Controllable Friction of an Epoxy Composite via Thermal Treatment
Abstract
:1. Introduction
2. Materials and Methods
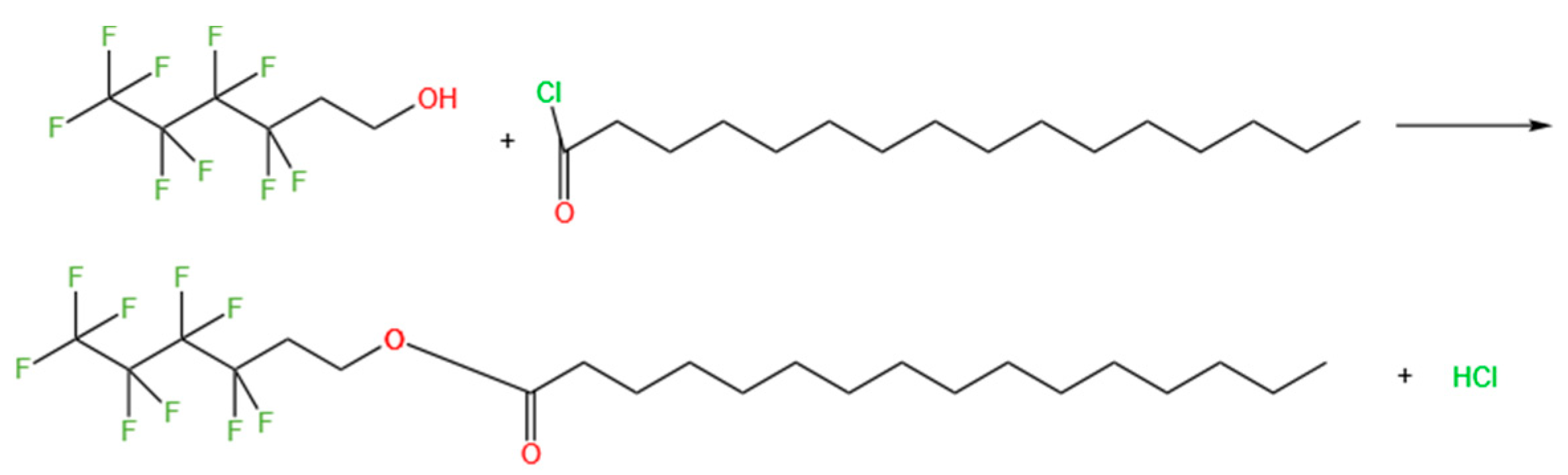
2.1. Preparation of 1H, 1H, 2H, 2H-Perfluorohexyl Hexadecanoate
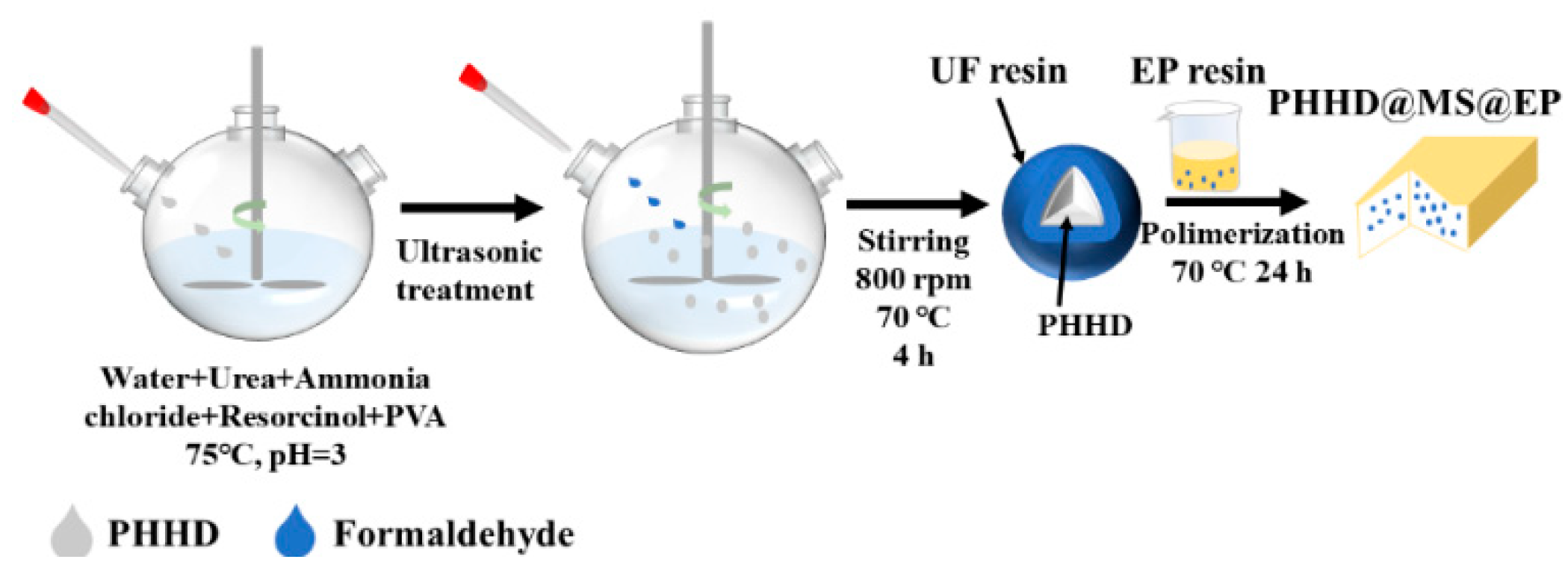
2.2. Preparation of Microcapsules
2.3. Preparation of Epoxy Composites
2.4. Friction Test of EP Composites
2.5. Durability Test of PHHD@MS@EP
2.6. Characterization
3. Results and Discussion
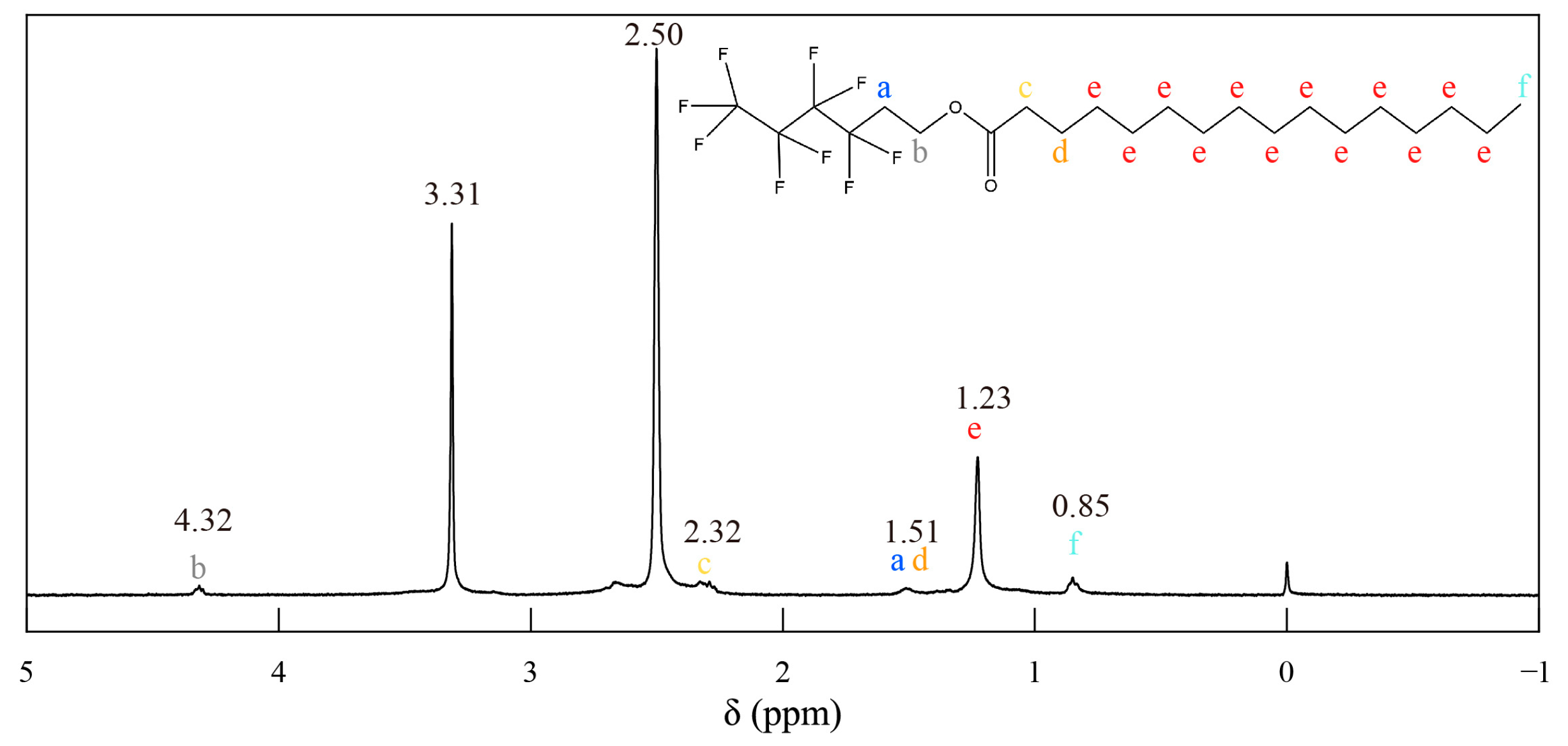
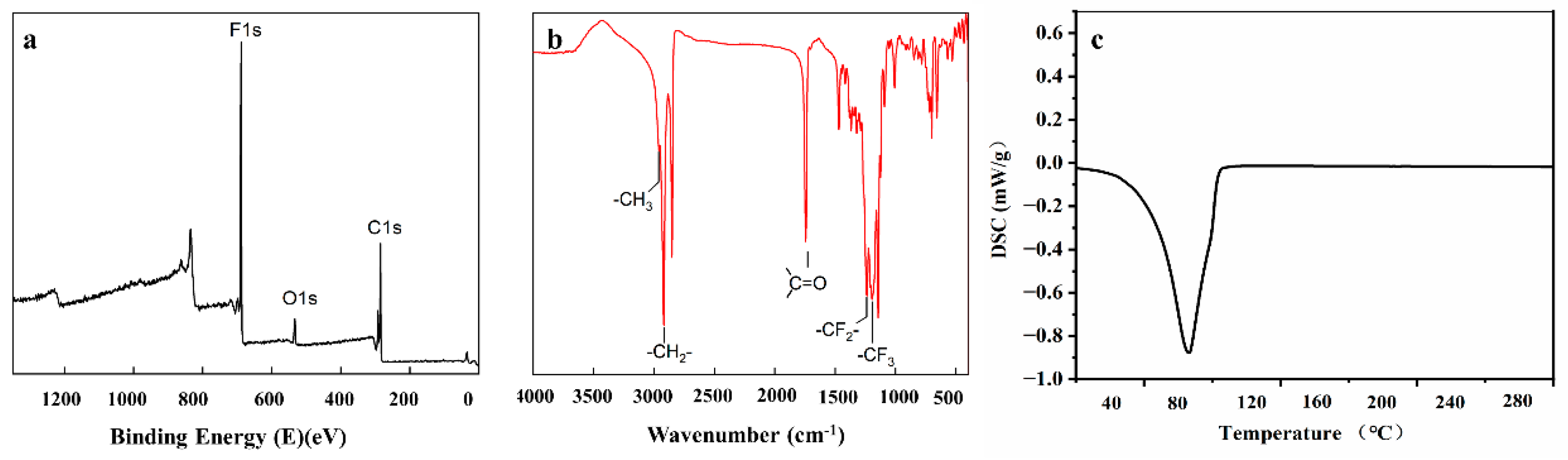
3.1. Fabrication and Characterization of PHHD
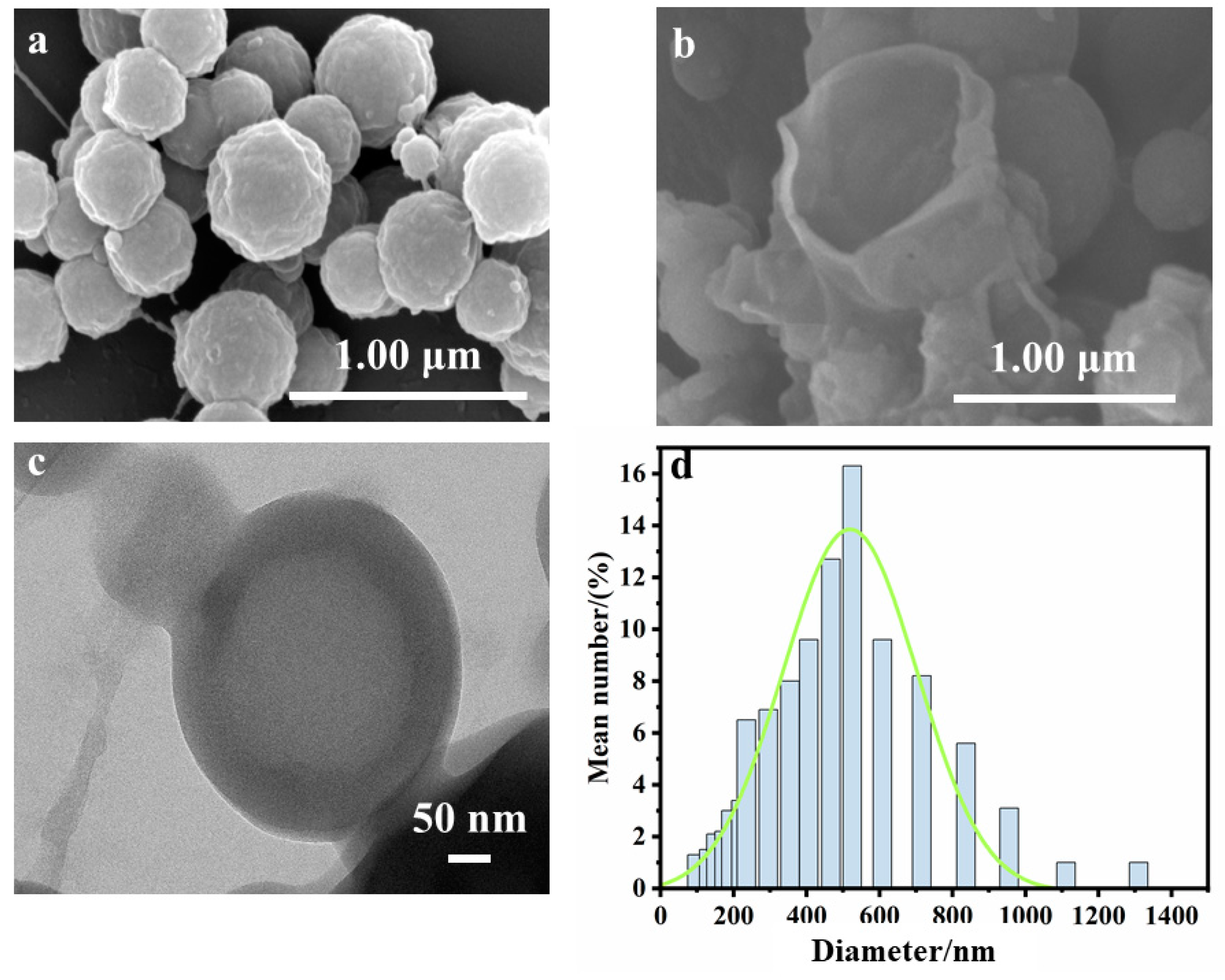
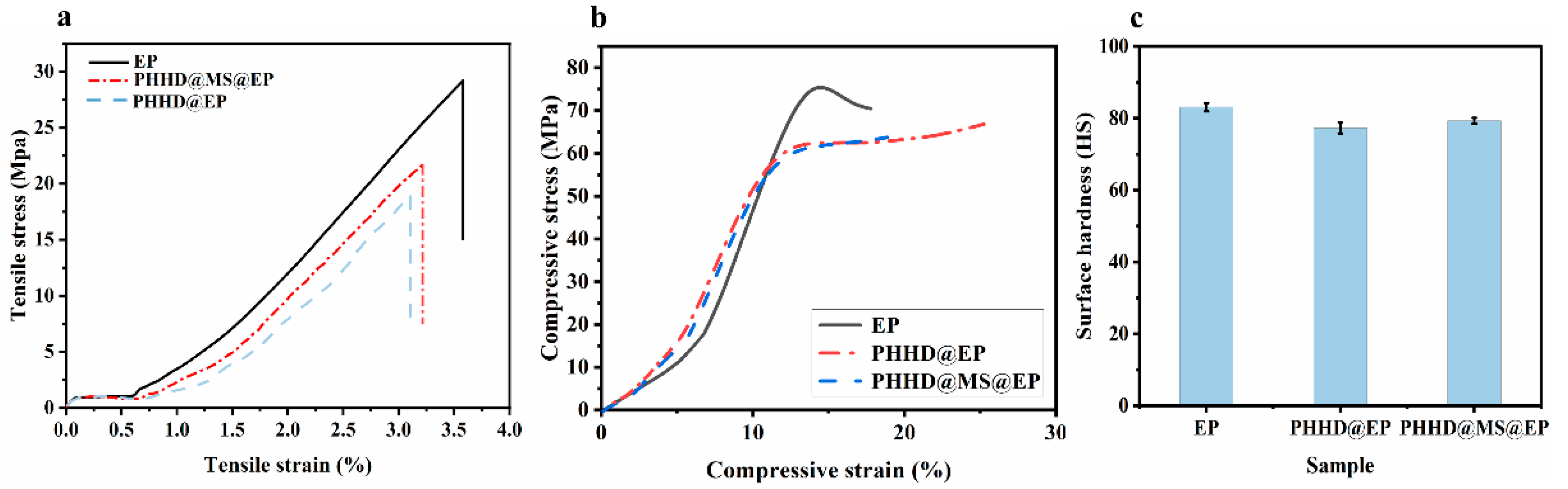
3.2. Fabrication and Characterization of Microcapsules and EP Composites
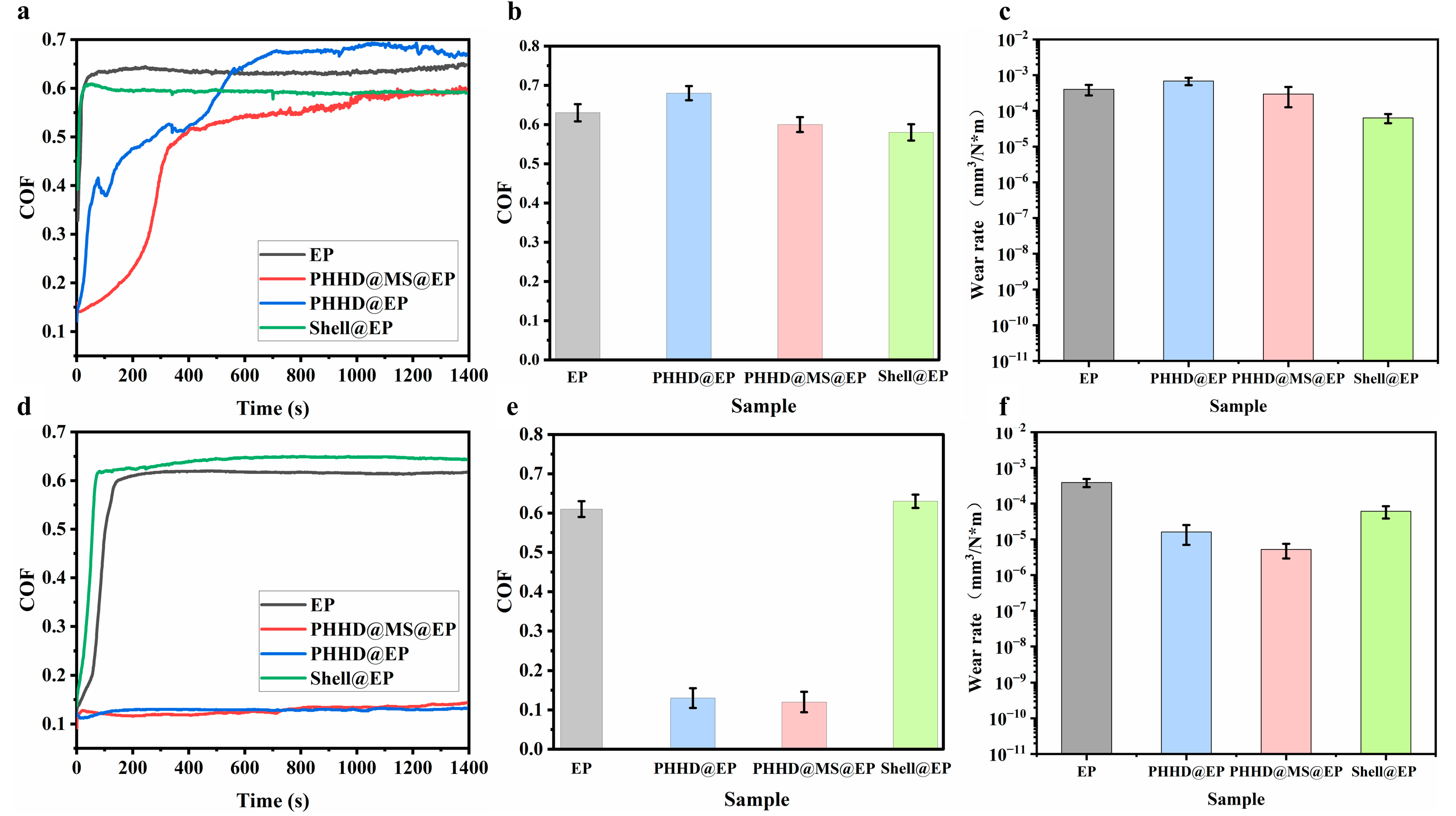
3.3. Friction and Wear Tests of EP Composites
3.4. Friction and Wear Test of PHHD@MS@EP
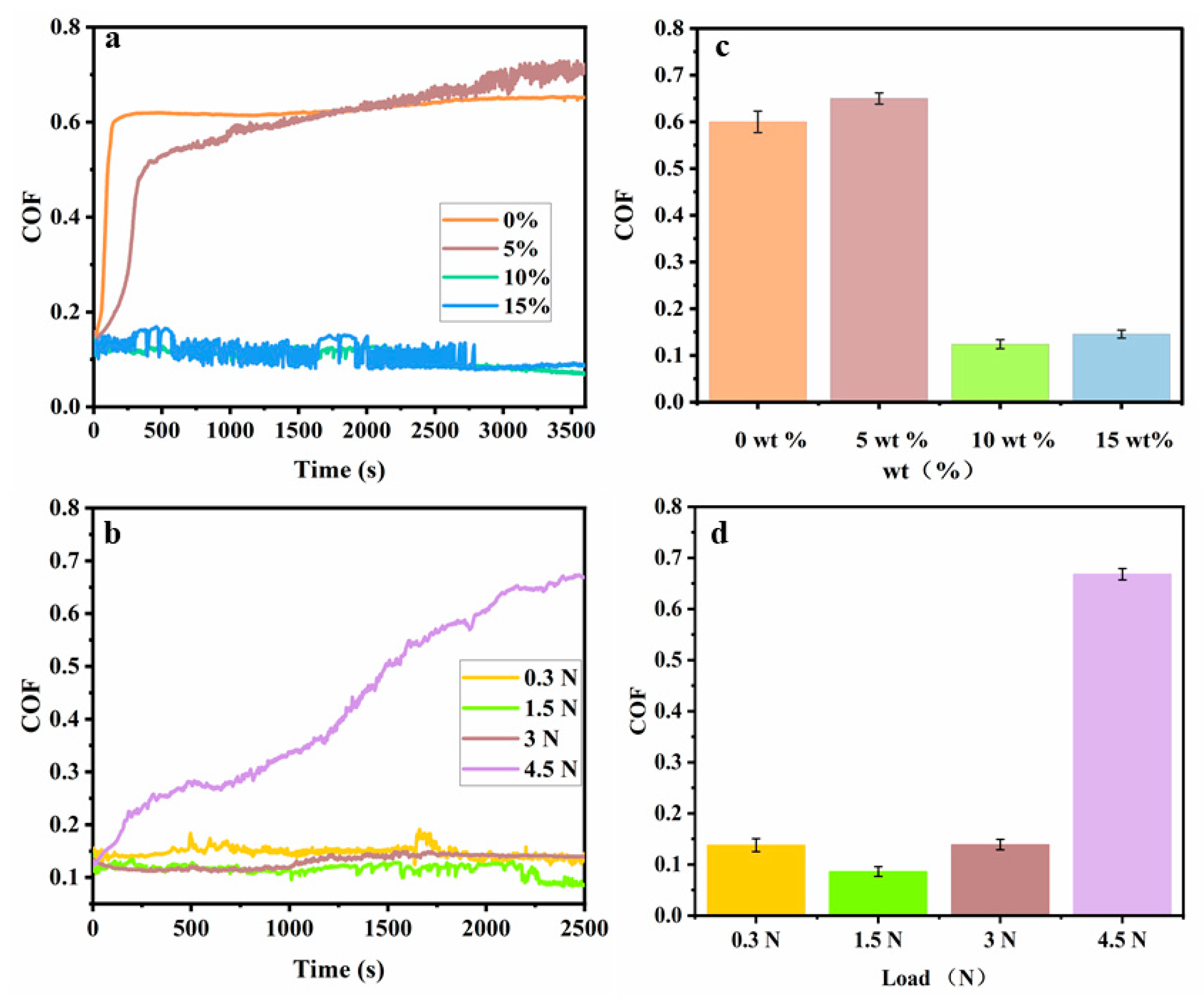
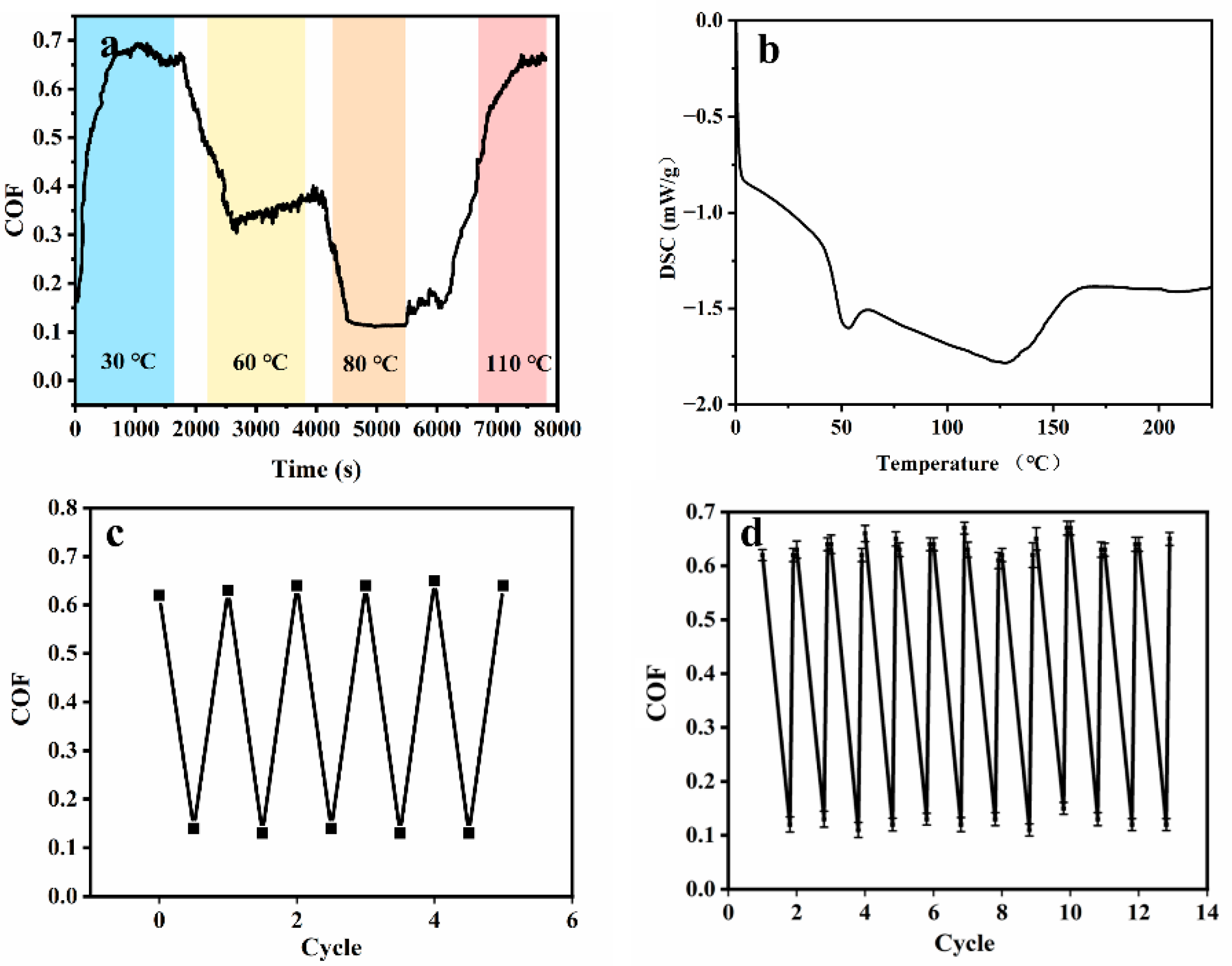
3.5. Friction-Switching Experiments Based on Thermal Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glavatskih, S.; Höglund, E. Tribotronics—Towards active tribology. Tribol. Int. 2008, 41, 934–939. [Google Scholar]
