Effect of Alginate Proportion in Glycerol-Reinforced Alginate–Starch Biofilms on Hydrogen Bonds by Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Mixtures Preparation
2.2. Raman Spectroscopy
2.3. Statistical Analysis
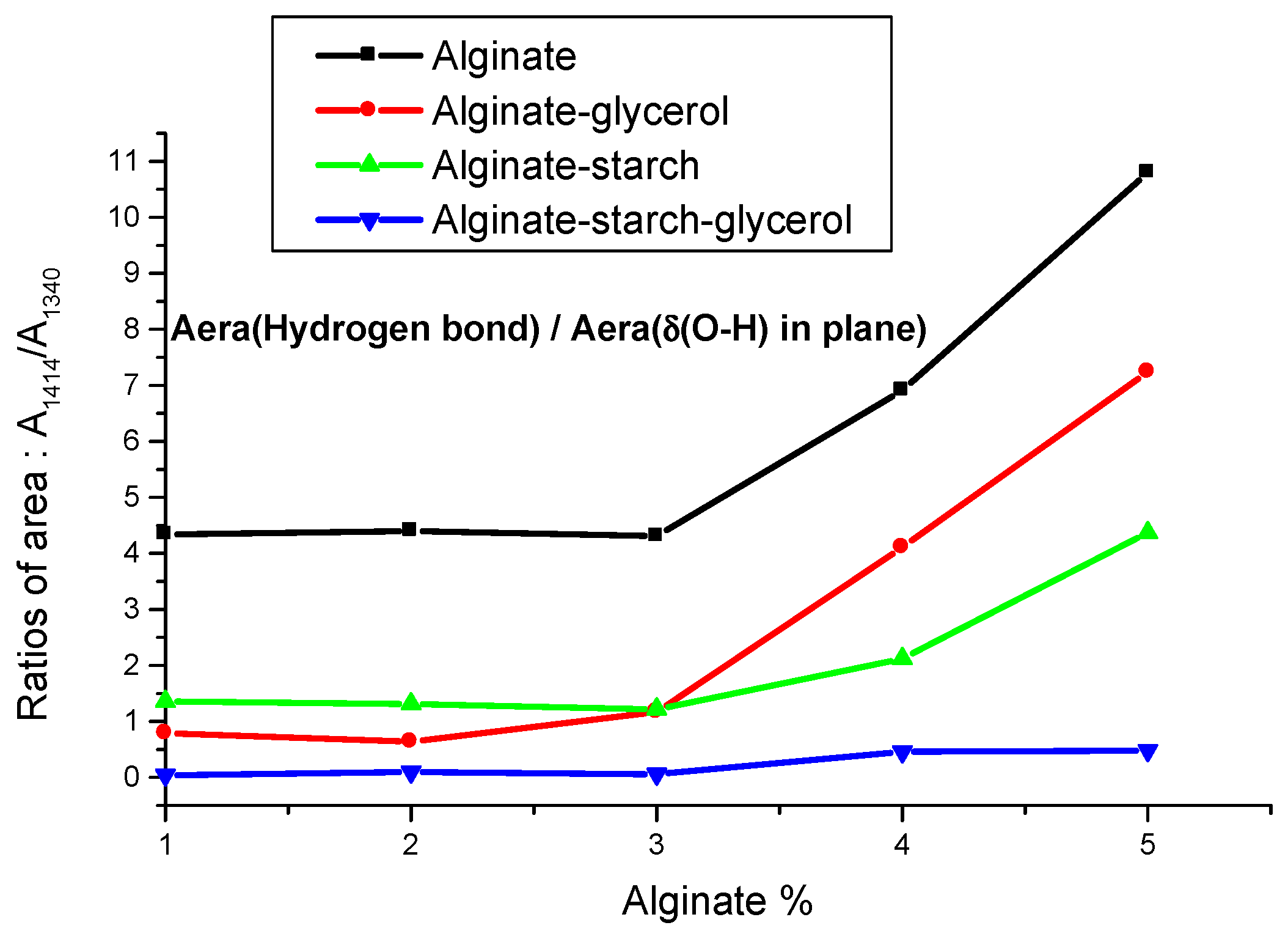
3. Results and Discussion
3.1. Alginate Films
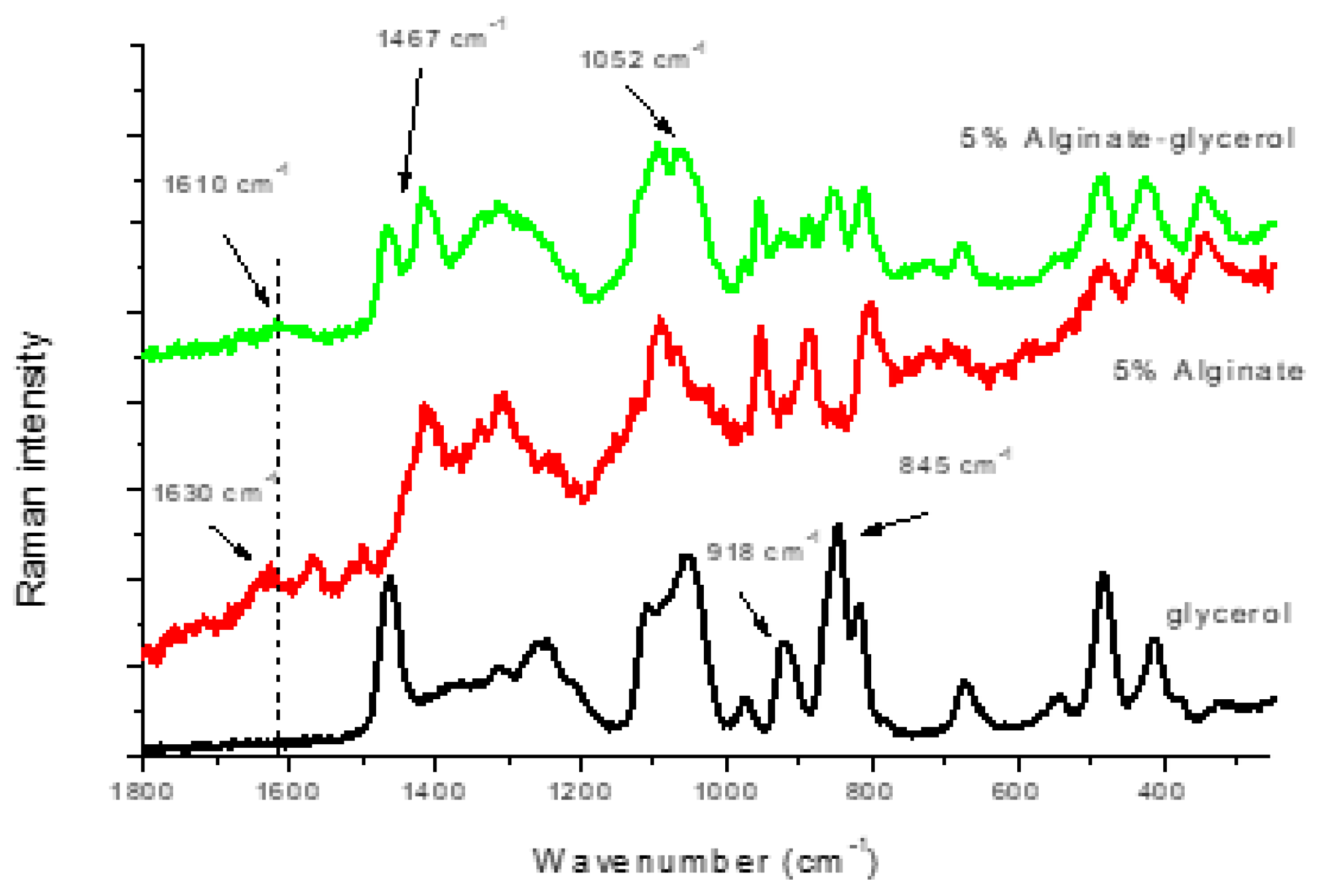
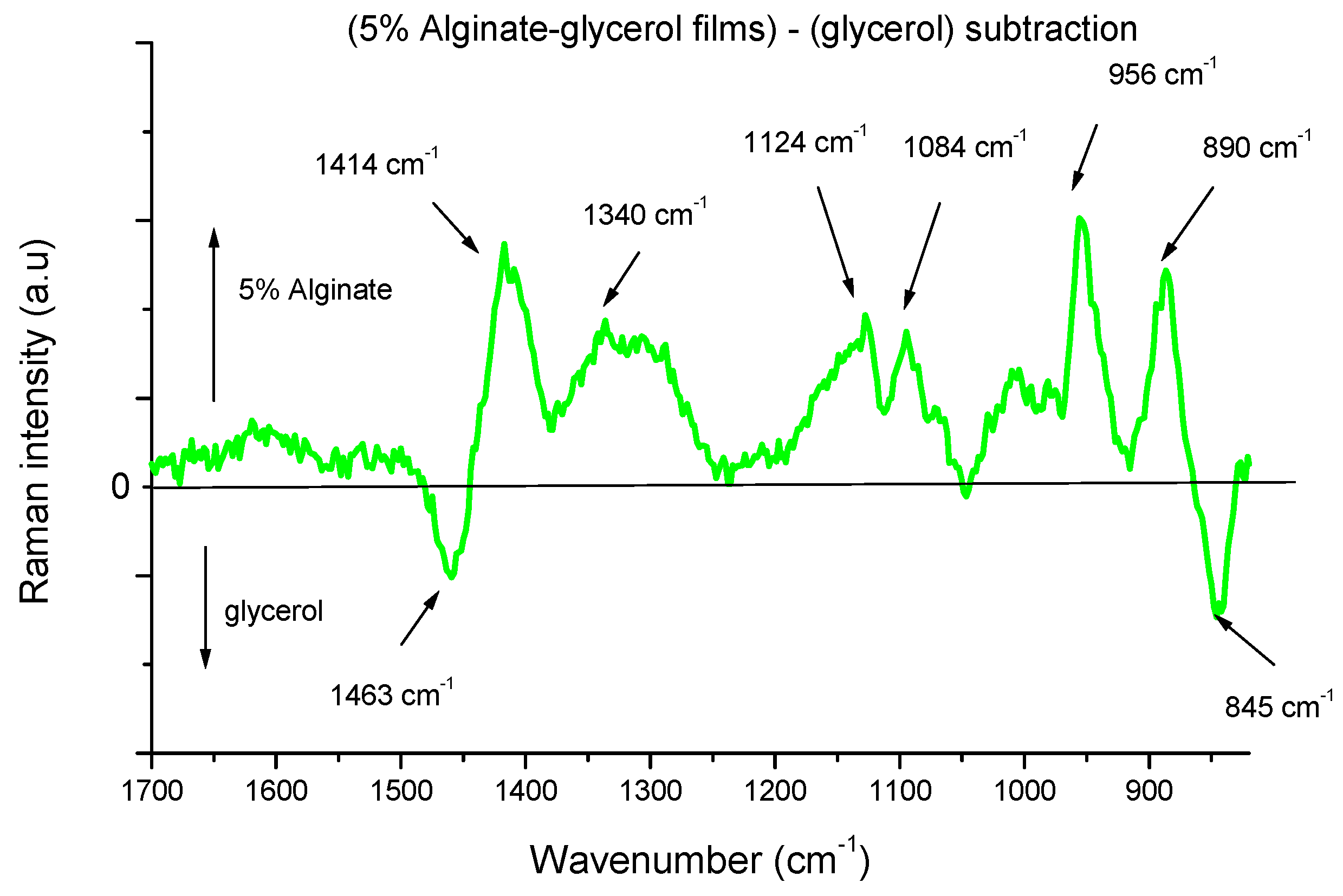
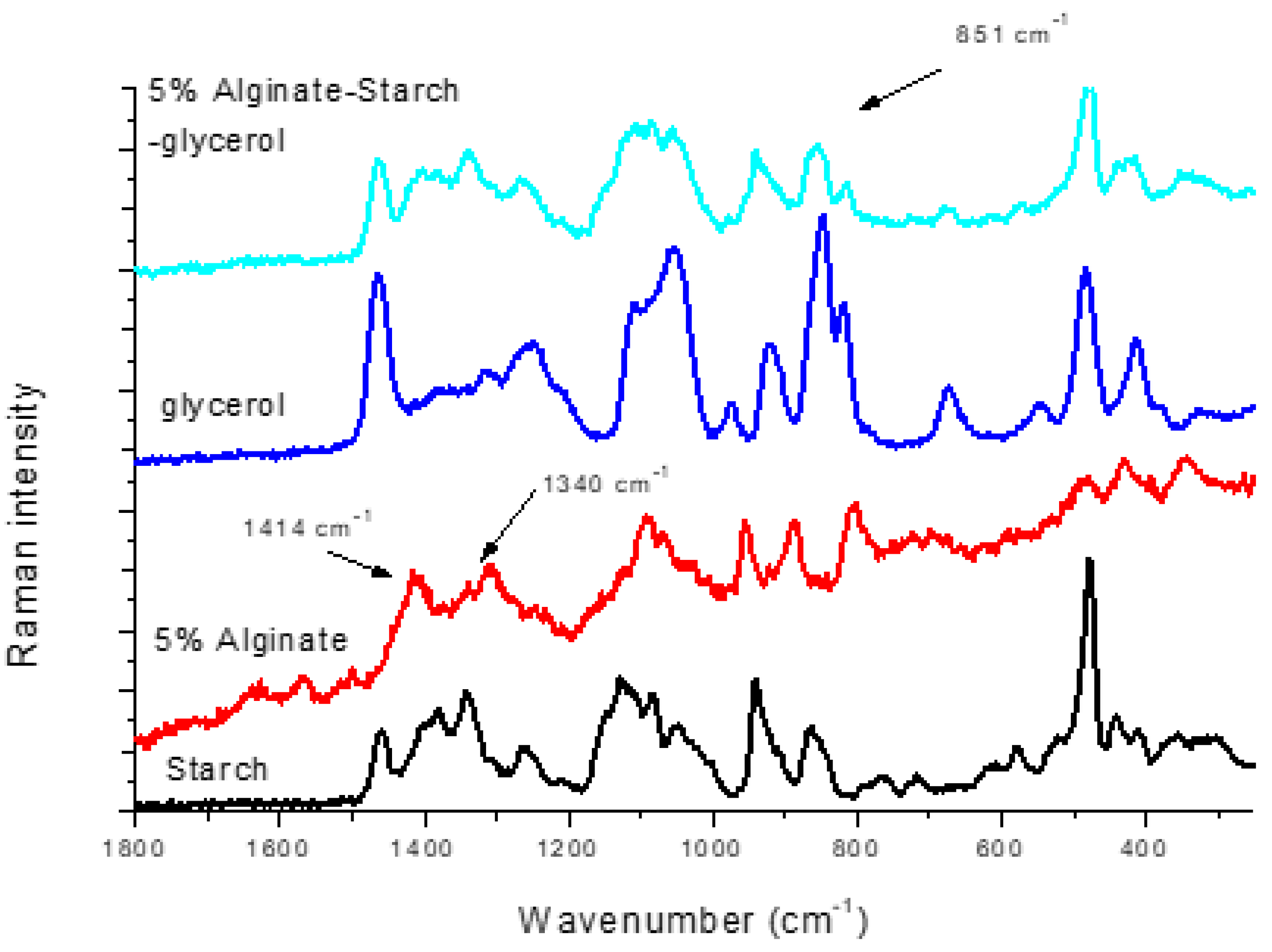
3.2. Alginate-Glycerol Films
3.3. Alginate–Starch Films
3.4. Alginate–Starch–Glycerol Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Starch-Based Adhesives for Wood/Wood Composite Bonding: Review. Open J. Polym. Chem. 2017, 7, 19–32. [Google Scholar] [CrossRef]
- Wang, H.; Liang, J.; Zhang, J.; Zhou, X.; Du, G. Performance of urea-formaldehyde adhesive with oxidized cassava starch. BioResources 2017, 12, 7590–7600. [Google Scholar] [CrossRef]
- Patel, A.K.; Michaud, P.; Petit, E.; De Baynast, H.; Grédiac, M.; Mathias, J.D. Developpment of a chitosan-based adhesive. Application to wood binding. J. Appl. Polym. Sci. 2013, 127, 5014–5021. [Google Scholar] [CrossRef]
- Al-Marsy, W.A.; Haider, S.; Mahmood, A.; Khan, M.; Adil, S.F.; Siddiqui, M.R.H. Evaluation of the Thermal and Morphological Properties of ϒ-Irradiated Chitosan-Glycerol-Based Polymeric Films. Processes 2021, 9, 1783–1796. [Google Scholar]
