Improvement of Luminescence and Photocatalytic Performance of ZnO:Eu3+ Nanocrystals Activated by Na+ Ions
Abstract
1. Introduction
2. Experimental
2.1. Samples Synthesis
2.2. Characterizations
2.3. Photocatalysis
3. Results and Discussion
3.1. XRD Analysis
3.2. Raman Analysis
3.3. XPS Analysis
3.4. UV-Visible Measurements
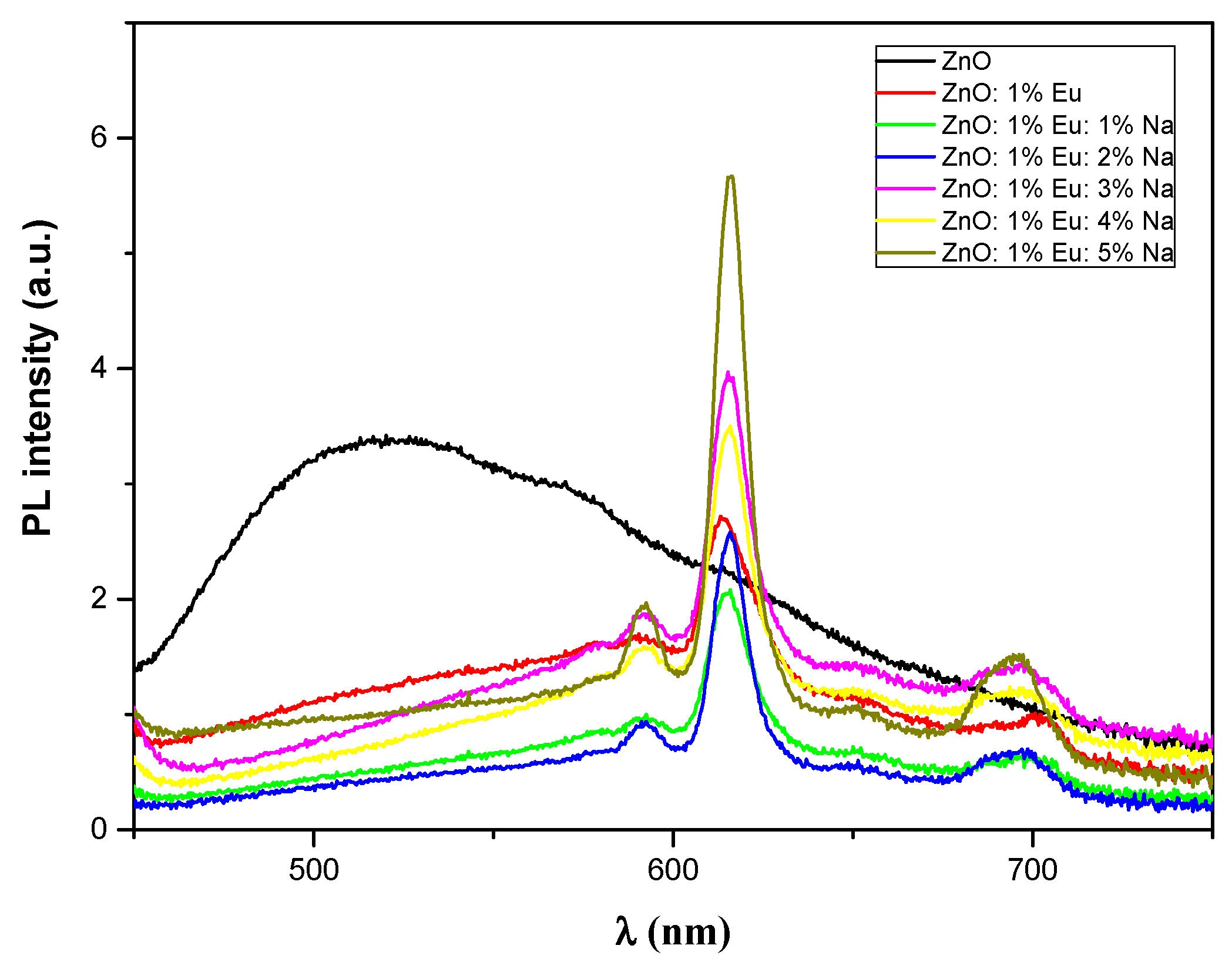
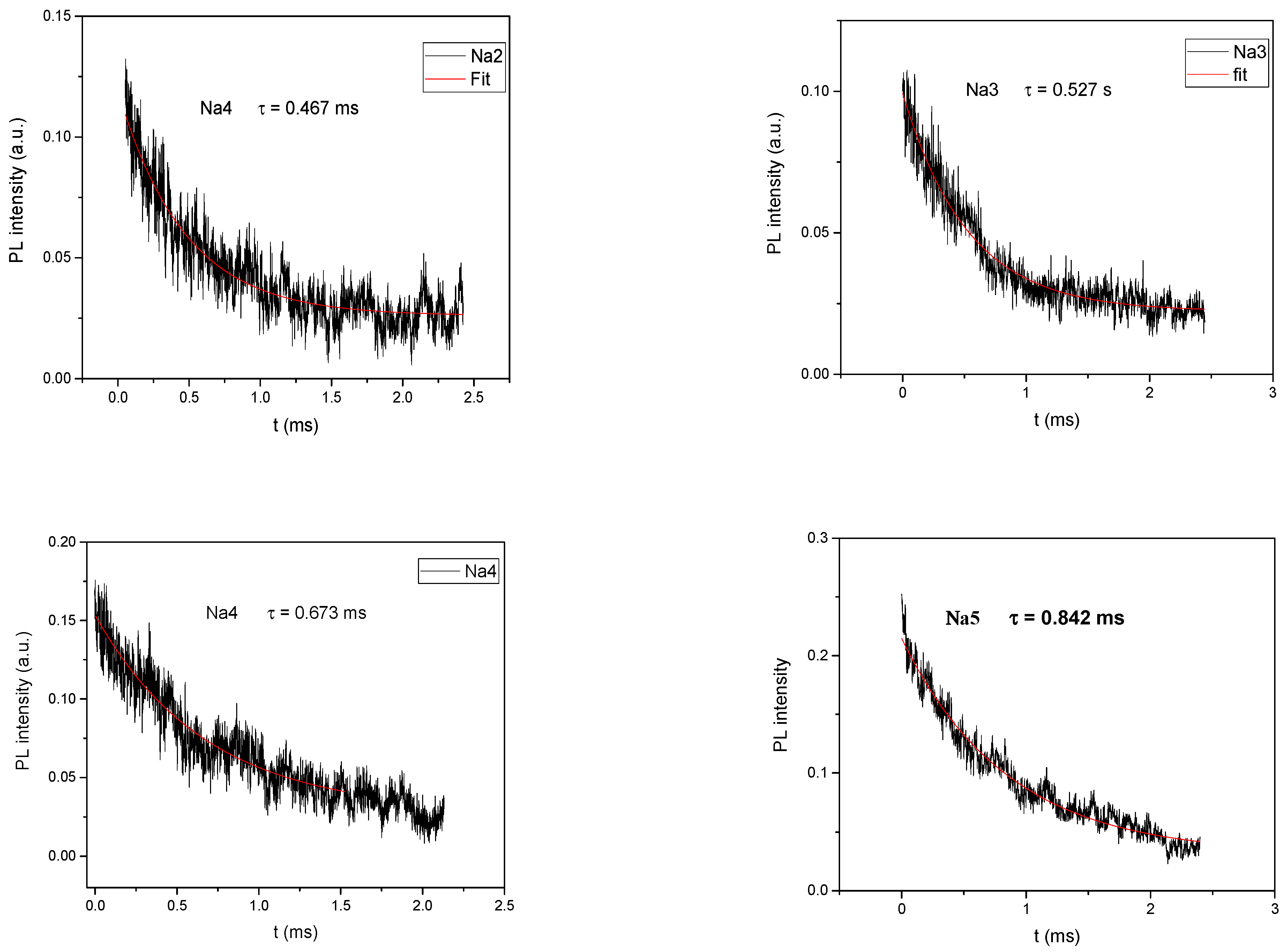
3.5. Luminescence Study
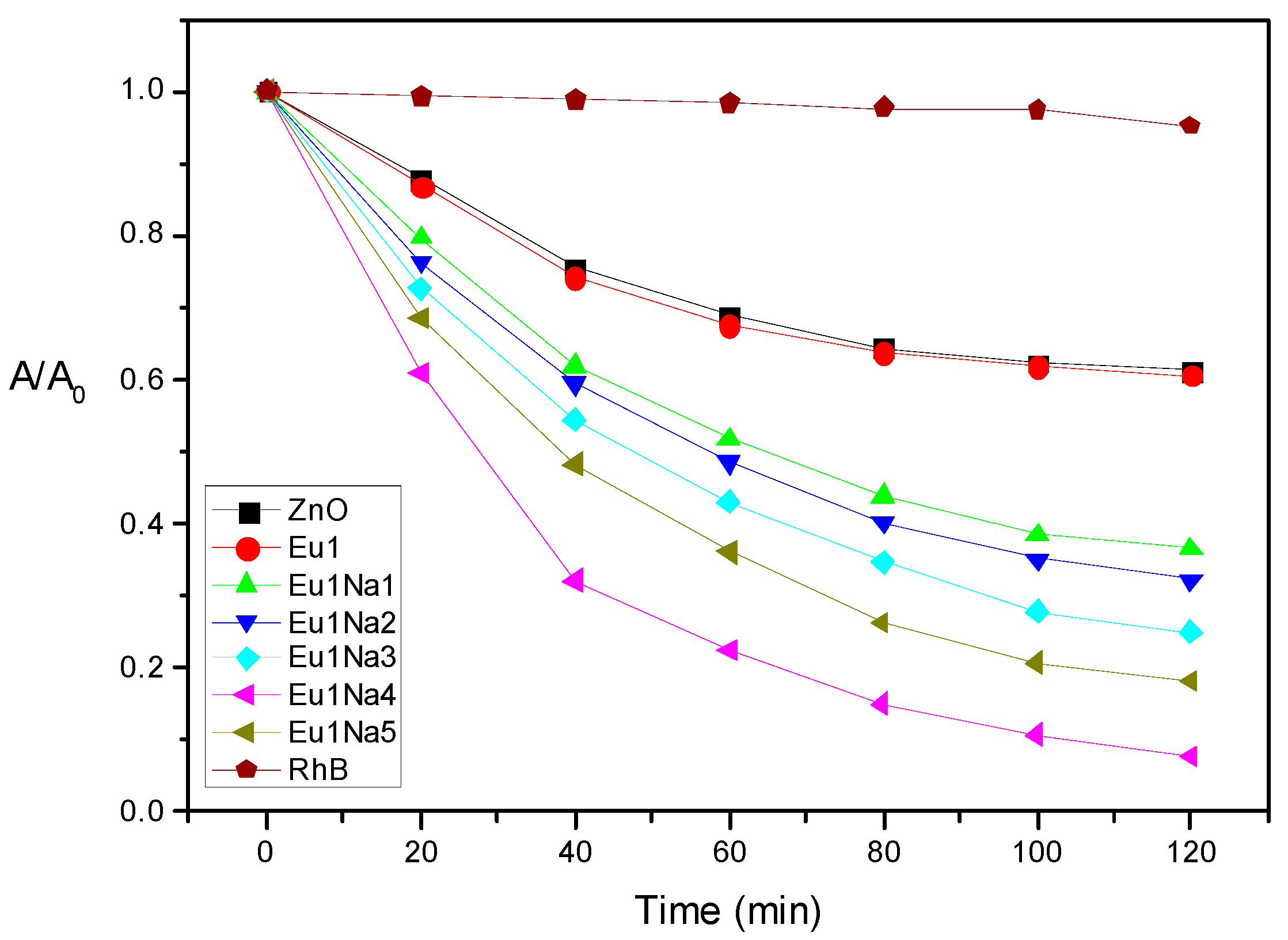
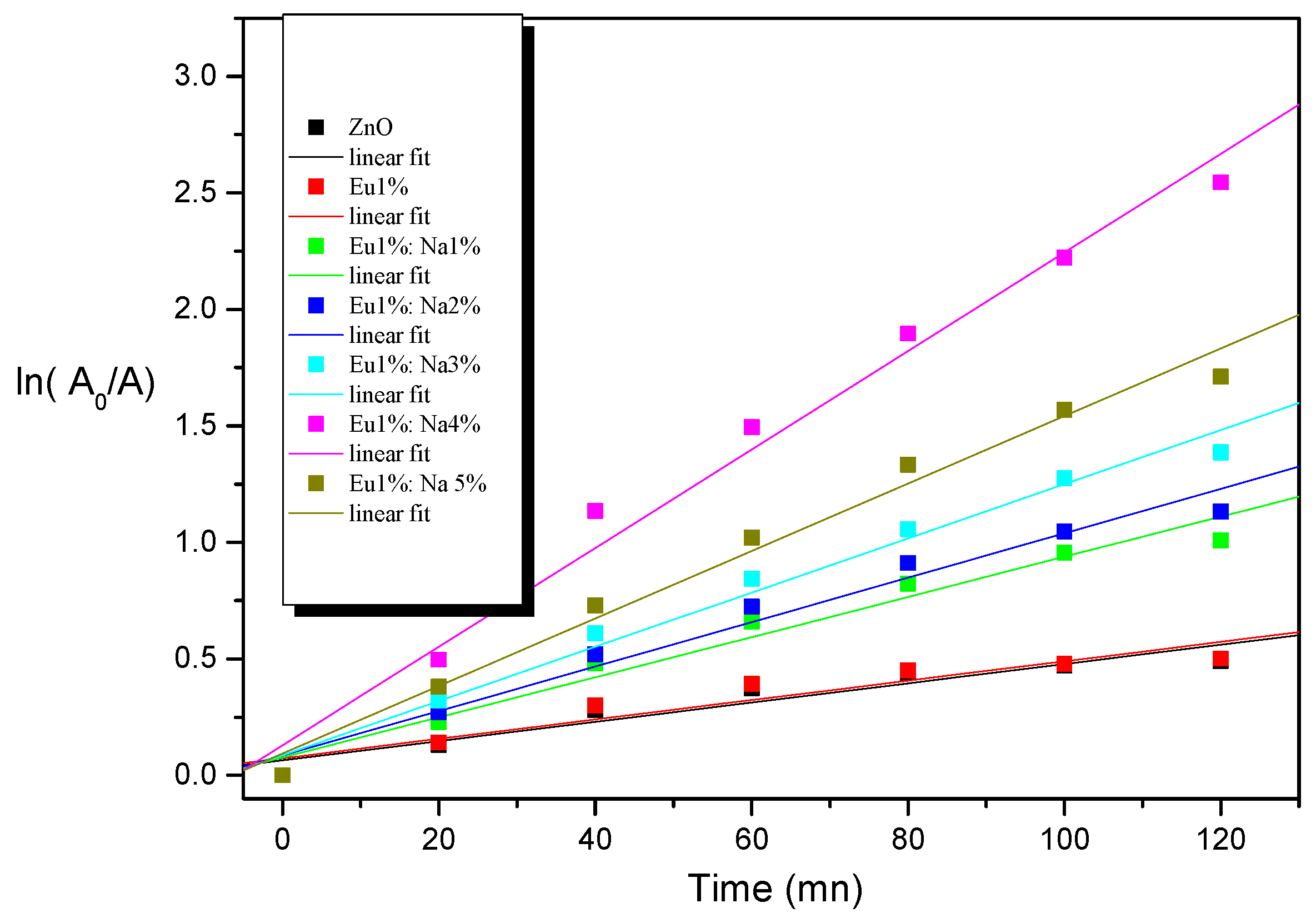
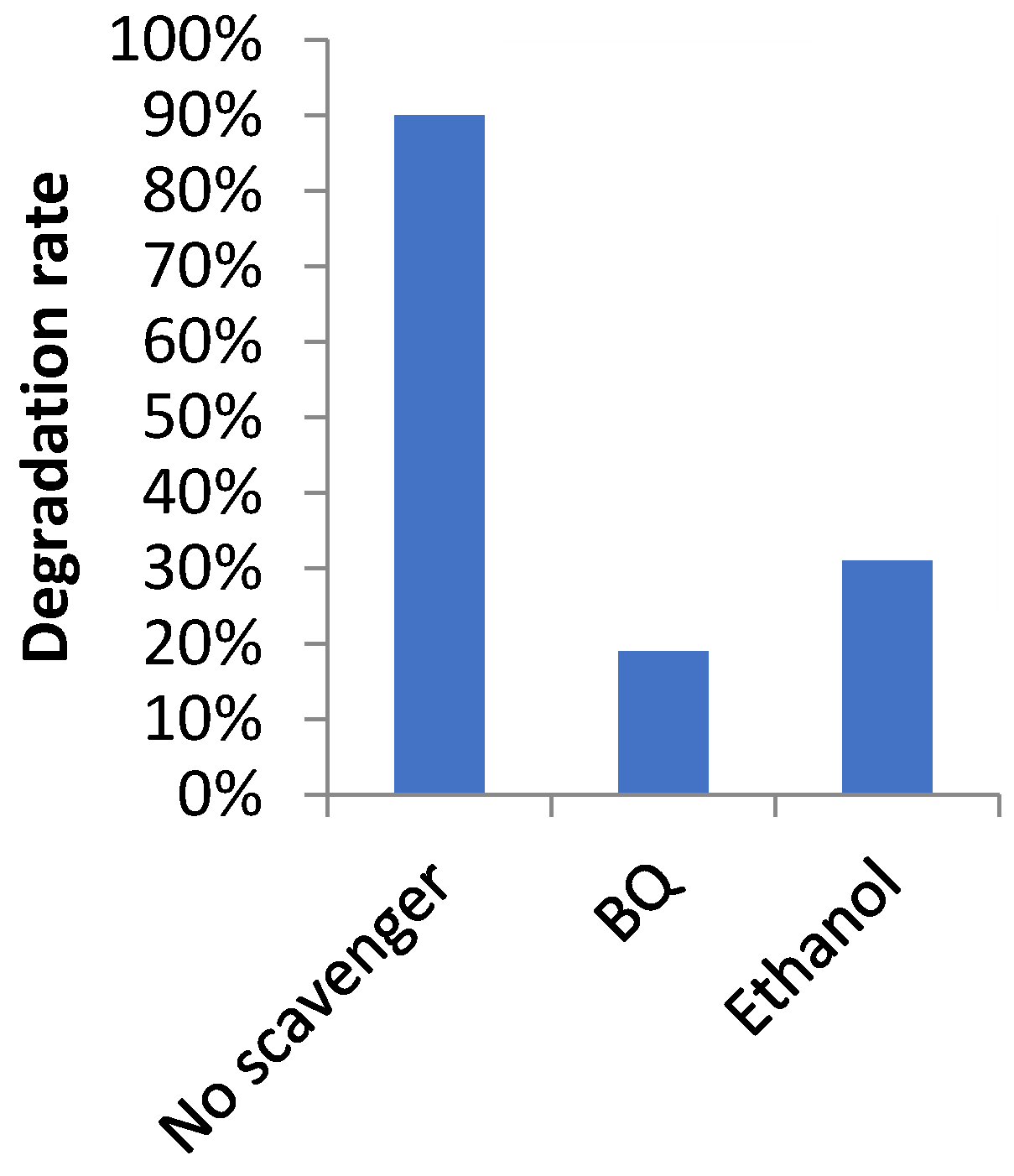
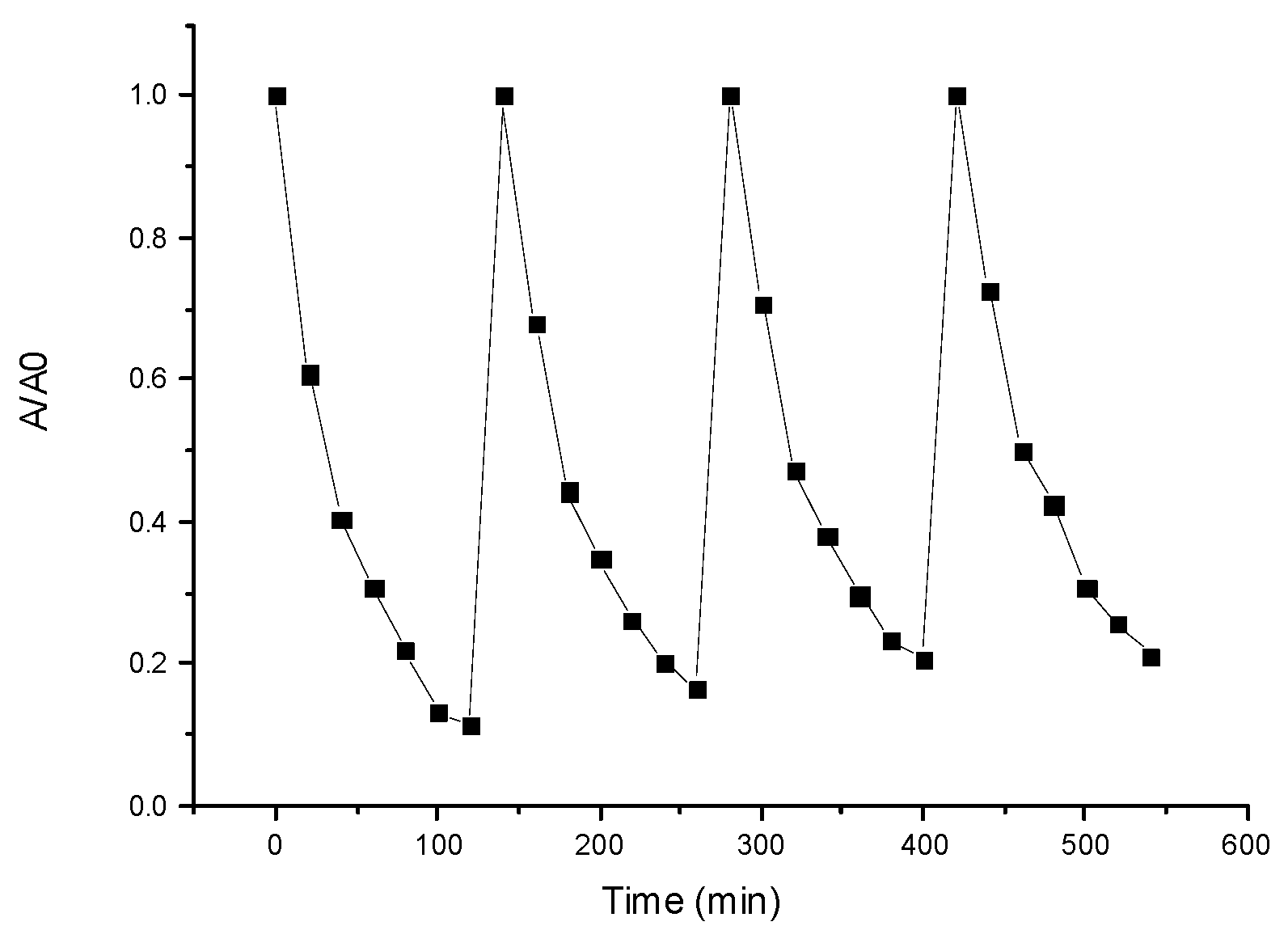
3.6. Photocatalytic Study
- ➣
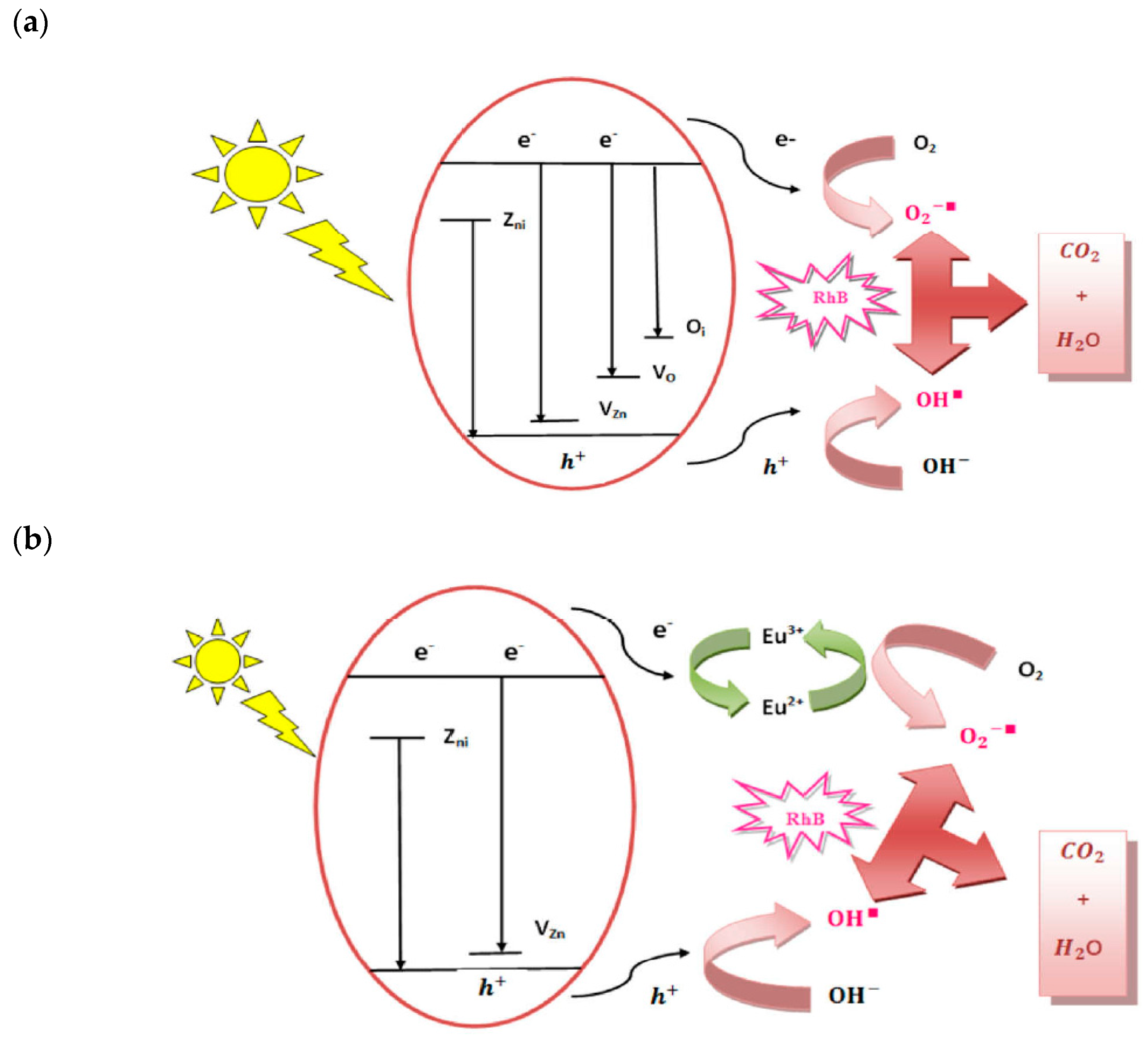
- Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liang, Z.; Jiang, K.; Lin, Y.; He, D.; Liu, J.; Wang, W.; Wang, J.; Deng, B.; Zhang, D.; et al. SrLaNaTeO6:Eu3+ red-emitting phosphors with high luminescence efficiency and high thermal stability for high CRI white LEDs. Ceram. Int. 2023, 49, 579–590. [Google Scholar] [CrossRef]
- Othmen, W.B.H.; Ali, M.B.; Bouslama, W.; Elhouichet, H. Solar driven photocatalytic properties of Sm3+ doped ZnO nanocrystals. Ceram. Int. 2020, 46, 18878. [Google Scholar] [CrossRef]
- Wangkhem, R.; Yaba, T.; Singh, N.S.; Ningthoujam, R.S. Red emission enhancement from CaMoO4:Eu3+ by co-doping of Bi3+ for near UV/blue LED pumped white pcLEDs: Energy transfer studies. J. Appl. Phys. 2018, 123, 124303. [Google Scholar] [CrossRef]
- Saad, M.; Elhouichet, H. Good optical performances of Eu3+/Dy3+/Ag nanoparticles co-doped phosphate glasses induced by plasmonic effects. J. Alloys Compd. 2019, 806, 1403. [Google Scholar] [CrossRef]
- Jlassi, I.; Mnasri, S.; Elhouichet, H. Concentration dependent spectroscopic behavior of Sm3+-doped sodium fluoro-phosphates glasses for orange and reddish-orange light emitting applications. J. Lumin. 2018, 199, 516–527. [Google Scholar] [CrossRef]
- Nasser, R.; Elhouichet, H. Production of acceptor complexes in sol-gel ZnO thin films by Sb doping. J. Lumin. 2018, 196, 11–19. [Google Scholar] [CrossRef]
