Abstract
Azorean Cryptomeria japonica is widely used for local wood production, generating large amounts of aerial-part biomass residues that can be a sustainable source of value-added bioproducts. This comparative study aimed (i) to determine the yield and chemical profile of the essential oils (EOs) extracted by hydrodistillation from Azorean C. japonica foliage, leaves, male cones (MCs), and female cones (FCs), and (ii) to investigate the antimicrobial and brine shrimp lethality (BSL) effects of the obtained EOs and some major components. The EOs yield revealed a wide range (ca. 1–3%, w/d.w.) and their chemical composition, analysed by GC–MS and GC–FID, showed the presence of seventy-one components. Monoterpene hydrocarbons (38–71%) dominated in all the studied EOs, mainly α-pinene (17–45% of total EOs), decreasing as follows: cone EOs > leaf EOs > foliage EOs. Oxygen-containing monoterpenes (mainly terpinen-4-ol) also dominated in cone EOs. Contrariwise, oxygen-containing sesquiterpenes (mainly elemol) and diterpene hydrocarbons (mainly phyllocladene) dominated in foliage/leaf EOs. The studied EOs exhibited activity against Gram-positive bacteria but no activity against Gram-negative bacteria. A similar trend was displayed by α-pinene. Only the cone EOs showed antifungal activity against Penicillium chrysogenum, but this was significantly lower than those of α-pinene and terpinen-4-ol. Moreover, FC EO should also be considered for further investigation due to its high toxicity on BSL bioassay. In conclusion, this study demonstrated the variability of yield, chemical profile, and bioactivities in the EOs from different parts of the Azorean C. japonica, expanding the knowledge of the potentialities of C. japonica aerial-part EOs, particularly the ones from the cone samples.
1. Introduction
The Azores archipelago is an autonomous region of Portugal located in the North Atlantic Ocean, 1500 km from Lisbon, Portugal and the European mainland. Forest occupies about one third of the Azorean territory. Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae), commonly called “criptoméria” in Portuguese, is a native species from Japan that was introduced in the Azores Islands in the mid-19th century. Currently, it is considered the most important forestry tree in the Azores archipelago, not only because of its economic value, occupying 60% of the total wood producing forest area [1], but also because its stands are a determinant element of the Azorean landscape, which attracts a substantial influx of tourists.
Cryptomeria japonica is a monoecious and very large evergreen conifer that may reach up to 70 m in height with a trunk diameter of up to 4 m. It is a fast-growing tree when planted in moist, well-drained, and deep forest soils [2]. In terms of appearance, C. japonica is a conical to pyramidal tree with a trunk clothed in a reddish-brown bark that is fibrous and peels off into vertical strips. The leaves are spirally arranged, 0.5–1 cm long, and needle-like in structure (Figure 1). C. japonica reproductive organs show two types of strobili (cones): female cones (FCs) and male cones (MCs) (Figure 1B,C). The FCs are brown (when mature), globular, up to 1–2 cm (0.39–0.79 in) in diameter, and composed of about 20–40 megasporophylls [3]. The MCs are numerous, ovoid or oblong, yellow or bright brown (when mature), arranged spirally, 2–3 mm long, and elongate up to 10 mm when ripe to shed pollen [4]. The pollen of C. japonica causes pollinosis, or hay fever, which is a serious health problem in Japan. Male-sterile C. japonica populations that release no pollen have recently gained interest as a potential measure to fight this problem [5].

Figure 1.
(A) Fresh leaves and strobili (female and male cones) of Azorean Cryptomeria japonica. (B–E) Dried Azorean C. japonica aerial parts utilised in this study: (B) female cones, (C) male cones, (D) leaves (needle-shaped), and (E) foliage. Bar = 1 cm.
Among woody plant species, conifers have evolved the capacity to produce a complex terpenoid mixture (oleoresin) accumulated in secretory structures (ranging from single cells or blisters to interconnected resin ducts) that acts as a strong defence system, thus contributing to their evolutionary diversification and colonization success. Resin ducts may be constitutive (CRD), that is, they are always present, or traumatic (TRD), in which case they are induced as response to biotic or mechanical damage [6]. As compiled by Vázquez-González [6], some studies suggest that TRD production might be a key mechanism involved in conifer resistance to both insects and pathogens. Cryptomeria and other conifer genera, such as Cupressus and Araucaria, are referred to as producing axial TRDs in their phloem tissue [6].
