Abstract
Pinto peanut (Arachis pintoi Krapov. & W.C. Greg.) is an herbaceous perennial plant which belongs to the Leguminosae family. This plant is well known for its use as a cover crop, but little information is available on the allelopathic potential of this legume. Therefore, this study aimed to explore the allelopathic effects of A. pintoi under various screening conditions and to analyze its potential allelochemicals using gas chromatography–mass spectrometry (GC-MS). In laboratory bioassays, aqueous extracts of A. pintoi powder exerted the average inhibition of the growth of Echinochloa crus-galli (55.1%), Oryza sativa (77.1%), and Vigna radiata (60.1%), respectively, of which the root lengths of the tested plants were the most suppressed. In greenhouse experiments, E. crus-galli was inhibited by 63.4% at 200 g/m2 of A. pintoi dried powder application. In field trials, A. pintoi also significantly reduced the growth of E. crus-galli and natural weeds. By incorporating a dose of 200 g/m2, the average inhibition of E. crus-galli was 43.9%, the dry weight of natural paddy weeds was 43.1%, and rice yield was simultaneously increased by 35%. The growth inhibitions of weeds and tested plants were proportional to the doses of A. pintoi applied and varied with the tested plant species. Among three different solvent extracts (methanol, hexane, and ethyl acetate), the hexane extract exerted the highest suppression against the growth of E. crus-galli and L. sativa by IC50 against root and shoot growth = 4.08 and 8.4 mg/mL and 1.7 and 1.54 mg/mL, respectively, followed by ethyl acetate extract, while the least effective was methanol extract. From those extracts, a total of 35 substances were detected by GC-MS analyses, including 14 newly identified constituents, such as phenolic acids, stearic acid, palmitic acid, fatty acids, pyranones, and benzofurans, which may be responsible for the herbicidal effects of A. pintoi. This study suggests that A. pintoi may be used as a source of bioherbicide to minimize the dependency on harmful synthetic herbicides and enhance rice yield.
1. Introduction
Weed interference is one of the most common abiotic threats to global crop production, as weeds can contaminate crops by spreading their seeds, thereby perpetuating the problem for subsequent growing seasons [1]. Weeds compete with crops, resulting in an annual reduction of approximately 10% in worldwide agricultural production [2]. Uncontrolled weeds can even lead to a complete loss of crop yields. Globally, there are approximately 30,000 species of weeds with detrimental effects on crop yield and quality [3,4]. In the United States, it is estimated that weeds cost USD 33 billion in lost crop production annually, and farmers spend over USD 6.2 billion each year to control weeds [5,6]. In Australia, the overall cost of weeds was about AUD 3.3 billion per year, with yield losses of 2.7 million tons of grains at a national level [6]. Over the last six decades since the discovery of 2,4-D, 2,4,5-T, and other synthetic herbicides, the use of herbicides has successfully decreased labor costs spent on weed control and increased global crop production [7]. In Vietnam, the use of synthetic herbicides for weed management in rice only significantly increased from the early 1990 and strikingly increased to 42,000 tons/year in 2013, equivalent to USD 300 million [8], and tremendously intensified in recent years [9]. In fact, modern agricultural systems have effectively employed synthetic herbicides to control weeds and minimized the need for weeding labor in agriculture. However, indiscriminate use or overuse of synthetic herbicides results in unwelcome side effects, such as environmental contamination, unsafe agricultural products, and human health concerns, as well as the development of herbicidal resistance in weeds [10,11]. To the best of our knowledge, there have been approximately 380 herbicide-resistant weed biotypes reported in 98 crops in over 72 countries globally [12,13]. This fact requires either increasing doses of applied synthetic herbicides in practice or developing new synthetic herbicides, which leads to further severe problems and threatens the sustainability of agricultural systems.