- Saad, M.; Stambouli, W.; Sdiri, N.; Elhouichet, H. Effect of mixed sodium and vanadium on the electric and dielectric properties of zinc phosphate glass. Mater. Res. Bull. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Bousslama, W.; Elhouichet, H.; Férid, M. Enhanced photocatalytic activity of Fe doped ZnO nanocrystals under sunlight irradiation. Optik 2017, 134, 88–98. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.H.; Elhouichet, H.; Férid, M. Preparation, characterization of Sb-doped ZnO nanocrystals and their excellent solar light driven photocatalytic activity. Appl. Surf. Sci. 2017, 393, 486–495. [Google Scholar] [CrossRef]
- Tabib, A.; Bouslama, W.; Sieber, B.; Addad, A.; Elhouichet, H.; Férid, M.; Boukherroub, R. Structural and optical properties of Na doped ZnO nanocrystals: Application to solar photocatalysis. Appl. Surf. Sci. 2017, 396, 1528–1538. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, G.; Chauhan, M.S. Europium (Eu3+)—Doped ZnO nanostructures: Synthesis, characterization, and photocatalytic, chemical sensing and preliminary assessment of magnetic properties. Ceram. Int. 2021, 47, 17023–17033. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.S.; Ahmed, E.; Ahmad, M.; Keller, V.; Khan, W.Q.; Khalid, N.R. Rare earth co-doped ZnO photocatalysts: Solution combustion synthesis and environmental applications. Sep. Purif. Technol. 2020, 237, 116328. [Google Scholar] [CrossRef]
- Zong, Y.; Li, Z.; Wang, X.; Ma, J.; Men, Y. Synthesis and high photocatalytic activity of Eu-doped ZnO nanoparticles. Ceram. Int. 2014, 40, 10375–10382. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, W.; Hu, Z.; Feng, Z.; Hu, P.; Wu, H.; Ma, L.; Pan, Y.; Zhu, Y.; Xiong, G. Dual-functional Bi3+, Dy3+ co-doping ZnWO4 for photoluminescence and photocatalysis. Appl. Phys. A 2018, 124, 526. [Google Scholar] [CrossRef]
- Bousslama, W.; Sieber, B.; Elhouichet, H.; Gelloz, B.; Addad, A.; Ferid, M. Enhancement of the intensity ratio of ultraviolet to visible luminescence with increased excitation in ZnO nanoparticles deposited on porous anodic alumina. J. Phys. D Appl. Phys. 2013, 46, 505104. [Google Scholar] [CrossRef]
