus polymyxa bacteriocins SRCAM 37 and similar proteins [32] with a conserved YGNGVXC domain. Subclass II.2 includes the thuricin group of Bacillus thuringiensis and B. cereus, containing a conserved DWTXWSXL motif. Subclass II.3 includes the lichenicidins (B. licheniformis), cereins, ericins, mersacidin, and haloduracins, and lacks conserved domains. Class III bacteriocins (>30 kDa) are large, heat-labile bacteriocins with phospholipase activity, such as the megacins (B. megaterium) [59,60,61]. The NH strain’s 50 kDa active protein (Figure 4) is consistent with a class III bacteriocin, but further purification and analysis are needed to check for phospholipase activity.
The mode of action of strain NH appears to be the lysis of the Listeria cells (Figure 2) and is thus bactericidal. Campylobacter jejuni fails to grow back in the presence of the NH CFCSAS precipitate dialysate, even after many days of microaerobic incubation, despite the presence of fresh growth media in these assays. Thus, the mode of action is likely bactericidal against C. jejuni, as well.
A previous report estimated that reducing Campylobacter in the poultry cecum by as little as 3 log CFU/g could reduce the risk of human disease by as much as 90–100% [62]. Given the very substantial 7 log reductions in C. jejuni resulting from exposure to strain NH culture filtrate (Figure 1), if the activity against Campylobacter exhibited by strain NH can be harnessed, this could represent an effective control strategy against this pathogen. To the best of our knowledge, this study is the first to demonstrate that A. aneurinilyticus is bactericidal towards human or foodborne pathogens, specifically C. jejuni and L. monocytogenes. However, the role of Aneurinibacillus spp. in the control of plant pathogens has been previously reported. An A. aneurinilyticus isolate that was shown to be an endophyte of Ocimum sanctum plants was found to inhibit the mycelia of the fungal pathogens Rhizoctonia solani, Fusarium oxysporium, and Phythia aphanidermatum. The A. aneurinilyticus isolate has also exhibited siderophore production and induction of systemic resistance in pea plants [63], including significant elevation of phenylalanine ammonia lyase (PAL), an enzyme involved in the production of phenolic metabolites such as flavonoids, isoflavonoids, and lignins [64]. A marine Aneurinibacillus isolate was found to produce a surfactant inhibitory to Escherichia coli, Klebsiella pneumoniae, Vibrio cholerae, Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus aureus. Based on GC-MS analysis, the lipopeptide consists of stearic acid methyl ester and four amino acids (serine, threonine, tyrosine, and valine) [65]. Aneurinibacillus migulanus strain Nagano is effective in the inhibition of a wide range of fungal plant pathogens, including Botrytis cinerea (Ascomycota), Phaeolus schweinitzii (Basidiomycota), and numerous Phytophthora species (Oomycetes). The authors used prediction algorithms such as ANTISMASH to identify gene clusters encoding bacteriocins, microcins, NRPS, lassopeptides, siderophores, and chitinases within the genome of A. migulanus strain Nagano and other species within the Aneurinibacillus genus [66].
The possible role of the flgM protein in the A. aneurinilyticus antagonism of Listeria monocytogenes and Campylobacter jejuni requires further investigation. Our attempts at flgM gene knockout in A. aneurinilyticus are ongoing and will be published separately. This gene has been known for many years to function in the regulation of flagellum assembly in Salmonella typhimurium. The flagellum is composed of a hook-basal body assembly and a filament. The hook-basal body, which includes a type III export apparatus, is comprised of class 2 gene products whose expression precedes the filament assembly, the latter being comprised of class 3 gene products. Class 3 genes are positively activated by fliA, encoding sigma factor 28, itself a class 2 gene product. FlgM negatively regulates class 3 gene expression by binding to and inhibiting sigma 28 [67,68]. This negative regulatory function is controlled by modulating the intracellular level of flgM upon the completion of the flagellar hook structure through the expulsion of flgM through the hook export apparatus [69,70]. FlgM export has also been demonstrated in Bacillus subtilis but is proteolytically degraded in the extracellular medium [71]. In contrast, we found the flgM protein to be stable in the A. aneurinibacillus extracellular medium and enriched in the active protein peak eluting from cation-exchange chromatography (Figure 6). When this peak was further separated via isoelectric focusing, the focused fractions with greater bactericidal activity also correlated with higher levels (more intense staining) of the flgM protein (Figure 7 and Figure 8) with equal (10 microgram) protein loading.