- Gumoska, A.; Robles, E.; Kowaluk, G. Evaluation of functional Features of Lignocellulosic Particle Composites Containing Biopolymer Binders. Materials 2021, 14, 7718. [Google Scholar] [CrossRef]
- Kadri, R.; Elkhoury, K.; Ben Messaoud, G.; Kahn, C.; Tamayol, A.; Mano, J.F.; Arab-Tehrany, E.; Sanchez-Gonzalez, L. Physicochemical Interactions in Nanofunctionalized Alginate/GeIMA IPN Hydrogels. Nanomaterials 2021, 11, 2256. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Mistra, M. Perspectives on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Chen, M.; Runge, T.; Wang, L.; Li, R.; Feng, J.; Shu, X.L.; Shi, Q.S. Hydrogen bonding impact on chitosan plasticization. Carbohydr. Polym. 2018, 200, 115–121. [Google Scholar] [CrossRef]
- Toxqui-Teran, A.; Leyva-Porras, C.; Ruiz-Cabrera, M.A.; Cruz-Alcantar, P.; Saavedra-Leos, M.Z. Thermal study of polyols for the technological application as plasticizers in food industry. Polymers 2018, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shao, C.; Miao, Z.; Lu, P. Development of leftover rice/gelatin interpenetrating polymer network films for food packaging. Green Process. Synth. 2021, 10, 37–48. [Google Scholar] [CrossRef]
