Abstract
Indigenous leafy vegetables are used mainly for human consumption since they provide health promoting phytonutrients and bioactive compounds such as antioxidants, flavonoids, minerals and vitamins. However, the phytonutrients and bioactive compounds in the leaves of these vegetables vary widely both quantitatively and qualitatively due to genetic and environmental factors. This study determined the diversity, molecular size variation and the relationships between the minerals and nutrients. Four common leafy vegetables and one standard were used in the study. The mean iron and manganese leaf content was 279.44 mg/kg DW and 247.86 mg/kg DW, respectively. The total phenolic content ranged between 0.37 and 0.50 mg GAE/g. Nineteen different bioactive compounds, varying widely in molecular size, were detected in the four common leafy vegetables. Jute mallow leaves contained only two bioactive molecules which included quercetin-3′-glucoside. None of the eight quercetin-related derivatives that were present among the leafy vegetables were detected in Swiss chard. In cowpea, 2,2 diphenyl-1-picrylhydrazyl free radical scavenging activity was four-fold higher than in pumpkin and Swiss chard leaves. These results demonstrated that the common leafy vegetables varied widely in mineral composition and bioactive compounds, suggesting that a combination of these vegetables in the human diet can provide a wider range of nutrients.
1. Introduction
In many parts of southern Africa, rural communities rely significantly on indigenous leafy vegetables as sources of affordable nutritious food in their daily meals that are often starch-based. Frequently, the leafy vegetables are often harvested from the wild during the summer (rain) season [1,2]. For instance, amaranth (Amaranthus cruentus), jute mallow (Corchorus olitorius) and spider plant (Cleome gynandra) are traditionally collected from the wild in South Africa and Zimbabwe, but cowpea leaf (Vigna anguiculata) and pumpkin leaf (Cucurbita pepo) are cultivated in the field, and the leaves are harvested during the summer (or cropping) season [3,4]. Each of these leafy vegetable types is often diverse in horticultural traits. For instance, the cultivated cowpea consists of both prostrate (indeterminate) and erect (determinate) types which differ from each other in many traits including duration to maturity and taste (or flavor perceived). The prostrate types are generally late maturing and produce relatively high dry matter per unit area [5,6,7]. In some areas in southern Africa, for instance in Zambia and Zimbabwe, pumpkin leaf is one of the most popular leafy vegetables during the rainy season [8]. In addition, the bioactive compounds (molecules) in these leafy vegetables vary widely in both quality and quantity depending on the genetic and environmental factors [9,10,11,12].
The leaves of these vegetables possess health-promoting phytonutrients and bioactive compounds such as antioxidants, flavonoids, minerals and vitamins [13,14]. For instance, the leaves contain phenolic compounds which have redox properties responsible for antioxidant activity [15]. Both phenolic and flavonoid compounds are secondary metabolites in the plant which contain an aromatic ring bearing at least one hydroxyl group, thus rendering them good electron donors in antioxidant activities [16,17,18,19]. In addition, some of the leafy vegetables possess sulfur- and nitrogen-containing secondary metabolites (glucosinolates) that confer a bitter taste and vary in different plant organs [20]. Moreover, some of the compounds can induce the synthesis of endogenous antioxidant molecules in cells of biological systems and exhibit free radical inhibition, oxygen scavenging and peroxide decomposition [20,21]. In some cases, the levels of nutrients in the common leafy vegetables are similar to those in exotic vegetables such as Swiss chard (Beta vulgaris L. var. cicla) [22]. Therefore, this study examined the comparative variation in the occurrence of leaf flavonoids, total phenolics and minerals in common leafy vegetables that are largely available to rural communities in southern Africa.
