Modified Periosteal Inhibition (MPI) Technique for Immediate Implants: A Multi-Center Retrospective Case Series Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Age >18 years old;
- General good health (ASA I–II);
- Adequate oral hygiene (full mouth plaque score ≤20%, full mouth bleeding score ≤20%);
- Presence of one or more hopeless teeth requiring extraction.
- Pregnancy or lactation;
- Untreated periodontitis;
- Osteometabolic disease;
- Intravenous bisphosphonate therapy;
- History of chemotherapy or radiation therapy in the neck–head area;
- Heavy smoking (>15 cigarettes per day);
- Absence of buccal bone plate.
- Age 31–77 years old;
- 7 men and 5 women.
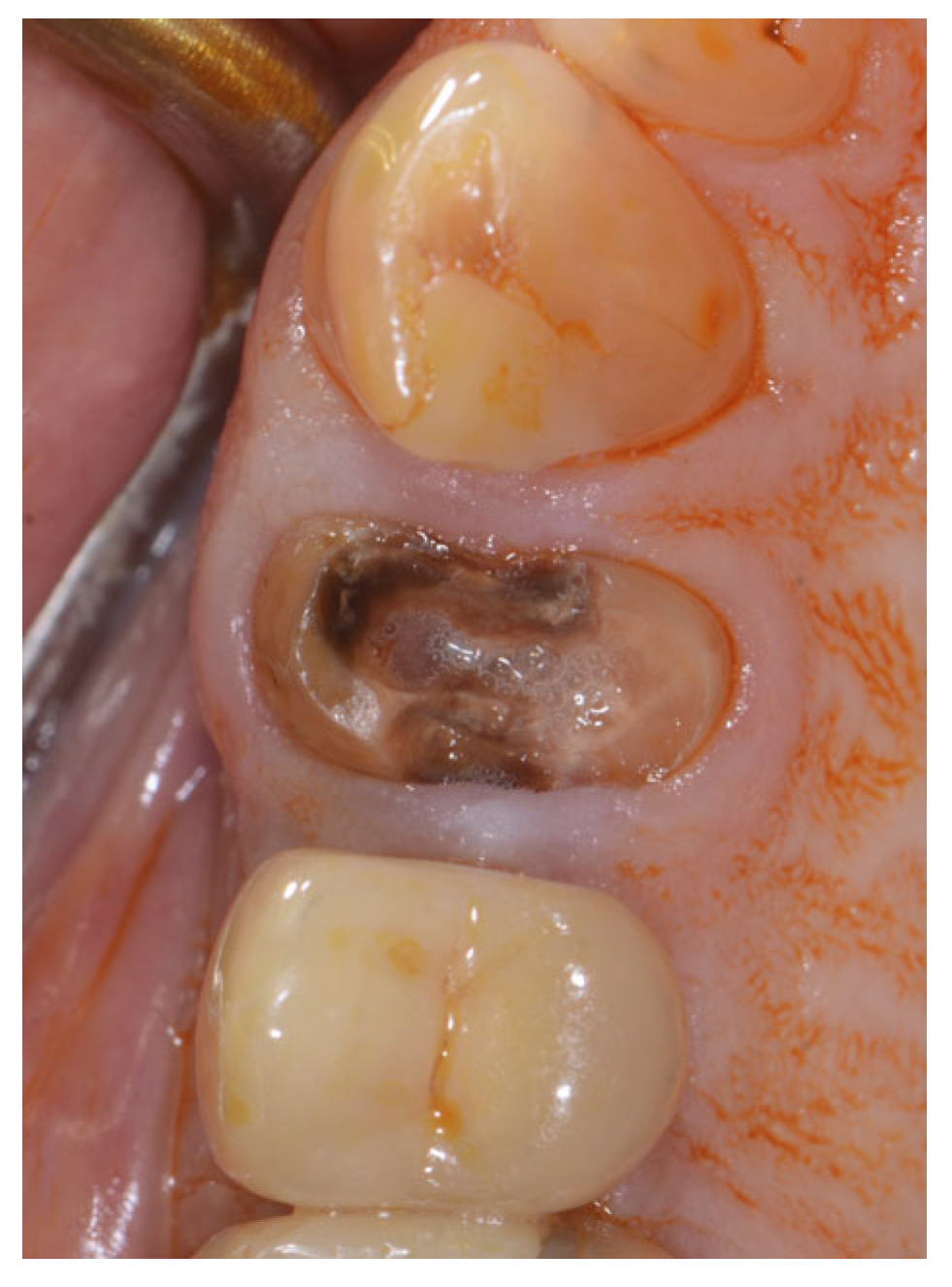
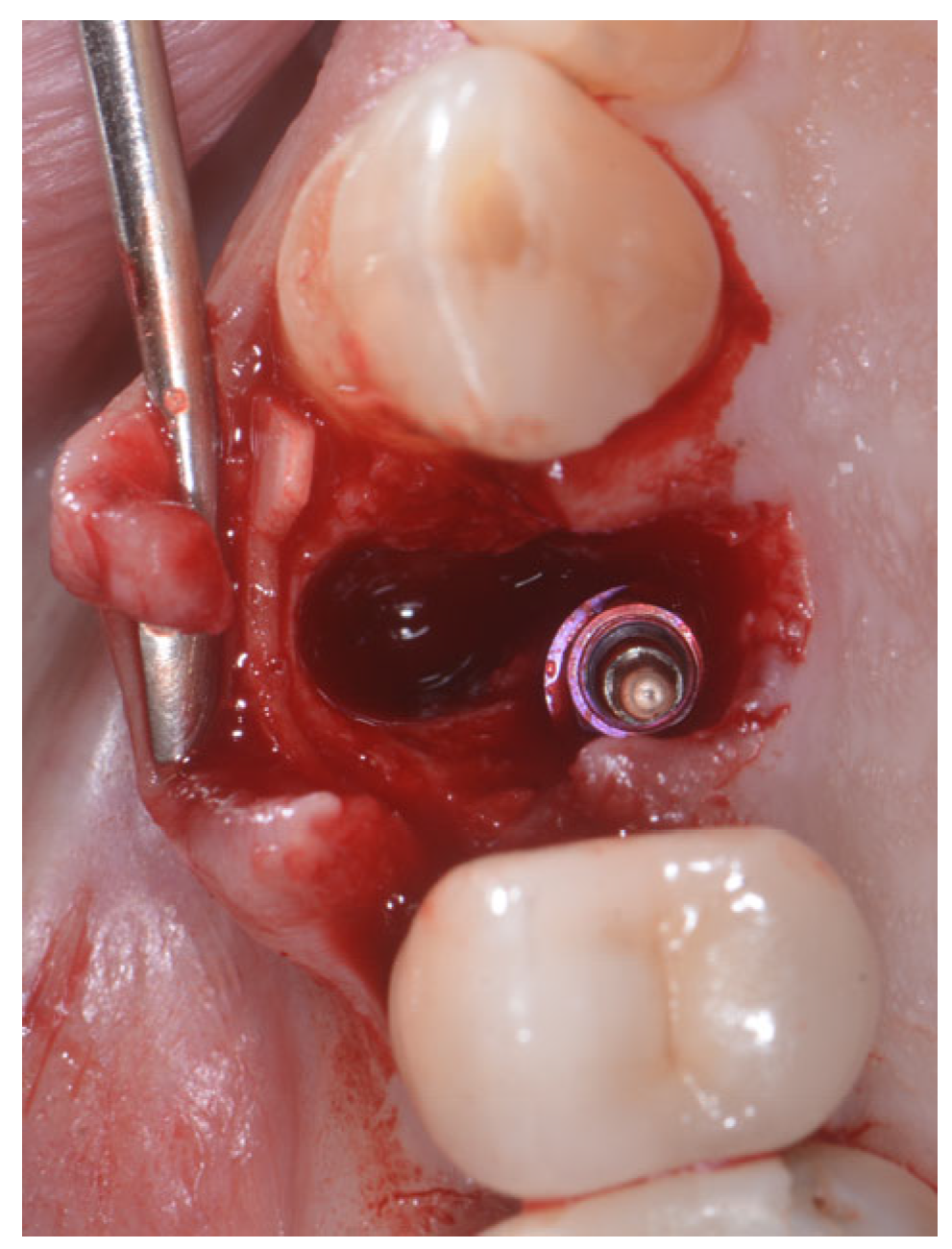
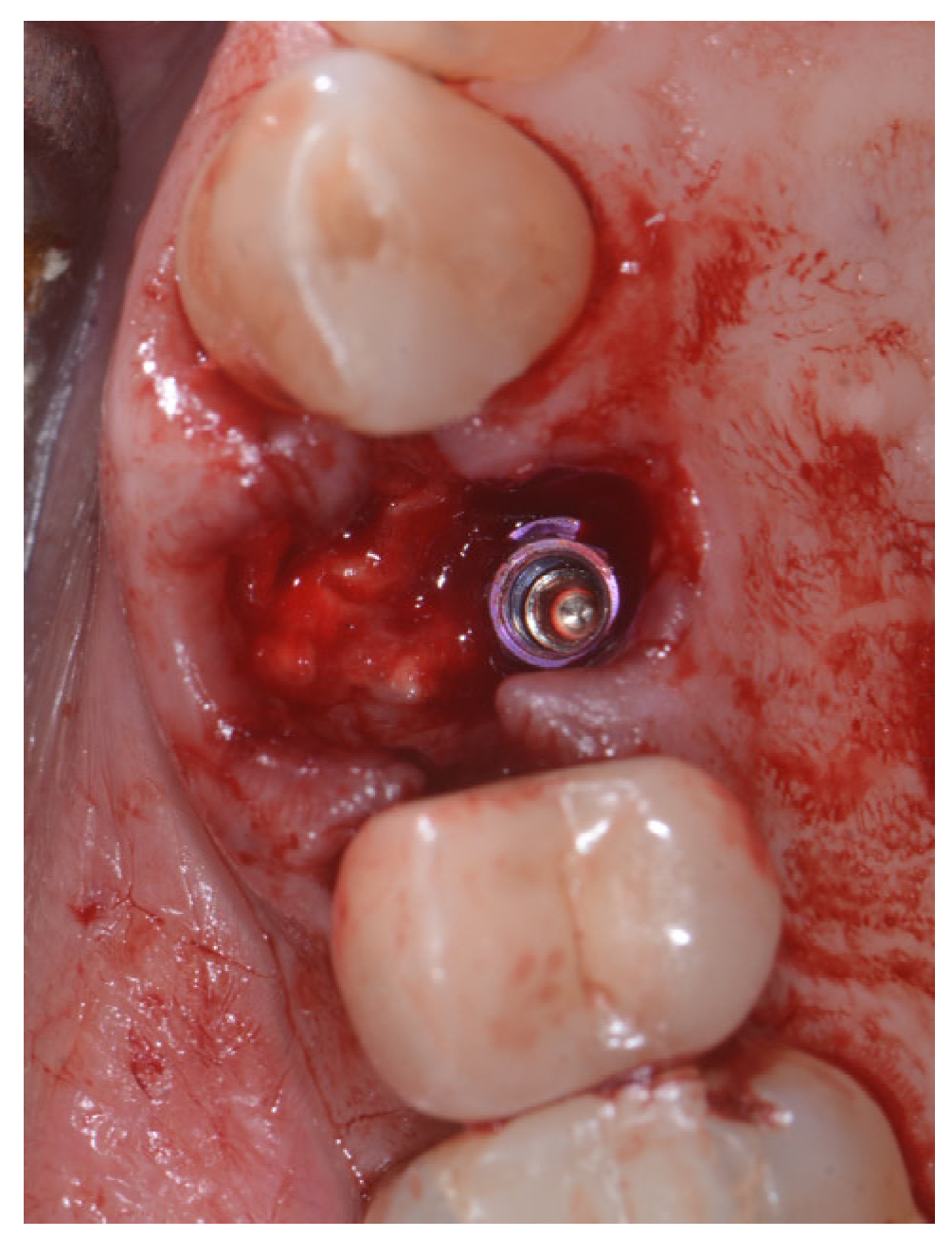
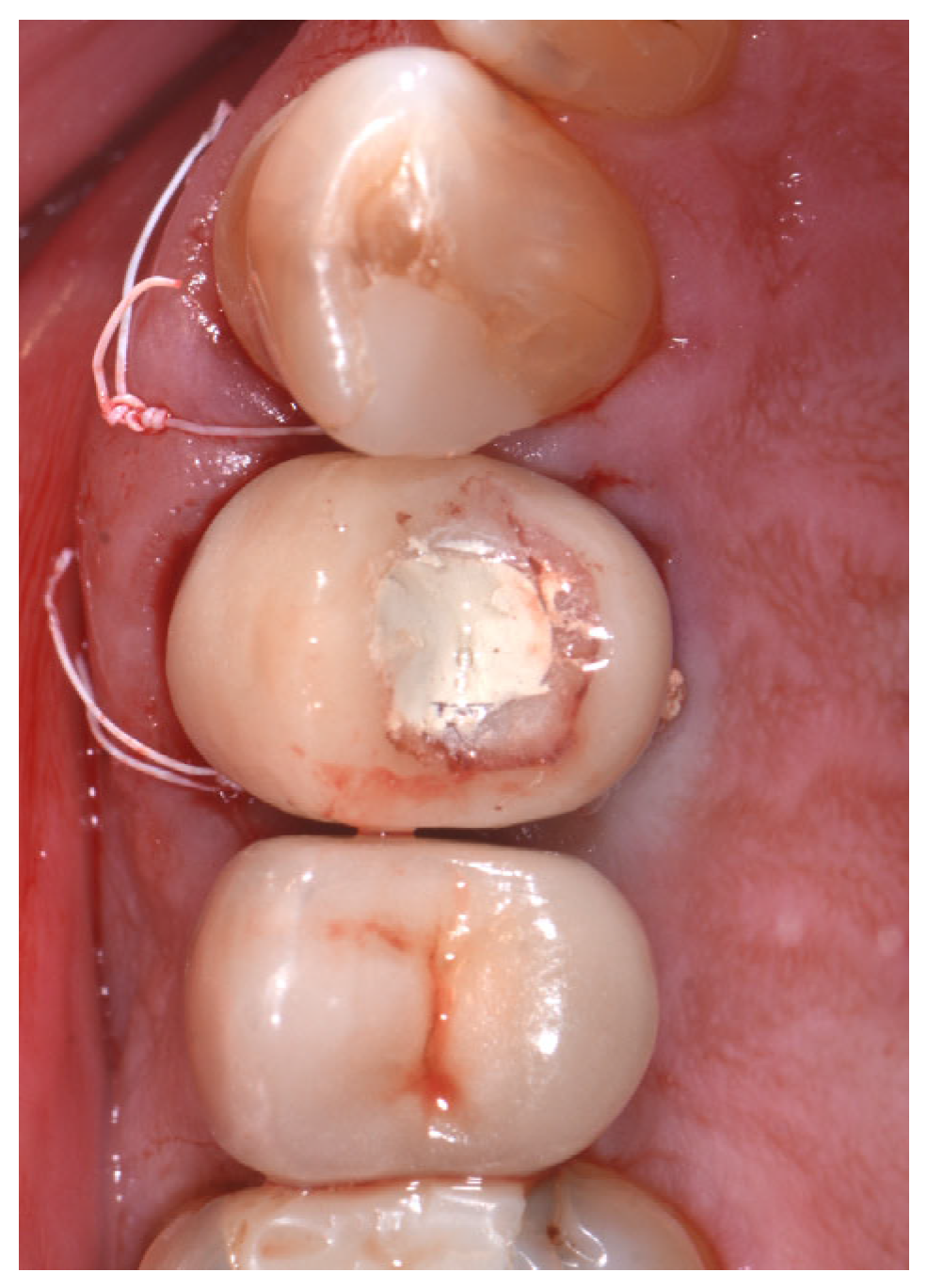
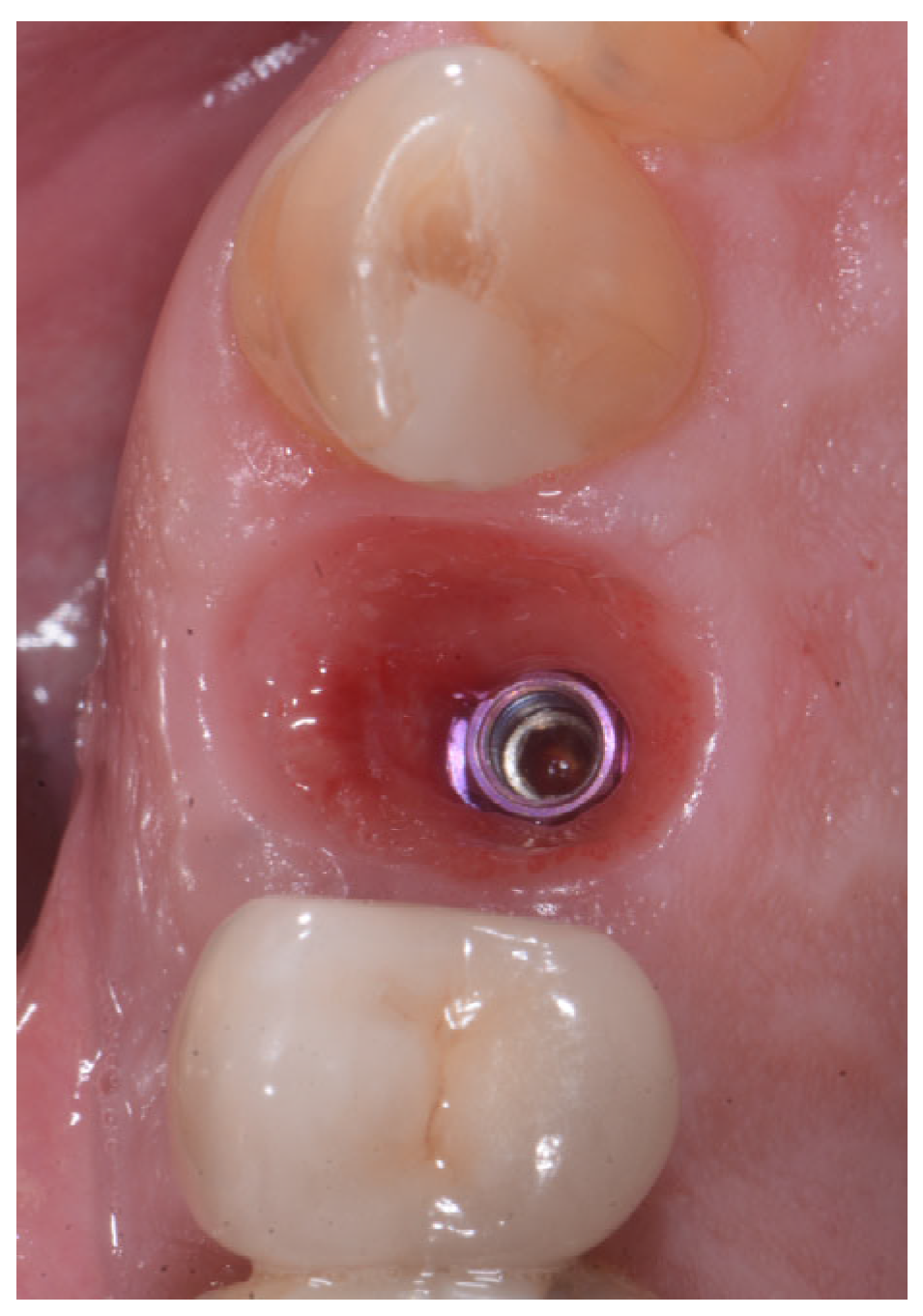
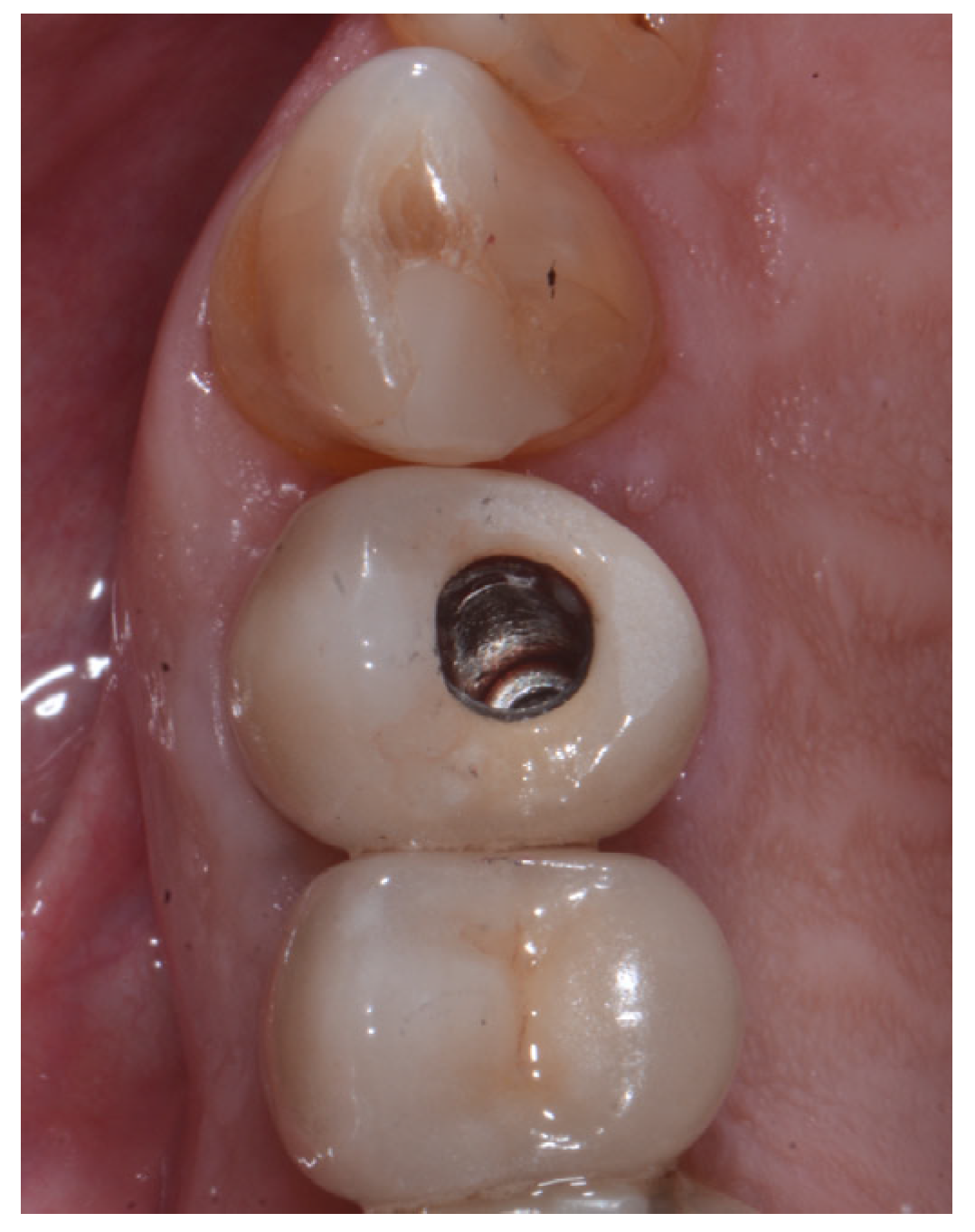
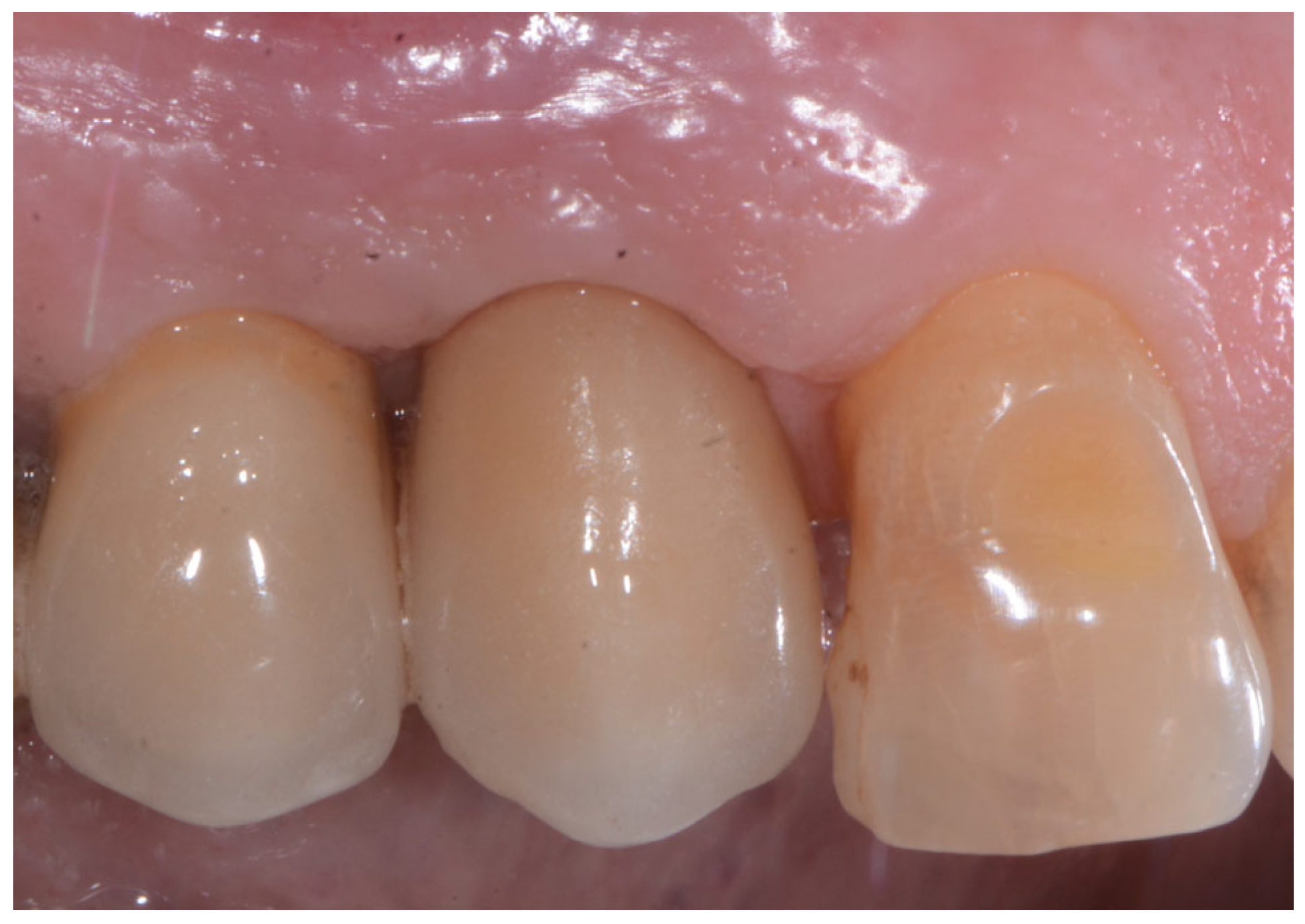
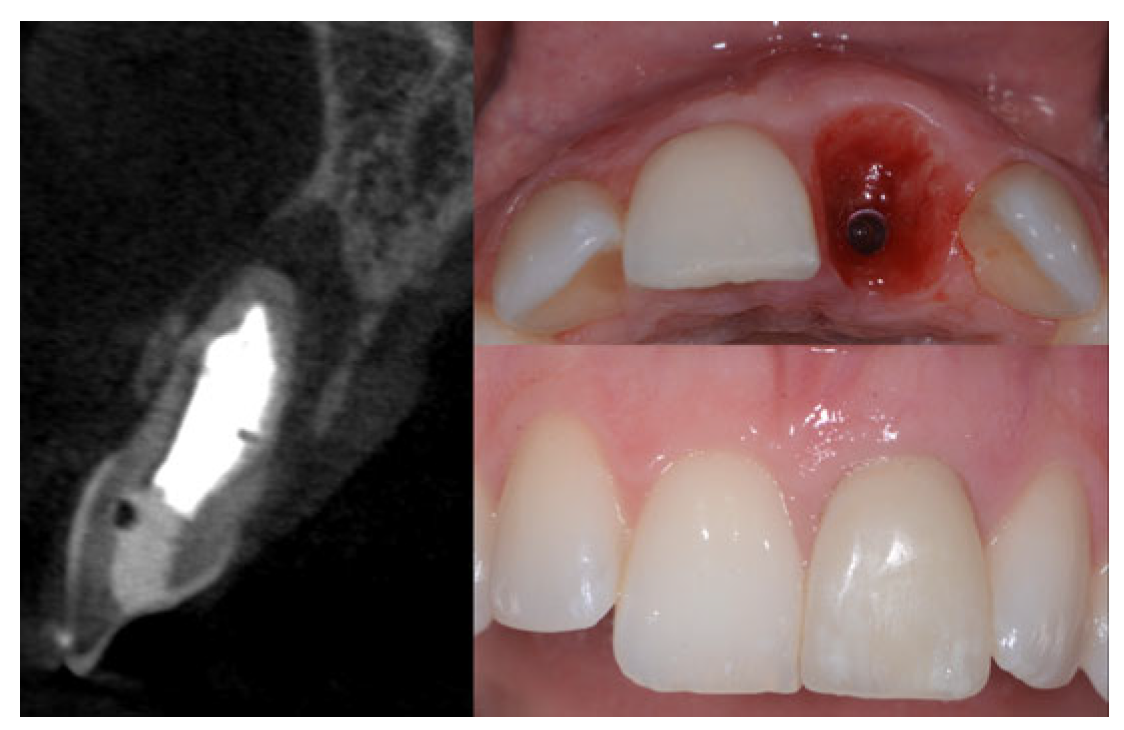
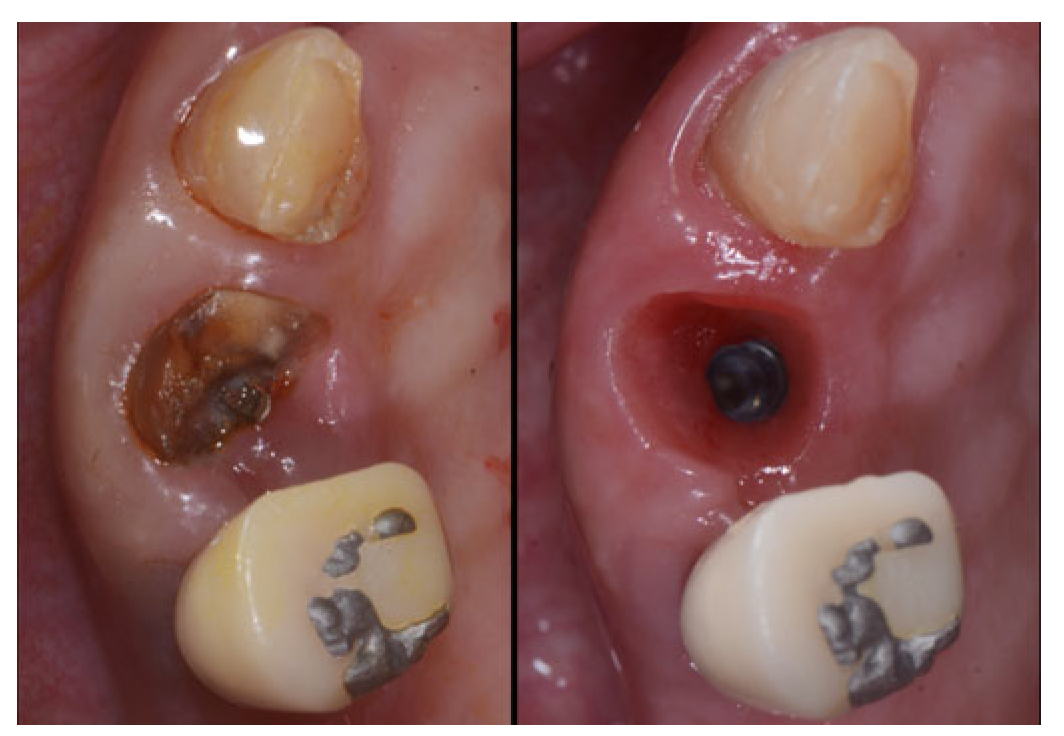
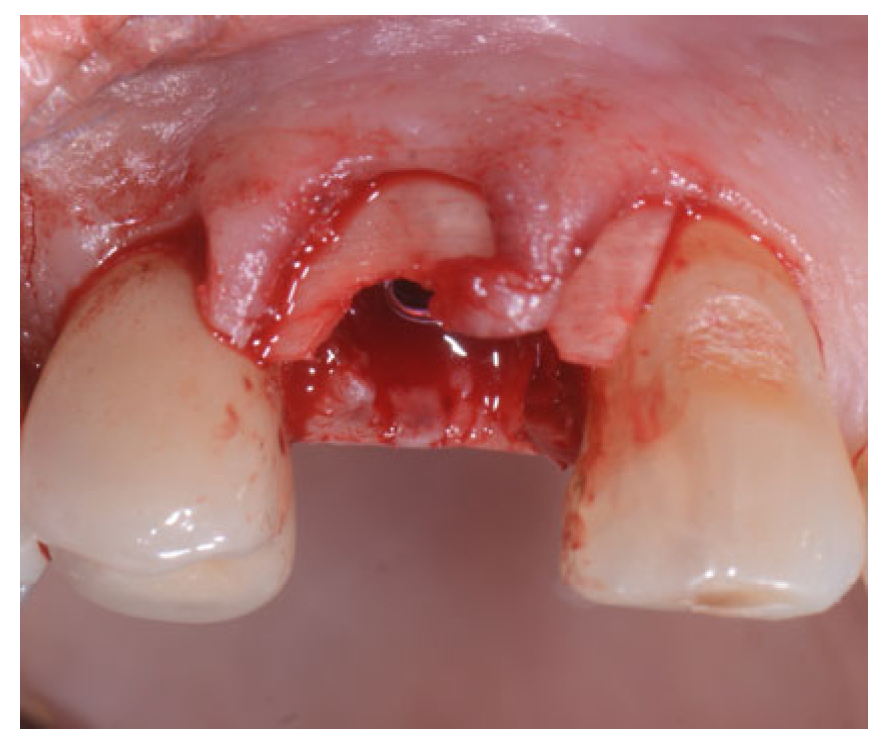
2.2. Surgical Technique
2.3. Indications
- Sockets that allow simultaneous implant placement;
- Sockets with intact buccal bone;
- Sockets with buccal fenestration.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Menchini-Fabris, G.-B.; Cosola, S.; Toti, P.; Hwang, M.H.; Crespi, R.; Covani, U. Immediate Implant and Customized Healing Abutment for a Periodontally Compromised Socket: 1-Year Follow-Up Retrospective Evaluation. J. Clin. Med. 2023, 12, 2783. [Google Scholar] [CrossRef]
