Assessment of Water Retention Capacity of Non-Ionic and Anionic Fluorinated Dust Suppressants on Coal Dust
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection and Analysis of Experimental Coal Samples
2.2. Experiments on the Preparation of Fluorine-Containing Solutions
2.3. Water Retention Experiment
3. Results and Discussion
3.1. Analysis of Water Retention in Anionic Fluorinated Solutions
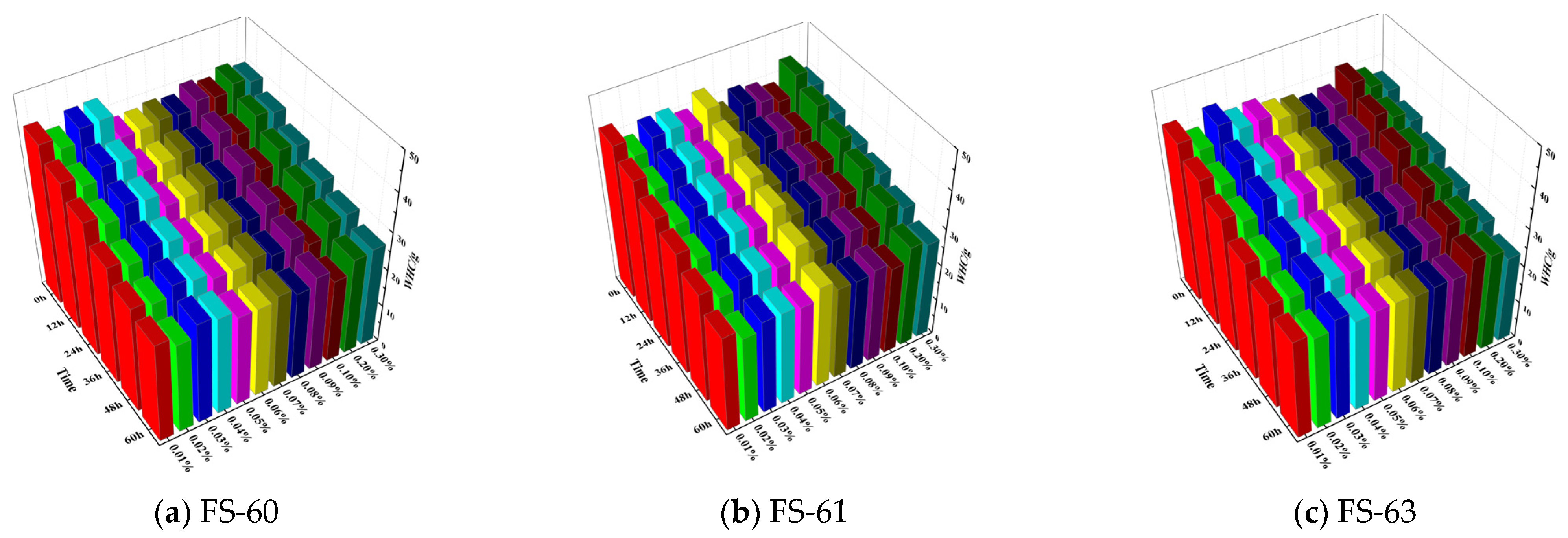
3.1.1. Study on the Change Law of Coal Dust Weight after the Action of Anionic Fluorine-Containing Solution
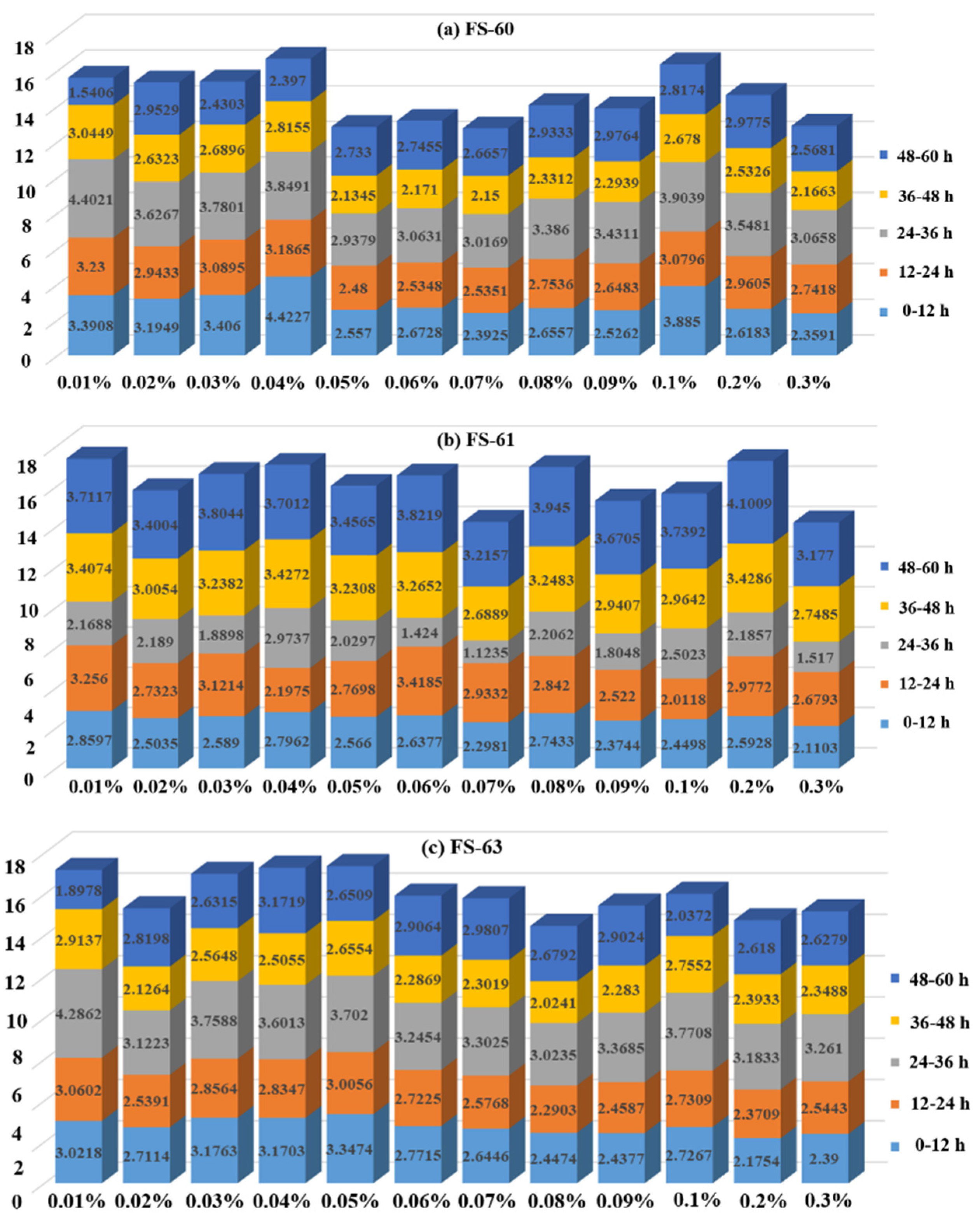
3.1.2. Analysis of Segmental Weight Loss Rate Variation of Anionic Fluorinated Solutions
