Exploring the Effects of Low-Level Laser Therapy on the Cytocompatibility and Osteo/Odontogenic Potential of Gingival-Derived Mesenchymal Stem Cells: Preliminary Report
Abstract
1. Introduction
2. Materials and Methods
2.1. GMSC Isolation and Culture
2.2. GMSC Characterization
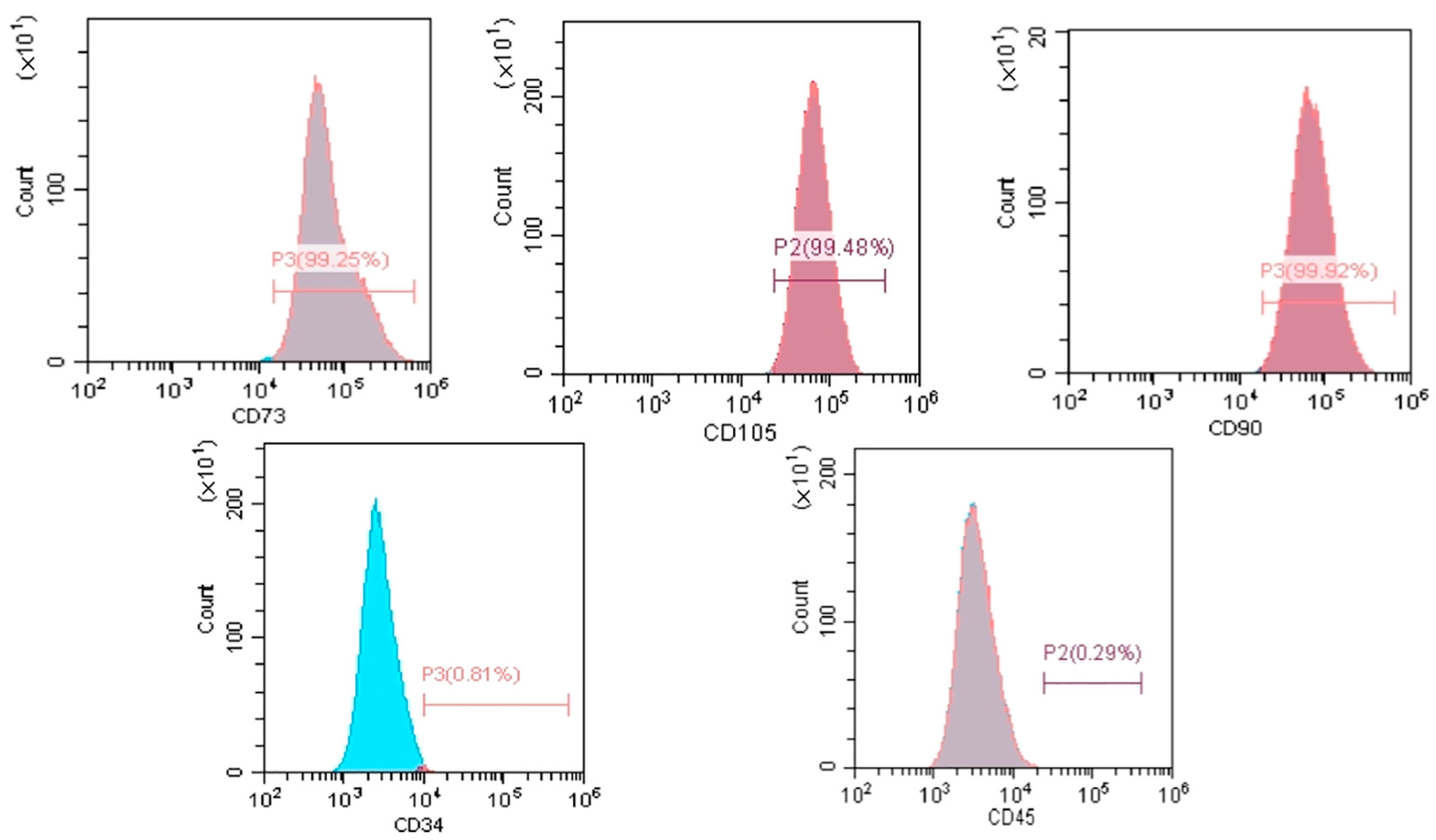
2.2.1. Surface Marker Analysis
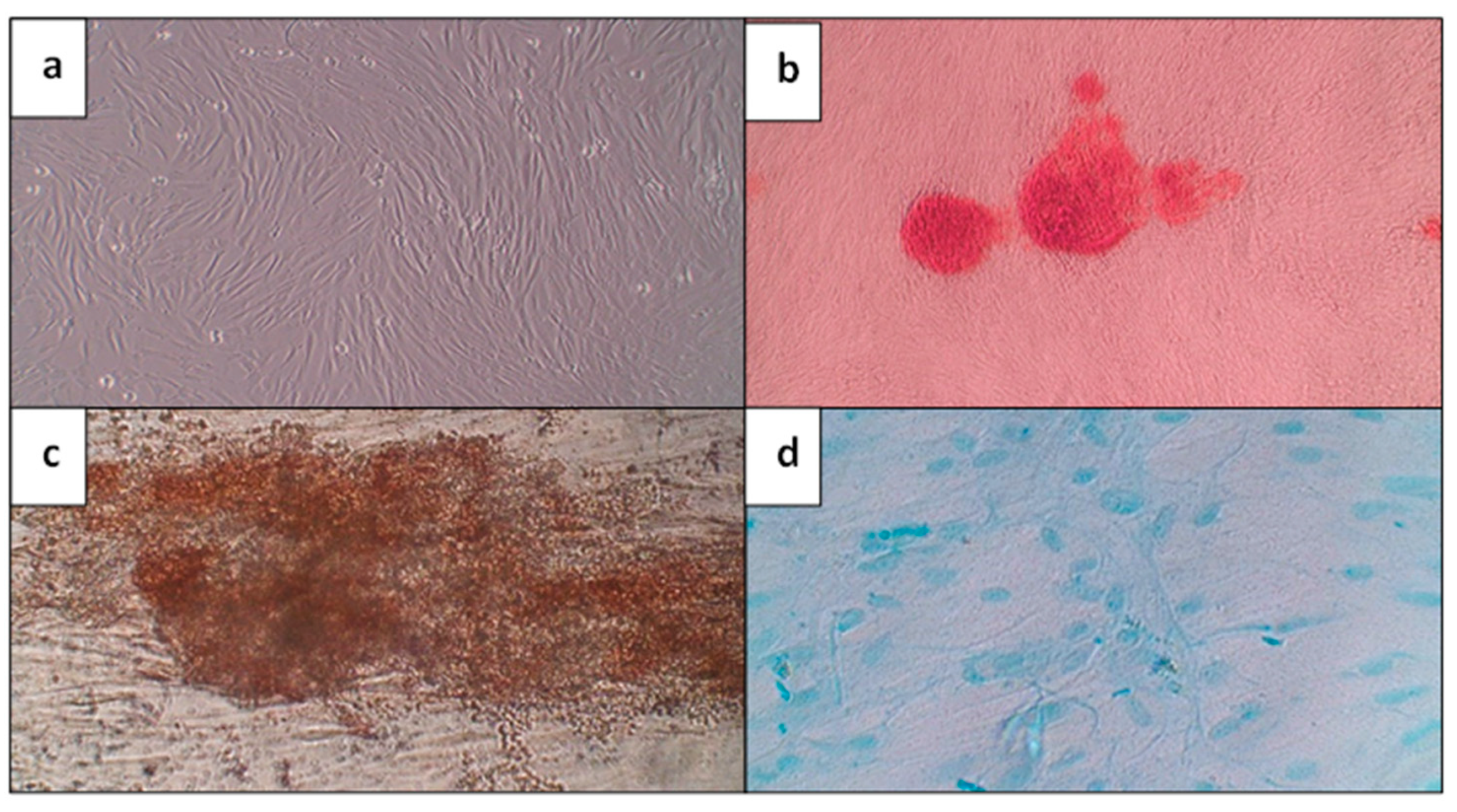
2.2.2. Multilineage Differentiation Potential
2.3. LLLT Protocol
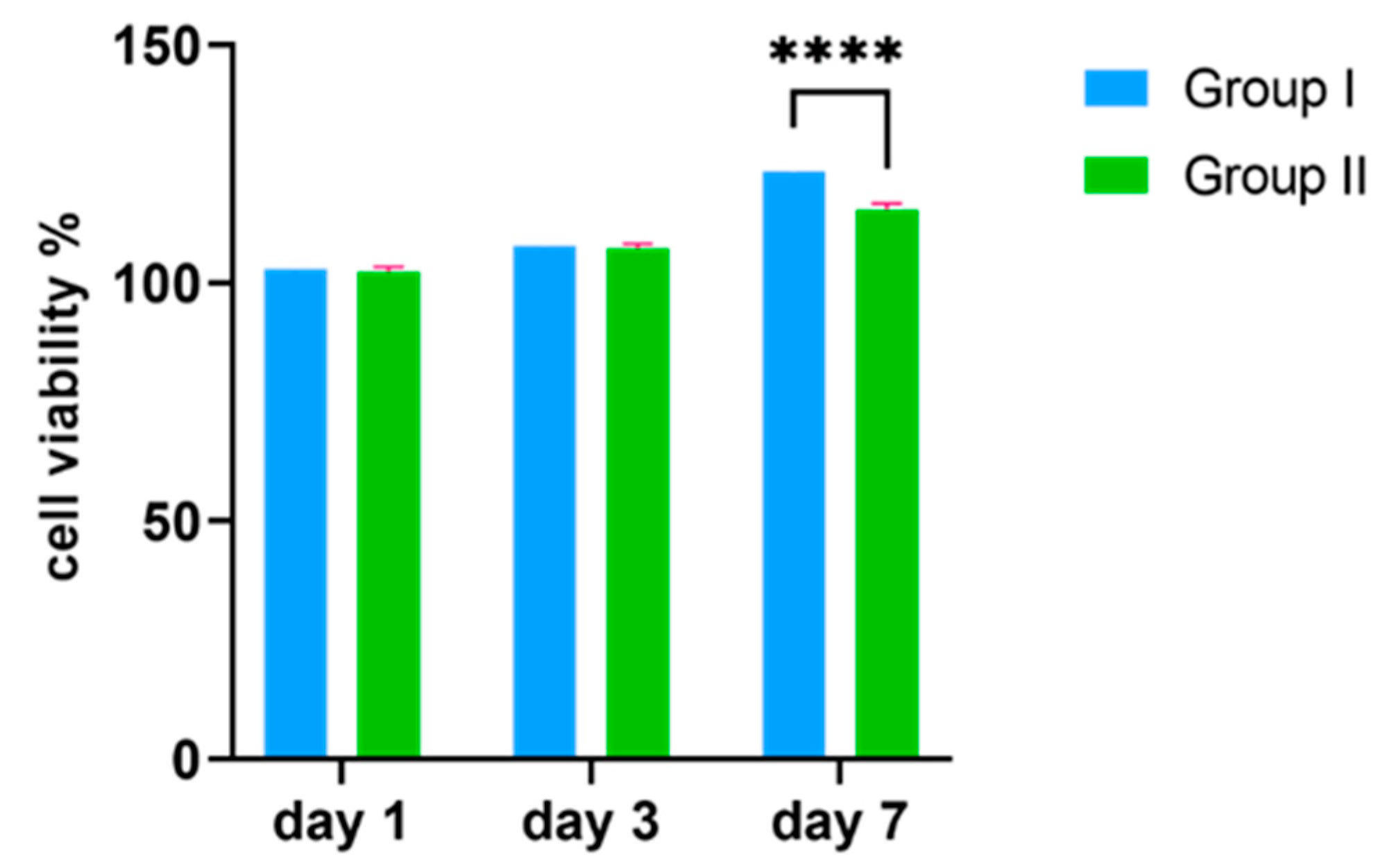
2.4. Cell Viability
2.5. Osteo/Odontogenic Differentiation of GMSCs
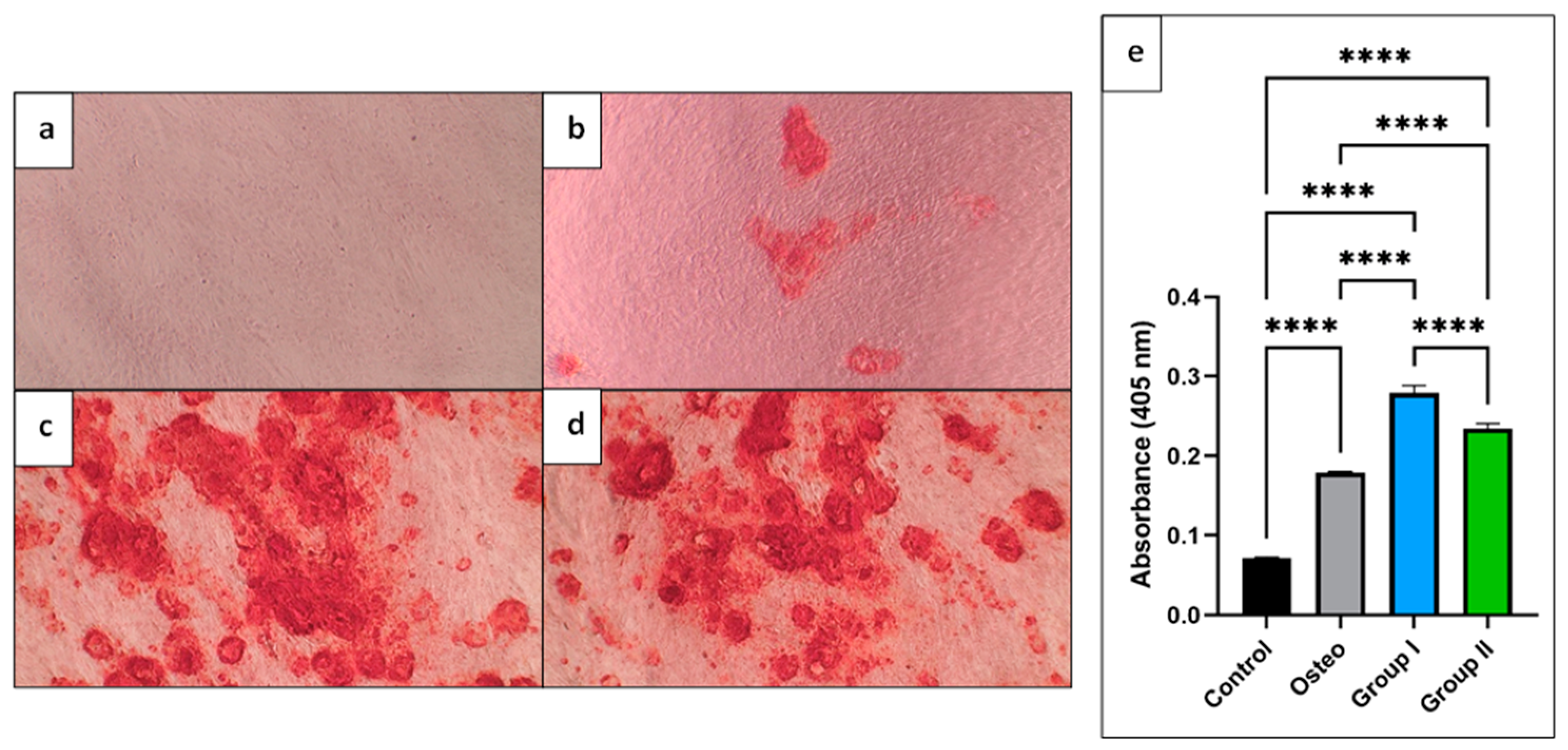
2.6. Alizarin Red S Staining
2.7. Quantitative Real-Time Polymerase Chain Reaction
- DMP1: 5′-AGGAAGTCTCGCATCT CAGAG-3′ (forward) and 5′-TGGAGTTGCTGTTTTCTGTAGAG-3′ (reverse);
- DSPP: 5′-TCACAAGGGAGAAGGGAATG-3′ (forward) and 5′-TGCCATTTGCTGTGATGTTT-3′ (reverse);
- Runx2: 5′-CACTGGCGCTGCAACAAGA-3′ (forward) and 5′-CATTCCGGAGCTCAGCAGAATAA-3′ (reverse);
- OC: 5′-CAGCAAAGGTGCAGCCTTG-3′ (forward) and 5′-TGGGGCTCCCAGCCATTG-3′ (reverse);
- β-actin: 5′-CCATCGTCCACCGCAAAT-3′ (forward) and 5′-CCTGTAACAACGCATCTCATA-3′ (reverse).
2.8. Statistical Analysis
3. Results
3.1. Morphological Characterization, Identification, and Multi-Differentiation Potential of GMSCs
3.2. MTT Assay
3.3. Alizarin Red S Assay
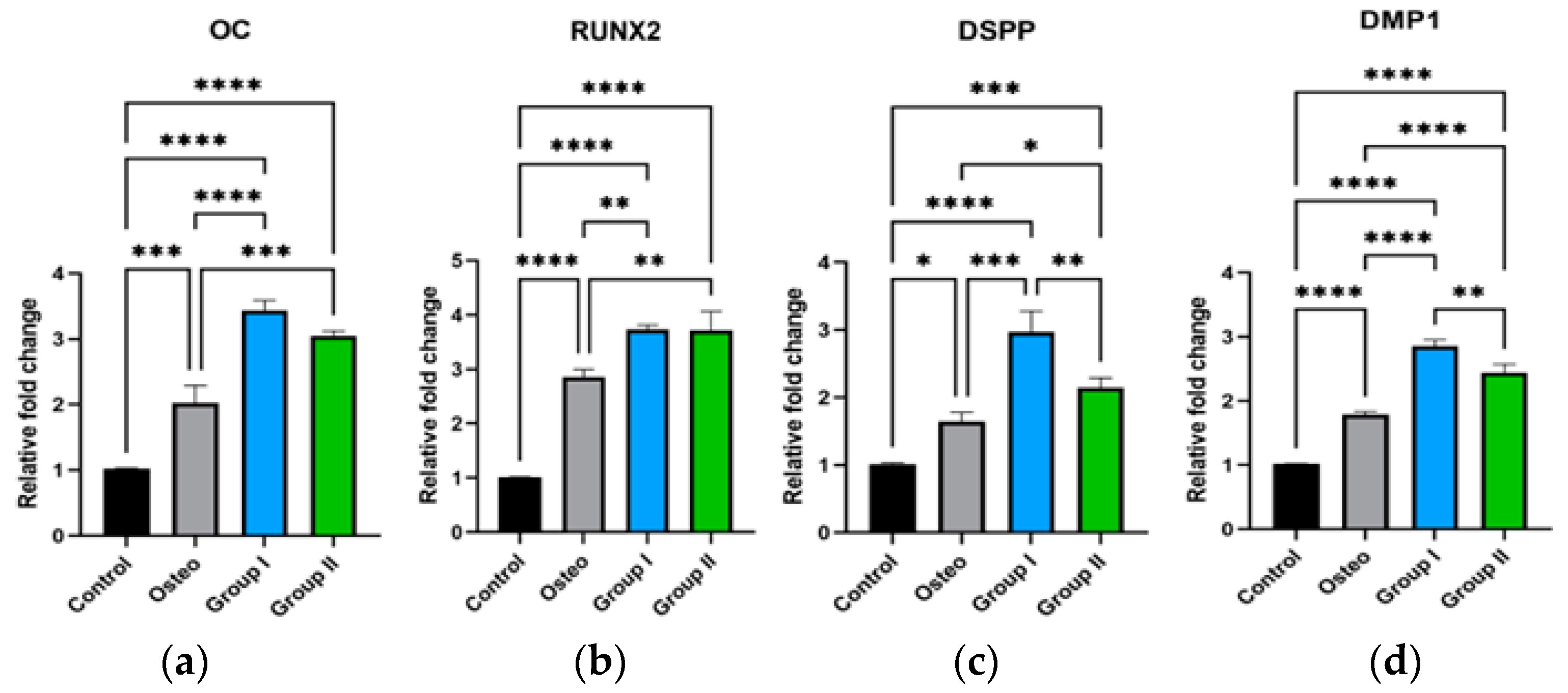
3.4. q-RT PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Moysidou, C.-M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 620962. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Chinigò, G.; Genova, T.; Munaron, L.; Mussano, F. Oral Cavity as a Source of Mesenchymal Stem Cells Useful for Regenerative Medicine in Dentistry. Biomedicines 2021, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the Apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef]
- Campanella, V. Dental Stem Cells: Current research and future applications. Eur. J. Paediatr. Dent. 2018, 19, 257. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chan, Y.H.; Hsieh, S.C.; Lew, W.Z.; Feng, S.W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef] [PubMed]