In addition to its role in flagellar assembly, flgM also functions in the control of autolysins, enzymes that digest the cell wall of the bacteria that produce them in order to remodel the wall for growth and cell division [72,73,74]. We speculate that the non-conservative amino acid substitutions identified here (Figure S3) in the strain NH and A. aneurinilyticus flgM sequences compared to other species may modify its role in autolysin regulation, perhaps contributing to the lysis of competing bacteria such as that of L. monocytogenes, as shown here (Figure 2).
5. Conclusions
We conclude that, in the present study, a new strain of the species Aneurinibacillus aneurinilyticus (A. aneurinilyticus strain NH) has been isolated and shown to secrete into the culture medium a bactericidal activity effective against Campylobacter jejuni and Listeria monocytogenes. This activity was found to be sensitive to protease, consistent with a protein, and is a relatively large 50 kDa molecule that may represent a new class III bacteriocin. However, more studies are needed to verify whether this molecule has phospholipase activity, consistent with known class III bacteriocins. This will require the purification of the molecule to homogeneity. On denaturing gels, the activity was correlated with the appearance of an 11 kDa protein. This protein was identified as flgM, a known regulator of flagellar assembly and autolysins.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app131810257/s1, Figure S1: (A) Heat map showing the pairwise % nucleotide sequence identities between the full-length 16s rRNA genes of strain NH and several Aneurinibacillus genus members (red) and the phylogenetic distance (blue) between these same sequences. (B) Phylogenetic tree showing the relative distance of these sequences; Figure S2: Smith–Waterman local alignment of flgM gene from Aneurinibacillus aneurinilyticus strain XH2 and the whole-genome sequence of strain NH; Figure S3: Protein sequence alignment of strain NH FlgM protein sequence with the FlgM of several Aneurinibacillus species. WP_021620954 = A. aneurinilyticus (line 2) and NH exhibit several non-conservative substitutions in comparison to the consensus sequence. These substitutions are H8Q, T39N, E51T, Q64K, and I76D. WP_043067642 = A. migulanus FlgM; WP_146810313 = A. danicus FlgM; MBN6186964 = Aneurinibacillus sp. BA2021; WP_220559155 = A. thermoaerophilus FlgM; flgM_XH2_transl = Aneurinibacillus.
Author Contributions
Conceptualization, J.L.; Investigation, P.M.R. and R.L.; Writing—review & editing, J.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by the Arkansas Biosciences Institute and the Arkansas Agricultural Experiment Station through the University of Arkansas System Division of Agriculture and the Hatch Program of the National Institute of Food and Agriculture, US Department of Agriculture. The funding organizations did not contribute to the design of the study or the resulting manuscript. Rohana Liyanage and Jackson O. Lay Jr. would like to acknowledge the statewide mass spectrometry facility and the COBRE grant NIH P30 GM103450.
Conflicts of Interest
The authors declare no conflict of interest in the current study.