- Wang, Q.H.; Zhang, N.; Qu, C.H.; Li, S.; Guo, L.H.; Yang, Z.H.; Zhang, X.R.; Wang, T.M. Photothermal effect of graphene oxide for 3D hybrid composites achieving controllable friction. Tribol. Int. 2022, 167, 107364–107372. [Google Scholar]
- Liu, D.Q.; Broer, D.J. Light controlled friction at a liquid crystal polymer coating with switchable patterning. Soft Matter 2014, 10, 7952–7958. [Google Scholar] [PubMed]
- Shao, Y.L.; Zhao, J.; Fan, Y.; Wan, Z.P.; Lu, L.S.; Zhang, Z.H.; Ming, W.H.; Ren, L.Q. Shape memory superhydrophobic surface with switchable transition between “Lotus Effect” to “Rose Petal Effect”. Chem. Eng. J. 2020, 382, 122989–122998. [Google Scholar]
- Bai, H.; Tian, X.L.; Zheng, Y.M.; Ju, J.; Zhao, Y. Direction controlled driving of tiny water drops on bioinspired artificial spider silks. Adv. Mater. 2010, 22, 5521–5525. [Google Scholar]
- Bowden, F.P.; Tabor, D.; Palmer, F. The Friction and Lubrication of Solids. Am. J. Phys. 1951, 19, 428–429. [Google Scholar]