- Wakankar, J.; Mangalekar, S.B.; Kamble, P.; Gorwade, N.; Vijapure, S.; Vhanmane, P. Comparative Evaluation of the Crestal Bone Level Around Pre- and Post-loaded Immediate Endoosseous Implants Using Cone-Beam Computed Tomography: A Clinico-Radiographic Study. Cureus 2023, 15, e34674. [Google Scholar] [CrossRef] [PubMed]
- Carosi, P.; Lorenzi, C.; Di Gianfilippo, R.; Papi, P.; Laureti, A.; Wang, H.-L.; Arcuri, C. Immediate vs. Delayed Placement of Immediately Provisionalized Self-Tapping Implants: A Non-Randomized Controlled Clinical Trial with 1 Year of Follow-Up. J. Clin. Med. 2023, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Dodi, A.; Petcu, L.C.; Nicolescu, M.I. Open Healing: A Minimally Invasive Protocol with Flapless Ridge Preservation in Implant Patients. Biology 2022, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Arcuri, L.; Carosi, P.; Nardi, A.; Kan, J. Clinical and radiological outcomes of novel digital workflow and dynamic navigation for single-implant immediate loading in aesthetic zone: 1-year prospective case series. Clin. Oral Implant. Res. 2021, 32, 1397–1410. [Google Scholar] [CrossRef]
- Schuh, P.L.; Wachtel, H.; Beuer, F.; Goker, F.; Del Fabbro, M.; Francetti, L.; Testori, T. Multi-Layer Technique (MLT) with Porcine Collagenated Cortical Bone Lamina for Bone Regeneration Procedures and Immediate Post-Extraction Implantation in the Esthetic Area: A Retrospective Case Series with a Mean Follow-Up of 5 Years. Materials 2021, 14, 5180. [Google Scholar] [CrossRef] [PubMed]
- Roberto, C.; Paolo, T.; Giovanni, C.; Ugo, C.; Bruno, B.; Giovanni-Battista, M.-F. Bone remodeling around implants placed after socket preservation: A 10-year retrospective radiological study. Int. J. Implant. Dent. 2021, 7, 74. [Google Scholar] [CrossRef]