- Soudi, A.; Yazdanian, M.; Ranjbar, R.; Tebyanian, H.; Yazdanian, A.; Tahmasebi, E.; Keshvad, A.; Seifalian, A. Role and application of stem cells in dental regeneration: A comprehensive overview. EXCLI J. 2021, 20, 454–489. [Google Scholar] [CrossRef]
- Grawish, M.E. Gingival-derived mesenchymal stem cells: An endless resource for regenerative dentistry. World J. Stem Cells 2018, 10, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef] [PubMed]
- Fonticoli, L.; Della Rocca, Y.; Rajan, T.S.; Murmura, G.; Trubiani, O.; Oliva, S.; Pizzicannella, J.; Marconi, G.D.; Diomede, F. A Narrative Review: Gingival Stem Cells as a Limitless Reservoir for Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 4135. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, A.E.; Xu, Q.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine—A Comprehensive Review. Front. Immunol. 2021, 12, 667221. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Mrozik, K.M.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Isolation and characterization of mesenchymal stem cell-like cells from healthy and inflamed gingival tissue: Potential use for clinical therapy. Regen. Med. 2012, 7, 819–832. [Google Scholar] [CrossRef]
- Jauregui, C.; Yoganarasimha, S.; Madurantakam, P. Mesenchymal Stem Cells Derived from Healthy and Diseased Human Gingiva Support Osteogenesis on Electrospun Polycaprolactone Scaffolds. Bioengineering 2018, 5, 8. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, G.; Li, D.; Chen, X.; Pang, J.; Ke, J. Isolation and Multiple Differentiation Potential Assessment of Human Gingival Mesenchymal Stem Cells. Int. J. Mol. Sci. 2014, 15, 20982–20996. [Google Scholar] [CrossRef]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef]
- Li, J.; Xu, S.Q.; Zhang, K.; Zhang, W.J.; Liu, H.L.; Xu, Z.; Li, H.; Lou, J.N.; Ge, L.H.; Xu, B.H. Treatment of gingival defects with gingival mesenchymal stem cells derived from human fetal gingival tissue in a rat model. Stem Cell. Res. Ther. 2018, 9, 27. [Google Scholar] [CrossRef]
- Li, D.; Zou, X.Y.; El-Ayachi, I.; Romero, L.O.; Yu, Z.; Iglesias-Linares, A.; Cordero-Morales, J.F.; Huang, G.T. Human Dental Pulp Stem Cells and Gingival Mesenchymal Stem Cells Display Action Potential Capacity In Vitro after Neuronogenic Differentiation. Stem Cell. Rev. Rep. 2019, 15, 67–81. [Google Scholar] [CrossRef]