- El Miri, N.; Aziz, F.; Aboulkas, A.; El Bouchti, M.; Ben Youcef, H.; El Achady, M. Effect of plasticizers on physiochemical properties of cellulose nanocrystals filled alginate bionanocomposite films. Adv. Polym. Technol. 2018, 37, 3171–3185. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Structural Features of Starch Granules I. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 149–192. [Google Scholar] [CrossRef]
- Carvaloh, A.J.F. Starch: Major sources, properties and applications as thermoplastic materials. In Monomers, Polymers and Composites from Renewable Resources, 3rd ed.; Belgacem, M.N., Gandini, A., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; pp. 321–342. [Google Scholar]
- Raus, R.A.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Muller, K.; Sckmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Canovas, G.V. Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT—Food Sci. Technol. 2008, 41, 359–366. [Google Scholar] [CrossRef]
- Kelis Cardoso, V.G.; Poppi, R.J. Cleaner and Faster method to detect adulteration in cassava starch using Raman spectroscopy and one-class support vector machine. Food Control 2021, 125, 107917. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, Y.S.; Zhao, J.S.; Li, Z.Y.; Chen, H.H. A pH-sensitive curcumin loaded microemulsion-filled alginate and porous starch composite gels: Characterization, in vitro release kinetics and biological activity. Int. J. Biol. Macromol. 2021, 182, 1863–1873. [Google Scholar] [CrossRef]