- Muñoz-Cámara, D.; Águila, O.G.-D.; Pardo-Zamora, G.; Camacho-Alonso, F. Immediate post-extraction implants placed in acute periapical infected sites with immediate prosthetic provisionalization: A 1-year prospective cohort study. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e720–e727. [Google Scholar] [CrossRef]
- Grassi, A.; Memè, L.; Orsini, G.; Licini, C.; Orilisi, G.; Bambini, F. A New Surgical Protocol For Horizontal Ridge Augmentation: Simplified Apposition Technique. Human Histologic and Radiographic Analysis after 2.5 Years of Follow-Up, A Case Report. Arch. Clin. Med. Case Rep. 2020, 4, 239–252. [Google Scholar] [CrossRef]
- Hürzeler, M.B.; Zuhr, O.; Schupbach, P.; Rebele, S.F.; Emmanouilidis, N.; Fickl, S. The socket-shield technique: A proof-of-principle report. J. Clin. Periodontol. 2010, 37, 855–862. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Toti, P.; Crespi, G.; Covani, U.; Furlotti, L.; Crespi, R. Effect of Different Timings of Implant Insertion on the Bone Remodeling Volume around Patients’ Maxillary Single Implants: A 2–3 Years Follow-Up. Int. J. Environ. Res. Public Health 2020, 17, 6790. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Fabris, G.B.M.; Crespi, G.; Toti, P.; Marconcini, S.; Covani, U. Effects of Different Loading Protocols on the Bone Remodeling Volume of Immediate Maxillary Single Implants: A 2- to 3-year Follow-up. Int. J. Oral Maxillofac. Implant. 2019, 34, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Berberi, A.N.; Tehini, G.E.; Noujeim, Z.F.; Khairallah, A.A.; Abousehlib, M.N.; Salameh, Z.A. Influence of Surgical and Prosthetic Techniques on Marginal Bone Loss around Titanium Implants. Part I: Immediate Loading in Fresh Extraction Sockets. J. Prosthodont. 