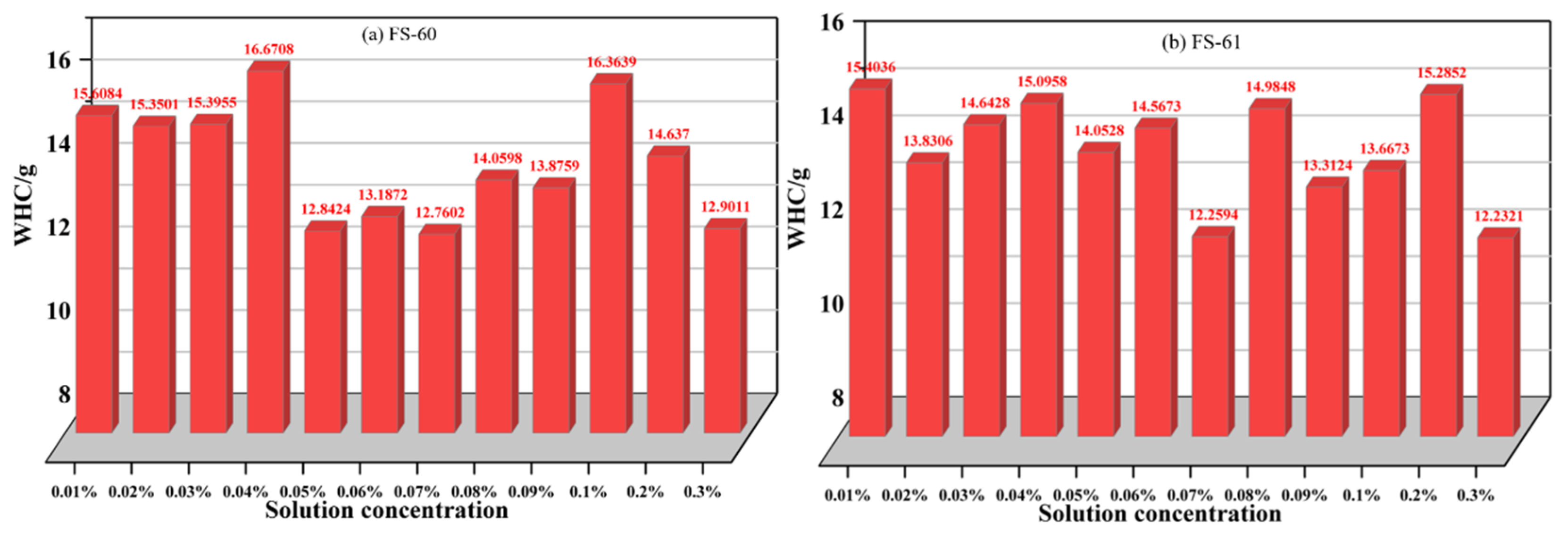
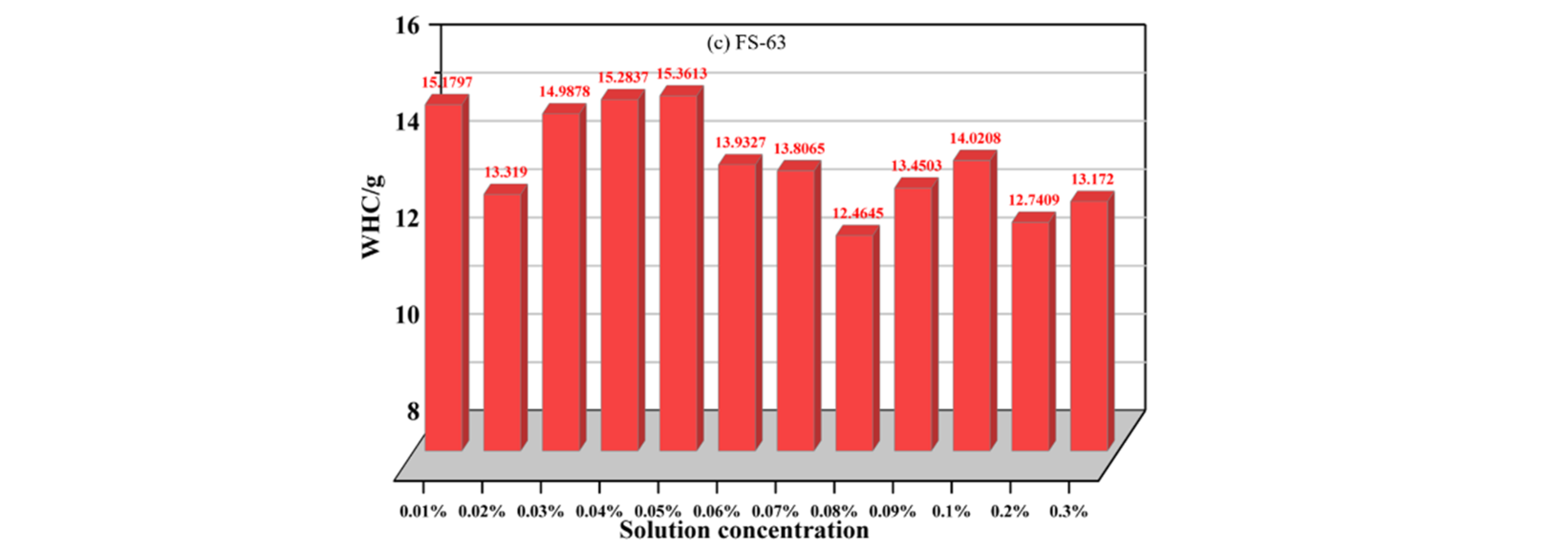
3.1.3. Analysis of Total Weight Loss Rate Variation of Anionic Fluorinated Solutions
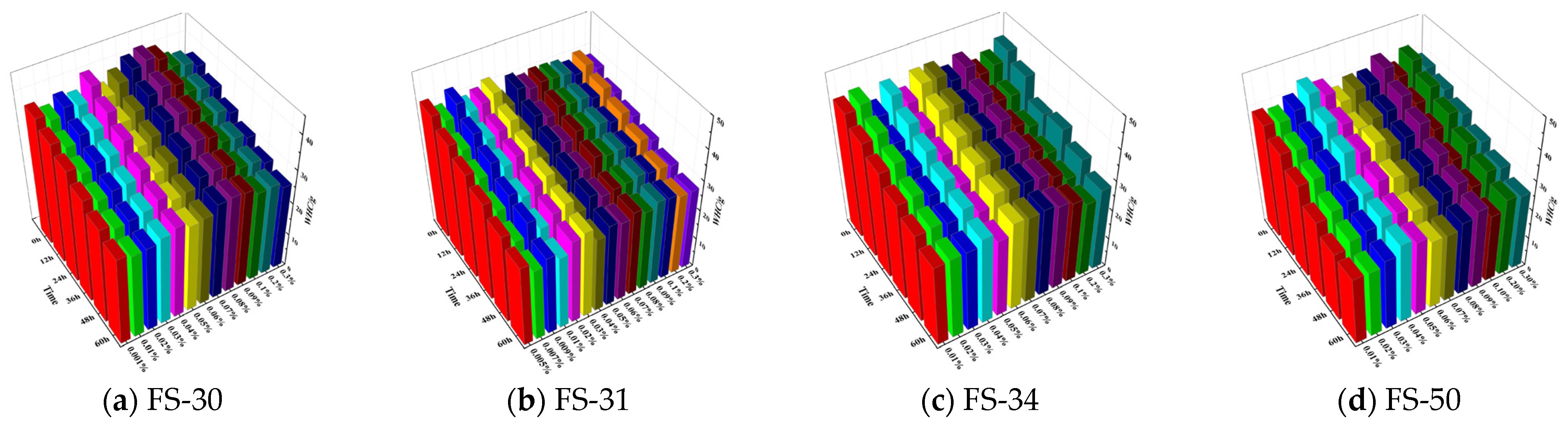
3.2. Analysis of Water Retention of Non-Ionic Fluorinated Dust Suppressants
3.2.1. Study on the Change Law of Coal Dust Weight after the Action of Anionic Fluorine-Containing Solution
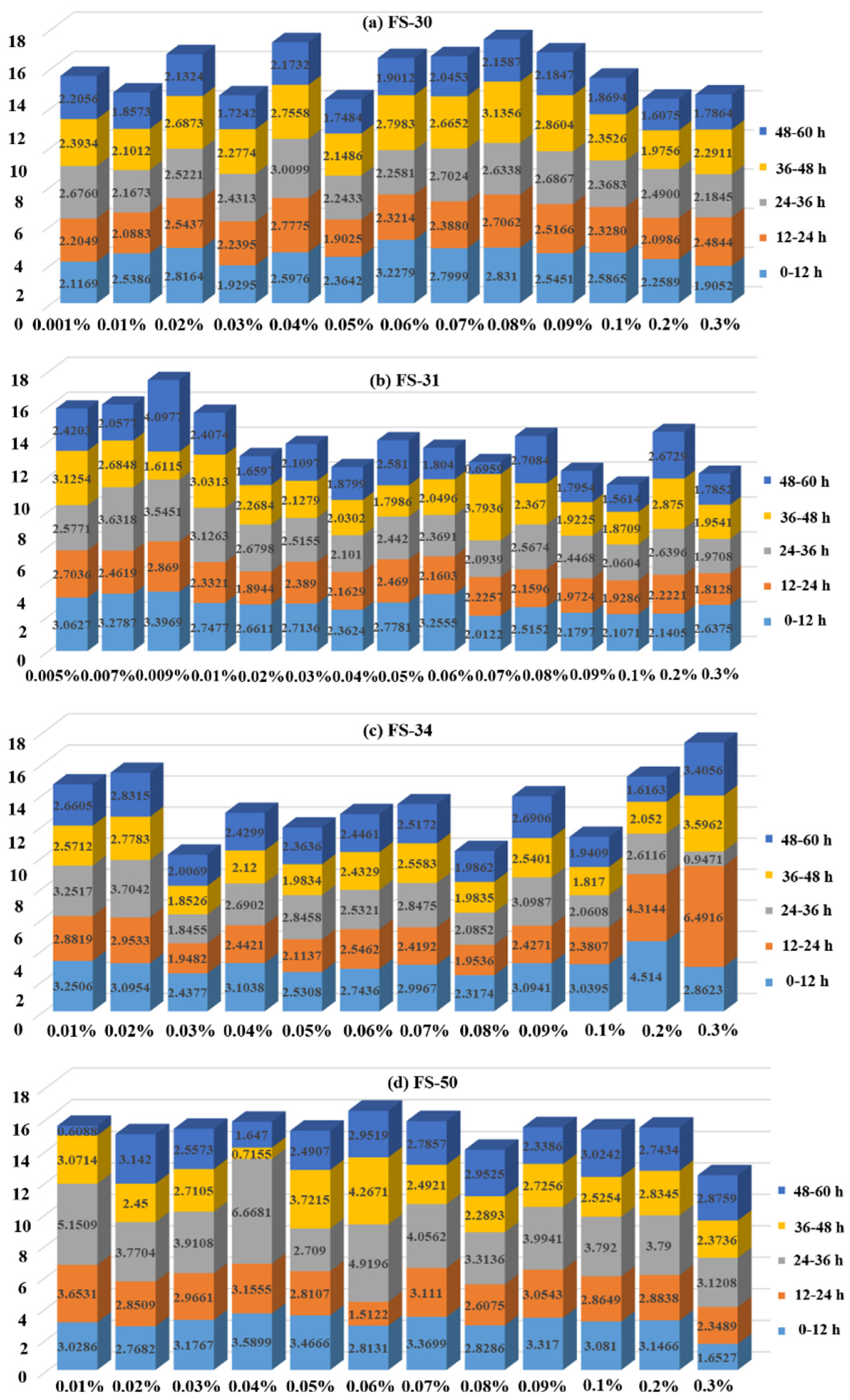
3.2.2. Analysis of Segmental Weight Loss Rate Variation of Non-Ionic Fluorinated Solutions
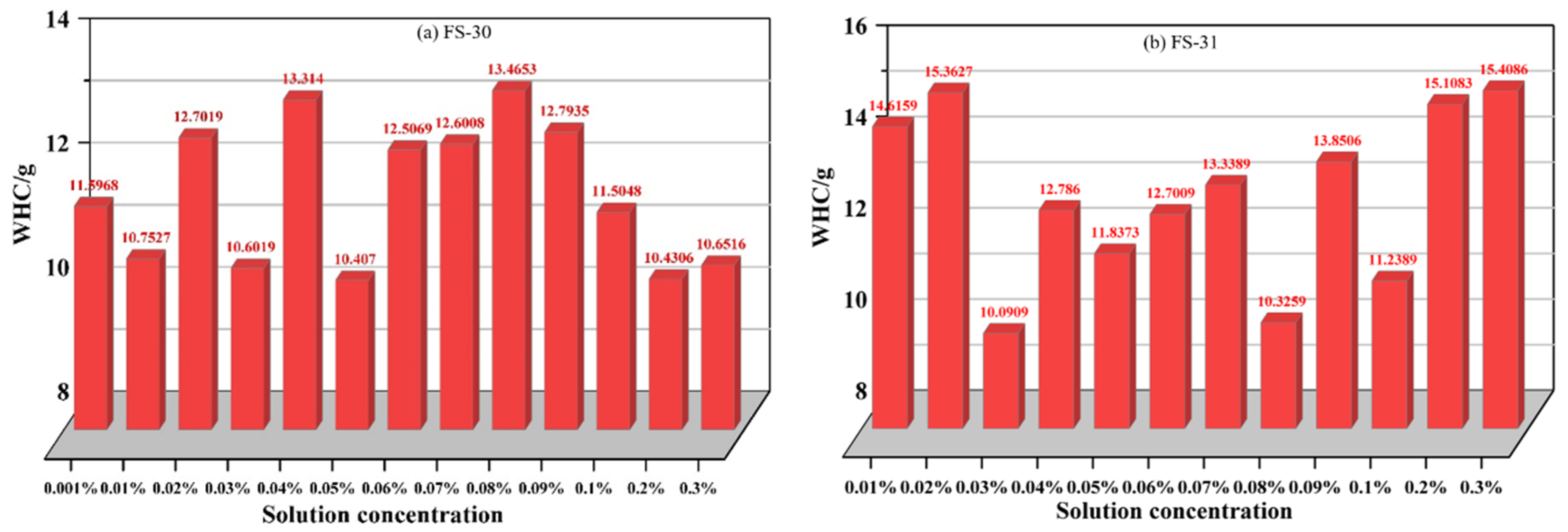
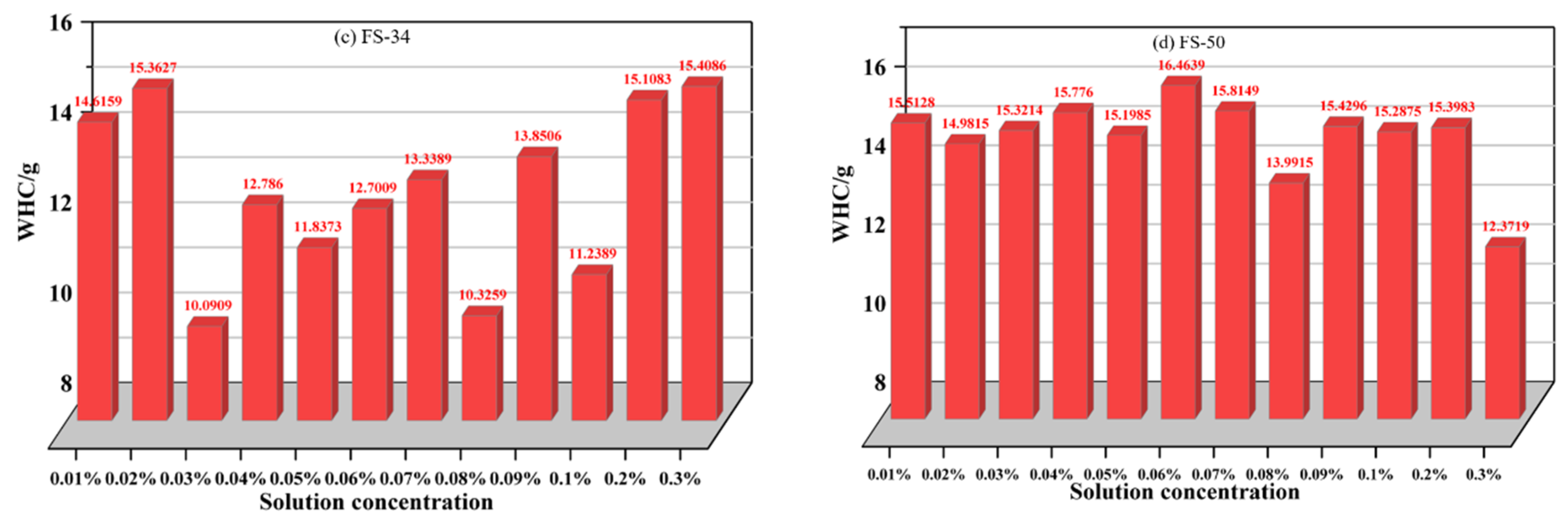