- Liu, D.Q.; Liu, L.; Onck, P.R.; Broer, D.J. Reverse switching of surface roughness in a self-organized polydomain liquid crystal coating. Proc. Natl. Acad. Sci. USA 2015, 112, 3880–3885. [Google Scholar]
- Li, F.D.; Hou, H.H.; Yin, J.; Jiang, X.S. Near-infrared light-responsive dynamic wrinkle patterns. Sci. Adv. 2018, 4, 5762–5770. [Google Scholar]
- Zhang, D.J.; Cheng, Z.J.; Kang, H.J.; Yu, J.Y.; Liu, Y.Y.; Jiang, L. A Smart Superwetting Surface with Responsivity in Both Surface Chemistry and Microstructure. Angew Chem. Int. Ed. Engl. 2018, 57, 3701–3705. [Google Scholar]
- Li, C.Z.; Jiao, Y.L.; Zhang, Y.Y.; Jiang, S.J.; Lv, X.D.; Wu, S.Z.; Li, J.W.; Hu, Y.L.; Ye, J.X.; Liu, K.; et al. Noncontact All-In-Situ Reversible Reconfiguration of Femtosecond Laser-Induced Shape Memory Magnetic Microcones for Multifunctional Liquid Droplet Manipulation and Information Encryption. Adv. Funct. 2021, 31, 2100543–2100553. [Google Scholar]
- Zhang, Y.N.; Jiang, J.; Ouyang, C.K.; Meng, Y.G.; Jia, W.P.; Ma, L.R.; Tian, Y. Effect of base oil lubrication properties on magnetorheological fluids. Smart Mater. Struct. 2021, 30, 95011–95022. [Google Scholar]