- Bousslama, W.; Elhouichet, H.; Gelloz, B.; Sieber, B.; Addad, A.; Moreau, M.; Férid, M.; Koshida, N. Structural and Luminescence Properties of Highly Crystalline ZnO Nanoparticles Prepared by Sol–Gel Method. Jpn. J. Appl. Phys. 2012, 51, 04DG13. [Google Scholar] [CrossRef]
- Abed, C.; Bouzid, C.; Elhouichet, H.; Gelloz, B.; Ferid, M. Mg doping induced high structural quality of sol–gel ZnO nanocrystals: Application in photocatalysis. Appl. Surf. Sci. 2015, 349, 855–863. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.H.; Elhouichet, H. Effect of Sb doping on the electrical and dielectric properties of ZnO nanocrystals. Ceram. Int. 2019, 45, 8000–8007. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Olaru, N.; Samoila, P.; Airinei, A.; Ignat, M.; Sacarescu, L.; Timpu, D. Novel rare earth (RE-La, Er, Sm) metal doped ZnO photocatalysts for degradation of Congo-Red dye: Synthesis, characterization and kinetic studies. J. Environ. Manag. 2019, 239, 225–234. [Google Scholar] [CrossRef]
- Trandafilovic, L.V.; Jovanovi’c, D.J.; Zhang, X.; Ptasinska, S.; Dramicanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO:Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.H.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solide State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Faraz, M.; Naqvi, F.K.; Shakira, M.; Khare, N. Synthesis of samarium-doped zinc oxide nanoparticles with improved photocatalytic performance and recyclability under visible light irradiation. New J. Chem. 2018, 42, 2295–2305. [Google Scholar] [CrossRef]
- George, A.; Sharma, S.K.; Chawla, S.; Malik, M.M.; Qureshi, M.S. Detailed of X-ray diffraction and photoluminescence studies of Ce doped ZnO nanocrystals. J. Alloys Compd. 2011, 509, 5942–5946. [Google Scholar] [CrossRef]