2014, 23, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Weinstein, T.; Scutellà, F.; Wang, H.-L.; Zucchelli, G. Implant placement in the esthetic area: Criteria for positioning single and multiple implants. Periodontology 2000 2018, 77, 176–196. [Google Scholar] [CrossRef]
- Gjelvold, B.; Kisch, J.; Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Clinical and radiographic outcome following immediate loading and delayed loading of single-tooth implants: Randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 549–558. [Google Scholar] [CrossRef]
- Quaranta, A.D.; Perrotti, V.D.; Putignano, A.M.; Malchiodi, L.M.; Vozza, I.D.; Guirado, J.L.D.C. Anatomical Remodeling of Buccal Bone Plate in 35 Premaxillary Post-Extraction Immediately Restored Single TPS Im-plants: 10-Year Radiographic Investigation. Implant. Dent. 2016, 25, 186–192. [Google Scholar] [CrossRef]
- Elad, A.; Rider, P.; Rogge, S.; Witte, F.; Tadić, D.; Kačarević, P.; Steigmann, L. Application of Biodegradable Magnesium Membrane Shield Technique for Immediate Dentoalveolar Bone Regeneration. Biomedicines 2023, 11, 744. [Google Scholar] [CrossRef]
- Park, W.-B.; Ko, J.-M.; Han, J.-Y.; Kang, P. Flap Extension Technique Using Intrasocket Granulation Tissue in Peri-Implant Osseous Defect: Case Series. Medicina 2022, 58, 1555. [Google Scholar] [CrossRef]
- Baskaran, P.; Prakash, P.; Appukuttan, D.; Mugri, M.H.; Sayed, M.; Subramanian, S.; Al Wadei, M.H.D.; Ahmed, Z.H.; Dewan, H.; Porwal, A.; et al. Clinical and Radiological Outcomes for Guided Implant Placement in Sites Preserved with Bioactive Glass Bone Graft after Tooth Extraction: A Controlled Clinical Trial. Biomimetics 2022, 7, 43. [Google Scholar] [CrossRef]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Ri genera® autologous micrografts in oral regeneration: Clinical, histological, and radiographical evaluations. Appl. Sci. 2020, 10, 5084. [Google Scholar] [CrossRef]