- Wang, Y.H.; Ouyang, J.H.; Liu, Z.G.; Wang, Y.M.; Wang, Y.J. Microstructure and high temperature properties of two-step voltage-controlled MAO ceramic coatings formed on Ti2AlNb alloy. Appl. Surf. Sci. 2014, 307, 62–68. [Google Scholar]
- Tan, X.; Hu, C.Z.; Li, X.; Liu, H.J.; Qu, J.H. Reversible superwettability switching of a conductive polymer membrane for oil-water separation and self-cleaning. J. Membr. Sci. 2020, 605, 118088–118098. [Google Scholar]
- Zhang, H.Y.; Lai, H.; Cheng, Z.J.; Zhang, D.J.; Liu, P.C.; Li, Y.F.; Liu, Y.Y. In-situ switchable superhydrophobic shape memory microstructure patterns with reversible wettability and adhesion. Appl. Surf. Sci. 2020, 525, 525–535. [Google Scholar]
- Sun, Q.Q.; Wang, D.H.; Li, Y.N.; Zhang, J.H.; Ye, S.J.; Cui, J.X.; Chen, L.Q.; Wang, Z.K.; Butt, H.J.; Vollmer, D.; et al. Surface charge printing for programmed droplet transport. Nat. Mater. 2019, 18, 936–941. [Google Scholar]
- Wu, P.; Zeng, C.; Guo, J.; Liu, G.; Zhou, F.; Liu, W. Achieving near-infrared-light-mediated switchable friction regulation on MXene-based double network hydrogels. Friction 2023. [Google Scholar] [CrossRef]