- Murugan Girija, D.; Kalachaveedu, M.; Ranga Rao, S.; Subbarayan, R. Transdifferentiation of human gingival mesenchymal stem cells into functional keratinocytes by Acalypha indica in three-dimensional microenvironment. J. Cell. Physiol. 2018, 233, 8450–8457. [Google Scholar] [CrossRef] [PubMed]
- Smojver, I.; Katalinić, I.; Bjelica, R.; Gabrić, D.; Matišić, V.; Molnar, V.; Primorac, D. Mesenchymal Stem Cells Based Treatment in Dental Medicine: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 1662. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Cavalcanti, A.S.; Saidy, N.T.; Schneidereit, D.; Friedrich, O.; Ravichandran, A.; De-Juan-Pardo, E.M.; Hutmacher, D.W. Convergence of 3D printed biomimetic wound dressings and adult stem cell therapy. Biomaterials 2021, 268, 120558. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Duan, R.; Li, H.; Jiang, L.; Tao, T.; Liu, X.; Zhu, L.; Li, Z.; Chen, B.; Zheng, S.; et al. Single-cell analysis of immune cells on gingiva-derived mesenchymal stem cells in experimental autoimmune uveitis. iScience 2023, 26, 106729. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Cullen, D.K.; Le, A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018, 8, 6634. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Z.; Xu, Q.; Sun, H.; Liu, X.; Yang, J.; Hong, R. The treatment of systematically transplanted gingival mesenchymal stem cells in periodontitis in mice. Exp. Ther. Med. 2019, 17, 2199–2205. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, A.L.; Shi, S.; Hill, C.; Wilder-Smith, P.; Krasieva, T.B.; Le, A.D. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012, 21, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Latif, N.; Abdulrahman, M.; Helal, M.; Grawish, M.E. Regenerative capacity of allogenic gingival margin- derived stem cells with fibrin glue on albino rats’ partially dissected submandibular salivary glands. Arch. Oral. Biol. 2017, 82, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S. Strategies to improve regenerative potential of mesenchymal stem cells. World J. Stem Cells 2021, 13, 1845–1862. [Google Scholar] [CrossRef]