- Bernardi, S.; Mummolo, S.; Varvara, G.; Marchetti, E.; Continenza, M.A.; Marzo, G.; Macchiarelli, G. Bio-morphological evaluation of periodontal ligament fibroblasts on mineralized dentin graft: An in vitro study. J. Biol. Regul. Homeost. Agents 2019, 33, 275–280. [Google Scholar]
- Memè, L.; Rocchetti, R.; Tiriduzzi, P.; Sampalmieri, F.; Putignano, A.; Procaccini, M.; Lo Muzio, L.; Bambini, F. Osteopontin, osteocalcin and OB-cadherin expression in synthetic nanohydroxyapatite vs. bovine hydroxyapatite cultured osteoblastic-like cells. J. Biol. Regul. Homeost. Agents 2014, 28, 523–529. [Google Scholar]
- Memè, L.; Santarelli, A.; Marzo, G.; Emanuelli, M.; Nocini, P.F.; Bertossi, D.; Putignano, A.; Dioguardi, M.; Lo Muzio, L.; Bambini, F. Novel hydroxyapatite biomaterial covalently linked to raloxifene. Int. J. Immunopathol. Pharmacol. 2014, 27, 437–444. [Google Scholar] [CrossRef]
- Memè, L.; Strappa, E.M.; Monterubbianesi, R.; Bambini, F.; Mummolo, S. SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study. Appl. Sci. 2022, 12, 1480. [Google Scholar] [CrossRef]
- Grassi, A.; Bernardello, F.; Cavani, F.; Palumbo, C.; Spinato, S. Lindhe Three-Punch Alveolar Ridge Reconstruction Technique: A Novel Flapless Approach in Eight Consecutive Cases. Int. J. Periodontics Restor. Dent. 2021, 41, 875–884. [Google Scholar] [CrossRef]
- Nguyen, V.; Von Krockow, N.; Pouchet, J.; Weigl, P.M. Periosteal Inhibition Technique for Alveolar Ridge Preservation as It Applies to Implant Therapy. Int. J. Periodontics Restor. Dent. 2019, 39, 737–744. [Google Scholar] [CrossRef]