Allelopathy is simply described as natural chemical interaction(s) between plants that can inhibit or enhance the growth of surrounding plant species [14,15]. In nature, green plants suppress other plants in the vicinity by releasing phytotoxic compounds (allelochemicals) into the environment from root exudates, aerial parts, and residues, etc. [16]. Therefore, the allelopathic traits of plants may be an effective tool for biological weed management in agricultural production [10]. Numerous plants in nature exert significant weed-suppressing ability; some of them have allelopathic potential to incorporate into paddy fields to manage weeds and significantly enhance rice yield. Examples include passion flower (Passiflora edulis), alfalfa (Medicago sativa L.), fragrant thoroughwort (Eupatorium cannabium), buckwheat (Fagopyrum esculentum), chinaberry (Melia azedarach), kava (Piper methysticum), galactia (Galactia pendula), neem (Azadirachta indica), billygoat weed (Ageratum conyzoides), and white lead-tree (Leucaena leucocephala) [17]. Some common allelochemicals found in plants are generally secondary metabolites, including phenolics, alkaloids, terpenoids, coumarins, flavonoids, steroids, etc. [18,19]. Allelopathic additives acquired from some plant biomass and their constituents may boost crop output while concurrently minimizing weed growth via mulch, cover crops, and green manures [11,17]. In recent decades, the allelopathic traits of plants have gained much attention from many researchers, since plants were identified to self-regulate density and distribution in nature with defense mechanisms by means of allelopathic interactions. Some allelochemicals are non-halgenated and water-soluble, and express short half-lives relative to synthetic herbicides; they are, therefore, considered safe for agricultural products and the environment. The use of allelopathic plants to manage weeds in agriculture may enhance crop yields and their quality, produce safer agricultural products, and minimize environmental costs. This is one of the most feasible considerations for sustainable agriculture production.
Arachis pintoi Krapov. & W.C. Greg. is an herbaceous perennial plant and is a member of Leguminosae family. This plant is considered to originate in South America and has widely spread to tropical and subtropical areas in many countries around the world. A. pintoi is a multi-use legume, providing a rich source of organic foliar material, and is used for soil coverage in gardens, forests, and low tillage systems, erosion control, animal feed, and for ornamental purposes due to its high biomass production, green manure, nitrogen fixation, and easy adaptability in nature [20,21]. A. pintoi has an axonomorphous root development without enlargements and a low stoloniferous growth habit. The leaves are compound and alternate with four obovate leaflets. The stems are angular, cylindrical, and hollow [21]. Some previous studies reported that Arachis species possessed phytosterols, triterpenes, fatty acids, alkaloids, phenolics, and flavonoids, etc., with a spectrum of biological activities, such as antifungal, antioxidative, antibacterial, antitumor, and anti-inflammatory activities [20,22,23].
Although extensive studies on the cover crop of A. pintoi have been carried out [20,21,24,25], little work has been conducted on evaluating the allelopathic potential of this legume. Therefore, the aims of this study were (i) to assess the allelopathic activity of A. pintoi aerial parts of powder against the germination and growth of E. crus-galli and various tested plants, especially on paddy weeds through bioassays and greenhouse and field trials; (ii) to determine total phenolic and flavonoid contents and inhibitory effects from the different solvent extracts (methanol, hexane and ethyl acetate); (iii) to analyze its potential allelochemicals using gas chromatography–mass spectrometry (GC-MS). Indeed, to develop a novel plant-based herbicide, the candidate plant must significantly inhibit problematic weeds without harmful effects on the main crops [26]. Therefore, (iv) the phenotypic responses and productivity of rice under the effects of the application of A. pintoi powder under a field trial were also examined.
2. Materials and Methods
2.1. Plant Material Collection
The above ground plant parts of A. pintoi, including leaves and stems (aerial), were collected at Thach Hoa commune, Thach That province, Hanoi, Vietnam, in 2019 (21°01′26.3″ N 105°30′20.1″ E). The materials were cleaned with tap water, then chopped into pieces of 2 cm in length, dried at 45 °C for 50 h until the humidity was less than 5%, and ground into a soft powder (approximately <4 mm) using a laboratory grinder. All materials were stored in the dark at 5 °C until use. The plant powders were used in bioassays, greenhouse experiments, and field trials.