Cryptomeria japonica aerial parts, as well as bark, are by-products of tree cutting, and the use of this large forest-based biomass as raw material for commercial applications is now considered to be an important research item. Indeed, C. japonica biomass residues (CJBR) can be used for producing eco-friendly and high value-added products [7,8,9], such as crude essential oils (EOs) and their fractions and/or individual components, with social, economic, and environmental impacts. However, as is well established, the yield and chemical profile of EOs and, consequently, their specific commercial application, quality, and price, depend on many factors besides the plant species, such as geographical location of the plants; environmental parameters; plant tissue, age, and developmental stage; post-harvest drying and storage; and extraction method [10,11]. EO extraction is widely carried out using traditional distillation methods, namely hydrodistillation (HD), water-steam distillation (WSD), and steam distillation (SD, commonly used for commercial scale production). Nevertheless, various innovative green techniques for volatiles isolation (such as non-thermal volatiles extractions) have been developed [12].
The chemical characterization and bioactivity assessment of CJBR EOs have focused mostly on leaves [7,9]. In fact, in our previous study the leaf EO chemical composition from Azorean C. japonica was compared with several C. japonica leaf EO samples from other geographical origins. It was clearly demonstrated by principal component analysis (PCA) and hierarchical cluster analysis (HCA) that, in the Azores, the C. japonica leaf EO chemotype is α-pinene type, while in most Asian countries the leaf EO chemotype is either ent-kaurene type or elemol + ent-kaurene type. In addition, monoterpene hydrocarbons dominated in Azorean C. japonica leaf EO, while oxygen-containing sesquiterpenes are the major terpene fraction in C. japonica leaf EOs from East Asia countries [7]. Concerning the biological activity of Azorean C. japonica leaf EO, previous investigations revealed their potential as a natural biocide, namely as a bactericide, fungicide [13], and molluscicide against Radix peregra (Müller), which is a crucial vector of fascioliasis, a disease with a high impact on public health, as well as on animal production losses and consequent economic costs [14]. Many other bioactivities of C. japonica leaf EO samples from other countries have been reported, including analgesic, anti-inflammatory, antimelanogenesis, antioxidant, antitussive, antiulcer, anxiolytic, cancer chemopreventive, neuropharmacological, whitening, antitermite, mosquito larvicidal, mosquito repellent, and silverfish repellent properties ([11] and the references therein).
However, the existing literature on the chemical composition of EOs from various C. japonica parts, such as FCs and MCs, and their biological potential remains limited. Furthermore, there is an ever-increasing demand for effective natural antimicrobial agents with applications in the fight against human and animal infectious diseases or in green plant protection and food preservation. According to the literature [15,16], crude EOs or their fractions/individual components, as well as synergistic combinations between EOs/components or with antibiotics, can play an important role in inhibiting microbial growth.
In this context, and knowing that bioactive secondary metabolites have different distributions in EOs from different plant parts, the aims of this comparative study were: (i) to determine the yield and chemical profile of the EOs extracted by HD from different Azorean C. japonica parts (foliage, leaves, FCs, and MCs) and (ii) to investigate the antimicrobial and brine shrimp lethality (BSL) effects of the obtained EOs and two pure EO components (α-pinene and terpinen-4-ol). The results will generate more data on the chemical and biological properties of this species to stimulate the development of CJBR’s commercial uses and, consequently, to contribute to the local circular economy.
2. Materials and Methods
2.1. Chemicals and Reagents
Anhydrous sodium sulphate (Na2SO4) and ethanol (purity of 96%) were obtained from Sigma–Aldrich (St. Louis, MO, USA). (−)-α-pinene (≥97%) and (−)-terpinen-4-ol (≥95%), used for the bioassays, were also purchased from Sigma–Aldrich and stored according to the supplier’s instructions. The antibiotics Kanamycin and Nystatin (Mycostatin®), used in the antibacterial and antifungal assays, respectively, were obtained from Sigma–Aldrich (St. Louis, MO, USA) and a local pharmacy, respectively. Sabouraud 4% dextrose agar (SDA) was purchased from Biokar Diagnostics (Beauvais Cedex, France).
2.2. Plant Material
Cryptomeria japonica aerial parts at dormant stage were harvested in January 2022 (winter season) from a 60-year-old tree population located on São Miguel Island (37°48′51.1″ N, 25°14′31.6″ W), Azores archipelago, Portugal. A voucher specimen was deposited in the herbarium of the University of the Azores under number AZB 4541. The fresh material collected was immediately brought to the laboratory at the same university and then was shade dried at room temperature (20 °C) in a well-ventilated area. The dried material was then separated into four samples: FCs, MCs, leaves (needle-shaped), and foliage, as illustrated in Figure 1B–E, respectively. The foliage sample was cut into small chips about 2 cm in length immediately prior to the distillation process.
2.3. EO Extraction by HD Method
The EOs from the different Azorean C. japonica parts were obtained by HD using a Clevenger-type extractor according to the European Pharmacopoeia [17]. The ratio of the sample to water was 1:10 g/mL, and the distillation time was approximately 6 h, except for the FCs (3 h), after the first drop of distillate fell. The isolated EOs were dried over anhydrous Na2SO4 and, after filtration, stored in sealed amber vials at 4 °C until further chemical analyses and biological assays. Each distillation was performed in triplicate, and the EO yield was calculated as a percentage (%, w/w) based on the plant material dry weight.