- Anand, V.; Sakthivelu, A.; Kumar, K.D.A.; Valanarasu, S.; Ganesh, V.; Shkir, M.; Kathalingam, A.; AlFaify, S. Novel rare earth Gd and Al co-doped ZnO thin films prepared by nebulizer spray method for optoelectronic applications. Superlattices Microstruct. 2018, 123, 311–322. [Google Scholar] [CrossRef]
- Mukherjee, S.; Katea, S.N.; Rodrigues, E.M.; Segre, C.U.; Hemmer, E.; Broqvist, P.; Rensmo, H.; Westin, G. Entrapped Molecule-Like Europium-Oxide Clusters in Zinc Oxide with Nearly Unaffected Host Structure. Small 2023, 19, 2203331. [Google Scholar] [CrossRef]
- Abed, C.; Ali, M.B.; Addad, A.; Elhouichet, H. Growth, Structural and optical properties of ZnO-ZnMgO-MgO nanocomposites and their photocatalytic activity under sunlight irradiation. Mater. Res. Bull. 2019, 110, 230–238. [Google Scholar] [CrossRef]
- Kumar, B.R.; Hymavathi, B. X-ray peak profile analysis of solid-state sintered alumina doped zinc oxide ceramics by Williamson–Hall and size-strain plot methods. J. Asian Ceram. Soc. 2017, 5, 94–103. [Google Scholar] [CrossRef]
- Bouslama, W.; Ali, M.B.; Sdiri, N.; Elhouichet, H. Conduction mechanisms and dielectric constant features of Fe doped ZnO nanocrystals. Ceram. Int. 2021, 47, 19106–19114. [Google Scholar] [CrossRef]
- Manjón, F.J.; Mari, B.; Serrano, J.; Romero, A.H. Silent Raman modes in zinc oxide and related nitrides. J. Appl. Phys. 2005, 97, 053516. [Google Scholar] [CrossRef]
- Nasser, R.; Song, J.; Elhouichet, H. Epitaxial growth and properties study of p-type doped ZnO:Sb by PLD. Superlattices Microstruct. 2021, 155, 106908. [Google Scholar] [CrossRef]
- Althumairi, N.A.; Baig, I.; Kayed, T.S.; Mekki, A.; Lusson, A.; Sallet, V.; Majid, A.; Fouzri, A. Characterization of Eu doped ZnO micropods prepared by chemical bath deposition on p-Si substrate. Vacuum 2022, 198, 110874. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Chen, X.; Qian, X.; Jiang, D.; Wu, Q.; Wang, J.; Xu, J.; Su, L. Color-tunable visible photoluminescence of Eu:CaF2 single crystals: Variations of valence state and local lattice environment of Eu ions. Opt. Express 2019, 27, 523–532. [Google Scholar] [CrossRef]