- Vidal Urquiza, T.K.; Perales Pérez, O.; Galvez Saldana, M. Effect of the cross-linking with calcium ions on the structural and Thermo-mechanical properties of alginate films. Mater. Res. Soc. Symp. Proc. 2011, 1355, 1136. [Google Scholar] [CrossRef]
- Lopez-Cordoba, A.; Deladino, L.; Martino, M. Corn starch-calcium alginate matrices for simultaneous carrying of zinc and yerba mate antioxidants. LWT—Food Sci. Technol. 2014, 59, 641–648. [Google Scholar] [CrossRef]
- Parad, N.D.T.; Tomar, V. Raman spectroscopy of algae: A review. J. Nanomed. Nanotechnol. 2012, 3, 131. [Google Scholar] [CrossRef]
- Kaczmarska, K.; Grabowska, B.; Spychaj, T.; Zdanowicz, M.; Sitarz, M.; Bobrowski, A.; Cukrowicz, S. Effect of microwave treatment on structure of binders based on sodium carboxymethyl starch: FT-IR, FT-Raman and XRD investigations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Bresson, S.; El Marssi, M.; Khelifa, B. Raman spectroscopy investigation of various saturated monoacid triglycerides. Chem. Phys. Lipids 2005, 134, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Bresson, S.; El Marssi, M.; Khelifa, B. Conformational influences of the polymorphic forms on the C=O and C-H stretching modes of five saturated monoacid triglycerides studied by Raman spectroscopy at various temperatures. Vib. Spectrosc. 2006, 40, 263–269. [Google Scholar] [CrossRef]