2.4. EO Composition Analysis
The isolated EOs were analysed by gas chromatography–mass spectrometry (GC–MS) and by gas chromatography with flame ionization detection (GC–FID) for component identification and quantification, respectively.
2.4.1. Gas Chromatography–Flame Ionization Detection (GC–FID)
A Perkin–Elmer Clarus 400 gas chromatograph (PerkinElmer, Waltham, MA, USA) with two flame ionization detectors, a data handling system, and a vaporizing injector port was used in GC–FID analyses. The chromatograph was fitted with two columns of varying polarities: a DB-1 fused-silica column (100% dimethylpolysiloxane, 30 m × 0.25 mm i.d., film thickness 0.25 μm) and a DB-17HT fused-silica column ((50% phenyl)-methylpolysiloxane, 30 m × 0.25 mm i.d., film thickness 0.15 µm), both from J & W Scientific Inc. (Rancho Cordova, CA, USA). The oven temperature was set at 3 °C/min from 45 °C to 175 °C and at 15 °C/min until reaching 300 °C, where it was maintained isothermally for the last 10 min. The injector and detector were set to 280 °C and 300 °C, respectively. The carrier gas used was hydrogen at 30 cm/s. The samples were injected using the split sampling technique at a ratio of 1:50. The volume of injection was 0.1 μL of an n-pentane-EO solution (1:1). The percentage composition of the volatiles was computed by the normalization method from the GC peak areas and calculated as mean values of two injections, from each sample, without using the response factors, in accordance with ISO 7609 [18].
2.4.2. Gas Chromatography–Mass Spectrometry (GC–MS)
A Perkin–Elmer Clarus 600 gas chromatograph, coupled with a DB-1 fused-silica column as detailed above and interfaced with a Perkin–Elmer 600T mass spectrometer (software version 5.4.2.1617, Perkin–Elmer, Shelton, CT, USA), was used in GC–MS analyses. Oven programming and injector temperatures were the same as for GC–FID analyses. The carrier gas was helium at a flow rate of 30 cm/s. The split ratio was 1:40. The transfer line and ion source temperatures were 280 °C and 220 °C, respectively. Electron impact (EI) mode at 70 eV, and mass scan range of 40–300 amu with a scan time of 1 s. Component identities were established by comparing their retention indices (RI), calculated as in ISO 7609 [18], relative to C9–C22 n-alkane (Sigma) indices, and with GC–MS spectra from a lab-made library, created using commercial available standards (Extrasynthese, Cymit Química, S.L.; Sigma–Aldrich; Fluka, Riedel-de Haën), laboratory-synthesised components, laboratory isolated compounds [19], and reference essential oils of Thymus caespititius [20], Juniperus cedrus [21], and C. japonica [22], in which component identities were confirmed by RI, GC–MS, and by 13C-NMR.
2.5. In Vitro Antimicrobial Activity
2.5.1. Microbial Strains and Culture Media
The microorganisms that were selected for the present analysis included one fungus (Penicillium chrysogenum Thom) and six bacteria. P. chrysogenum was isolated from an infected citrus fruit and identified by macro- and micro-morphological characteristics based on mycological keys [23,24]. The bacterial strains were obtained from the Microbiology Laboratory, Department of Biology, University of the Azores. Among the selected bacteria, three were Gram-positive, namely, Bacillus subtilis (Ehrenberg) Cohn (DSM10) and Bacillus licheniformis (Weigmann) Chester (DSM13), which represent food spoilage Bacillales members [25], and Microccocus luteus (Schroeter) Cohn (DSM20030), a clinically potential opportunistic pathogen [26]. The other three were Serratia marcescens Bizio (DSM48), Entereobacter cloacae (Jordan) Hormaeche & Edwards (DSM30054), and Escherichia coli (Migula) Castellani & Chalmers (DSM498), which represent foodborne pathogenic Gram-negative bacteria [27]. Nutrient agar was employed to culture bacterial strains while SDA was cast off for the development and culturing P. chrysogenum fungus. Bacterial inoculums were prepared by the direct inoculation of colonies in 1 mL of sterile saline solution and adjusted to the 0.5 standard of the McFarland scale. The fungal spore suspension was prepared by scraping a 7-day-old pure culture of P. chrysogenum using a sterile loop and adjusted to 5 × 104 spores/mL using a haemocytometer (Hirschmann, Eberstadt, Germany). After the fungus inoculum, the SDA plates were incubated overnight before loading of samples.