References
- Tack, D.M.; Marder, E.P.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Hurd, S.; Scallan, E.; Lathrop, S.; Muse, A.; Ryan, P.; et al. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2015–2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, J.M.; Matthew Kuhlmann, F.; Sheikh, A. Acute Bacterial Gastroenteritis. Gastroenterol. Clin. N. Am. 2021, 50, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Batz, M.B.; Hoffmann, S.; Morris, J.G. Ranking the Disease Burden of 14 Pathogens in Food Sources in the United States Using Attribution Data from Outbreak Investigations and Expert Elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Batz, M.B.; Morris, J.G. Annual Cost of Illness and Quality-Adjusted Life Year Losses in the United States Due to 14 Foodborne Pathogens. J. Food Prot. 2012, 75, 1292–1302. [Google Scholar] [CrossRef]
- Acheson, D.; Allos, B.M. Campylobacter jejuni Infections: Update on Emerging Issues and Trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cruz, A.; Muñoz, P.; Mohedano, R.; Valerio, M.; Marín, M.; Alcalá, L.; Rodriguez-Créixems, M.; Cercenado, E.; Bouza, E. Campylobacter Bacteremia: Clinical Characteristics, Incidence, and Outcome over 23 Years. Medicine 2010, 89, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Klem, F.; Wadhwa, A.; Prokop, L.J.; Sundt, W.J.; Farrugia, G.; Camilleri, M.; Singh, S.; Grover, M. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome after Infectious Enteritis: A Systematic Review and Meta-Analysis. Gastroenterology 2017, 152, 1042–1054.e1. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and Alternative Strategies for the Prevention, Control, and Treatment of Antibiotic-Resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Casagrande Proietti, P.; Guelfi, G.; Bellucci, S.; De Luca, S.; Di Gregorio, S.; Pieramati, C.; Franciosini, M.P. Beta-Lactam Resistance in Campylobacter coli and Campylobacter jejuni Chicken Isolates and the Association between BlaOXA-61 Gene Expression and the Action of β-Lactamase Inhibitors. Vet. Microbiol. 2020, 241, 108553. [Google Scholar] [CrossRef]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni Is Not Merely a Commensal in Commercial Broiler Chickens and Affects Bird Welfare. mBio 2014, 5, e01364-14. [Google Scholar] [CrossRef]
- Menzies, P.I. Vaccination Programs for Reproductive Disorders of Small Ruminants. Anim. Reprod. Sci. 2012, 130, 162–172. [Google Scholar] [CrossRef]
- Courtice, J.M.; Mahdi, L.K.; Groves, P.J.; Kotiw, M. Spotty Liver Disease: A Review of an Ongoing Challenge in Commercial Free-Range Egg Production. Vet. Microbiol. 2018, 227, 112–118. [Google Scholar] [CrossRef]
- Yaeger, M.J.; Wu, Z.; Plummer, P.J.; Sahin, O.; Ocal, M.M.; Beyi, A.F.; Xu, C.; Zhang, Q.; Griffith, R.W. Experimental Evaluation of Tulathromycin as a Treatment for Campylobacter jejuni Abortion in Pregnant Ewes. Am. J. Vet. Res. 2020, 81, 205–209. [Google Scholar] [CrossRef]
- Sahin, O.; Plummer, P.J.; Jordan, D.M.; Sulaj, K.; Pereira, S.; Robbe-Austerman, S.; Wang, L.; Yaeger, M.J.; Hoffman, L.J.; Zhang, Q. Emergence of a Tetracycline-Resistant Campylobacter jejuni Clone Associated with Outbreaks of Ovine Abortion in the United States. J. Clin. Microbiol. 2008, 46, 1663–1671. [Google Scholar] [CrossRef]
- Friedman, C.R.; Hoekstra, R.M.; Samuel, M.; Marcus, R.; Bender, J.; Shiferaw, B.; Reddy, S.; Ahuja, S.D.; Helfrick, D.L.; Hardnett, F.; et al. Risk Factors for Sporadic Campylobacter Infection in the United States: A Case-Control Study in FoodNet Sites. Clin. Infect. Dis. 2004, 38, S285–S296. [Google Scholar] [CrossRef] [PubMed]