- Grassi, A.; Memè, L.; Strappa, E.M.; Martini, E.; Bambini, F. Modified Periosteal Inhibition (MPI) Technique for Extraction Sockets: A Case Series Report. Appl. Sci. 2022, 12, 12292. [Google Scholar] [CrossRef]
- Rossi, R.; Memè, L.; Strappa, E.M.; Bambini, F. Restoration of Severe Bone and Soft Tissue Atrophy by Means of a Xeno-genic Bone Sheet (Flex Cortical Sheet): A Case Report. Appl. Sci. 2023, 13, 692. [Google Scholar] [CrossRef]
- Rossi, R.; Modoni, M.; Monterubbianesi, R.; Dallari, G.; Memè, L. The ‘Guided Tissue Regeneration (GTR) Effect’ of Guided Bone Regeneration (GBR) with the Use of Bone Lamina: A Report of Three Cases with More than 36 Months of Follow-Up. Appl. Sci. 2022, 12, 11247. [Google Scholar] [CrossRef]
- Bambini, F.; Orilisi, G.; Quaranta, A.; Memè, L. Biological Oriented Immediate Loading: A New Mathematical Implant Vertical Insertion Protocol, Five-Year Follow-Up Study. Materials 2021, 14, 387. [Google Scholar] [CrossRef]
- Berglundh, T. Dimension of the periimplant mucosa Biological width revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Nardi, D.; Piattelli, A. Immediate provisionalization of implants placed in fresh extraction sockets using a definitive abutment: The chamber concept. Int. J. Periodontics Restor. Dent. 2013, 33, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.; Linder, E.; Wennström, J.; Lindhe, J. The influence of Bio-Oss Collagen on healing of an extraction socket: An experimental study in the dog. Int. J. Periodontics Restor. Dent. 2008, 28, 123–135. [Google Scholar]