2. Materials and Methods
2.1. Location and Planting
The experiment was conducted in the field during the summer season (October– March) in the field at Thohoyandou, (22°95′ S; 30°48′ E, 595 m a.s.l.) The mean daily temperatures ranged from 25 °C to 40 °C in summer and from 18 to 26 °C in winter. About 95% of the rainfall occurs during October and March; however, it is highly seasonal. The annual mean rainfall is about 650 mm. The location at Thohoyandou is characterized by well drained soils which are deep (>1500 mm) and dystrophic, with apedal structure, and they are classed as Hutton [23].
The seeds of each of the four common traditional leafy vegetables were manually sown in the field (Table 1). Swiss chard was used as a standard or reference material to measure and compare the quality of the common leafy vegetables. Although exotic, it is widely adapted to South Africa, and it tolerates both cool weather and heat. In addition, it contains several polyphenolic antioxidants, vitamins and minerals [24]. For each vegetable, the seeds were sown in a plot consisting of a row measuring 2.0 m long and spaced 30.0 cm apart. Standard management practices for leafy vegetables were used. In this study, no fertilizers were used. Leaf samples were picked six weeks after germination at random from five chosen plants in the inner rows and bulked for analysis.

Table 1.
Four common leafy vegetables that were used in the study.
2.2. Determination of Mineral Content
The leaves were collected at random from five plants in the inner rows, bulked and then washed with distilled water before being dried overnight at 75 °C. The dried leaf samples were milled and sieved using a 1 mm stainless steel sieve and then ashed. Approximately 2 mL of hydrochloric acid (HCl) was added to the samples [25]. The samples were then slowly evaporated in a water bath until dry. A total of 2.5 mL of a 1:9 HCl solution was added to each sample, and it was then filtered through Advantec 5B: 90 MM filter papers. The Varian 720 Inductively Coupled Plasma Emission Spectrometer (ICP-OES, Frankfurt, Germany) was used to analyze the filtrate after diluting it with deionized water at a ratio of 5:20.
2.3. Preparation, Quantification and Identification of Phenolic Compounds
To prepare the phenolic extracts, 2 g of the milled sample was refluxed in 20 mL of acidified methanol (1% HCL) for 2 h at 60 ± 5 °C followed by centrifuging at 5000 rpm for 20 min and separating the supernatants. The supernatants were used to analyze the total phenolic content (TPC), total flavonoid content (TFC) analysis and antioxidant activity. The liquid chromatograph–mass spectrometer (LC-MS) was used to identify and quantify the TPC [26]. A Waters Synapt G2 quadrupole time-of-flight MS (Milford, MA, USA) (fitted with a Waters ultra-pressure LC and photo diode array detector) was used to separate and identify the phenolic compounds in the extracts. To achieve the best sensitivity, the mass spectrometer was optimized (with cone voltage set at 15 V, with nitrogen used as the desolvation gas at 650 L/h and a desolvation temperature of 275 °C). For the analysis of phenolic compounds, the separation was accomplished on a Waters HSS T3, 150 2.1 mm column using a gradient.
2.4. Determination of Total Phenolics
Using the method previously described by [25], the total phenolic content (TPC) was analyzed. Approximately 0.1 mL of acidified methanolic extract was mixed with 5 mL of distilled water in a 50 mL volumetric flask. Folin–Ciocalteu’s reagent (2.5 mL) and 7.5 mL 15% sodium carbonate solution were added, mixed thoroughly, made up to 50 mL and allowed to react for 30 min. All the reagents were purchased from MERCK (PTY) LTD, Modderfontein, 1645, South Africa. The reaction mixture absorbance was read at 760 nm using a 96-well microplate spectrophotometer (Biowave II, 80-3003-75, Biochrom LTD, Cambridge, UK). The result was expressed as mg of gallic acid equivalent (GAE) per g of the sample, and standard curves (Supplementary Figures S1 and S2) were prepared.