- Mossong, J.; Mughini-Gras, L.; Penny, C.; Devaux, A.; Olinger, C.; Losch, S.; Cauchie, H.-M.; van Pelt, W.; Ragimbeau, C. Human Campylobacteriosis in Luxembourg, 2010–2013: A Case-Control Study Combined with Multilocus Sequence Typing for Source Attribution and Risk Factor Analysis. Sci. Rep. 2016, 6, 20939. [Google Scholar] [CrossRef] [PubMed]
- Ravel, A.; Hurst, M.; Petrica, N.; David, J.; Mutschall, S.K.; Pintar, K.; Taboada, E.N.; Pollari, F. Source Attribution of Human Campylobacteriosis at the Point of Exposure by Combining Comparative Exposure Assessment and Subtype Comparison Based on Comparative Genomic Fingerprinting. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef]
- Rosner, B.M.; Schielke, A.; Didelot, X.; Kops, F.; Breidenbach, J.; Willrich, N.; Gölz, G.; Alter, T.; Stingl, K.; Josenhans, C.; et al. A Combined Case-Control and Molecular Source Attribution Study of Human Campylobacter Infections in Germany, 2011–2014. Sci. Rep. 2017, 7, 5139. [Google Scholar] [CrossRef]
- Bily, L.; Petton, J.; Lalande, F.; Rouxel, S.; Denis, M.; Chemaly, M.; Salivat, G.; Fravalo, P. Quantitative and Qualitative Evaluation of Campylobacter Spp. Contamination of Turkey Cecal Contents and Carcasses during and Following the Slaughtering Process. J. Food Prot. 2010, 73, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Jacobs-Reitsma, W.; Lyhs, U.; Wagenaar, J. Campylobacter in the Food Supply. In Campylobacter, 2nd ed.; American Society of Microbiology: Washington, DC, USA, 2008; pp. 627–644. [Google Scholar]
- Rosenquist, H.; Sommer, H.M.; Nielsen, N.L.; Christensen, B.B. The Effect of Slaughter Operations on the Contamination of Chicken Carcasses with Thermotolerant Campylobacter. Int. J. Food Microbiol. 2006, 108, 226–232. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; Jacobs-Reitsma, W.; Hofshagen, M.; Newell, D. Poultry Colonization with Campylobacter and Its Control at the Primary Production Level. In Campylobacter, 3rd ed.; American Society of Microbiology: Washington, DC, USA, 2008; pp. 667–678. [Google Scholar]
- Sahin, O.; Zhang, Q.; Meitzler, J.C.; Harr, B.S.; Morishita, T.Y.; Mohan, R. Prevalence, Antigenic Specificity, and Bactericidal Activity of Poultry Anti-Campylobacter Maternal Antibodies. Appl. Environ. Microbiol. 2001, 67, 3951. [Google Scholar] [CrossRef]
- Wyszyńska, A.; Raczko, A.; Lis, M.; Jagusztyn-Krynicka, E.K. Oral Immunization of Chickens with Avirulent Salmonella Vaccine Strain Carrying C. jejuni 72Dz/92 CjaA Gene Elicits Specific Humoral Immune Response Associated with Protection against Challenge with Wild-Type Campylobacter. Vaccine 2004, 22, 1379–1389. [Google Scholar] [CrossRef]
- Nothaft, H.; Davis, B.; Lock, Y.Y.; Perez-Munoz, M.E.; Vinogradov, E.; Walter, J.; Coros, C.; Szymanski, C.M. Engineering the Campylobacter jejuni N-Glycan to Create an Effective Chicken Vaccine. Sci. Rep. 2016, 6, 26511. [Google Scholar] [CrossRef] [PubMed]
- Layton, S.L.; Morgan, M.J.; Cole, K.; Kwon, Y.M.; Donoghue, D.J.; Hargis, B.M.; Pumford, N.R. Evaluation of Salmonella-Vectored Campylobacter Peptide Epitopes for Reduction of Campylobacter jejuni in Broiler Chickens. Clin. Vaccine Immunol. 2011, 18, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Wanford, J.J.; Lango-Scholey, L.; Nothaft, H.; Hu, Y.; Szymanski, C.M.; Bayliss, C.D. Random Sorting of Campylobacter jejuni Phase Variants Due to a Narrow Bottleneck during Colonization of Broiler Chickens. Microbiology 2018, 164, 896–907. [Google Scholar] [CrossRef]