- El Hadri, M.; Achahbar, A.; El Khamkhami, J.; Khelifa, B.; Faivre, V.; Abbas, O.; Bresson, S. Lyotropic behavior of Gelucire 50/13 by XRD, Raman and IR spectroscopies according to hydration. Chem. Phys. Lipids 2016, 200, 11–23. [Google Scholar] [CrossRef]
- Bresson, S.; Rousseau, D.; Ghosh, S.; El Marssi, M.; Faivre, V. Raman spectroscopy of the polymorphic forms and liquid state of cocoa butter. Eur. J. Lipid Sci. Technol. 2011, 113, 992–1004. [Google Scholar] [CrossRef]

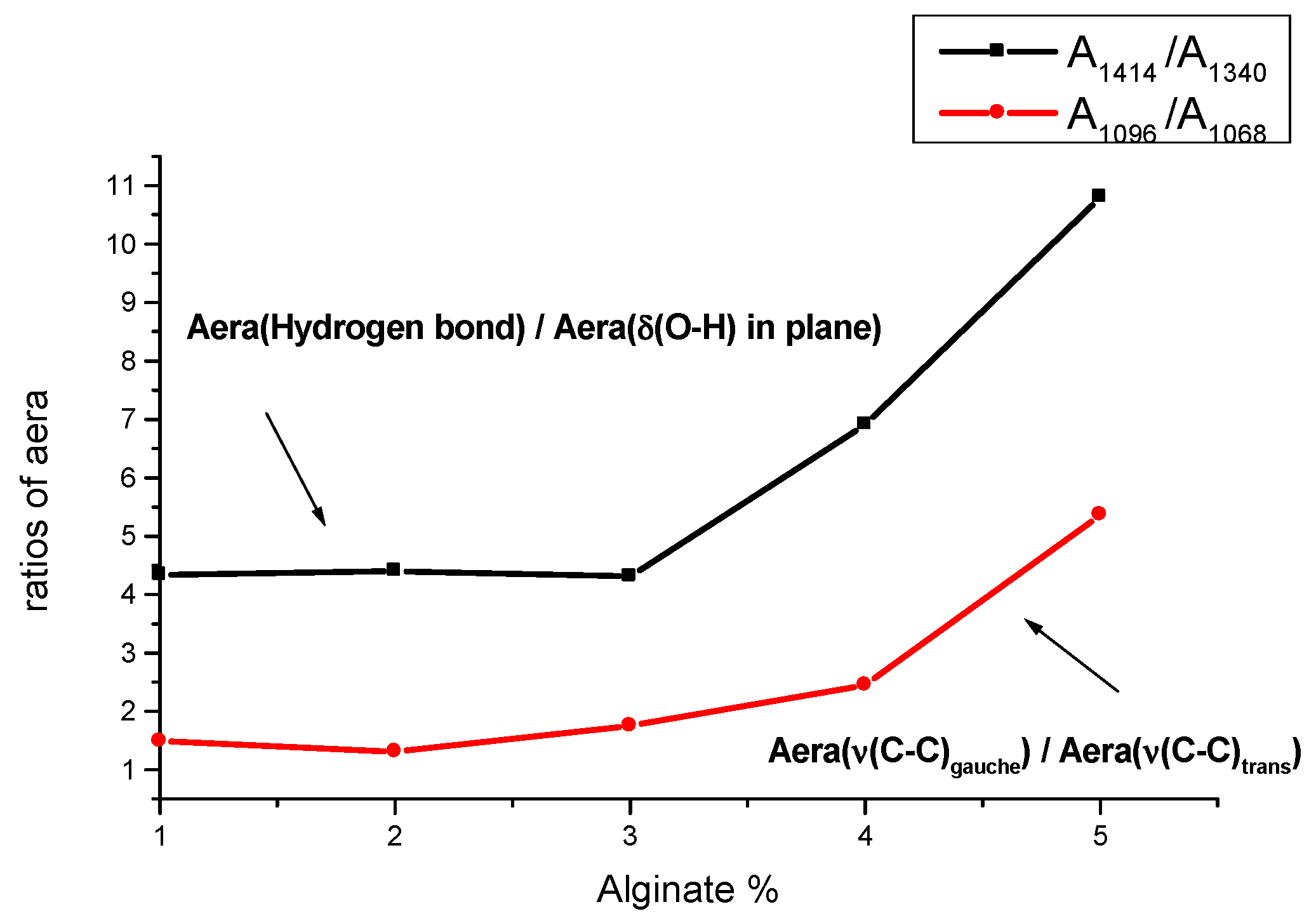
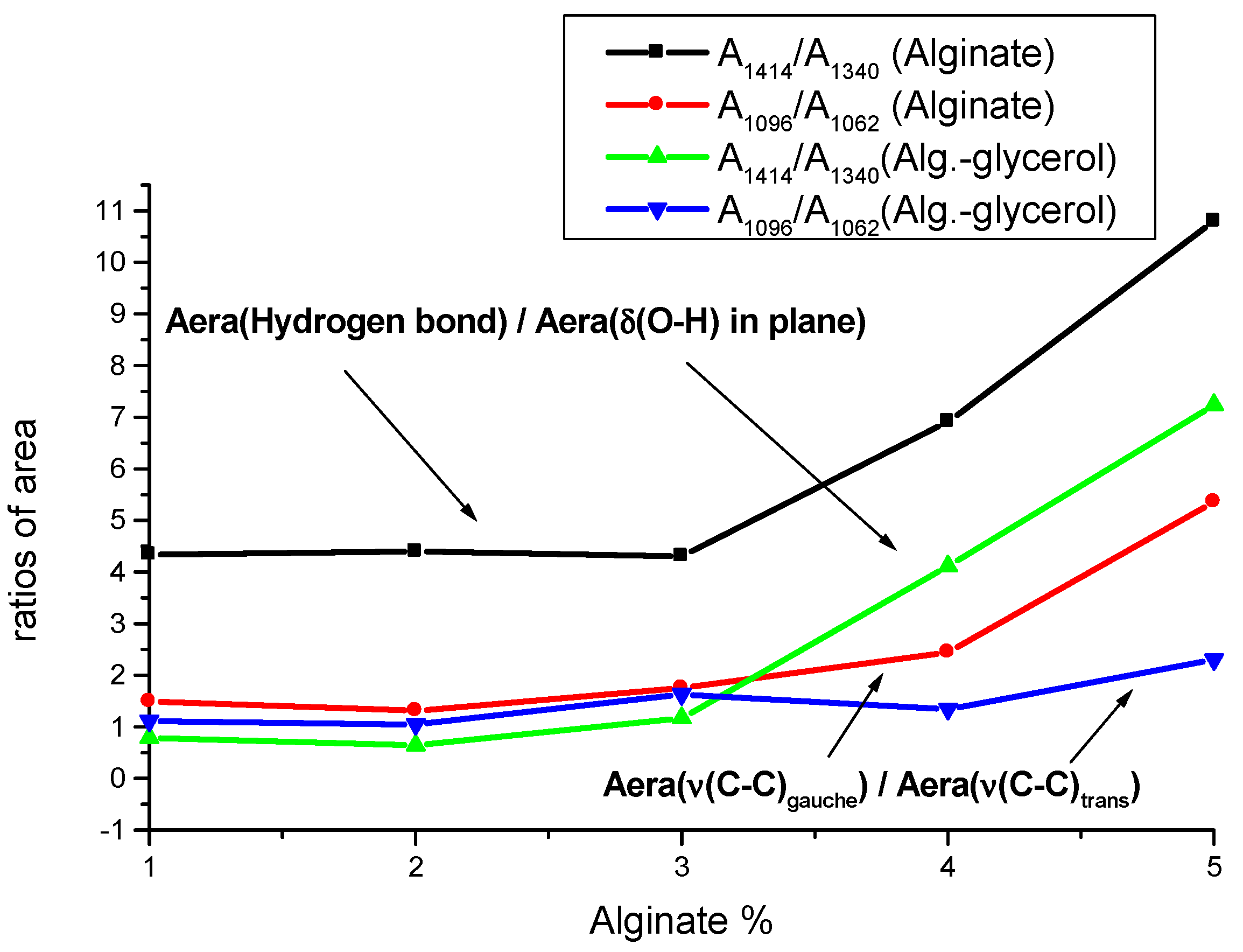
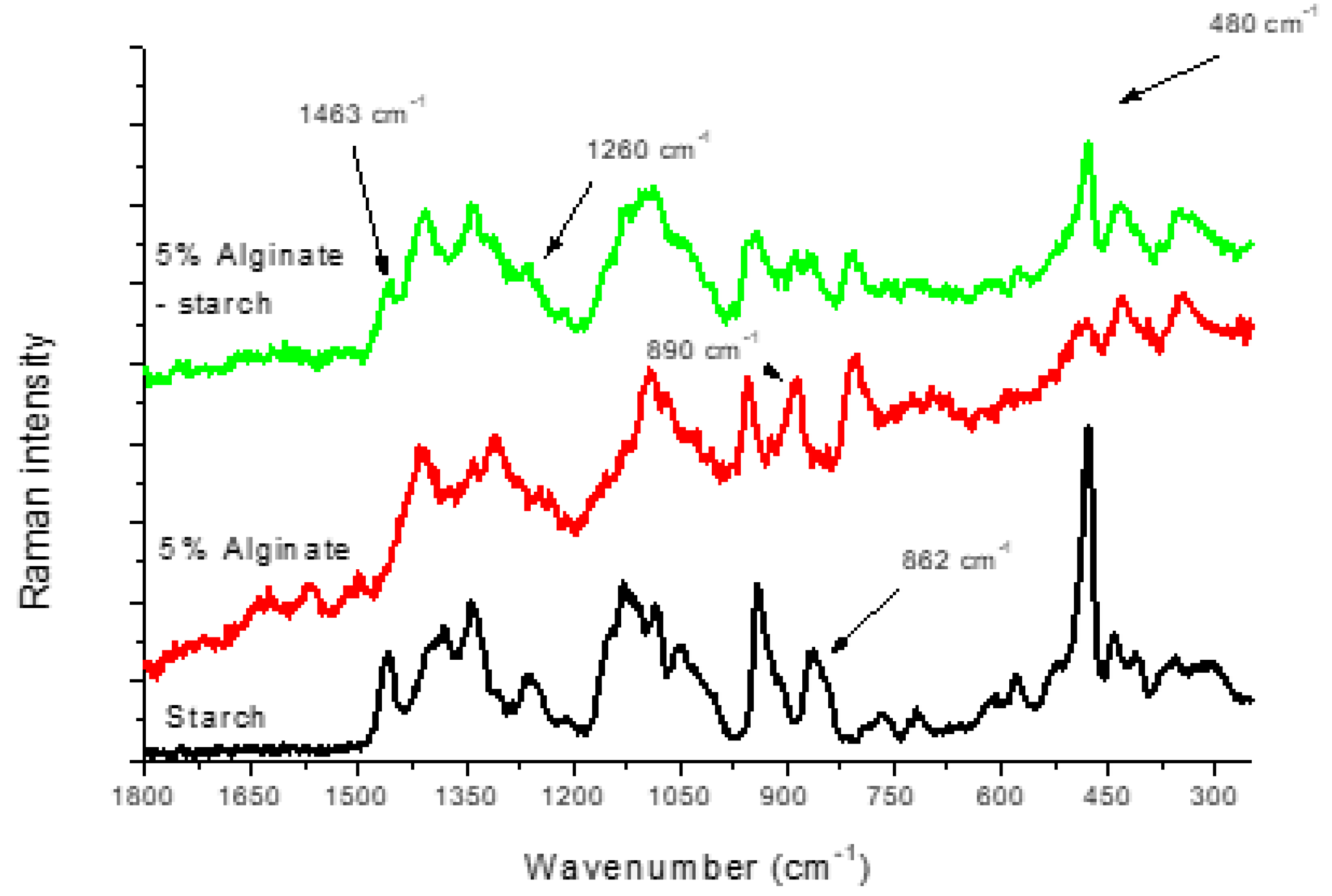

| Alginate 1% | Alginate 2% | Alginate 3% | Alginate 4% | Alginate 5% | Assignments |
|---|---|---|---|---|---|
| 345 | 345 | 345 | 345 | 343 | δ(C-O) out of plane |
| 430 | 431 | 431 | 430 | 428 | δ(C-O) in plane |
| 497 | 496 | 496 | 498 | 491 | δ(C-O) in plane |
| 733 | 730 | 730 | 731 | 710 | δ(O-H) out of plane |
| 806 | 806 | 806 | 806 | 805 | ν(C-O-C) |
| 889 | 889 | 889 | 889 | 885 | ν(C-O-C) |
| 920 * | 920 * | 921 * | 920 * | 922 | ν(C-O-C) |
| 955 | 954 | 954 | 953 | 954 | ν(C-O-C) |
| 982 | 983 | 983 | 982 | 980 | ν(C-O-C) |