2.5.2. Disc Diffusion Method (DDM)
The antimicrobial activity of the EOs and two pure EO components (α-pinene and terpinen-4-ol) was evaluated by DDM according to the Kirby–Bauer method [28], with some modifications. Briefly, 5 µL of an undiluted EO or EO component was loaded onto a 6 mm diameter sterile paper disk and placed on target inoculated Muller–Hinton agar or SDA plates. The plates were then incubated for 24 h at 25 °C for P. chrysogenum, 28 °C for Gram-positive bacteria, and 37 °C for Gram-negative bacteria. After incubation, the diameters of inhibition growth zones were measured in mm, including the diameter of the disc. All tests were performed in triplicate. The antimicrobial activity of the positive controls (Kanamycin and Nystatin) was also recorded using the same procedure as above. Inoculated plates without samples were used as controls.
2.6. Brine Shrimp Lethality Bioassay
The toxicity of the EOs and two pure EO components (α-pinene and terpinen-4-ol) was monitored by an in vivo assay using nauplii of brine shrimp (Artemia salina Leach). The BSL bioassay was performed according to the Meyer et al. [29] method, with some modifications. A. salina cysts were purchased at a pet shop and were hatched in artificial seawater for 48 h. A stock solution of each EO or EO component was prepared by dissolving 150 mg of sample in ethanol up to a final volume of 0.5 mL. Then, the stock solution was diluted to 1 mg/mL in water and sonicated. Afterwards, 100 μg/mL of the EO or EO component emulsion was prepared with artificial seawater. Control samples of artificial seawater and ethanol (<0.1%, v/v) were also prepared to correct values with the natural mortality rate. Ten to fifteen nauplii were brought into contact with the EOs or EO components and with the control in microwell plates. After 24 h of contact, the mortality rate of the nauplii was calculated. All the experiments were performed in triplicate.
2.7. Statistical Analysis
Variables were tested for normal distribution by using the Shapiro–Wilk test, and when this condition was not met, data were transformed. One-way analysis of variance (ANOVA) and the least significant difference (LSD) test were performed to verify statistical significances between EOs and EO components. The statistical significance of differences among mean values was established at p < 0.05. The data were expressed as mean ± standard deviation (SD) for each of the assays. All analyses were performed by using SPSS version 27.0 software (SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
3.1. EO Extraction and Yield
The EOs from the dried Azorean C. japonica aerial parts were extracted by HD with a Clevenger-type apparatus, since it is a technique included in the European Pharmacopoeia. All EOs had a density lower than water (ca. 0.90 g/mL), a strong odor, with that from cones being the most pleasant, and a yellowish color, except for MC EO, which was pale yellow. The EO yields ranged approximately from 1 to 3% (w/w), as summarised in Table 1. The highest EO yield was found in leaves, followed by foliage, MCs, and FCs, with significant differences among them (F = 249.46; df = 3; p = 0.022). Since all samples came from the same tree population, these variations could potentially be attributed to anatomical differences, particularly in the quantity of the secretory structures that accumulate the volatile components of the EO [6].

Table 1.
Yield of the essential oil from Azorean Cryptomeria japonica female cones (FCs), male cones (MCs), leaves, and foliage.
3.2. EO Chemical Composition
The chemical composition of the EOs obtained from different Azorean C. japonica parts are detailed in Table 2, and their chromatographic profiles are shown in Figure 2A–D.

Table 2.
Percentage of essential oil (EO) composition from Azorean Cryptomeria japonica female cones (FCs), male cones (MCs), leaves, and foliage.
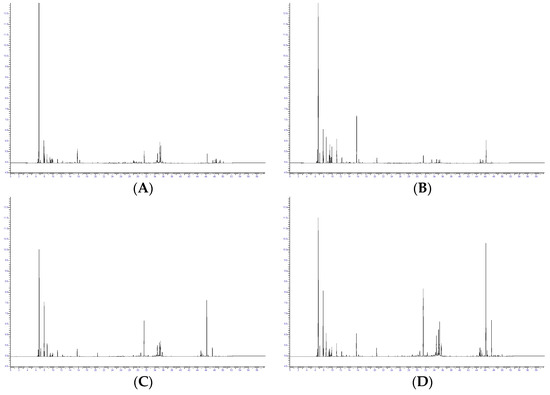
Figure 2.
Gas chromatography with flame ionization detection profiles, taken on the DB-1 column, of the essential oil from different Azorean Cryptomeria japonica parts: (A) female cones, (B) male cones, (C) leaves, and (D) foliage.
GC–MS and GC–FID analysis resulted in the identification of seventy-one components in the EOs isolated from the different Azorean C. japonica parts which accounted for up to 92 to 98% of the total EOs and were grouped into six classes: monoterpene hydrocarbons, oxygen-containing monoterpenes, sesquiterpene hydrocarbons, oxygen-containing sesquiterpenes, diterpene hydrocarbons, and oxygen-containing diterpenes (Table 2).