- Andrello, C.; Gouder, T.; Favergeon, L.; Desgranges, L.; Tereshina-Chitrova, E.; Havela, L.; Konings, R.J.M.; Eloirdi, R. In-situ high-resolution photoelectron spectroscopy study on interaction of sodium with UO2+x film (0 ≤ x ≤ 1). J. Nucl. Mater. 2021, 545, 152646. [Google Scholar] [CrossRef]
- Poornaprakash, B.; Chalapathi, U.; Sekhar, M.C.; Rajendar, V.; Vattikuti, S.P.; Reddy, M.S.P.; Suh, Y.; Park, S.-H. Effect of Eu3+ on the morphology, structural, optical, magnetic, and photocatalytic properties of ZnO nanoparticles. Superlattices Microstruct. 2018, 123, 154–163. [Google Scholar] [CrossRef]
- Bian, M.; Zhang, H.; Zhang, J.; Li, Z. Effects of post-annealing on photoluminescence of Eu-doped ZnO microsphere for single-component white-light materials. Optik 2020, 209, 164602. [Google Scholar] [CrossRef]
- Manikandan, A.; Manikandan, E.; Meenatchi, B.; Vadivel, S.; Jaganathan, S.K.; Ladchumananandasivam, R.; Henini, M.; Maaza, M.; Aanand, J.S. Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: Synthesis structural optical and antibacterial studies. J. Alloys Compd. 2017, 723, 1155–1161. [Google Scholar] [CrossRef]
- Yu, K.S.; Shi, J.Y.; Zhang, Z.L.; Liang, Y.M.; Liu, W. Synthesis, Characterization, and Photocatalysis of ZnO and Er-Doped ZnO. J. Nanomater. 2013, 2013, 75. [Google Scholar] [CrossRef]
- Ghosh, S.; Nambissan, P.M.G.; Thapa, S.; Mandal, K. Defect dynamics in Li substituted nanocrystalline ZnO: A spectroscopic analysis. Physica B 2014, 454, 102–109. [Google Scholar] [CrossRef]
- Frodason, Y.K.; Johansen, K.M.; Galeckas, A.; Vines, L. Broad luminescence from donor-complexed LiZn and NaZn acceptors in ZnO. Phys. Rev. B 2019, 100, 184102. [Google Scholar] [CrossRef]
- Shen, H.; Shi, X.; Wang, Z.; Hou, Z.; Xu, C.; Duan, L.; Zhao, X.; Wu, H. Defects control and origins of blue and green emissions in sol-gel ZnO thin films. Vacuum 2022, 202, 111201. [Google Scholar] [CrossRef]
- Ennouri, M.; Gelloz, B.; Elhouichet, H. Impact of Ag species on luminescence and spectroscopic properties of Eu3+ doped fluoro-phosphate glasses. J. Non-Cryst. Solids 2021, 570, 120938. [Google Scholar] [CrossRef]