| 1034 | 1034 | 1035 | 1034 | 1032 | ν(C-O-C)) |
| 1067 | 1068 | 1068 | 1069 | 1067 | νas(C-C)T |
| 1096 | 1097 | 1096 | 1096 | 1094 | ν(C-C)G |
| 1130 | 1129 | 1130 | 1128 | 1127 | νs(C-C)T, ν(C-O)T |
| 1162 * | 1165 * | 1164 * | 1162 * | 1160 * | νs(C-C)T, ν(C-O)G |
| 1270 * | 1291 * | 1271 * | 1291 * | 1275 * | νs(C-C)G |
| 1307 | 1317 | 1307 | 1315 | 1310 | δ(O-H) in plane |
| 1344 | 1341 | 1344 | 1340 | 1340 | δ(O-H) in plane |
| 1361 * | 1361 * | 1364 * | 1360 * | 1360 * | δ(O-H) in plane |
| 1414 | 1414 | 1413 | 1414 | 1412 | νs(COO−), H- bond |
| 1500 | hydrogen bond | ||||
| 1570 | hydrogen bond | ||||
| 1611 | 1612 | 1613 | 1612 | νas(COO−) | |
| 1630 | νas(COO−) | ||||
| 2935 | 2935 | 2935 | 2935 | 2934 | ν(CH-OH) |
| 5% Alginate | Glycerol | Starch | 5% Alginate–Glycerol | 5% Alginate–Starch | 5% Alginate–Glycerol–Starch | Assignments |
|---|---|---|---|---|---|---|
| 343 | 346 | 348 | 343 | δ(C-O) out of plane | ||
| 376 | δ(C-O) in plane | |||||
| 414 | 409 | 413 | 415 | δ(C-O) in plane | ||
| 428 | 425 | δ(C-O) in plane | ||||
| 441 | 440 | 440 | δ(C-O) in plane | |||
| 483 | 480 | 480 | 480 | δ(C-O) in plane | ||
| 491 | 494 | 488 | δ(C-O) in plane | |||
| 520 | 521 | 521 | ν(C-O-C) | |||
| 546 | 550 | ν(C-O-C), δ(O-H) | ||||
| 577 | 577 | 576 | δ(O-H) out of plane | |||