The major compounds (≥4%) identified in the FC EO were α-pinene (45%), β-eudesmol (7%), α-eudesmol (6%), sabinene, elemol, terpinen-4-ol, and γ-eudesmol (4% each), while those from MC EO were α-pinene (38%), terpinen-4-ol (12%), sabinene (6%), γ-terpinene, β-myrcene, and phyllocladene (5% each). On the other hand, leaf EO and foliage EO shared the same major compounds (≥4%): α-pinene (26 vs. 17%), sabinene (14 vs. 8%), elemol (12 vs. 14%), phyllocladene (7 vs. 12%), α-eudesmol (5 vs. 6%), β-eudesmol (4 vs. 5%), and γ-eudesmol (both 4%). In comparison with previous reports [22] on foliage EOs obtained by SD from Azorean C. japonica samples, the terpenoid compounds including α-pinene, sabinene, elemol, α-eudesmol, and phyllocladene were also clearly the most abundant constituents of these EOs.
Our results also revealed that α-pinene (17–45%) dominated in all the studied EOs and decreased as follows: FC EO > MC EO > leaf EO > foliage EO. Interestingly, phyllocladene content (2–12%) displayed the opposite trend. Similarly, as observed for α-pinene, the terpinen-4-ol content (3–12%) decreased as follows: MC EO > FC EO > foliage EO > leaf EO, whereas the elemol content (2–14%) seems to vary in an inverse way to α-pinene, decreasing as follows: foliage EO > leaf EO > FC EO > MC EO. These results agree with what was found and reported from previous studies in Figueiredo et al. [22]. In fact, their research [22] also stated that the ratio between the EO main compounds seemed to be mainly determined by the type of plant material (branches and foliage with more or less strobili vs. wood).
Overall, it should be highlighted that monoterpene hydrocarbons constituted the major fraction in all the studied EOs (38–71% of the total EOs), decreasing as follows: MC EO > FC EO > leaf EO > foliage EO, which is a similar order to the one reported for α-pinene content. The oxygen-containing monoterpenes fraction was also higher in strobili (FC and MC) EOs, as compared to the EOs from foliage/leaves, particularly in MC EOs due to their highest content of terpinen-4-ol (12% vs. 3–4%). Contrariwise to strobili EOs, those from leaves and foliage were particularly rich in oxygen-containing sesquiterpenes (mainly elemol) and in diterpene hydrocarbons (mainly phyllocladene). In fact, as already mentioned, the elemol and phyllocladene contents seem to vary in an inverse way to α-pinene. Oxygen-containing diterpenes were present in very low amounts in FC, MC, and leaf EOs (≤1%), whereas the presence of nezukol (3%) in foliage EO has yet to be evidenced. Sesquiterpene hydrocarbons were also less abundant (0.2–3%), particularly in MC EO. Indeed, contrariwise to the monoterpenes class, with the oxygen-containing monoterpenes accounting for only 4–15% of the total EOs, the sesquiterpenes class mainly consists of oxygen-containing components (6–31% of the total EOs).
Our results for EO chemical compositions from different Azorean C. japonica parts were in good agreement with those reported by Garcia et al. [30] for different C. japonica parts collected on Corsica (France), except regarding the diterpene hydrocarbons class, which is mainly phyllocladene in Azorean samples and kaurene in Corsican ones [30]. This difference is probably due to genetic differences and/or different environmental conditions.
3.3. In Vitro Antimicrobial Activity
In the present study, the DDM, as a simple, low cost, rapid, and well-known antimicrobial screening procedure [31], was used to compare the growth-inhibitory activity of the Azorean C. japonica EOs, EO components (α-pinene and terpinen-4-ol), and antimicrobial drugs (Kanamycin and Nystatin) against the selected microorganisms, as illustrated in Table 3.

Table 3.
In vitro antimicrobial activity (growth inhibition zone) of the essential oils (EOs) from Azorean Cryptomeria japonica female cones (FCs), male cones (MCs), leaves, and foliage and some of their components.