3.2.3. Analysis of the Variation in Total Weight Loss Rate of Non-Ionic Fluorinated Solutions
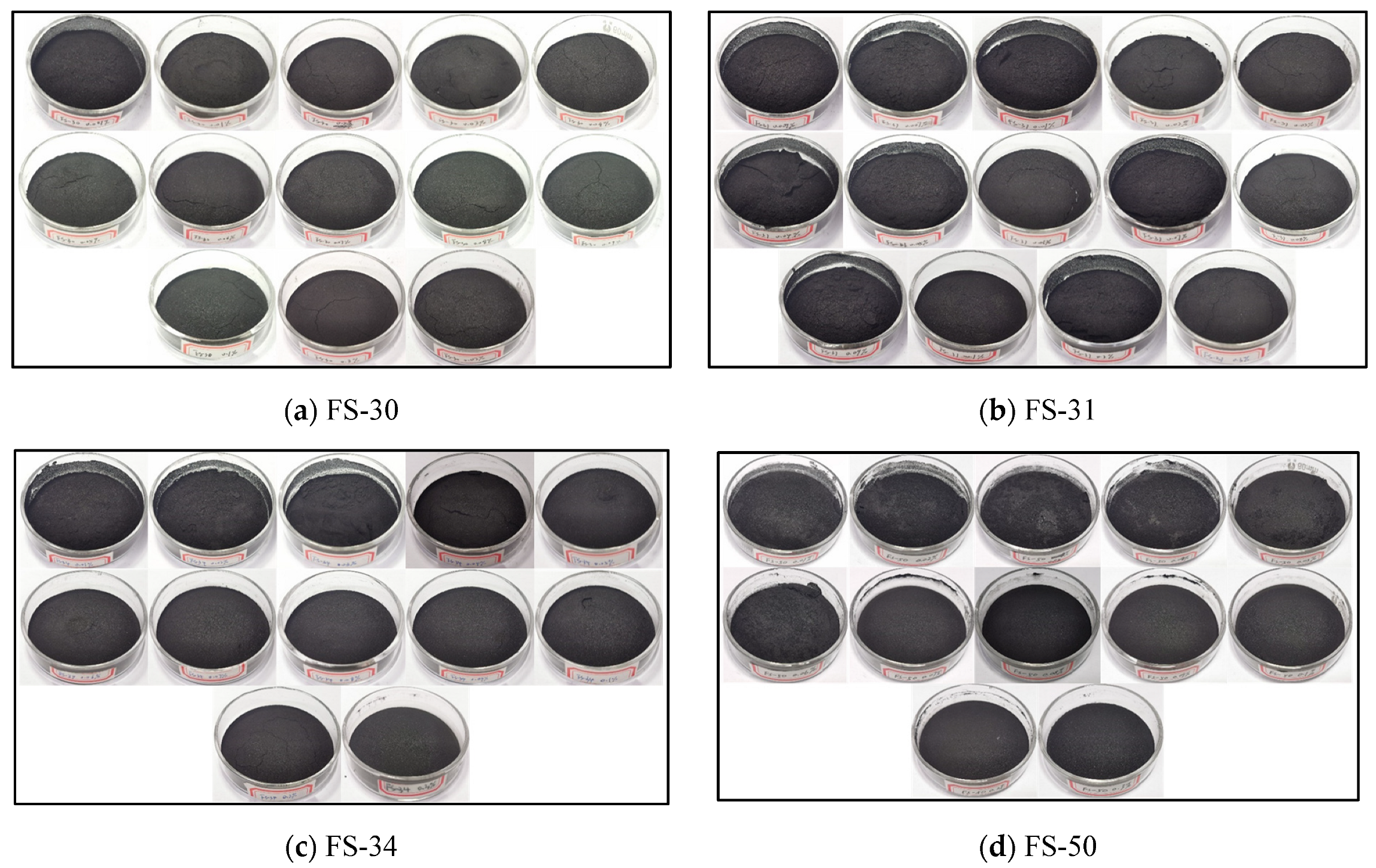
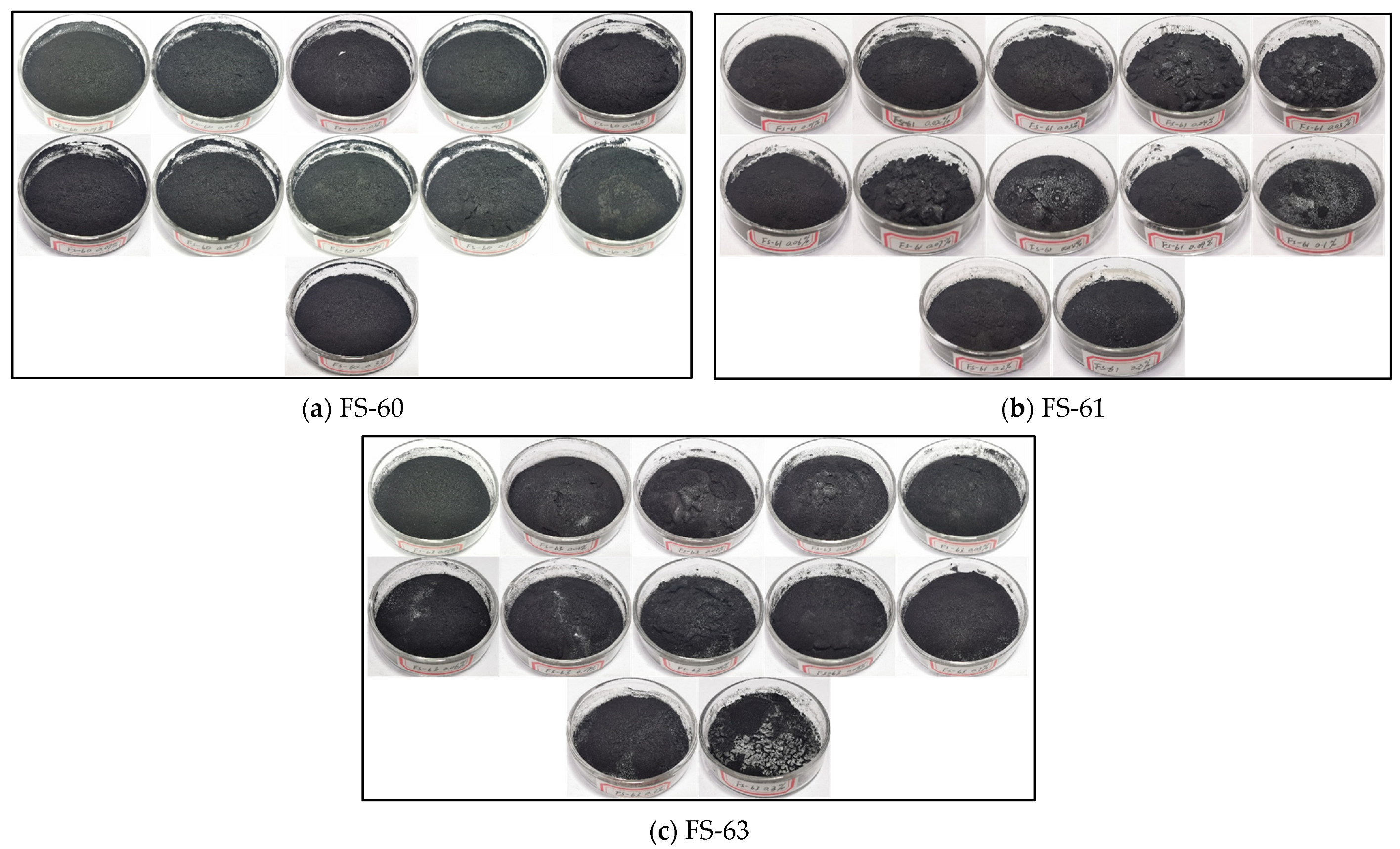
3.3. Analysis of Surface Morphological Changes in Coal Dust Samples after the Experiment
3.3.1. Analysis of Coal Dust Surface Morphology after the Action of Non-Ionic Fluorine-Containing Solution
3.3.2. Analysis of Coal Dust Surface Morphology after the Action of Anionic Fluorine-Containing Solution
3.4. Analysis of Water Retention of Coal Dust by Different Fluorine-Containing Solutions
4. Conclusions
- (1)
- The water retention of non-ionic fluorinated solutions is significantly better than that of anionic fluorinated solutions, and