- Subba, T.A.; Varma, S.; Thomas, B.; Rao, S.; Kumar, M.; Talwar, A.; Shashidhar, K. Comparison of Cellular and Differentiation Characteristics of Mesenchymal Stem Cells Derived from Human Gingiva and Periodontal Ligament. J. Int. Soc. Prev. Community Dent. 2022, 12, 235–244. [Google Scholar] [CrossRef]
- Theocharidou, A.; Bakopoulou, A.; Kontonasaki, E.; Papachristou, E.; Hadjichristou, C.; Bousnaki, M.; Theodorou, G.; Papadopoulou, L.; Kantiranis, N.; Paraskevopoulos, K.; et al. Odontogenic differentiation and biomineralization potential of dental pulp stem cells inside Mg-based bioceramic scaffolds under low-level laser treatment. Lasers Med. Sci. 2017, 32, 201–210. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Wu, F. Low-level laser irradiation enhances the proliferation and osteogenic differentiation of PDLSCs via BMP signaling. Lasers Med. Sci. 2022, 37, 941–948. [Google Scholar] [CrossRef]
- El Nawam, H.; El Backly, R.; Zaky, A.; Abdallah, A. Low-level laser therapy affects dentinogenesis and angiogenesis of in vitro 3D cultures of dentin-pulp complex. Lasers Med. Sci. 2019, 34, 1689–1698. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Tim, C.R.; Bossini, P.S.; Kido, H.W.; Malavazi, I.; von Zeska Kress, M.R.; Carazzolle, M.F.; Parizotto, N.A.; Rennó, A.C. Effects of low level laser therapy on inflammatory and angiogenic gene expression during the process of bone healing: A microarray analysis. J. Photochem. Photobiol. B: Biol. 2016, 154, 8–15. [Google Scholar] [CrossRef]
- Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G.; et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. [Google Scholar] [CrossRef] [PubMed]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; Genot, M.T.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 2: Proposed applications and treatment protocols. Support. Care Cancer 2016, 24, 2793–2805. [Google Scholar] [CrossRef]