2.2. Tested Plants and Laboratory Bioassays
The bioassay-tested plant seeds, including seeds of E. crus-galli, were collected at the experimental farm of Agricultural Genetics Institute, Hanoi, Vietnam (AGI), in 2019. O. sativa seeds of the Khang Dan 18 variety, and commercial seeds of V. radiata were provided by Department of Genetic Engineering, AGI.
The laboratory bioassay experiments were performed at the laboratory of the Department of Genetic Engineering, AGI, Hanoi, Vietnam. In order to ensure the good quality of the E. crus-galli seeds for bioassays, the unfilled and empty seeds were discarded by floating in tap water. The seeds were then treated with H2SO4 (90%) for 30 s to scarify the seed coats and were rinsed many times with distilled water. The tested seeds were then air-dried and hermetically stored at −2 °C to keep the seeds fresh. Before germination tests, the germination percentage of all tested seeds of weeds and cultivated plant species was randomly checked and found to be greater than 90%, respectively [27]. The bioassays were conducted using the method of Fujii et al. [28] with some minor modifications [29,30]. Briefly, agar (5%) was dissolved in distilled water (5 g/L), and then boiled to prepare the growth medium. The powders of A. pintoi were then added to agar medium at various concentrations (50 g/L, 25 g/L, 12.5 g/L, and 6.2 g/L) and mixed thoroughly. The growth media were sterilized by autoclaving at 121 °C for 20 min, kept cool at 30–35 °C at room temperature, and then transferred into plastic pots (250 mL).
Twenty healthy seeds of each tested plant (E. crus-galli, O. sativa, and V. radiata) were sown evenly in the pots, then placed in the growth chamber (25 °C, light: 9:00 to 17:00 day/night cycle, humidity 70%, 4000 lux). Three replicates of all treatments were conducted using a completely random design, and the distilled water treatment was used as the control. After one week, the number of germinated seeds, shoot and root length, and fresh and dry weight of all tested plants were measured and calculated using the following formula [30]:
Inhibition (%) = [1 − (sample/control)] × 100
2.3. Greenhouse Experiments
The experiments were performed at the greenhouse of the Faculty of Agronomy, Vietnam National University of Agriculture, Hanoi, Vietnam, in 2019. Three kilograms of commercially sterilized soil (pH 4.5–5.8, EC 1.0 ± 0.2, N 1100 ± 100 mg/kg−1, P2O4 400 ± 100 mg kg−1) which did not contain any microorganisms or weed seeds, were used in this experiment. The soil was air-dried and put in plastic containers (7000 mL, 25 cm in diameter) and then saturated with 1000 mL of tap water. Twenty healthy seeds of E. crus-galli were sown at a depth of 1.5 cm. After 2 days, the ground powders of A. pintoi at doses of 200, 150, 100, and 50 g/m2 were applied evenly by scattering on the surface of the containers. Each treatment was performed at least thrice. The water was applied to the containers twice, on the first day and 15 days after the powders were applied to ensure that the water level was about 2 cm above the surface of the soil. The treatments using only tap water were used as the control [29,30]. The greenhouse temperature was set up around 25–30 °C. After 30 days, the number of E. crus-galli plants, shoot length, and fresh and dry weight were collected and determined.
2.4. Field Trials
2.4.1. Evaluation of Allelopathic Potential of A. pintoi against the Growth of Echinochloa crus-galli
The field experiments were conducted at the Experimental Station of AGI, Lai Yen Commune, Hanoi city, Vietnam (21°00′46.5″ N 105°42′00.9″ E), from June to October 2019. Each treatment was performed with three replicates. The experimental rice field was divided into 15 experimental small plots with a size of 1 m × 1 m2. Each plot was judiciously shielded by the plastic board in order to prevent cross-contamination with other plots. Rice seeds (Khang dan 18), a mega variety, were sown in bed trays for 2 weeks. On 20 June 2019, the healthy rice seedlings (one seedling/clumps) were then manually transplanted in each plot, with row spacing (20 × 20 cm) and seedling distances (20 × 10 cm), respectively. Simultaneously, the 2-week-old E. crus-galli seedlings taken from the bed trays were interplanted in the middle of each rice row. The ground powders of A. pintoi, similar to that applied in the greenhouse experiment, at 200 g, 150 g, 100 g, and 50 g/m2, were applied to the field by scattering them on the soil surface of the plots at 2 days after transplanting rice and E. crus-galli [31,32]. The control treatments were not subjected to any weed management, and no herbicide was used for all plots. The fertilizers and pesticides were used following the conventional methods in Vietnam. After 1 month, the plant height, fresh weights, and total dry weights of E. crus-galli were determined and compared with the control.