All the studied EOs showed weak to moderate antibacterial activity against Gram-positive bacteria as compared to the positive control (inhibition zones of 7–13 mm and 30–39 mm, respectively). However, there is no significant difference in antimicrobial activity between the studied plant parts regarding these bacteria, namely, B. subtilis (F = 1.12; df = 3; p = 0.392), B. licheniformis (F = 1.26; df = 3; p = 0.351), and M. luteus (F = 2.40; df = 3; p = 0.135), as we can see in Figure 3. Moreover, all the studied EOs were inactive against Gram-negative bacteria. Previous reports [9] on antibacterial activity of foliage EOs obtained by SD from Azorean C. japonica samples revealed no activity against B. subtilis and E. coli. However, it should be noted that the comparison of antimicrobial efficacy data between studies is difficult when different raw material, microbial strains, antimicrobial evaluation methods, and experimental conditions (e.g., culture medium, amount of EO tested, and incubation conditions) were used, among other factors [32]. Our results also revealed that the antibacterial action of the studied EOs is in accordance with their major component, α-pinene, that also exhibited superior efficacy against Gram-positive bacteria over negative ones (inhibition zones of 14–18 mm and 7–8 mm, respectively), possibly due to the difference in the cell wall structures of the two bacteria groups. Indeed, Gram-negative bacteria have an outer membrane rich in lipopolysaccharide molecules, which hinder the entry of hydrophobic components into the bacterial cell [33]; thus, Gram-positive bacteria, which lack this outer membrane, were found to be more sensitive to the studied EOs and pure α-pinene when compared with the negative ones. A similar trend was reported by other researchers investigating the antimicrobial activity of other EOs [10,15]. However, this pattern is not always observed for all EOs/EO molecules, as documented in the review by Angane et al. [34] and in the present study. Indeed, contrary to α-pinene action, the oxygen-containing monoterpene terpinen-4-ol was more effective against Gram-negative bacteria than Gram-positive bacteria (inhibition zones of 19–31 mm and 7–11 mm, respectively). According to the literature, terpinen-4-ol has gained attention for its potential as a broad-spectrum antibacterial agent [35] for therapeutic application. For example, a recent study [36] showed that this compound has strong bactericidal activity against Staphylococcus aureus, probably by interfering with the synthesis of the bacterial cell wall, resulting in the loss of cytoplasmic material. Interestingly, the present study revealed that the studied EOs, even the MC EO, which presented the highest terpinen-4-ol content (12%), were inactive against all the selected Gram-negative bacteria, as mentioned above. This indicates the possibility of an antagonistic interaction between terpinen-4-ol and other Azorean C. japonica EO components in the antibacterial effect, which should be considered for further investigation.
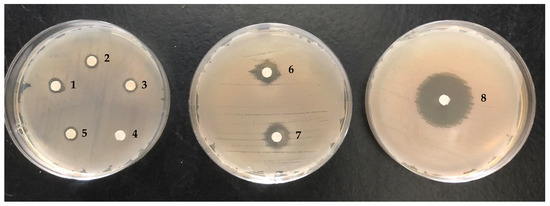
Figure 3.
Examples from images of the antimicrobial activity experiments by disc diffusion method against Bacillus licheniformis. Legend: 1—female cones; 2—male cones; 3—foliage; 4—negative control; 5—leaves; 6—α-pinene; 7—terpinen-4-ol; 8—kanamycin.
Regarding the antifungal activity, only the EOs from FCs and MCs were slightly active against P. chrysogenum, probably due to the higher concentration of monoterpene hydrocarbons and oxygen-containing monoterpenes in the EOs from these plant parts. In fact, α-pinene and terpinen-4-ol showed higher antifungal activity than the positive control (F = 24.28; df = 2; p = 0.001). Previous studies reported strong in vitro growth-inhibitory activity of terpinen-4-ol against several mycotoxigenic plant pathogens, including Penicillium sp. [37]. Our results also revealed that P. chrysogenum is equally susceptible to α-pinene as Gram-positive bacteria.
3.4. Brine Shrimp Lethality Bioassay
The BSL bioassay, as a simple, economic, and rapid screening procedure, was also used to determine the toxicity of the Azorean C. japonica EOs and some of their components (Figure 4). FC EO was found to be the most toxic EO against A. salina nauplii, with a 70.6 ± 4.2% mortality rate, followed by leaf (53.9 ± 10.6%), MC (38.8 ± 13.1%), and foliage (34.1 ± 8.6%) EOs, with significant differences among them (F = 10.62; df = 5; p < 0.001). α-Pinene, the major component in all the studied EO samples, and terpinen-4-ol do not seem to be involved in toxicity against brine shrimp (36.6 ± 6.2% and 22.7 ± 12.4% of mortality rate, respectively). The highest toxicity of FC EO could be due to other major components or due to a synergistic effect of its minor components, which will require further investigation. Considering that the BSL bioassay is an adequate method for preliminary toxicity testing of EOs in tumor cell lines [38], future studies should consider FC EO for further investigation of its potential pharmacological properties to assess their application in industry.
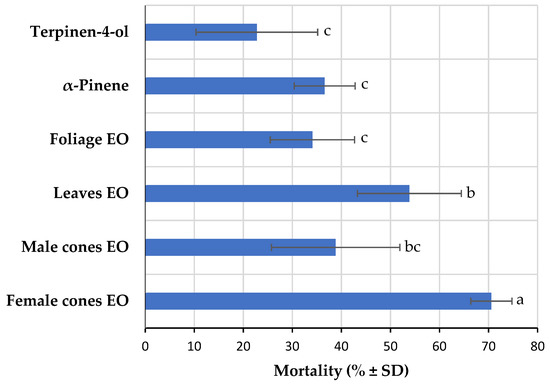
Figure 4.