- Cardoso Bezerra, S.J.; Fioranelli Vieira, G.; Eduardo Cde, P.; de Freitas, P.M.; Aranha, A.C. Laser Phototherapy (660 nm) Can Be Beneficial for Reducing Gingival Inflammation in Prosthodontics. Case Rep. Dent. 2015, 2015, 132656. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Ajlal, S. Applications of Light Amplification by Stimulated Emission of Radiation (Lasers) for Restorative Dentistry. Med. Princ. Pract. 2016, 25, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Junqueira Mde, A.; Marques, N.C.; Machado, M.A.; Santos, C.F.; Oliveira, T.M.; Sakai, V.T. Effects of low-level laser therapy on stem cells from human exfoliated deciduous teeth. J. Appl. Oral. Sci. 2016, 24, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, A.; Abbasi, R.; Najafi, R.; Rezaei-soufi, L.; Karkehabadi, H. Effect of diode low level laser and red light emitting diode irradiation on cell proliferation and osteogenic/odontogenic differentiation of stem cells from the apical papilla. BMC Oral. Health 2022, 22, 543. [Google Scholar] [CrossRef]
- Rathod, A.; Jaiswal, P.; Bajaj, P.; Kale, B.; Masurkar, D. Implementation of Low-Level Laser Therapy in Dentistry: A Review. Cureus 2022, 14, e28799. [Google Scholar] [CrossRef]
- Meesuk, L.; Suwanprateeb, J.; Thammarakcharoen, F.; Tantrawatpan, C.; Kheolamai, P.; Palang, I.; Tantikanlayaporn, D.; Manochantr, S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci. Rep. 2022, 12, 19509. [Google Scholar] [CrossRef]
- Widholz, B.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Westhauser, F. Pooling of Patient-Derived Mesenchymal Stromal Cells Reduces Inter-Individual Confounder-Associated Variation without Negative Impact on Cell Viability, Proliferation and Osteogenic Differentiation. Cells 2019, 8, 633. [Google Scholar] [CrossRef]
- Du, L.; Yang, P.; Ge, S. Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method. J. Dent. Sci. 2016, 11, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell. Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; Raafat, S.; Elashiry, M.; El-Banna, A.; Schäfer, E. Effect of Different Sealers on the Cytocompatibility and Osteogenic Potential of Human Periodontal Ligament Stem Cells: An In Vitro Study. J. Clin. Med. 2023, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Mastrangelo, F.; Gastaldi, G.; Tettamanti, L.; Bukvic, N.; Cantore, S.; Cocco, T.; Saini, R.; Desiate, A.; Gherlone, E.; et al. Osteogenic differentiation and gene expression of dental pulp stem cells under low-level laser irradiation: A good promise for tissue engineering. J. Biol. Regul. Homeost. Agents 2015, 29, 813–822. [Google Scholar]