2.4.2. Evaluation of Allelopathic Potential of A. pintoi against the Growth of Natural Paddy Weeds
This experiment aimed to assess allelopathic effects of A. pintoi powders against the growth of natural paddy weeds and their potential impact on rice yield. The experiment was carried out at the same rice field as mentioned above from January to May 2020. The experimental plots arrangement was similar to the field experiment described earlier but without being interplanted with the E. crus-galli seedlings. A total of 21 experimental plots were prepared as presented above. On the 27th of February 2020, rice seedlings were transplanted into the plots. In order to assess rice yield among the treatments, the hand-weeding and herbicide treatments with Butachlor (Butoxim 60EC) (Saigon, Plant Protection company, Ho Chi Minh City, Vietnam) at a concentration of 1.2 L ha−1) were also included. The control treatments did not receive any weed management (herbicide or A. pintoi powders). All treatments were treated fertilizer according to conventional methods (N, P2O5, and K2O at the rate of 8.0, 6.0, and 6.0 g/m2) one day before the soil was saturated with water. All treatments were replicated three times. One month after application, weed emergence was randomly determined in each treatment in an area of 50 × 50 cm with three replications. At harvest, rice yield was also calculated [7,33].
2.5. Extracts from Arachis pintoi Powders
The experiments were conducted at the Laboratory of Plant Physiology and Biochemistry, Department of Development Technology, Hiroshima University, Japan in 2019. The extract procedures were conducted following the method of Quan et al. [34]. Briefly, to prepare the crude extract, 1.0 kg of A. pintoi powder was immersed in 3 L of methanol (MeOH, 100%) at room temperature (24 ± 28 °C) for 2 weeks. The supernatant was then filtered using Whatman filter paper under vacuum at 40° to remove the powder residues using a rotary evaporator (SB-350-EYELA, Tokyo Rikai Co., Ltd., Tokyo, Japan) to yield 68.9 g of the methanolic crude extract. This crude extract was subsequently suspended in distilled water (200 mL) and subjected to liquid–liquid phase (1:1, v/v) extraction with hexane, followed by ethyl acetate, respectively. The collected extracts were filtered and evaporated to yield 7.3 g and 4.5 g of hexane and ethyl acetate extracts, respectively. After that, the methanolic, hexane, and ethyl acetate extracts were used to examine the allelopathic activity against germination and growth of tested plants including L. sativa and E. crus-galli. The phytochemical profiles of these obtained extracts were also determined.
2.6. Determination of Total Phenolic and Flavonoid Contents
The total phenolic contents (TPCs) of the extracts were recorded using the Folin–Cicalteau methods [35]. In brief, a mixed solution consisting of 20 µL of the extract was blended with 100 µL of Folin–Ciocalteu reagent (10%) and further added to 80 μL of Na2CO3 (75%). The absorbance was determined at 765 nm by using a spectrophotometer after 30 min at room temperature. Subsequently, the milligram of gallic acid equivalent (GAE)/mL was determined as an expression of TPC.
The total flavonoid contents (TFC) were determined using the method previously reported by Bueno-Costa et al. [36] with some minor modifications. The record was expressed as milligrams of rutin equivalent per one gram of sample dry weight (mg RE/g DW). The mixture included 100 μg/mL of the sample, and 100 μL AlCl3 (2%, w/v) was kept for 15 min before being determined by a microplate spectrophotometer (Multiskan™ GO Microplate Spectrophotometer, Thermo Fisher Scientific, Osaka, Japan) at 430 nm.