- Cayrou, C.; Barratt, N.A.; Ketley, J.M.; Bayliss, C.D. Phase Variation during Host Colonization and Invasion by Campylobacter jejuni and Other Campylobacter Species. Front. Microbiol. 2021, 12, 705139. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Jang, M.J.; Kim, S.Y.; Yang, Y.; Pavlidis, H.O.; Ricke, S.C. Potential for Prebiotics as Feed Additives to Limit Foodborne Campylobacter Establishment in the Poultry Gastrointestinal Tract. Front. Microbiol. 2019, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Solis de los Santos, F.; Hume, M.; Venkitanarayanan, K.; Donoghue, A.M.; Hanning, I.; Slavik, M.F.; Aguiar, V.F.; Metcalf, J.H.; Reyes-Herrera, I.; Blore, P.J.; et al. Caprylic Acid Reduces Enteric Campylobacter Colonization in Market-Aged Broiler Chickens but Does Not Appear to Alter Cecal Microbial Populations. J. Food Prot. 2010, 73, 251–257. [Google Scholar] [CrossRef]
- Guyard-Nicodème, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sánchez, J.; Vesseur, P.; Martín, Á.; Medel, P.; et al. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef]
- Svetoch, E.A.; Stern, N.J.; Eruslanov, B.V.; Kovolev, Y.N.; VOLODINA, L.I.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Pokhilenko, V.D.; Borzenkov, V.N.; et al. Isolation of Bacillus circulans and Paenibacillus polymyxa Strains Inhibitory to Campylobacter jejuni and Characterization of Associated Bacteriocins. J. Food Prot. 2005, 68, 11–17. [Google Scholar] [CrossRef]
- Stern, N.J.; Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Pokhilenko, V.D.; Levchuk, V.P.; Svetoch, O.E.; Seal, B.S. Isolation of a Lactobacillus salivarius Strain and Purification of Its Bacteriocin, Which Is Inhibitory to Campylobacter jejuni in the Chicken Gastrointestinal System. Antimicrob. Agents Chemother. 2006, 50, 3111–3116. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- US FDA. Get the Facts about Listeria. 2020. Available online: https://www.fda.gov/animal-veterinary/animal-health-literacy/get-facts-about-listeria#statistics (accessed on 3 September 2023).
- Scallan, E.; Hoekstra, R.M.; Mahon, B.E.; Jones, T.F.; Griffin, P.M. An Assessment of the Human Health Impact of Seven Leading Foodborne Pathogens in the United States Using Disability Adjusted Life Years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef]
- FAO. Listeria Monocytogenes in Ready-to-Eat (RTE) Foods: Attribution, Characterization, and Monitoring; Microbiological Risk Assessment Series, No. 38; FAO and WHO: Rome, Italy, 2022. [Google Scholar]
- Koopmans, M.; Brouwer, M.; Vazquez-Boland, J.; van de Beek, D. Human Listeriosis. Clin. Microbiol. Rev. 2023, 36, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Thonnings, S.; Knudsen, J.D.; Schonheyder, H.C.; Sogaard, M.; Arpi, M.; Gradel, K.O.; Østergaard, C.; Østergaard, C.; Arpi, M.; Gradel, K.O.; et al. Antibiotic Treatment and Mortality in Patients with Listeria monocytogenes Meningitis or Bacteraemia. Clin. Microbiol. Infect. 2016, 22, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.C.; Collins, J.P.; Griffin, P.M.; Shah, H.J.; Boyle, M.M.; Cieslak, P.R.; Dunn, J.; Lathrop, S.; McGuire, S.; Rissman, T.; et al. Decreased Incidence of Infections Caused by Pathogens Transmitted Commonly Through Food During the COVID-19 Pandemic—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2017-2020. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- CDC. Listeria (Listeriosis). 2023. Available online: https://www.cdc.gov/listeria/outbreaks/deli-11-22/ (accessed on 3 September 2023).
- USDA FSIS. FSIS Directive 10,240.4. 2022. Available online: https://www.fsis.usda.gov/policy/fsis-directives/10240.4 (accessed on 3 September 2023).