| 710 | 711 | 720 | 720 | 720 | δ(O-H) out of plane | |
| 765 | 762 | 763 | δ(O-H) out of plane | |||
| 805 | 795 | ν(C-O-C) | ||||
| 818 | 814 | 814 | ν(C-O-C) | |||
| 845 | 848 | 847 | 850 | 850 | τ(CH2-OH) | |
| 865 | 861 | 867 | 866 | τ(CH2-OH) | ||
| 885 | 890 | 890 | 889 | ν(C-O-C) | ||
| 904 | ν(C-O-C) | |||||
| 922 | 920 | 921 | 922 | 923 | 923 | ν(C-O-C) |
| 940 | 941 | 941 | ν(C-O-C) | |||
| 954 | 956 | 956 | ν(C-O-C) | |||
| 971 | ν(C-O-C) | |||||
| 980 | 980 | 976 | 976 | ν(C-O-C) | ||
| 1002 | ν(C-O-C) | |||||
| 1025 | 1020 | ν(C-O-C) | ||||
| 1032 | 1040 | 1041 | 1032 * | ν(C-O-C) | ||
| 1055 | 1052 | 1051 | 1052 | ν(C-O-C) | ||
| 1067 | 1062 | νas(C-C)T | ||||
| 1094 | 1088 | 1083 | 1096 | 1082 | 1084 | ν(C-C)G |
| 1112 | 1112 | 1108 | 1109 | ν(C-C)G | ||
| 1127 | 1132 | 1124 | 1130 | 1127 | νs(C-C)T, ν(C-O)T | |
| 1160 * | 1155 | 1154 | 1154 | νs(C-C)T, ν(C-O)G | ||
| 1202 * | 1203 | 1203 | 1203 | νs(C-C) | ||
| 1252 | 1258 | 1256 | 1259 | τ(CH2) | ||
| 1275 * | 1275 * | νs(C-C)G | ||||
| 1310 | 1315 | 1303 * | 1315 | 1311 | 1308 | δ(O-H) in plane |
| 1340 | 1344 | 1340 | 1340 | 1340 | 1340 | δ(O-H) in plane |
| 1360 * | 1361 * | 1362 * | δ(O-H) in plane | |||
| 1374 | 1380 | 1379 | 1381 | δ(O-H) in plane | ||
| 1412 | 1414 | 1404 * | 1412 | 1414 | 1418 | νs(COO−), H- bond |
| 1463 | 1461 | 1465 | 1460 | 1460 | δ(CH2) | |