- Saghaei Bagheri, H.; Rasta, S.H.; Mohammadi, S.M.; Rahimi, A.A.R.; Movassaghpour, A.; Nozad Charoudeh, H. Low-Level Laser Irradiation Modulated Viability of Normal and Tumor Human Lymphocytes In Vitro. J. Lasers Med. Sci. 2020, 11, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; López-García, S.; García-Bernal, D.; Sanz, J.L.; Lozano, A.; Pecci-Lloret, M.P.; Melo, M.; López-Ginés, C.; Forner, L. Cytocompatibility and bioactive properties of the new dual-curing resin-modified calcium silicate-based material for vital pulp therapy. Clin. Oral. Investig. 2021, 25, 5009–5024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, S.; Li, X.; Wang, S.; Zhang, T.; Huo, N.; Duan, R.; Shi, Q.; Zhang, J.; Xu, J. Local transplantation of GMSC-derived exosomes to promote vascularized diabetic wound healing by regulating the Wnt/β-catenin pathways. Nanoscale Adv. 2023, 5, 916–926. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rouabhia, M.; Ortiz, J.; Gaviria, D.; Alfonso, C.; Muñoz, A.; Inostroza, C. Low-Level Laser Irradiation Promotes Proliferation and Differentiation on Apical papilla Stem Cells. J. Lasers Med. Sci. 2021, 12, e75. [Google Scholar] [CrossRef]
- Tam, S.Y.; Tam, V.C.W.; Ramkumar, S.; Khaw, M.L.; Law, H.K.W.; Lee, S.W.Y. Review on the Cellular Mechanisms of Low-Level Laser Therapy Use in Oncology. Front. Oncol. 2020, 10, 1255. [Google Scholar] [CrossRef]
- Gao, X.; Chen, T.; Xing, D.; Wang, F.; Pei, Y.; Wei, X. Single cell analysis of PKC activation during proliferation and apoptosis induced by laser irradiation. J. Cell. Physiol. 2006, 206, 441–448. [Google Scholar] [CrossRef]
- Rola, P.; Włodarczak, S.; Lesiak, M.; Doroszko, A.; Włodarczak, A. Changes in Cell Biology under the Influence of Low-Level Laser Therapy. Photonics 2022, 9, 502. [Google Scholar] [CrossRef]