2.7. Inhibitory Effects of Different Extracts of Arachis pintoi against the Growth of the Tested Plants
The tested plants used in this experiment were E. crus-galli and L. sativa, which were provided by the Laboratory of Plant Physiology and Biochemistry, Department of Development Technology, Hiroshima University, Japan, in 2020. Before experiments, the E. crus-galli seeds were soaked in 0.5% aqueous sodium hypochlorite for 30 min and then washed with distilled water multiple times. They were dried in an oven at 35 °C for 5 days, then stored in a fridge at −20 °C until used. Before conducting experiments, the healthy seeds were selected and incubated in 250 mL of distilled water (30 °C) for 2 days. The allelopathic bioassays were performed following the method previously presented by Minh et al. [37] and Quan et al. [38] with some minor modifications. Germination and growth of the tested plants were conducted using Nunc™ 12-well plates (Thermo Fisher Scientific, Jiangsu, China) in a growth chamber (Biotron NC system, Nippon Medical and Chemical Instrument, Co, Ltd., Osaka, Japan) by applying a photoperiod of 16 h day/8 h night at 30 °C for 5 days. Each well of NuncTM 12-well plates was firstly lined with a layer of 0.5% agar gel (1 mL). Each sample was diluted in separate solvents (EtOAc, hexane, and MeOH as mentioned above) to obtain different concentrations (0.5, 1.0, 2.5 and 5.0 mg/mL), respectively. An aliquot of 0.3 mL of each crude extract was separately penetrated to a filter paper disc (20 mm diameter), then evaporated in an oven at 40 °C for 30 min in order to subtract the effects of the solvents. The control treatments consisting of EtOAc, hexane, and MeOH were conducted similarly. The treated paper discs were directly placed on the surface of the well followed by pipetting 0.2 mL of distilled water. A total of 6 seeds of E. crus-galli and L. sativa were used in each experiment. After 5 days, the germination rates, shoot and root length of the tested plants were determined. The percentage of inhibition and stimulation were calculated over the controls. The IC50 value demonstrated a concentration that provided 50% suppression and was recorded.
2.8. Identification of Allelochemicals by GC-MS
The allelochemicals present in A. pintoi extracts (EtOAc, hexane, and MeOH) were identified by GC-MS analysis following the previously described method [37]. The GC-MS system (JMS-T100 GCV, JEOL Ltd., Tokyo, Japan) was used with an autosampler coupled with a 30 m × 0.25 mm I.D. × 0.25 μm film thickness DB-5MS column (Agilent Technologies, J & W Scientific Products, Folsom, CA, USA). Each extract of A. pintoi at a concentration (1000 μg/mL) was used for the initial injection. Helium was used as a carrier gas at a split ratio of 5:1. The oven conditions for GC were as follows. The initial temperature was 50 °C without hold time, and the boosted temperature was increased up to 300 °C at 10 °C/min and then held for 20 min. The detector temperature and injection port were set at 300 °C and 320 °C, respectively. The detected compounds were identified based on their retention time, molecular weight, and mass spectrum. The outcomes were compared to the standard sources comprising the library of JEOL’s GC-MS Mass Center System Version 2.65a, the online database of National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda, MD, USA (PubChem-https://pubchem.ncbi.nlm.nih.gov, accessed on 15 December 2022), and the National Institute of Standards and Technology, U.S. Department of Commerce (NIST-https://webbook.nist.gov, accessed on 12 January 2023) [37,38].
2.9. Statistical Analysis
The data were elaborated on the Minitab 1.8 and IRRISTAT version 2010 software products. All experiments were randomly implemented at least thrice, and the results were expressed as means ± standard errors (SE). The IC50 value was the amount required to inhibit 50% growth of the tested plants. Significant differences among assays were evaluated by one-way ANOVA using Tukey’s test at p < 0.05, and CV(%) was analyzed using IRRISTAT ver 2010.