2.5. Determination of Total Flavonoid Content
The total flavonoid content was determined according to [26] Obeng (2020) using a microplate spectro-photometer (Biowave II, 80-3003-75, Biochrom LTD, Cambridge, UK). Approximately 0.1 mL of the extract was mixed with 4.9 mL of distilled water, and 0.3 mL 5% w/v) NaNO2 was added. About 0.3 mL of 10% (w/v) AlCl3 and 2 mL of 1 M NaOH were added after 5 and 6 min, respectively, and the volume was immediately increased to 10 mL with distilled water. After vortexing the mixture, the absorbance was measured at 510 nm. Using catechin hydrate as a standard, a calibration curve was prepared. The result was expressed in milligrams of catechin equivalents per gram of sample.
2.6. Determination of Antioxidant Activity
In addition, the method described by [27,28] was used to measure the antioxidant activity using the 2,2 diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity and the ferric-reducing antioxidant power (FRAP). All the reagents were purchased from MERCK (PTY) LTD, Modderfontein, 1645, South Africa. In a test tube, 10 µL of acidified methanolic extract was mixed with 90 µL of distilled water and 3.9 mL of methanolic 0.1 mM DPPH solution, vortexed and stored in the dark for 30 min. Equation (1) below was used to calculate the percentage inhibition of the DPPH from the absorbance of the reaction mixture measured at 515 nm using a microplate spectro-photometer (Biowave II, 80-3003-75, Biochrom LTD, Cambridge, UK) [25]:
where Abs sample is the absorbance of the extract and Abs control is the absorbance of the DPPH solution without the extract. Similarly, the FRAP assay was determined as previously described [25]. The extract volume (100 µL) was fine tuned to 1 mL using methanol. The tube was filled with 2.5 mL of phosphate buffer (2.5 mL 0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide, and it was vortexed before being heated in a water bath for 20 min at 50 °C. After the incubation period, 2.5 mL of 10% (w/v) trichloroacetic acid was added, and the tube was then spun at 5000 rpm for 20 min. About 2.5 mL of distilled water and 0.5 mL of ferric chloride solution containing 0.1% (w/v) were added to the supernatant. At 700 nm, the absorbance was measured using a microplate spectro-photometer (Biowave II, 80-3003-75, Biochrom LTD, Cambridge, UK). Higher absorbance indicates higher reducing power.
% Inhibition of DPPH = [(Abs blank − Abs sample)/Abs blank] × 100
2.7. Experimental Design and Data Analysis
A field experiment was laid as a completely randomized block design with three replications. Quantitative data sets were subjected to analysis of variance using SAS software (version 9.4) followed by Fisher’s least significance difference test for mean separation [29]. To determine the association between variables, Pearson’s correlation coefficient test was conducted using SAS software (version 9.4) [29]. To determine the significant variables (mineral content, total phenols and antioxidant activity) that contributed to the variation between the leafy vegetables, principal component analysis (PCA) was performed.
3. Results
3.1. Leaf Mineral Content
The leaves of the vegetables showed a diverse range of leaf mineral content, total phenolic compounds and antioxidant activity. Mg ranged between 0.37 and 1.29 (g/kg DW) with a mean value of 0.63 ± 0.38 g/kg DW (Table 2). The mean Fe and Mn leaf content was 279.44 mg/kg DW and 247.86 mg/kg DW, respectively. The TPC ranged between 0.37 and 0.50 mg GAE/g (Table 2). However, Mg was significantly (p ≤ 0.05) higher (11.5 g/kg DW) in Swiss chard when compared to each of the leafy vegetables separately, but the spider plant attained at least 20.0% higher Ca and P leaf content than the standard (check) (Table 3). Nonetheless, Swiss chard was superior in terms of all the micro-elements that were evaluated in this study. For instance, the Mn leaf content (134.0 mg/kg DW) in the spider plant was at least 60% less than in Swiss chard (Table 3). Similarly,) there was a >25% deficit in the Fe leaf content (289.3 mg/kg DW) in the spider plant in comparison with Swiss chard.

Table 2.
The range of mean values for leaf mineral content, total phenolic content and antioxidant activity among common traditional leafy vegetables.