- Nouri, A.; Beniaiche, A.; Soucase, B.M.; Guessas, H.; Azizi, A. Photoluminescence study of Eu+3 doped ZnO nanocolumns prepared by electrodeposition method. Optik 2017, 139, 104–110. [Google Scholar] [CrossRef]
- Gao, M.; Yan, C.; Li, B.Z.; Zhou, L.J.; Yao, J.C.; Zhang, Y.J.; Liu, H.L.; Cao, L.H.; Cao, Y.T.; Yang, J.H.; et al. Strong red emission and catalytic properties of ZnO by adding Eu2O3 shell. J. Alloys Compd. 2017, 724, 537–542. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.P.; Srivastava, M.; Srivastava, A.; Singh, P.; Srivastava, A.; Srivastava, S.K. Structural and photoluminescence properties of thermally stable Eu3+activated CaWO4 nanophosphor via Li+ incorporation. J. Lumin. 2018, 203, 507–514. [Google Scholar] [CrossRef]
- Othmen, W.B.H.; Sieber, B.; Cordier, C.; Elhouichet, H.; Addad, A.; Gelloz, B.; Moreau, M.; Barras, A.; Ferid, M.; Boukherroub, R. Iron addition induced tunable band gap and tetravalent Fe ion in hydrothermally prepared SnO2 nanocrystals: Application in photocatalysis. Mater. Res. Bull. 2016, 83, 481. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Meng, S.; Fu, X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3. Appl. Catal. B Environ. 2014, 150–151, 564–573. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Satoshi, I.; Lee, K.T.; Mohamed, A.R. Sunlight photocatalytic activity enhancement and mechanism of novel europium-doped ZnO hierarchical micro/nanospheres for degradation of phenol. Appl. Catal. B Environ. 2014, 148–149, 258–268. [Google Scholar] [CrossRef]
- Salah, N.; Hameed, A.; Aslam, M.; Abdelwahab, M.S.; Babkair, S.S.; Bahabri, F.S. Flow controlled fabrication of N doped ZnO thin films and estimation of their performance for sunlight photocatalytic decontamination of water. Chem. Eng. J. 2016, 291, 115–127. [Google Scholar] [CrossRef]
- Fu, M.; Li, Y.; Wu, S.; Lu, P.; Liu, J.; Dong, F. Sol–gel preparation and enhanced photocatalytic performance of Cu-doped ZnO nanoparticles. Appl. Surf. Sci. 2011, 258, 1587–1591. [Google Scholar] [CrossRef]