3. Results
3.1. Inhibitory Effects of Arachis pintoi against the Growth of Tested Plants
In laboratory bioassays, A. pintoi demonstrated strong inhibition against the growth of E. crus-galli. At doses of 50 g/L and 25 g/L, the length of shoot and root, fresh weight, and dry weight of E. crus-galli were remarkably suppressed, in which the root length was the most inhibited (88.3%), followed by the dry weight (62.3%), fresh weight (58.3%), and shoot length (53.7%), while the germination was less influenced (12.9%). At the lowest dose (6.2 g/L), only the root length of E. crus-galli was inhibited (35.2%), and both shoot length and dry weight were stimulated by 8.8% and 19.1%, respectively (Table 1). However, A. pintoi slightly enhanced the germination rate of E. crus-galli by a range of 3.9% to 7.2%. Similarly, the length and fresh weight of O. sativa were significantly suppressed at doses of 50 g/L by over 89.0%, followed by the dry weight (71.1%) and shoot length (69.7%), and the germination rate was the least affected (66.1%). At the 25 g/L dose, the shoot length, root length, fresh weight, and dry weight of O. sativa were remarkably inhibited by 33.0, 84.2, 44.6, and 29.6%, respectively. The lower doses applied at 12.5 g/L and 6.2 g/L exerted either inhibitory or stimulatory effects. In the V. radiata test shown in Table 1, the growth of shoot length, root length, fresh weight, and dry weight were significantly suppressed at three doses of application (12.5, 25.0, and 50.0 g/L) ranging from 28.5 to 69.7%, 26.4 to 72.9%, and 32.7 to 71.1%, and 31.7 to 71.4%, respectively. At the lowest dose applied (6.2 g/L), only the root length of V. radiata was inhibited by 13.4%, and other growth parameters showed either negligible inhibition or promotion (Table 1).

Table 1.
Herbicidal effects of Arachis pintoi powders on the germination and growth of the tested plants under laboratory conditions.
3.2. Inhibitory Effects of Arachis pintoi against the Growth of Echinochloa crus-galli under Greenhouse Conditions
Table 2 shows that A. pintoi powders caused a significant reduction in the growth of E. crus-galli at all applied doses. At the applied doses of 50, 100, and 150 g/m2, the germination rate was significantly inhibited from 42.6 to 47.7%, respectively. The highest reduction was found in the dose of 200 g/m2 by over 60%. Similarly, at the applied doses of 100, 150, and 200 g/m2, the shoot length was significantly inhibited by 21.8, 48.3, and 53.2%, respectively. Both fresh and dry weights were remarkably reduced from 31.7 to 71.8% and 39.8 to 67.4%, respectively. On the average inhibition, it showed that A. pintoi powders reduced E. crus-galli growth by 63.4% at the applied dose of 200 g/m2, followed by 53.0% (150 g/m2), 34.7% (100 g/m2) while the lowest was 16.7% (50 g/m2) (Table 2).

Table 2.
Inhibitory effects of Arachis pintoi on the germination and growth of Echinochloa crus-galli under greenhouse conditions.
3.3. Inhibitory Effects of Arachis pintoi against Echinochloa crus-galli in Paddy Field Condition
In this experiment, the inhibitory effects of A. pintoi powders against the growth of E. crus-galli under field trial were evaluated. Generally, the growth of E. crus-galli was reduced compared with the control at all applied doses. At 200 g/m2, the shoot length and fresh and dry weight were significantly minimized from 40.6% to 46.4%, in which the dry weight was the most inhibited. At the applied dose of 150 g/m2, E. crus-galli growths (shoot length and fresh and dry weight) were reduced from 20.6% to 31.1%. The shoot length of E. crus-galli was suppressed (11.6%), and both fresh weight and dry weight were reduced over 10.0% at the lower doses of 100 and 50 g/m2. However, the shoot length was negligibly inhibited, and the dry weight was promoted by over 6.0% (Table 3).

Table 3.
Inhibitory effect of Arachis pintoi against the growth of Echinochloa crus-galli under field trials.