Table 3.
Mean separation for leaf mineral content among four common traditional leafy vegetables and one standard (Swiss chard).
3.2. Occurrence of Bioactive Molecules
There were 18 distinct bioactive molecules (bio-compounds) that were detected in the leaves of the four common leafy vegetables. The bio-compounds varied widely in molecular size. Both the largest (755, 2135) and smallest (191, 0184) bioactive compounds occurred in the spider plant while the check leafy vegetable (Swiss chard) possessed an additional three unique compounds namely apiin, apigein-7-6″-malonylneohespiridosine and isohamnetin-3-4′-glucoside (Table 4). In addition, the spider plant leaves possessed the highest number of unique bioactive molecules (five) followed by Swiss chard (three) (Table 4). In contrast, jute mallow leaves contained only two bioactive molecules which included quercetin-3′-glucoside. At least one unique bioactive molecule was detected each in cowpea (chartreusin) and pumpkin (3-desmethyl-5-deshydroxyseleroin). None of the eight quercetin-related derivatives that were present variably among the four common leafy vegetables were detected in Swiss chard. Moreover, most of the quercetin derivatives were detected in the spider plant leaves (Table 4) (Supplementary Figure S3).

Table 4.
Occurrence of leaf flavonoids and total phenolic content among four common traditional leafy vegetables and one standard (Swiss chard).
3.3. Relationships between the Flavonoids, Minerals and Phenolic Compounds
There was a highly significant (p ≤ 0.01) positive relationship between Zn and Al (Table 5). However, a negative but highly significant (p ≤ 0.01) relationship was detected between Fe and DPPH among the leafy vegetables (Table 5). Fe also showed a negative but not significant (p ≥ 0.05) relationship with both TFC and TPC. The principal component (PC) analysis indicated that the first two PCs accounted for 79.74% of the total variation (Table 6). Both Cu and Mg were highly associated with PC1 but in PC 3 and PC 4 were dominated by TPC (0.64) and Ca (0.72), respectively. Further biplot analysis indicated that the spider plant was highly associated with Ca, N, P and FRAP. Cowpea showed a strong association with DPPH (Figure 1). Acute angles between each pair among the six mineral elements that were clustered in the top right quadrant indicated positive correlation (Figure 1). Furthermore, DPPH was closely associated with cowpea leaves, but both TPC and Mn showed no strong associations with any of the leafy vegetables.

Table 5.
Relationships of leaf minerals, total phenolic contents and antioxidant activity among common traditional leafy vegetables (Swiss chard = check).

Table 6.
Principal component analysis and eigenvalues for leaf minerals, total phenolic content and antioxidant activity among common traditional leafy vegetables.
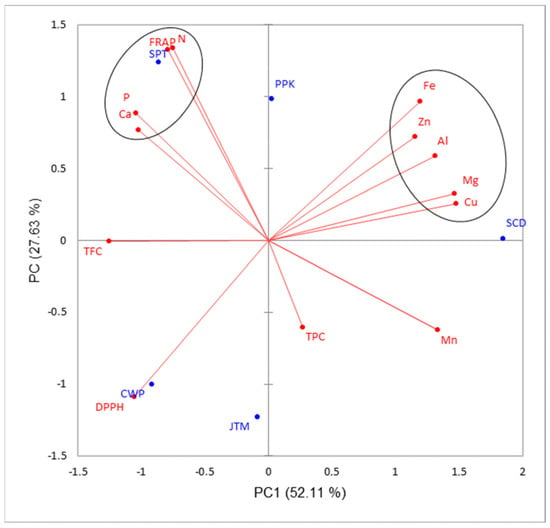
Figure 1.