- Alkallas, F.H.; Trabelsi, A.B.G.; Nasser, R.; Mansour, S.A.; Song, J.-M.; Elhouichet, H. Promising Cr-Doped ZnO Nanorods for Photocatalytic Degradation Facing Pollution. Appl. Sci. 2022, 12, 34. [Google Scholar] [CrossRef]
- Hanifehpour, Y.; Soltani, B.; Amani-Ghadim, A.R.; Hedayati, B.; Khomami, B.; Joo, S.W. Synthesis and characterization of samarium-doped ZnS nanoparticles: A novel visible light responsive photocatalyst. Mater. Res. Bull. 2016, 76, 411–421. [Google Scholar] [CrossRef]
- Nasser, R.; Elhouichet, H.; Ferid, M. Effect of Mn doping on structural, optical and photocatalytic behaviors of hydrothermal Zn1−xMnxS nanocrystals. Appl. Surf. Sci. 2015, 351, 1122–1130. [Google Scholar] [CrossRef]
- Guesmi, A.; Bouzidi, C.; Elhouichet, H. Spectroscopic properties of Dy3+ doped ZnO for white luminescence applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 177, 164–169. [Google Scholar] [CrossRef]
- Kumar, S.G.; Kavitha, R. Lanthanide ions doped ZnO based photocatalysts. Sep. Purif. Technol. 2021, 274, 118853. [Google Scholar] [CrossRef]
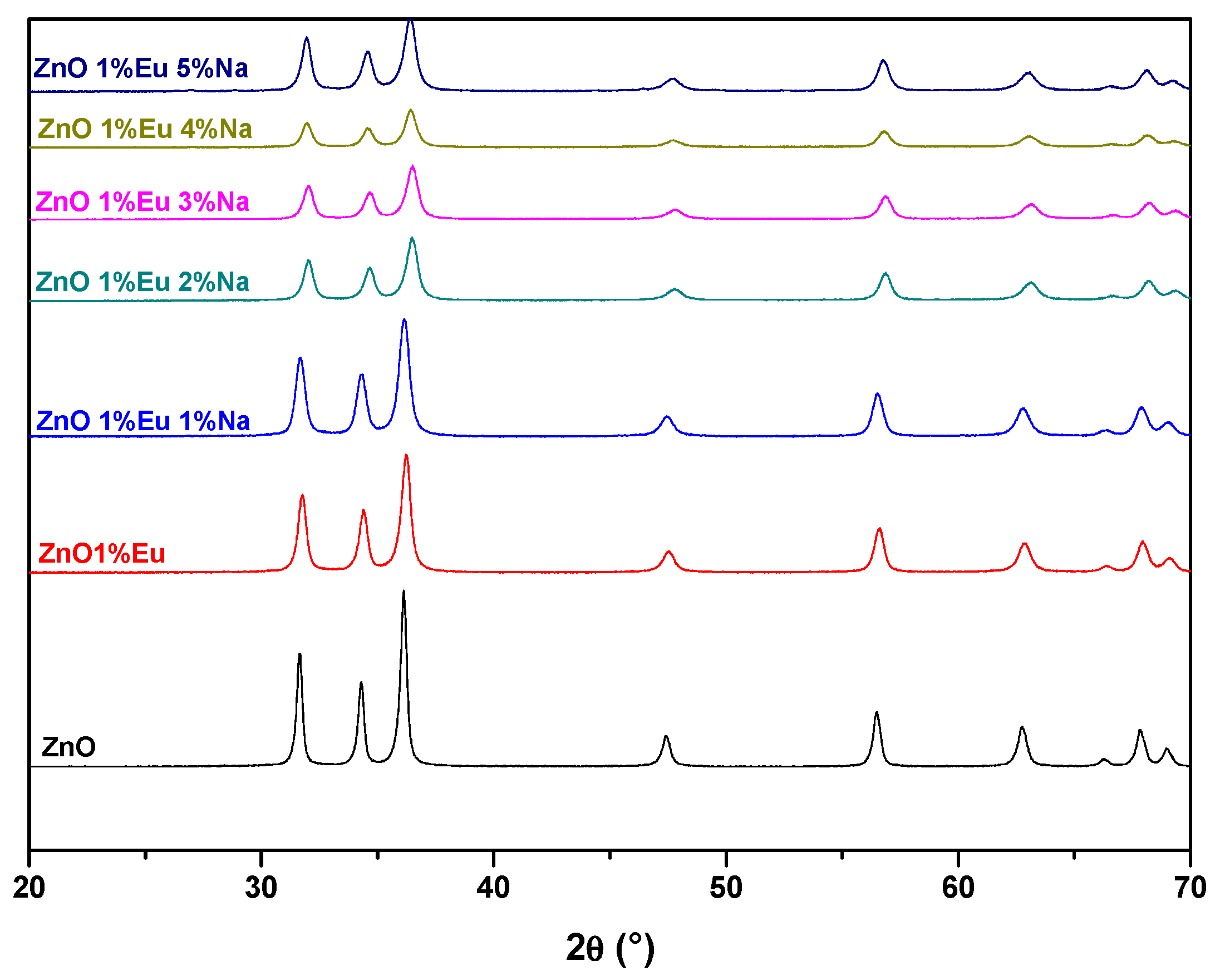


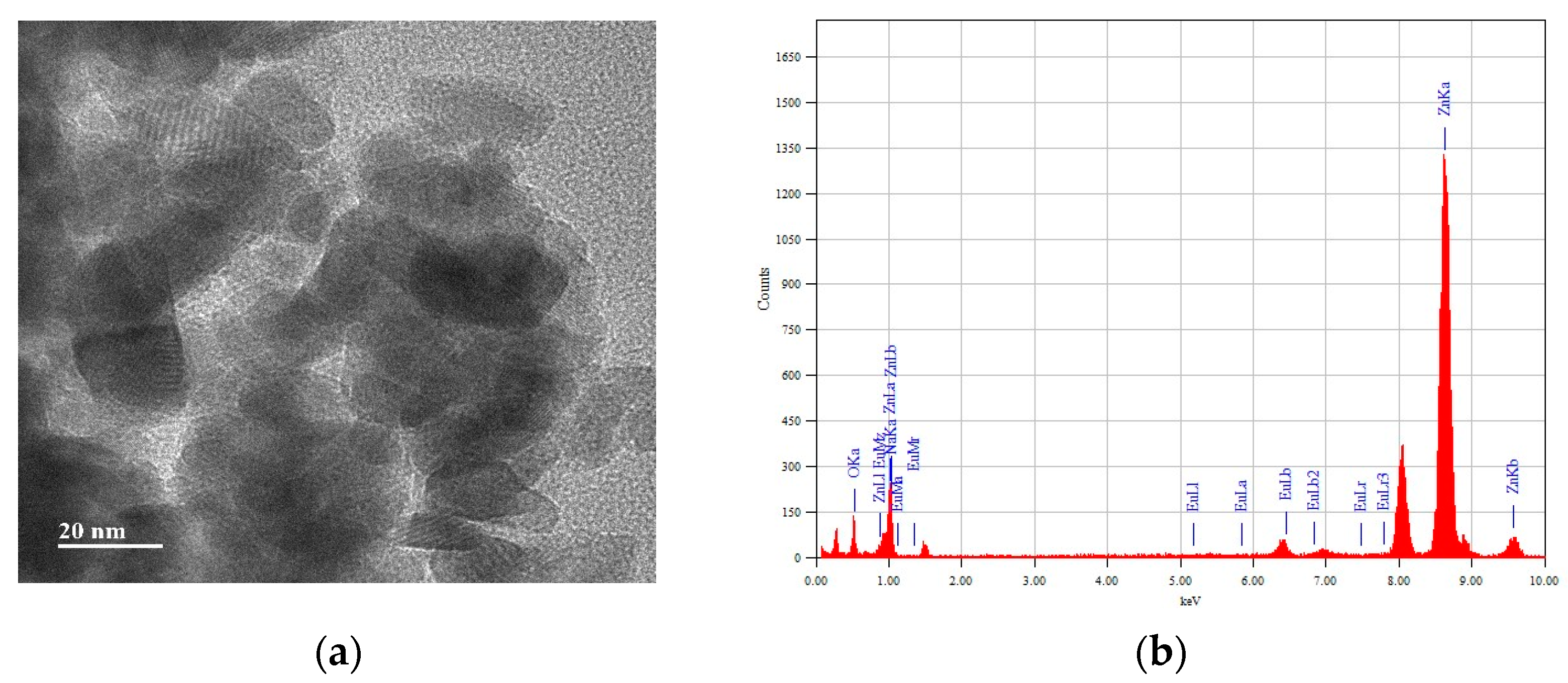


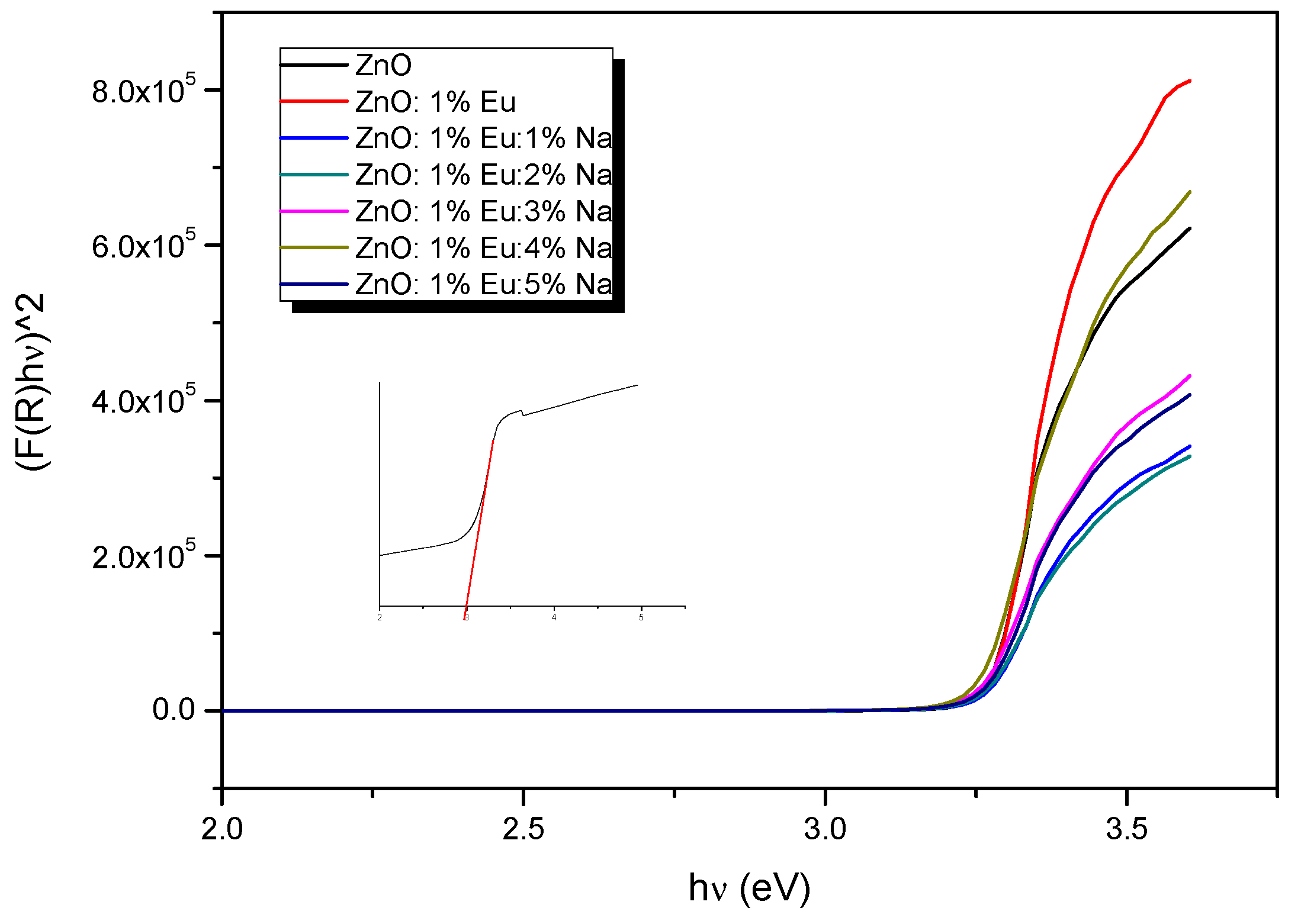
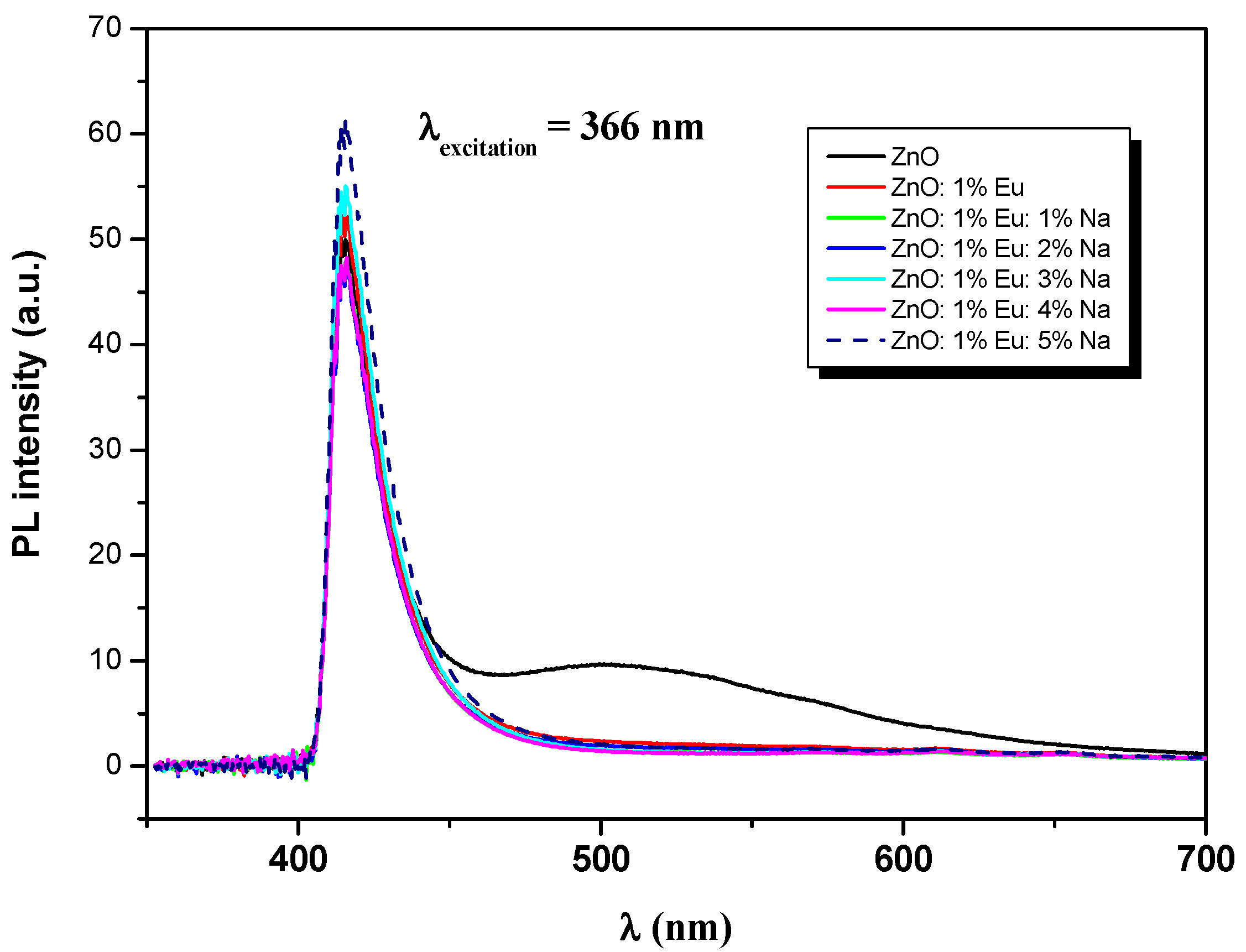

| Samples | ZnO | ZnEu | ZnEuNa1 | ZnEuNa2 | ZnEuNa3 | ZnEuNa4 | ZnEuNa5 |
|---|---|---|---|---|---|---|---|
| (Å) | 2.824 | 2.815 | 2.822 | 2.793 | 2.792 | 2.797 | 2.800 |
| (Å) | 2.612 | 2.606 | 2.609 | 2.585 | 2.585 | 2.594 | 2.591 |
| a (Å) | 3.261 | 3.251 | 3.258 | 3.225 | 3.224 | 3.230 | 3.233 |
| c (Å) | 5.225 | 5.212 | 5.219 | 5.171 | 5.171 | 5.188 | 5.182 |
| c/a | 1.602 | 1.603 | 1.601 | 1.603 | 1.603 | 1.606 | 1.602 |
| V (Å3) | 48.142 | 47.712 | 47.994 | 46.598 | 46.558 | 46.884 | 46.912 |
| Sample | ZnO | ZnEu | ZnEuNa1 | ZnEuNa2 | ZnEuNa3 | ZnEuNa4 | ZnEuNa5 | Zn 5Na [10] |
|---|---|---|---|---|---|---|---|---|
| DD-Sr (nm) | 27.49 | 18.65 | 15.94 | 15.42 | 15.2 | 15.30 | 15.22 | - |