- Lutz, R.; Sendlbeck, C.; Wahabzada, H.; Tudor, C.; Prechtl, C.; Schlegel, K.A. Periosteal elevation induces supracortical peri-implant bone formation. J. Craniomaxillofacial Surg. 2017, 45, 1170–1178. [Google Scholar] [CrossRef]
- Tan-Chu, J.H.P.; Tuminelli, F.J.; Kurtz, K.S.; Tarnow, D.P. Analysis of buccolingual dimensional changes of the extraction socket using the “ice cream cone” flapless grafting technique. Int. J. Periodontics Restor. Dent. 2014, 34, 399–403. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Ridge preservation with the use of Bio-Oss collagen: A 6-month study in the dog. Clin. Oral Implant. Res. 2009, 20, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Marconcini, S.; Denaro, M.; Cosola, S.; Gabriele, M.; Toti, P.; Mijiritsky, E.; Proietti, A.; Basolo, F.; Giammarinaro, E.; Covani, U. Myofibroblast Gene Expression Profile after Tooth Extraction in the Rabbit. Materials 2019, 9, 12–22. [Google Scholar] [CrossRef]
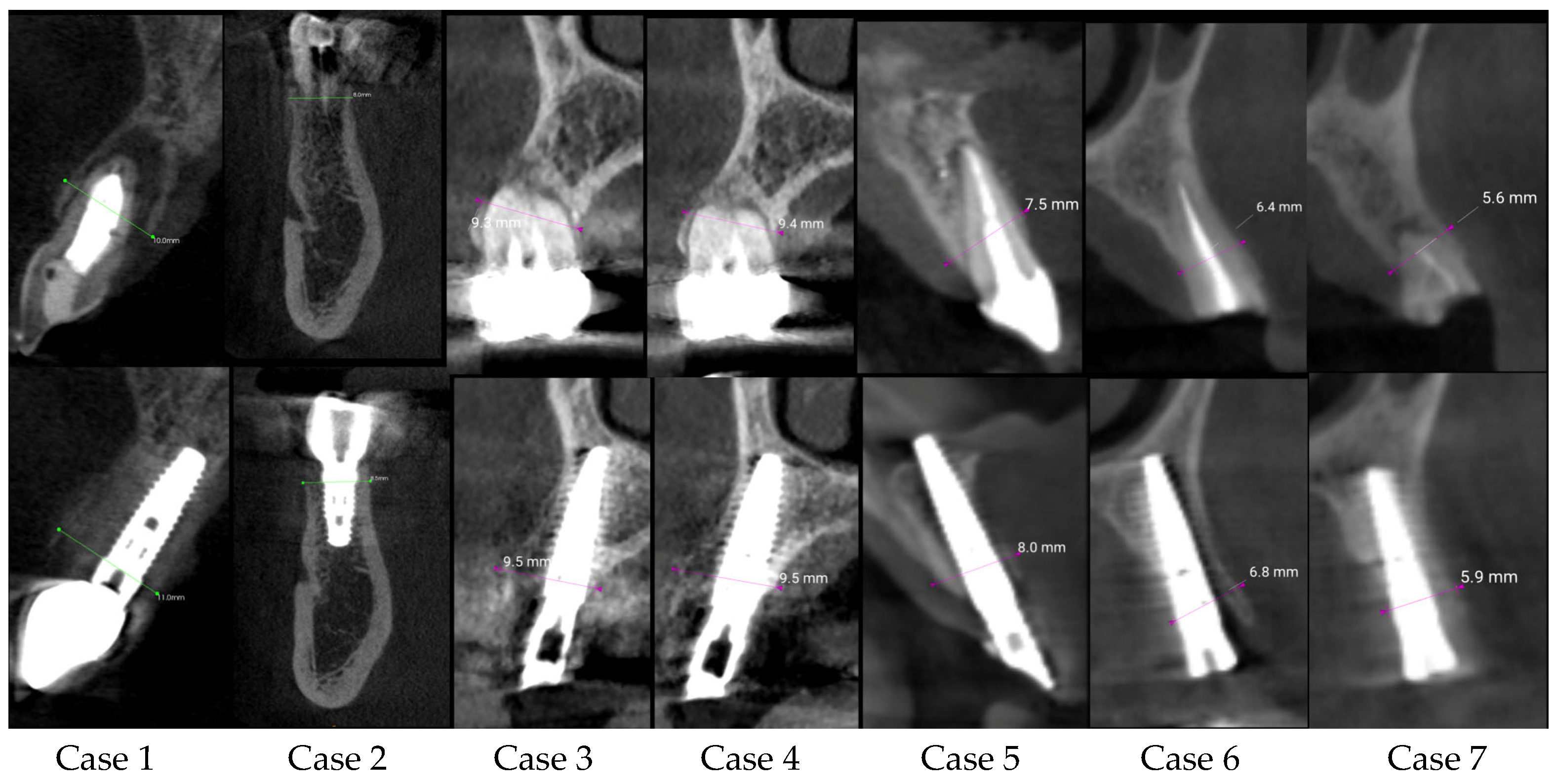
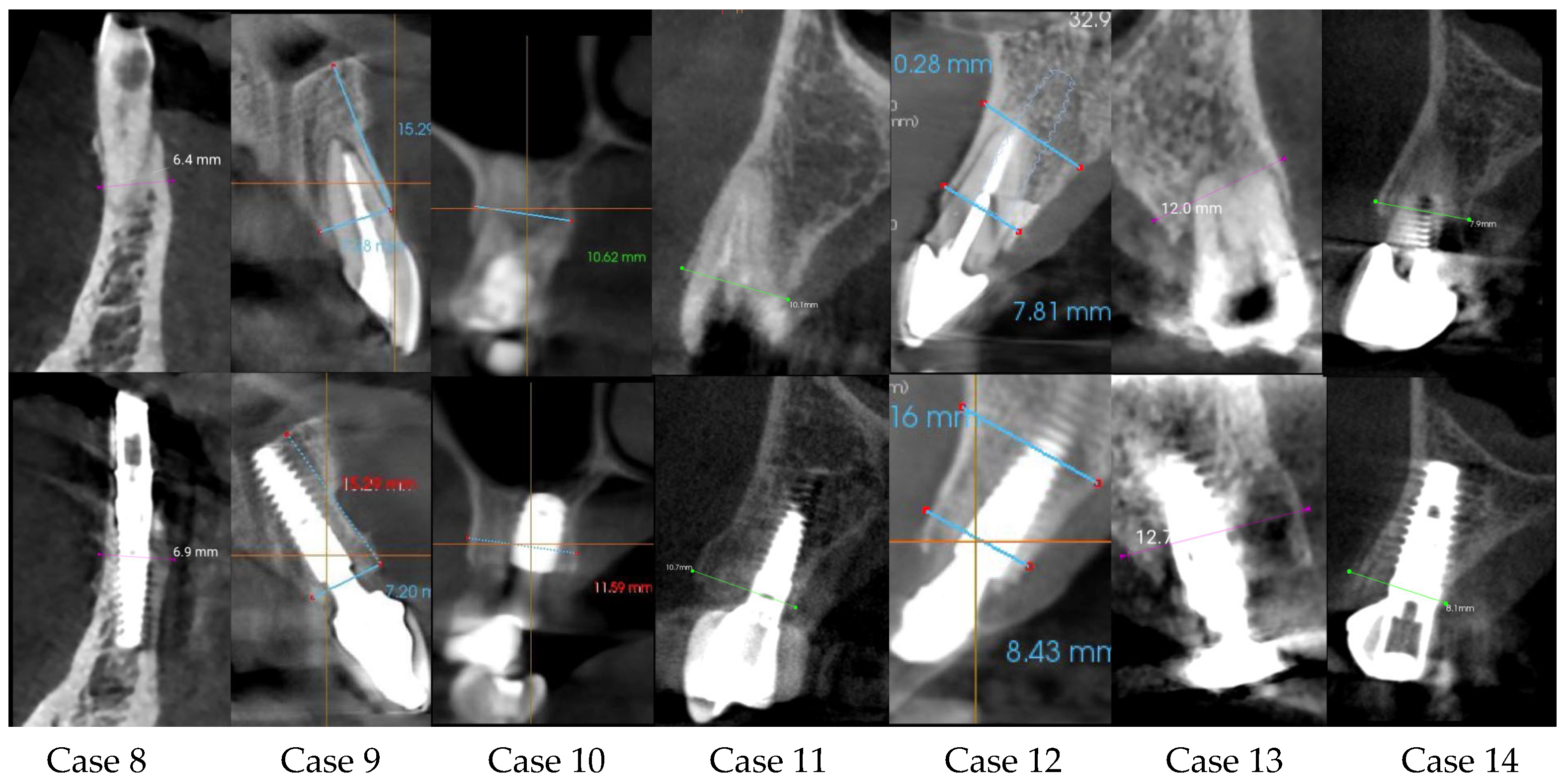
| Center/Case | Sex/Age | Tooth Number | Ridge Thickness before (mm) | Ridge Thickness after (mm) | Follow-Up Time | Difference | Immediate Loading |
|---|---|---|---|---|---|---|---|
| C1 case 1 | Female/31 | 21 | 10.00 | 11.00 | 6 months | 1.00 | yes |
| C1 case 2 | Male/63 | 45 | 8.00 | 8.50 | 12 months | 0.50 | no |
| C2 case 3 | Female/50 | 15 | 9.00 | 9.50 | 6 months | 0.50 | yes |
| C2 case 4 | Female/50 | 14 | 9.40 | 9.50 | 6 months | 0.10 | yes |
| C2 case 5 | Female/71 | 21 | 7.50 | 8.00 | 6 months | 0.50 | yes |
| C2 case 6 | Female/63 | 23 | 6.40 | 6.80 | 6 months | 0.40 | yes |
| C2 case 7 | Female/63 | 24 | 5.60 | 5.90 | 6 months | 0.30 | no |
| C2 case 8 | Male/68 | 42 | 6.40 | 6.90 | 6 months | 0.50 | yes |