- Cios, A.; Ciepielak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ouchi, T.; Cao, Y.; Zhao, Z.; Men, Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell. Dev. Biol. 2021, 9, 654559. [Google Scholar] [CrossRef] [PubMed]
- Amid, R.; Kadkhodazadeh, M.; Gilvari Sarshari, M.; Parhizkar, A.; Mojahedi, M. Effects of Two Protocols of Low-Level Laser Therapy on the Proliferation and Differentiation of Human Dental Pulp Stem Cells on Sandblasted Titanium Discs: An In Vitro Study. J. Lasers Med. Sci. 2022, 13, e1. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A. Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J. Photochem. Photobiol. B: Biol. 2016, 162, 577–582. [Google Scholar] [CrossRef]
- Chen, J.; Sang, Y.; Li, J.; Zhao, T.; Liu, B.; Xie, S.; Sun, W. Low-level controllable blue LEDs irradiation enhances human dental pulp stem cells osteogenic differentiation via transient receptor potential vanilloid 1. J. Photochem. Photobiol. B: Biol. 2022, 233, 112472. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sardana, S.; Hamblin, M.R. Role of opsins and light or heat activated transient receptor potential ion channels in the mechanisms of photobiomodulation and infrared therapy. J. Photochem. Photobiol. 2023, 13, 100160. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Marques, L.; Holgado, L.A.; Francischone, L.A.; Ximenez, J.P.; Okamoto, R.; Kinoshita, A. New LLLT protocol to speed up the bone healing process-histometric and immunohistochemical analysis in rat calvarial bone defect. Lasers Med. Sci. 2015, 30, 1225–1230. [Google Scholar] [CrossRef]
- de Oliveira, G.J.P.L.; Aroni, M.A.T.; Pinotti, F.E.; Marcantonio, E.; Marcantonio, R.A.C. Low-level laser therapy (LLLT) in sites grafted with osteoconductive bone substitutes improves osseointegration. Lasers Med. Sci. 2020, 35, 1519–1529. [Google Scholar] [CrossRef]
- Yaralı Çevik, Z.B.; Karaman, O.; Topaloğlu, N. Photobiomodulation therapy at red and near-infrared wavelengths for osteogenic differentiation in the scaffold-free microtissues. J. Photochem. Photobiol. B: Biol. 2023, 238, 112615. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Wang, Y.; Li, T.; Liu, P.; Han, Z.; Sun, Y.; Liu, Y. Study on the Osteogenic Differentiation of hUCMSCs in Silk Fibroin/Polycaprolactone Membrane Under the Intervention of Photobiomodulation. IEEE J. Sel. Top. Quantum Electron. 2023, 29, 3200367. [Google Scholar] [CrossRef]