Toxicity of the essential oils (EOs) from different Azorean Cryptomeria japonica parts and some of their components against Artemia salina nauplii at 100 µg/mL. Within each bar, means followed by the same letter are not significantly different at p < 0.05.
4. Conclusions
The available literature on the chemical compositions of EOs from different C. japonica parts and their biological potential is still scarce. Therefore, this study compared the yield, chemical composition, antimicrobial activity, and BSL effect of EOs from different parts of a 60-year-old C. japonica tree population planted in Azores archipelago.
This study confirms that all the selected plant parts (leaves, foliage, and reproductive structures) produce EOs; however, a substantial variation was noticed in their quantity. A considerable chemical variability was also observed, namely, the α-pinene and terpinen-4-ol contents were lower in foliage/leaves than in strobili EOs, while the elemol and phyllocladene amount showed an opposite tendency. It should also be noted that the studied EOs from different Azorean C. japonica parts were richer in phyllocladene as compared with those of C. japonica from Corsica (France), probably due to genetic differences and/or different environmental conditions.
A variation in the biological potential of the studied EOs is also confirmed. Regarding the antimicrobial activity, the bioactive EOs exhibited, in general, a weaker inhibitory effect against the selected microorganisms as compared to the tested EO components (α-pinene and terpinen-4-ol). Moreover, both EO compounds showed better antifungal activity than the positive control, and terpinen-4-ol exhibited a very strong activity against Gram-negative bacteria, even better than that of the positive control in the case of the E. cloacae strain. Concerning the BSL effect, the most toxic EOs were from FCs, followed by leaf samples.
Overall, among the studied samples, only the strobili (FC and MC) EOs showed antifungal activity against P. chrysogenum. In addition, FC EO is the most promising source of multi-bioactivities since it also showed the best antibacterial activities and the highest toxicity. Thus, future investigations into the potential pharmacological properties of FC EO are required to assess their application in industry. In addition, further ongoing studies will involve fractionating C. japonica EOs to enhance the content of their active components, thereby increasing their antimicrobial activity, as well as to provide data on synergistic effects between C. japonica EOs/fractions and selected antibiotics.
Author Contributions
Conceptualization, A.L., F.A., A.J. and E.L.; methodology, A.L., F.A., A.J., T.R. and A.C.F.; software, A.L., F.A. and A.C.F.; writing—original draft preparation, A.L.; writing—review and editing, A.L., F.A., A.J., T.R., J.B., A.C.F. and E.L.; supervision, J.B. and E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by DRCT funds, under the project M1.1.C/PROJ.EXPLORATÓRIOS/003/2022—PotBioCJap, and CESAM UIDB/50017/2020 + UIDP/50017/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Marques, S.A. from São Miguel Island, Azores, who provided the Cryptomeria japonica samples. Filipe Arruda thanks FRCT for Doctoral Research Scholarships (ref. M3.1.a/F/008/2021). Thanks are due to FCT/MCTES for financial support to CESAM UIDB/50017/2020 + UIDP/50017/2020.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
BSL: brine shrimp lethality; 13C-NMR, carbon-13 nuclear magnetic resonance; DDM, disc diffusion method; d.w, dry weight; EO, essential oil; FCs, female cones; FID, flame ionization detector; GC, gas chromatography; MCs, male cones; MS, mass spectrometry; RI, retention indices; SDA, Sabouraud dextrose agar.