- Spanu, C.; Jordan, K. Listeria monocytogenes Environmental Sampling Program in Ready-to-Eat Processing Facilities: A Practical Approach. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2843–2861. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Verwiel, Y.; Bahri Khomami, M.; Roseboom, T.J.; Painter, R.C. Nutrition and Listeriosis during Pregnancy: A Systematic Review. J. Nutr. Sci. 2018, 7, e25. [Google Scholar] [CrossRef]
- Shida, O.; Takagi, H.; Kadowaki, K.; Komagata, K. Proposal for Two New Genera, Brevibacillus Gen. Nov. and Aneurinibacillus Gen. Nov. Int. J. Syst. Evol. Microbiol. 1996, 46, 939–946. [Google Scholar] [CrossRef]
- Rubinelli, P.M.; Kim, S.A.; Park, S.H.; Roto, S.M.; Nealon, N.J.; Ryan, E.P.; Ricke, S.C. Differential Effects of Rice Bran Cultivars to Limit Salmonella Typhimurium in Chicken Cecal in vitro Incubations and Impact on the Cecal Microbiome and Metabolome. PLoS ONE 2017, 12, e0185002. [Google Scholar] [CrossRef]
- Stempler, O.; Baidya, A.K.; Bhattacharya, S.; Malli Mohan, G.B.; Tzipilevich, E.; Sinai, L.; Mamou, G.; Ben-Yehuda, S. Interspecies Nutrient Extraction and Toxin Delivery between Bacteria. Nat. Commun. 2017, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Khochamit, N.; Siripornadulsil, S.; Sukon, P.; Siripornadulsil, W. Antibacterial Activity and Genotypic–Phenotypic Characteristics of Bacteriocin-Producing Bacillus subtilis KKU213: Potential as a Probiotic Strain. Microbiol. Res. 2015, 170, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Duong-Ly, K.C.; Gabelli, S.B. Salting out of Proteins Using Ammonium Sulfate Precipitation. In Laboratory Methods in Enzymology: Protein Part C; Lorsch, J., Ed.; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2014; Volume 541, pp. 85–94. [Google Scholar]
- Jungbauer, A.; Hahn, R. Ion-Exchange Chromatography. In Guide to Protein Purification; Methods in Enzymology; Academic Press: Oxford, UK, 2009; Volume 463, pp. 349–371. [Google Scholar]
- O’Bryan, C.A.; Hemminger, C.L.; Rubinelli, P.M.; Koo, O.K.; Story, R.S.; Crandall, P.G.; Ricke, S.C. The Efficacy of a Commercial Antimicrobial for Inhibiting Salmonella in Pet Food. Agric. Food Anal. Bacteriol. 2015, 5, 65–72. [Google Scholar]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Liyanage, R.; Gupta, C.; Lay, J.O.; Pereira, A.; Rojas, C.M. The Arabidopsis Proteins AtNHR2A and AtNHR2B Are Multi-Functional Proteins Integrating Plant Immunity with Other Biological Processes. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Baker, G.C.; Smith, J.J.; Cowan, D.A. Review and Re-Analysis of Domain-Specific 16S Primers. J. Microbiol. Methods 2003, 55, 541–555. [Google Scholar] [CrossRef]
- Stackebrandt, E. Taxonomic Parameters Revisited: Tarnished Gold Standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
- Abriouel, H.; Franz, C.M.A.P.; Omar, N.B.; Gálvez, A. Diversity and Applications of Bacillus Bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef]
- Chatterjee, C.; Paul, M.; Xie, L.; van der Donk, W.A. Biosynthesis and Mode of Action of Lantibiotics. Chem. Rev. 2005, 105, 633–684. [Google Scholar] [CrossRef]
- LeMarrec, C.; Hyronimus, B.; Bressollier, P.; Verneuil, B.; Urdaci, M.C. Biochemical and Genetic Characterization of Coagulin, a New Antilisterial Bacteriocin in the Pediocin Family of Bacteriocins, Produced by Bacillus coagulans I4. Appl. Environ. Microbiol. 2000, 66, 5213–5220. [Google Scholar] [CrossRef]
- Ozaki, M.; Higashi, Y.; Saito, H.; An, T.; Amano, T. Identity of Megacin A with Phospholipase A. Biken J. 1966, 9, 201–213. [Google Scholar]