- Figueredo, C.A.; Abdelhay, N.; Ganatra, S.; Gibson, M.P. The role of Dentin Sialophosphoprotein (DSPP) in craniofacial development. J. Oral. Biol. Craniofac Res. 2022, 12, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Dussold, C.; Gerber, C.; White, S.; Wang, X.; Qi, L.; Francis, C.; Capella, M.; Courbon, G.; Wang, J.; Li, C.; et al. DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res. 2019, 7, 12. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Vale, G.C.; Mayer, M.P.A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral. Biol. 2021, 128, 105174. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhazmi, Y.A.; Aljabri, M.Y.; Raafat, S.N.; Gomaa, S.M.; Shamel, M. Exploring the Effects of Low-Level Laser Therapy on the Cytocompatibility and Osteo/Odontogenic Potential of Gingival-Derived Mesenchymal Stem Cells: Preliminary Report. Appl. Sci. 2023, 13, 8490. https://doi.org/10.3390/app13148490
Alhazmi YA, Aljabri MY, Raafat SN, Gomaa SM, Shamel M. Exploring the Effects of Low-Level Laser Therapy on the Cytocompatibility and Osteo/Odontogenic Potential of Gingival-Derived Mesenchymal Stem Cells: Preliminary Report. Applied Sciences. 2023; 13(14):8490. https://doi.org/10.3390/app13148490
Chicago/Turabian StyleAlhazmi, Yaser A., Mohammed Y. Aljabri, Shereen N. Raafat, Shaimaa M. Gomaa, and Mohamed Shamel. 2023. "Exploring the Effects of Low-Level Laser Therapy on the Cytocompatibility and Osteo/Odontogenic Potential of Gingival-Derived Mesenchymal Stem Cells: Preliminary Report" Applied Sciences 13, no. 14: 8490. https://doi.org/10.3390/app13148490
APA StyleAlhazmi, Y. A., Aljabri, M. Y., Raafat, S. N., Gomaa, S. M., & Shamel, M. (2023). Exploring the Effects of Low-Level Laser Therapy on the Cytocompatibility and Osteo/Odontogenic Potential of Gingival-Derived Mesenchymal Stem Cells: Preliminary Report. Applied Sciences, 13(14), 8490. https://doi.org/10.3390/app13148490