- Hou, Y.; Weng, D.; Zhang, Z.; Yu, Y.; Chen, L.; Wang, J. Near-Infrared Light Responsive Surface with Switchable Wettability in Microstructure and Surface Chemistry. Langmuir 2023, 39, 6276–6286. [Google Scholar] [PubMed]
- Wang, H.; Zhang, Z.; Zheng, J.; Zhao, J.; Liang, Y.; Li, X.; Ren, L. Multifunctional superhydrophobic surface with dynamically controllable micro/nanostructures for droplet manipulation and friction control. Chem. Eng. J. 2021, 417, 127944–127952. [Google Scholar]
- Patric, C.; Maria, J.B.P.; Francis, P.; Bernard, R.; Elias, F. Multiple emulsion technology for the design of microspheres containing peptides and oligopeptides. Adv. Drug. Deliv. Rev. 1997, 28, 85–96. [Google Scholar]
- Brwon, E.N.; Kessler, M.R.; Sottos, N.R.; White, S.R. In situ poly(ureaformaldehyde) microencapsulation of dicyclopentadiene-2. J. Microencapsul. 2003, 20, 719–730. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Caruso, M.M.; McIlroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Ren, Y.L.; Gao, K.; Ying, S.J.; Zhao, Y.; Zhang, L.; Guo, D.; Xie, G.X. Significant