| C3 case 9 | Male/62 | 21 | 7.18 | 7.20 | 8 months | 0.02 | yes |
| C3 case 10 | Male/58 | 17 | 10.52 | 11.69 | 5 months | 1.17 | yes |
| C1 case 11 | Male/68 | 24 | 10.10 | 10.70 | 6 months | 0.60 | yes |
| C3 case 12 | Female/46 | 11 | 7.81 | 8.43 | 7 months | 0.62 | yes |
| C2 case 13 | Male/77 | 24 | 12.00 | 12.70 | 6 months | 0.70 | yes |
| C1 case 14 | Male/74 | 13 | 7.9 | 8.1 | 4 months | 0.20 | no |
| Before | After | p-Value | ||
|---|---|---|---|---|
| Ridge thickness (mm) | 14 | 8.4 ± 1.8 | 9.0 ± 2.0 | p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, A.; Memè, L.; Rossi, R.; Faustini, F.; Marinotti, F.; Bambini, F.; Mummolo, S. Modified Periosteal Inhibition (MPI) Technique for Immediate Implants: A Multi-Center Retrospective Case Series Study. Appl. Sci. 2023, 13, 9034. https://doi.org/10.3390/app13159034
Grassi A, Memè L, Rossi R, Faustini F, Marinotti F, Bambini F, Mummolo S. Modified Periosteal Inhibition (MPI) Technique for Immediate Implants: A Multi-Center Retrospective Case Series Study. Applied Sciences. 2023; 13(15):9034. https://doi.org/10.3390/app13159034
Chicago/Turabian StyleGrassi, Andrea, Lucia Memè, Roberto Rossi, Fabio Faustini, Fabio Marinotti, Fabrizio Bambini, and Stefano Mummolo. 2023. "Modified Periosteal Inhibition (MPI) Technique for Immediate Implants: A Multi-Center Retrospective Case Series Study" Applied Sciences 13, no. 15: 9034. https://doi.org/10.3390/app13159034
APA StyleGrassi, A., Memè, L., Rossi, R., Faustini, F., Marinotti, F., Bambini, F., & Mummolo, S. (2023). Modified Periosteal Inhibition (MPI) Technique for Immediate Implants: A Multi-Center Retrospective Case Series Study. Applied Sciences, 13(15), 9034. https://doi.org/10.3390/app13159034