Principal component analysis biplot of PC1 and PC2 illustrating the variation among common traditional leafy vegetables estimated using the data set of leaf minerals, total phenolic content and antioxidant activity. Long lines indicate high correlation values, while acute angles indicate positive correlation between each pair of variables. CWP = cowpea; JTM = jute mallow; PPK = pumpkin; SCD = Swiss chard (check); SPT = spider plant; Ca = calcium; Mg = magnesium; P = phosphorus; N = nitrogen; Al = aluminum; Cu = copper; Fe = iron; Mn = manganese; Zn = zinc; FC = total flavonoid content; TPC = total phenolic content; DPPH = 2,2 diphenyl-1-picrylhydrazyl free radical scavenging activity; FRAP = ferric-reducing antioxidant power.
4. Discussion
The intensity of both micro- and macro-elements in the four common leafy vegetables was comparable with those reported in similar studies [30]. The observed differences in selected specific minerals were likely due to the environmental, ontogenic and genetic factors. For instance, the application of calcium ammonium nitrate fertilizer enhanced leaf Ca content, but farmyard manure decreased Fe content in spider plant [31]. In another study involving parsley (Petroselinum crispum), baby greens accumulated more Ca and Mg than microgreens [32].
The superiority of micro-element content in the Swiss chard leaves relative to the common leafy vegetables which was observed in this study was consistent with findings from other similar studies [33]. However, in this study, the common leafy vegetables showed superiority in macro-element content suggesting that consumers can derive optimum benefits from combining (or including) the various types of leafy vegetables. It should be emphasized that the threshold quantities of individual nutrient elements that are necessary for human consumption per designated period are more important than the mere presence of the elements in the leaves in the vegetables. Studies have also elucidated the necessity of balancing the nutrient elements in diets and their role particularly in conferring strong bones (teeth), transmitting nerve impulses and forming integral constituents of different hormones [34]. Microelements also participate in the formation of erythrocyte cells while macro-minerals, such as Ca and Mg, have a high potential to control blood pressure and the immune system [34].
The results also showed that no single leafy vegetable type contained all the desirable minerals, flavonoids and phenolic compounds suggesting that perhaps human intervention, particularly through genetic enhancement, can develop novel genotypes of the leafy vegetables that possess preferred combinations of the nutrients. One of the possible reasons for the observed absence of specific mineral elements or bioactive compounds in some of the leafy vegetables could be attributed to the existence of inverse relationships between them in the vegetable species. Nonetheless, such inverse relationships can be broken through plant breeding techniques such as mutation and probably the CRSPR technology [35,36].
The results also revealed the availability of a wide range of quercetin-related compounds in all the leafy vegetables particularly in spider plant. These findings agreed with the results that were reported for a similar study [37]. The role of quercetin as an antioxidant was documented widely as a potent antioxidant flavonoid (or more specifically, a flavanol) which occurs also in onion, broccoli and citrus fruits and is protective against tissue injury caused by drug toxicities [38,39,40,41]. However, it is unclear if this desirable group of nutrients can be introgressed into the other leafy vegetables without diminishing the already existing beneficial nutrient elements in such vegetables. Another drawback in the study was that fresh leaf samples were processed for the analysis such that the effects (on the quality and levels of these nutrients) of homemade processing of the vegetables for long term preservation remain unclear. Previously, contrasting findings were reported with regard to this vegetable home processing [42,43,44]. Perhaps future investigations could focus on this aspect. Similarly, the effects of fertilizing vegetable plants with chemicals or organic manure could be of interest to researchers and users. In addition, the absence of some of the flavonoids that were detected in similar studies in spider plant might have been due to the small number of standards that were used in the current study. Moreover, the alteration of regulatory networks in plants due to UV-B radiation was observed previously [45].
The positive significant correlations between individual minerals (for instance, Zn and Al) indicated that there was a relationship between the pair, and likely selection for increased content of either mineral might inadvertently result in elevating the content of the other one. However, such a conclusion will need to be approached with caution since several species of leafy vegetables were involved in this study. The relationships between the same pairs of candidate minerals may be relatively weaker in a specific leafy vegetable species but masked by a combined correlation analysis of parameters among all the species. Therefore, in future, separate correlation studies between the different parameters involving many plants within each leaf, vegetable species will be merited and might produce unequivocal conclusions regarding the relationships.