enhancement of tribological properties of microcapsule/epoxy composites in the presence of high loads by incorporating PTFE. Wear 2023, 15, 204563–204574. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; Gong, H.J.; Wang, Z.; Zhu, L.N.; Xie, G.X. Preparation of self-lubricating porous alumina ceramics with PMMA /PAO6 microcapsules and their tribological properties. Ceram. Int. 2022, 48, 8031–8038. [Google Scholar] [CrossRef]
- Zhang, W.L.; Qi, X.W.; Yang, X.; Dong, Y.; Fan, B.L.; Liang, L. Fabrication of high-stability Ni-PSF@PAO40 microcapsules and their lubricating properties in polyamide 6. Friction 2022, 10, 1985–1999. [Google Scholar] [CrossRef]
- Hou, Y.C.; Weng, D.; Yu, Y.D.; Wang, J.D.; Chen, L. Near infrared light responsive surface with self-healing superhydrophobicity in surface chemistry and microstructure. Appl. Surf. Sci. 2022, 598, 153772–153781. [Google Scholar] [CrossRef]
- Ma, Y.J.; Li, Z.K.; Wang, H.Y.; Li, H.Y. Synthesis and optimization of polyurethane microcapsules containing [BMIm]PF6 ionic liquid lubricant. J. Colloid Interface Sci. 2019, 534, 469–479. [Google Scholar] [CrossRef]