References
- Dias, E.; Araújo, C.; Mendes, J.; Elias, R.; Mendes, C.; Melo, C. Espécies Florestais das Ilhas—Açores. In Árvores e Florestas de Portugal; Silva, J.S., Ed.; Público, Comunicação Social, SA/Fundação Luso-Americana/Liga para a Protecção da Natureza: Lisboa, Portugal, 2007; Volume 6, pp. 199–254. [Google Scholar]
- Nagakura, J.; Shigenaga, H.; Akama, A.; Takahashi, M. Growth and transpiration of Japanese cedar (Cryptomeria japonica) and Hinoki cypress (Chamaecyparis obtusa) seedlings in response to soil water content. Tree Physiol. 2004, 24, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Kuriyama, I. Cedar (Cryptomeria japonica) Oils. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; pp. 317–324. [Google Scholar]
- Farjon, A. Plate 371. Cryptomeria japonica. Curtis’s Bot. Mag. 1999, 16, 212–228. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ueno, S.; Wei, F.J.; Matsumoto, A.; Uchiyama, K.; Ujino-Ihara, T.; Hakamata, T.; Fujino, T.; Kasahara, M.; Bino, T.; et al. Identification and genetic diversity analysis of a male-sterile gene (MS1) in Japanese cedar (Cryptomeria japonica D. Don). Sci. Rep. 2021, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, C.; Zas, R.; Erbilgin, N.; Ferrenberg, S.; Rozas, V.; Sampedro, L. Resin ducts as resistance traits in conifers: Linking dendrochronology and resin-based defences. Tree Physiol. 2020, 40, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Arruda, F.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Variations in essential oil chemical composition and biological activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from different geographical origins—A critical review. Appl. Sci. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Janeiro, A.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Biological activities of organic extracts and specialized metabolites from different parts of Cryptomeria japonica (Cupressaceae)—A critical review. Phytochemistry 2023, 206, 113520. [Google Scholar] [CrossRef] [PubMed]
- Ruas, A.; Graça, A.; Marto, J.; Gonçalves, L.; Oliveira, A.; Nogueira da Silva, A.; Pimentel, M.; Moura, A.M.; Serra, A.T.; Figueiredo, A.C.; et al. Chemical characterization and bioactivity of commercial essential oils and hydrolates obtained from Portuguese forest logging and thinning. Molecules 2022, 27, 3572. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Arruda, F.; Rosa, J.S.; Rodrigues, A.; Oliveira, L.; Lima, A.; Barroso, J.G.; Lima, E. Essential oil variability of Azorean Cryptomeria japonica leaves under different distillation methods, Part 1: Color, yield and chemical composition analysis. Appl. Sci. 2022, 12, 452. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Moiteiro, C.; Esteves, T.; Ramalho, L.; Rojas, R.; Alvarez, S.; Zacchino, S.; Bragança, H. Essential oil characterization of two Azorean Cryptomeria japonica populations and their biological evaluations. Nat. Prod. Commun. 2013, 8, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Arruda, F.; Lima, A.; Oliveira, L.; Rodrigues, T.; Janeiro, A.; Rosa, J.S.; Lima, E. Essential oil variability of Azorean Cryptomeria japonica leaves under different distillation methods, Part 2: Molluscicidal activity and brine shrimp lethality. Separations 2023, 10, 241. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.; Yiap, B.C.; Ping, H.C.; Lim, S.H. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol, J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Directorate for the Quality of Medicines. In European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourg, France, 2010; p. 241. [Google Scholar]
- ISO 7609; Essential Oils—Analysis by Gas Chromatography on Capillary Columns—General Method. ISO: Geneva, Switzerland, 1985.
- Ascensão, L.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Schripsem, J.; Deans, S.G.; Scheffer, J.C.J. Plectranthus madagascariensis: Morphology of the glandular trichomes, essential oil composition and its biological activity. Int. J. Plant Sci. 1998, 159, 31–38. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Vila, R.; Tomi, F.; Figueiredo, A.C.; Barroso, J.G.; Cañigueral, S.; Casanova, J.; Cunha, A.P.; Adzet, T. Variability of essential oils of Thymus caespititius from Portugal. Phytochemistry 1997, 45, 307–331. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Salgueiro, L.; Barroso, J.G.; Figueiredo, A.C.; Pedro, L.G.; Fontinha, S.S.; Bighelli, A.; Casanova, J.; Looman, A.; Scheffer, J.J.C. Composition of the essential oil of Juniperus cedrus Webb & Berth. grown on Madeira. Flavour. Fragr. J. 2002, 17, 111–114. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Moiteiro, C.; Rodrigues, M.C.S.M.; Almeida, A.J.R.M. Essential oil composition from Cryptomeria japonica D. Don grown in Azores: Biomass valorization from forest management. Nat. Prod. Commun. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food- and Airborne Fungi, 6th ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; p. 389. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd; CBS–KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010; p. 390. [Google Scholar]
- André, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, Q.; Yang, Z.; Liang, Z. Clinical characteristics of patients with Micrococcus luteus bloodstream infection in a chinese tertiary-care hospital. Pol. J. Microbiol. 2021, 70, 321–326. [Google Scholar] [CrossRef]
- Khater, D.F.; Lela, R.A.; El-Diasty, M.; Moustafa, S.A.; Wareth, G. Detection of harmful foodborne pathogens in food samples at the points of sale by MALDT-TOF MS in Egypt. BMC Res. Notes 2021, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Garcia, G.; Garcia, A.; Gibernau, M.; Bighelli, A.; Tomi, F. Chemical compositions of essential oils of five introduced conifers in Corsica. Nat. Prod. Res. 2017, 31, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Van de Vel, E.; Sampers, I.; Raes, K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit. Rev. Food Sci. Nutr. 2019, 59, 357–378. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The antibacterial mechanism of terpinen-4-ol against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an antibacterial and antibiofilm agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Niksic, H.; Becic, F.; Koric, E.; Gusic, I.; Omeragic, E.; Muratovic, S.; Miladinovic, B.; Duric, K. Cytotoxicity Screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci. Rep. 2021, 11, 13178. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).