- Von Tersch, M.A.; Carlton, B.C. Bacteriocin from Bacillus megaterium ATCC 19213: Comparative Studies with Megacin A-216. J. Bacteriol. 1983, 155, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Balikó, G.; Csorba, A.; Tungalag, C.; Medzihradszky, K.; Alföldi, L. Cloning and Characterization of the DNA Region Responsible for Megacin A-216 Production in Bacillus megaterium 216. J. Bacteriol. 2008, 190, 6448–6457. [Google Scholar] [CrossRef] [PubMed]
- Romero-Barrios, P.; Hempen, M.; Messens, W.; Stella, P.; Hugas, M. Quantitative Microbiological Risk Assessment (QMRA) of Food-Borne Zoonoses at the European Level. Food Control 2013, 29, 343–349. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S.; Sharma, S. Decoding the Plant Growth Promotion and Antagonistic Potential of Bacterial Endophytes from Ocimum sanctum L. against Root Rot Pathogen Fusarium oxysporum in Pisum sativum. Front. Plant Sci. 2022, 13, 813686. [Google Scholar] [CrossRef]
- Solekha, R.; Susanto, F.A.; Joko, T.; Nuringtyas, T.R.; Purwestri, Y.A. Phenylalanine Ammonia Lyase (PAL) Contributes to the Resistance of Black Rice against Xanthomonas oryzae Pv. oryzae. J. Plant Pathol. 2020, 102, 359–365. [Google Scholar] [CrossRef]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Aneurinifactin, a New Lipopeptide Biosurfactant Produced by a Marine Aneurinibacillus aneurinilyticus SBP-11 Isolated from Gulf of Mannar: Purification, Characterization and Its Biological Evaluation. Microbiol. Res. 2017, 194, 1–9. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Rekik, I.; Bełka, M.; Ibrahim, A.F.; Luptakova, L.; Jaspars, M.; Woodward, S.; Belbahri, L. Strain-Level Diversity of Secondary Metabolism in the Biocontrol Species Aneurinibacillus Migulanus. Microbiol. Res. 2016, 182, 116–124. [Google Scholar] [CrossRef]
- Gillen, K.L.; Hughes, K.T. Molecular Characterization of flgM, a Gene Encoding a Negative Regulator of Flagellin Synthesis in Salmonella Typhimurium. J. Bacteriol. 1991, 173, 6453–6459. [Google Scholar] [CrossRef][Green Version]
- Ohnishi, K.; Kutsukake, K.; Suzuki, H.; Lino, T. A Novel Transcriptional Regulation Mechanism in the Flagellar Regulon of Salmonella Typhimurium: An Anti-Sigma Factor Inhibits the Activity of the Flagellum-Specific Sigma Factor, ΣF. Mol. Microbiol. 1992, 6, 3149–3157. [Google Scholar] [CrossRef]
- Hughes, K.T.; Gillen, K.L.; Semon, M.J.; Karlinsey, J.E. Sensing Structural Intermediates in Bacterial Flagellar Assembly by Export of a Negative Regulator. Science 1993, 262, 1277–1280. [Google Scholar] [CrossRef]
- Kutsukake, K. Excretion of the Anti-Sigma Factor through a Flagellar Substructure Couples Flagellar Gene Expression with Flagellar Assembly in Salmonella Typhimurium. Mol. Gen. Genet. MGG 1994, 243, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R.A.; Kearns, D.B. FlgM Is Secreted by the Flagellar Export Apparatus in Bacillus subtilis. J. Bacteriol. 2015, 197, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Helmann, J.D.; Márquez, L.M.; Chamberlin, M.J. Cloning, Sequencing, and Disruption of the Bacillus subtilis Sigma 28 Gene. J. Bacteriol. 1988, 170, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Magaña, L.M.; Chamberlin, M.J. Characterization of the SigD Transcription Unit of Bacillus subtilis. J. Bacteriol. 1994, 176, 2427–2434. [Google Scholar] [CrossRef]
- Van Tilburg, A.Y.; Fülleborn, J.A.; Reder, A.; Völker, U.; Stülke, J.; van Heel, A.J.; Kuipers, O.P. Unchaining Mini Bacillus Strain PG10: Relief of FlgM-Mediated Repression of Autolysin Genes. Appl. Environ. Microbiol. 2021, 87, e01123-21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).