While these results indicated the relative superiority of the leafy vegetables for specific minerals and bio-compounds, it appears that future studies need to consider the ontogenic stages probably by bulking leaf samples harvested at different growth stages prior to the analysis. At the juvenile phase, the concentration of bio-compounds may increase partly due to a high uptake rate of the young roots as well as the high relative growth rate of the plants. Moreover, such studies can also benefit from the inclusion of a wide range of internal standards to enhance the detection of a broad spectrum of bio-compounds. Nonetheless, in this study, the important bio-compounds, including eight quercetin-related derivatives, were detected in most of the leafy vegetable species. In conclusion, the common leafy vegetables were comparatively rich in macro-elements. The findings of this study were significant for end-users in the common leafy vegetable value chain. The empirical evidence presented in this paper supported the need to widen the range of leafy vegetables to optimize the spectrum of beneficial nutrients. The spider plants possessed a superior spectrum of desirable nutrient elements suggesting that it can be recommended highly to consumers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13148503/s1.
Author Contributions
Conceptualization, E.T.G. and F.T.; methodology, F.T.; software, N.N.; formal analysis, N.N.; investigation, F.T.; writing, F.T., writing—review and editing, E.T.G.; supervision, E.T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
There are supplementary data used in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van der Walt, A.M.; Loots, D.T.; Ibrahima, M.I.M.; Bezuidenhout, C.C. Minerals, Trace Elements and Antioxidant Phytochemicals in Wild African Dark-Green Leafy Vegetables (Morogo). S. Afr. J. Sci. 2009, 105, 444–448. [Google Scholar]
- van Rensburg, W.S.J.; van Averbeke, W.; Slabbert, R.; Faber, M.; van Jaarsveld, P.; van Heerden, I.; Wenhold, F.; Oelofse, A. African Leafy Vegetables in South Africa. Water S. Afr. 2007, 33, 317–326. [Google Scholar] [CrossRef]
- Akundabweni, L.S.; Peter-Paul, C.; Singh, B.B. Evaluation of Elite Lines of Cowpea (Vigna unguiculata (L.) Walp.) for Leaf/Fodder Plus Grain (i.e., Dual Purpose). Trop. Agric. 1989, 67, 133–136. [Google Scholar]
- Maseko, I.; Ncube, B.; Mabhaudhi, T.; Tesfay, S.; Chimonyo, V.G.P.; Araya, H.T.; Fessehazion, M.; Du Plooy, C.P. Nutritional Quality of Selected African Leafy Vegetables Cultivated Under Varying Water Regimes and Different Harvests. S. Afr. J. Bot. 2019, 126, 78–84. [Google Scholar] [CrossRef]
- Nkongolo, K.K. Genetic Characterization of Malawian Cowpea (Vigna unguiculata (L.) Walp.) Landraces: Diversity and Gene flow Among Accessions. Euphytica 2003, 129, 219–228. [Google Scholar] [CrossRef]
- Keller, G.B.; Mndiga, H.; Maass, B.L. Diversity and Genetic Erosion of Traditional Vegetables in Tanzania from the Farmer’s Point of View. Plant Genet. Res. 2005, 3, 400–413. [Google Scholar] [CrossRef]
- Keding, G.; Weinberger, K.; Swai, I.; Mndiga, H. Diversity, Traits and Use of Traditional Vegetables in Tanzania; Technical Bulletin No. 40. AVRDC; The World Vegetable Center: Shanhua, Taiwan, 2007; Volume 53, pp. 400–413. [Google Scholar]