water retention performance gradually increases with increasing concentration within a certain range. Among the four non-ionic fluorinated solutions, FS-30 solution has the better overall water retention, followed by FS-31 solution, FS-34 solution, and FS-50 solution.
- (2)
- To meet the requirements for water retention of coal dust, the optimum concentration of anionic fluoride containing solution FS-30 is preferably 0.05%, and the optimum concentration of anionic fluoride solution FS-31 is 0.03%. The optimum concentration of the anionic fluoride solution FS-34 is 0.03%. The optimum concentration of anionic fluoride solution FS-50 is 0.3%. The optimum concentration of 0.07% for non-ionic fluoride solution FS-60. The optimum concentration is 0.3% for non-ionic fluoride solution FS-61. The optimum concentration is 0.08% for non-ionic fluoride solution FS-63.
- (3)
- The surface morphology of the coal dust after the solution action can be analyzed, the surface of the coal dust after the action of the non-ionic fluorine solution is more compact and detailed, and with the increase in concentration, the surface of the graininess is less obvious. FS-50 solution showed a better effect among the four non-ionic fluorine solutions, the surface of the coal dust samples showing no obvious cracks or fine lines. This indicates that the wetting and agglomeration properties of the non-ionic fluoride-containing solutions are better than those of the anionic fluoride-containing solutions.
- (4)
- The water loss at different time periods shows that the four non-ionic fluoride solutions and the anionic fluoride solutions FS-60 and FS-63 all have the highest water loss in the 24–36 h time period, while the anionic fluoride solution FS-61 has the highest water loss in the 36–48 h time period. Therefore, to prevent the risk of secondary dusting, emphasis should be placed on timely wetting of coal dust agglomerates at 24 h and 36 h after the solution has been applied to the coal dust.