| (10−3) | 1.81 | 1.60 | 1.55 | 2.01 | 2.52 | 3.13 | 2.90 | 1.49 |
| DW-H (nm) | 47.63 | 25.06 | 20.26 | 21.19 | 22.83 | 26.20 | 24.57 | 24.5 |
| Samples | ZnO | ZnEu | ZnEuNa1 | ZnEuNa2 | ZnEuNa3 | ZnEuNa4 | ZnEuNa5 | Zn5Na [10] | ZnEu [13] |
|---|---|---|---|---|---|---|---|---|---|
| Eg (eV) | 3.27 | 3.28 | 3.27 | 3.25 | 3.24 | 3.23 | 3.26 | 3.38 | 3.31 |
| Eu (eV) | 0.092 | 0.074 | 0.094 | 0.102 | 0.108 | 0.103 | 0.100 | 0.108 | - |
| Samples | ZnO | ZnEu | ZnEuNa1 | ZnEuNa2 | ZnEuNa3 | ZnEuNa4 | ZnEuNa5 |
|---|---|---|---|---|---|---|---|
| PL lifetime τ (ms) | - | 0.173 | 0.366 | 0.467 | 0.527 | 0.673 | 0.842 |
| Kinetic constant (10−3 min−1) | 4.14 | 4.16 | 8.61 | 9.54 | 11.63 | 21.16 | 14.49 |
| photodegradation percentage (%) | 40 | 40 | 64 | 68 | 75 | 93 | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouslama, W.; Nasser, R.; Gelloz, B.; Trabelsi, A.B.G.; Alkallas, F.H.; Song, J.-M.; Srasra, E.; Elhouichet, H. Improvement of Luminescence and Photocatalytic Performance of ZnO:Eu3+ Nanocrystals Activated by Na+ Ions. Appl. Sci. 2023, 13, 8448. https://doi.org/10.3390/app13148448
Bouslama W, Nasser R, Gelloz B, Trabelsi ABG, Alkallas FH, Song J-M, Srasra E, Elhouichet H. Improvement of Luminescence and Photocatalytic Performance of ZnO:Eu3+ Nanocrystals Activated by Na+ Ions. Applied Sciences. 2023; 13(14):8448. https://doi.org/10.3390/app13148448
Chicago/Turabian StyleBouslama, Wiem, Ramzi Nasser, Bernard Gelloz, Amira Ben Gouider Trabelsi, Fatemah Homoud Alkallas, Ji-Ming Song, Ezzeddine Srasra, and Habib Elhouichet. 2023. "Improvement of Luminescence and Photocatalytic Performance of ZnO:Eu3+ Nanocrystals Activated by Na+ Ions" Applied Sciences 13, no. 14: 8448. https://doi.org/10.3390/app13148448
APA StyleBouslama, W., Nasser, R., Gelloz, B., Trabelsi, A. B. G., Alkallas, F. H., Song, J.-M., Srasra, E., & Elhouichet, H. (2023). Improvement of Luminescence and Photocatalytic Performance of ZnO:Eu3+ Nanocrystals Activated by Na+ Ions. Applied Sciences, 13(14), 8448. https://doi.org/10.3390/app13148448