- Oluoch, M.O. Production Practices of Pumpkins for Improved Productivity. In Proceedings of the Technical Consultation Workshop, Arusha, Tanzania, 7–8 December 2009. [Google Scholar]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica Foods: Bioavailability in Food and Significance for Human Health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional Quality of Ten Leafy Vegetables Harvested at Two Light Intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Moussa, M.I.D.; Alashi, A.M.; Sossa-Vihotogbe, C.N.A.; Akponikpe, P.B.I.; Baco, M.N.; Djenontin, A.J.; Aluko, R.E.; Akissoe, N.H. Proximate Composition, Mineral Profile and Trypsin-Inhibitory of West African Leafy Vegetables: Influence of Urea Micro-Dosing and Harvest Time. Pol. J. Food Nutr. Sci. 2020, 70, 179–188. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Vijayalakshmi, R.; Singh, V. Peroxidase Activity, Chlorophylls and Antioxidant Profile of Two Leaf Vegetables (Solanum nigrum L. and Amaranthus cruentus L.) Under Six Pre-treatment Methods Before Cooking. Int. J. Food Sci. Technol. 2008, 43, 173–178. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Nutrients, Minerals, Pigments, Phytochemicals, and Radical Scavenging Activity in Amaranthus blitum Leafy vegetables. Sci. Rep. 2020, 10, 3868. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res.-Fund. Mol. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-Radical Scavenging Capacity and Antioxidant Activity of Selected Plant Species from the Canadian Prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; Hady, S. Antioxidant and Structure–Activity Relationships (SARs) of Some Phenolic and Anilines Compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Bioactive Compounds in Cranberries and Their Biological Properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Sandhu, S.K. Therapeutic and Nutraceutical Potential of Bioactive Compounds Extracted from Fruit Residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337. [Google Scholar]
- Afolayan, A.J.; Jimoh, F. Nutritional Quality of Some Wild Leafy Vegetables in South Africa. Int. J. Food Sci. Nutr. 2009, 60, 424–431. [Google Scholar] [CrossRef]
- Mzezewa, J.; Misi, T.; van Rensburg, L.D. Characterization of Rainfall at a Semi-Arid Ecotope in the Limpopo Province (South Africa) and Its Implications for Sustainable Crop Production. Water S. Afr. 2009, 36, 19–26. [Google Scholar]
- Gamba, M.; Raguindin, P.F.; Asllanaj, E.; Merlo, F.; Glisic, M.; Minder, B.; Bussler, W.; Metzger, B.; Hua Kern, H.; Muka, T. Bioactive Compounds and Nutritional Composition of Swiss Chard (Beta vulgaris L. var. cicla) and flavescens. Crit. Rev. Food Sci. Nutr. 2020, 61, 3465–3480. [Google Scholar] [CrossRef] [PubMed]
- Meda, N.T.R.; Bangou, M.J.; Bakasso, S.; Millogo-Rasolodimby, J.; Nacoulma, O.G. Antioxidant Activity of Phenolic and Flavonoid Fractions of Cleome gynandra and Maerua angolensis of Burkina Faso. J. Appl. Pharm. Sci. 2013, 3, 036–042. [Google Scholar] [CrossRef]
- Obeng, E.; Kpodo, F.M.; Tettey, C.O.; Essuman, E.K.; Adzinyo, O.A. Antioxidant, total phenols and proximate constituents of four tropical leafy vegetables. Sci. Afr. 2020, 7, e00227. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Response of Nutrients, Minerals, Antioxidant Leaf Pigments, Vitamins, Polyphenol, Flavonoid and Antioxidant Activity in Selected Vegetable Amaranth Under Four Soil Water Content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef]
- Meir, S.; Kanner, J.; Akiri, B.; Philosoph-Hadas, S. Determination and Involvement of Aqueous Reducing Compounds in Oxidative Defense Systems of Various Senescing Leaves. J. Agric. Food Chem. 1995, 43, 1813–1819. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-Radical Scavenging Capacity and Reducing Power of Wild Edible Mushrooms from Northeast Portugal: Individual Cap and Stipe Activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis System User’s Guide: Statistical Version, 9th ed.; SAS Institute: Cary, NC, USA, 2003. [Google Scholar]