- (5)
- Overall, the regularity results show that the concentration and type of fluorinated solution used can significantly influence the water retention of coal dust. The non-ionic fluorinated solution FS-50 with a concentration of 0.3% appears to be the most effective at increasing water retention, likely due to the formation of stable micelles that reduce the surface energy and increase the wettability of the coal dust particles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Tao, Q.; Yuan, C.; Zheng, Y.; Zhang, G.; Liu, J. Investigation on the structure evolution of pre and post explosion of coal dust using X-ray diffraction. Int. J. Heat Mass Transf. 2018, 120, 1162–1172. [Google Scholar] [CrossRef]
- Xi, Z.; Jin, L.; Liew, J.R.; Li, D. Characteristics of foam sol clay for controlling coal dust. Powder Technol. 2018, 335, 401–408. [Google Scholar] [CrossRef]
- Wang, P.; Tan, X.; Zhang, L.; Li, Y.; Liu, R. Influence of particle diameter on the wettability of coal dust and the dust suppression efficiency via spraying. Process Saf. Environ. Prot. 2019, 132, 189–199. [Google Scholar] [CrossRef]
- Yu, H.; Wang, C.; Pang, L.; Cui, Y.; Chen, D. Inhibiting effect of coal fly ash on minimum ignition temperature of coal dust clouds. J. Loss Prev. Process Ind. 2019, 61, 24–29. [Google Scholar] [CrossRef]
- Chang, P.; Zhao, Z.; Xu, G.; Ghosh, A.; Huang, J.; Yang, T. Evaluation of the coal dust suppression efficiency of different surfactants: A factorial experiment. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124686. [Google Scholar] [CrossRef]
- Xiaonan, W. Study on Synergistic Effect of Surfactant Compound on Coal Dust Wettability. Ph.D. Thesis, Anhui University of Science& Technology, Huainan, China, 2020. [Google Scholar]
- Liu, X.; Chang, P.; Wang, E.; Zhang, Z.; Yang, S. Numerical study of the respirable coal dust removal performance of a vortex ventilation system at an excavation face. Energies 2018, 11, 2449. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, B.; Wang, J.; Wang, H.; Wang, F. Experimental investigation on the changes of the wettability and surface characteristics of coal dust with different fractal dimensions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 148–157. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Chen, K.; Niu, K.; Wu, G.; Wei, X.; Wang, H. Preparation and characterization of a composite dust suppressant for coal mines. Polymers 2020, 12, 2942. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Y.; Lei, Y.; Zheng, X.; Luo, W.; Song, W.; Wang, Z.; Han, F. Study on coal dust crusting for coal pile based on a compound binder. Powder Technol. 2020, 376, 149–166. [Google Scholar] [CrossRef]
- Yunlong, M. Experimental Study on Compound Dust Suppressor for Mine Based on Molecular Modification Technology. Master’s Thesis, Shandong University of Science and Technology, Qingdao, China, 2019. [Google Scholar]
- Liang, W.J.; Lan, T.; Zhang, Z.X.; Ren, S.D.; Wang, Y.L. Preparation and application of new environmental protection coal dust suppressor. China Coal 2021, 47, 57–65. [Google Scholar] [CrossRef]
- Hu, J. Development and Performance of Compound Environmental Dust Suppressor. Master’s Thesis, Shandong University of Science and Technology, Qingdao, China, 2019. [Google Scholar]
- Tao, F. Experimental Study on Environmental Protection Molecular Modified Dust Suppressor for Mine. Master’s Thesis, Shandong University of Science and Technology, Qingdao, China, 2018. [Google Scholar]
- Hetang, W.; Sheng, H.; Qi, Z.; Xia, Z. Experimental study on synthesis of biological dust suppressor by microbial fermentation. J. China Coal Soc. 2021, 46, 477–488. [Google Scholar]
- Li, S.; Tian, J.; Xie, H.; Ma, Y.L. Study on preparation of wetting dust suppressor from paper waste and its properties. Saf. Coal Mines 2019, 50, 14–16+20. [Google Scholar] [CrossRef]
- Yaheng, P.; Jian, C.; Jian, W.; Chunhui, P. Preparation and properties of chitosan—Based dust suppressor. Coal Chem. Ind. 2021, 44, 123–126. [Google Scholar] [CrossRef]
- Li, J.; Sun, S.; Wang, Y.; Wang, Z. Experimental study on coal dust pollution law of railway tunnel. J. Lanzhou Jiaotong Univ. 2005, 24, 52–54. [Google Scholar]
- Li, K.; Yang, L.; Jiang, F.; Tang, Y. Preparation and study of modified lignosulfonate dust suppressor for coal. Environ. Eng. 2012, 30, 66–69. [Google Scholar] [CrossRef]
- Jia, Z.A.; Jihong, S.; Chao, W.; Jianglong, C. Preparation and property analysis of compound environmental dust suppressor for mine. Coal Chem. Ind. 2021, 44, 94–98. [Google Scholar] [CrossRef]
- Frey, F.; Weidner, E.; Pedersen, B.; Boysen, H.; Burghammer, M.; Hoelzel, M. Pyroxene from martian meteorite nwa856: Structural investigations by x-ray and neutron diffraction. Z. Für Krist. 2010, 225, 287–297. [Google Scholar] [CrossRef]