| 1500 | hydrogen bond | |||||
| 1570 | hydrogen bond | |||||
| 1612 | νas(COH) | |||||
| 1630 | νas(COO−) | |||||
| 2882 | νs(CH2) | |||||
| 2905 | 2902 | 2905 | 2903 | νas(CH2) | ||
| 2934 | 2935 | ν(CH-OH), νas(CH2) | ||||
| 2945 | 2946 | 2946 | 2946 | νas(CH2) | ||
| 2961 | ν(CH-OH) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadri, R.; Bresson, S.; Aussenac, T. Effect of Alginate Proportion in Glycerol-Reinforced Alginate–Starch Biofilms on Hydrogen Bonds by Raman Spectroscopy. Appl. Sci. 2023, 13, 8846. https://doi.org/10.3390/app13158846
Kadri R, Bresson S, Aussenac T. Effect of Alginate Proportion in Glycerol-Reinforced Alginate–Starch Biofilms on Hydrogen Bonds by Raman Spectroscopy. Applied Sciences. 2023; 13(15):8846. https://doi.org/10.3390/app13158846
Chicago/Turabian StyleKadri, Rana, Serge Bresson, and Thierry Aussenac. 2023. "Effect of Alginate Proportion in Glycerol-Reinforced Alginate–Starch Biofilms on Hydrogen Bonds by Raman Spectroscopy" Applied Sciences 13, no. 15: 8846. https://doi.org/10.3390/app13158846
APA StyleKadri, R., Bresson, S., & Aussenac, T. (2023). Effect of Alginate Proportion in Glycerol-Reinforced Alginate–Starch Biofilms on Hydrogen Bonds by Raman Spectroscopy. Applied Sciences, 13(15), 8846. https://doi.org/10.3390/app13158846