- Omondi, E.O.; Engels, C.; Nambafu, G.; Schreiner, M.; Neugart, S.; Abukutsa-Onyango, M.; Winkelmann, T. Nutritional Compound Analysis and Morphological Characterization of Spider Plant (Cleome gynandra)—An African Indigenous Leafy Vegetable. Food Res. Int. 2017, 100, 284–295. [Google Scholar] [CrossRef]
- Hutchinson, M.J. The Effect of Farmyard Manure and Calcium Ammonium Nitrate Fertilizers on Micronutrient Density (Iron, Zinc, Manganese, Calcium and Potassium) and Seed Yields of Solanum villosum (Black Nightshade) and Cleome gynandra (Cat’s Whiskers) on Eutric Nitisol. J. Agric. Sci. Technol. 2011, 13, 35–52. [Google Scholar]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Giordano, M.; Kyriacou, M.C.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Mineral and Antioxidant Attributes of Petroselinum crispum at Different Stages of Ontogeny: Microgreens vs. Baby Greens. Agronomy 2021, 11, 857. [Google Scholar] [CrossRef]
- Mavengahama, S.; de Clercq, W.P.; McLachlan, M. Trace Element Composition of Two Wild Vegetables in Response to Soil-Applied Micronutrients. S. Afr. J. Sci. 2014, 110, 1–5. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects and Nanoencapsulation. Trend. Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Thangwana, A.; Gwata, E.T.; Zhou, M.M. Impact of Chemical Mutagenesis Using Ethyl Methane Sulphonate on Tepary Bean Seedling Vigour and Adult Plant Performance. Heliyon 2021, 7, e06103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Malzahn, A.A.; Sretenovic, S.; Qi, Y. The Emerging and Uncultivated Potential of CRISPR Technology in Plant Science. Nat. Plants 2019, 5, 778–794. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Baldermann, S.; Ngwene, B.; Wasonga, J.; Schreiner, M. Indigenous Leafy Vegetables of Eastern Africa—A Source of Extraordinary Secondary Plant Metabolites. Food Res. Int. 2017, 100, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H. Antibacterial and Antioxidant Activities of Quercetin Oxidation Products from Yellow Onion (Allium cepa) Skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a Quercetin-rich Onion Skin eExtract on 24 h Ambulatory Blood Pressure and Endothelial Function in Overweight-to-Obese Patients with (pre-)Hypertension: A Randomised Double-Blinded Placebo-Controlled Cross-Over Trial. Br. J Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Al-Zharani, M.; Mubarak, M.; Rudayni, H.A.; Al-Doaiss, A.A.; Abd-Elwahab, M.M.; Al-Eissa, M.S. Quercetin as a Dietary Supplementary Flavonoid Alleviates the Oxidative Stress Induced by Lead Toxicity in Male Wistar Rats. Nutrients 2023, 15, 1888. [Google Scholar] [CrossRef]
- Shahnaz, A.; Khan, K.M.; Sheikh, M.A.; Shahid, M. Effect of Peeling and Cooking on Nutrients in Vegetables. Pak. J. Nutr. 2003, 2, 189–191. [Google Scholar]
- Gupta, S.; Gowri, B.S.; Lakshmi, A.J.; Prakash, J. Retention of Nutrients in Green Leafy Vegetables on Dehydration. J. Food Sci. Technol. 2013, 50, 918–925. [Google Scholar] [CrossRef]
- Li, Y.; Qin, W.; Fu, X.; Zhang, Y.; Hassani, D.; Kayani, S.I.; Xie, L.; Liu, H.; Chen, T.; Yan, X.; et al. Transcriptomic Analysis Reveals the Parallel Transcriptional Regulation of UV-B-Induced Artemisinin and Flavonoid Accumulation in Artemisia annua L. Plant Physiol. Biochem. 2021, 163, 189–200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).