- Xiong, W.T.; Li, X.L.; Chen, X.C.; Zhang, C.H.; Luo, J.B. Preparation and Tribological Properties of Self-Lubricating Epoxy Resins with Oil-Containing Nanocapsules. ACS Appl. Mater. Interfaces 2022, 14, 18954–18964. [Google Scholar] [CrossRef]
- Hao, Y.; Zhou, X.Y.; Shao, J.J.; Zhu, Y.K. The influence of multiple fillers on friction and wear behavior of epoxy composite coatings. Surf. Coat. Technol. 2019, 362, 213–219. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.Y.; He, F.; Chen, H.; Xie, G.X.; Wei, B.; Luo, J.B.; He, B.; Wu, Z. Wear in-situ self-healing polymer composites incorporated with bifunctional microcapsules. Compos. B Eng. 2022, 232, 109566–109575. [Google Scholar] [CrossRef]
- Li, H.Y.; Ma, Y.J.; Li, Z.K.; Cui, Y.X.; Wang, H.Y. Synthesis of novel multilayer composite microcapsules and their application in self-lubricating polymer composites. Compos. Sci. Technol. 2018, 164, 120–128. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, S.; Li, Z.K.; Zhu, Y.J.; Wang, H.Y. Polysulfone/SiO2 Hybrid Shell Microcapsules Synthesized by the Combination of Pickering Emulsification and the Solvent Evaporation Technique and Their Application in Self-Lubricating Composites. Langmuir 2017, 33, 14149–14155. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Liu, Y.; Chen, L.; Weng, D.; Ma, Y.; Yu, Y.; Wu, Y.; Wang, J. Controllable Friction of an Epoxy Composite via Thermal Treatment. Appl. Sci. 2023, 13, 9899. https://doi.org/10.3390/app13179899
Hou Y, Liu Y, Chen L, Weng D, Ma Y, Yu Y, Wu Y, Wang J. Controllable Friction of an Epoxy Composite via Thermal Treatment. Applied Sciences. 2023; 13(17):9899. https://doi.org/10.3390/app13179899
Chicago/Turabian StyleHou, Yacong, Yubo Liu, Lei Chen, Ding Weng, Yuan Ma, Yadong Yu, Yang Wu, and Jiadao Wang. 2023. "Controllable Friction of an Epoxy Composite via Thermal Treatment" Applied Sciences 13, no. 17: 9899. https://doi.org/10.3390/app13179899
APA StyleHou, Y., Liu, Y., Chen, L., Weng, D., Ma, Y., Yu, Y., Wu, Y., & Wang, J. (2023). Controllable Friction of an Epoxy Composite via Thermal Treatment. Applied Sciences, 13(17), 9899. https://doi.org/10.3390/app13179899