- Zilei, W.; Xiaoming, J. Properties of fluorinated surfactants and the effect of melon ring on their properties. Chem. Res. Appl. 2022, 34, 2114–2120. [Google Scholar]
- Yutang, Z.; Yujia, T.; Yong, J. Research progress in the synthesis and application of fluorinated surfactants. Leather Sci. Eng. 2020, 30, 26–32. [Google Scholar] [CrossRef]
- Kronlund, D.; Bergbreiter, A.; Meierjohann, A.; Kronberg, L.; Lindén, M.; Grosso, D.; Smått, J.-H. Hydrophobization of marble pore surfaces using a total immersion treatment method—Product selection and optimization of concentration and treatment time. Prog. Org. Coat. 2015, 85, 159–167. [Google Scholar] [CrossRef]
- Xu, Z.; Peng, S.; Zhou, H.; Guo, Y.; Zhou, G.; Xu, X. Progress in synthesis and electrowettability of fluorine-containing polymer coatings. Mater. Guide 2022, 36, 235–249. [Google Scholar]
- Liu, N.; Yu, Z.; Liang, Y.; Zhang, H. Effects of mixed surfactants on heat transfer performance of pulsed spray cooling. Int. J. Heat Mass Transf. 2019, 144, 118593. [Google Scholar] [CrossRef]
- Liu, N.; Yu, Z.; Zhu, T.; Yin, X.; Zhang, H. Effects of microstructured surface and mixed surfactants on the heat transfer performance of pulsed spray cooling. Int. J. Therm. Sci. 2020, 158, 106530. [Google Scholar] [CrossRef]
- Xiaoyan, L.; Fodi, W.; Yin, Z.; Quanhong, H. Structure and properties analysis of medium—Resistant liquid fluorinated elastomers. J. Compos. 2022, 39, 1592–1600. [Google Scholar] [CrossRef]
- Yi, M.; Hong, S.; Kim, J.-R.; Kang, H.; Lee, J.; Yu, K.; Kee, S.; Lee, W.; Lee, K. Modification of a pedot:Pss hole transport layer for printed polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 153, 117–123. [Google Scholar] [CrossRef]
- Chuanrong, Z.; Xiangcheng, W.; Guangyu, Z.; Xiaogang, W. Surface, interfacial activity and wettability of sulfonate fluorinated surfactants. Oilfield Chem. 2020, 37, 701–706. [Google Scholar] [CrossRef]
- Kun, W.; Xiaoming, J. Effect of light on surface properties of fluorinated surfactants. Image Sci. Photochem. 2021, 39, 492–496. [Google Scholar]
- Lei, L.; Yanling, W.; Bin, L.; Yongfei, L.; Longhao, T. Preparation of fluoropolymer emulsion and its application at solid-liquid interface. Mater. Guide 2021, 35, 586–593. [Google Scholar]
- Zhichao, H.; Hui, L.; Tingjian, Z.; Xiaonan, L. Research and application progress of waterborne fluorine-containing coatings. Yunnan Chem. Ind. 2021, 48, 21–26. [Google Scholar]
- Goryeo, W.; Rui, J.X.; Yunzi, L. Applications of synthetic biology in the production of fluorine-containing compounds. Synth. Biol. 2020, 1, 358–371. [Google Scholar]
- Yichao, S.; Xiaoling, Z.; Yong, J. Progress in synthesis and application of nonionic fluorinated surfactants. Leather Sci. Eng. 2018, 28, 24–31. [Google Scholar] [CrossRef]
- Lu, Z.; Lei, Z.; Zafar, M.N. Synthesis and performance characterization of an efficient environmental-friendly sapindus mukorossi saponins based hybrid coal dust suppressant. J. Clean. Prod. 2021, 306, 127261. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate Analysis of Coal. China Coal Industry Association: Beijing, China, 2019.

| Mad/% | Ad/% | Vd/% | Cdaf/% |
|---|---|---|---|
| 8.05 | 7.17 | 28.20 | 56.59 |
| Experimental Reagents | Short Form | Active Component | Molecular Formula | Type |
|---|---|---|---|---|
| Perfluoropropyl acrylate | FS-30 | 24–26% | CH2=CHC(O)OC3F7 | Non-ionic |
| Perfluorooctyl acrylate | FS-31 | 24–26% | CH2=CHC(O)OC8F17 | Non-ionic |
| Perfluorooctylsulfonyl phenoxyethyl methacrylate | FS-34 | 24–26% | C8F17SO2OC6H4OH | Non-ionic |
| Perfluoropropanoic acid | FS-50 | 26–28% | CF3CF2COOH | Non-ionic |
| Sodium perfluorooctanesulfonate | FS-60 a | 39–41% | C8F17SO3Na | Anionic |
| Perfluoroisopropyl acrylate | FS-61 | 13–15% | C4F9COOCH3 | Anionic |
| Perfluorooctanesulfonate carbamate acid | FS-63 b | 34–36% | C9H6F17NO2S | Anionic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Xu, M.; Zhou, B.; Yang, M.; Li, X.; Yue, Q. Assessment of Water Retention Capacity of Non-Ionic and Anionic Fluorinated Dust Suppressants on Coal Dust. Appl. Sci. 2023, 13, 9118. https://doi.org/10.3390/app13169118
Wang K, Xu M, Zhou B, Yang M, Li X, Yue Q. Assessment of Water Retention Capacity of Non-Ionic and Anionic Fluorinated Dust Suppressants on Coal Dust. Applied Sciences. 2023; 13(16):9118. https://doi.org/10.3390/app13169118
Chicago/Turabian StyleWang, Kai, Min Xu, Biao Zhou, Mengjiao Yang, Xiaoxuan Li, and Qihang Yue. 2023. "Assessment of Water Retention Capacity of Non-Ionic and Anionic Fluorinated Dust Suppressants on Coal Dust" Applied Sciences 13, no. 16: 9118. https://doi.org/10.3390/app13169118
APA StyleWang, K., Xu, M., Zhou, B., Yang, M., Li, X., & Yue, Q. (2023). Assessment of Water Retention Capacity of Non-Ionic and Anionic Fluorinated Dust Suppressants on Coal Dust. Applied Sciences, 13(16), 9118. https://doi.org/10.3390/app13169118