3.4. Inhibitory Effects of Arachis pintoi against the Natural Paddy Weeds and Rice Yield under Field Condition
In this field experiment, the dry weight of natural paddy weeds was suppressed at all applied doses of A. pintoi powders, while the rice yield was promoted. Some major natural weed species were observed, including Leptochloa chinensis L. (Chinese sprangletop), Cyperus difformis L. (small flower umbrella sedge), Rotala indica Koehne var. uliginosa Koehne (spike-flowered rotala), Sphenochlea zeylanica Gaertn. (goose weed), Jussiacea decurrens DC. (winged water primrose), Dactyloctenium aegyptium (L.) Beauv. (crowfootgrass), and Fimbristylis miliacea (L.) Vahl (globe fringerush) [33]. The highest inhibition of dry weight was 43.1% at 200 g/m2, followed by 34.2% (150 g/m2), and 21.9% (100 g/m2), and the lowest inhibition was 14.5% at 50 g/m2. Rice yield was promoted at all applied doses. Rice yield was significantly increased by over 35% at 200 g/m2 of application, followed by 26.49% (150 g/m2), and 19.19% (100 g/m2), and rice yield was slightly promoted by 2.99% at a dose of 50 g/m2, respectively. Interestingly, at the highest doses applied (200 g/m2), rice yield was significantly increased by 35.13%, higher than hand-weeding (20.15%) and herbicide application (26.86%), respectively (Table 4).

Table 4.
Allelopathic effects of Arachis pintoi powder on the growth of natural paddy weeds and rice yield in field trial.
3.5. Inhibitory Effects of Different Extracts of Arachis pintoi on the Growth of the Tested Plants
Figure 1 presents the inhibitory effects of extracts with different polarity solvents including methanol, hexane, and ethyl acetate (EtOAc). It was exhibited that all extracts disclosed some reduction in germination and shoot and root lengths of noxious E. crus-galli weed and L. sativa. Generally, the strength of the inhibitory effect was proportional to the dose.
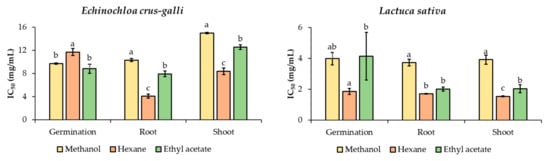
Figure 1.
Inhibitory effects of Arachis pintoi extracts on the growth of Echinochloa crus-galli and Lactuca sativa. Different letters (a, b, c) enclosed with columns within a parameter (germination, root, and shoot) are significantly different (p < 0.05).
We then compared the inhibitory effects among the different solvent extracts by applying IC50 values. These imply the extract quality that is required to reduce 50% of the shoot and root growth of E. crus-galli and L. sativa. Therefore, a lower IC50 value denotes a higher herbicidal activity. Among the extracted solvents, ethyl acetate extract (EtOAc) exhibited the greatest inhibition on germination of E. crus-galli (IC50 = 8.82 mg/mL), followed by methanol extract (IC50 = 9.71 mg/mL), and hexane extract (IC50 = 11.71 mg/mL). Conversely, hexane extract showed the highest suppression against the root and shoot length of E. crus-galli (IC50 = 4.08 and 8.40 mg/mL, respectively); the next most effective was EtOAc extract (IC50 = 7.94 and 12.55 mg/mL, respectively), and methanol was the least (Figure 1). For germination suppression of L. sativa, hexane extract showed the highest inhibitory effect (IC50 = 1.86 mg/mL), followed by methanol (IC50 = 3.98 mg/mL), and the lowest was the EtOAc extract (IC50 = 4.14 mg/mL). Also, the hexane extract displayed the strongest inhibition on the elongation of root and shoot of L. sativa (IC50 = 1.70 and 1.54 mg/mL, respectively), followed by the EtOAc extract (IC50 = 2.01 and 2.03 mg/mL, respectively). Consequently, the suppressive strength can be ranked in the following order: hexane extract > EtOAc extract > methanol extract. In short, all extracts exerted strong inhibition; however, among the solvent extracts, hexane showed the most inhibition on the growth of the tested plants (Figure 1).
3.6. Determination of Total Phenolic and Flavonoid Contents of the Different Extracts from Arachis pintoi
In this experiment, we evaluated the total phenolic (TPC) and flavonoid (TFC) contents in three different extract phases from A. pintoi powders (methanol, hexane, and ethyl acetate extracts). As shown in Figure 2, the highest TPC was found in the methanol extract (2.67 mg GAE/g DW), followed by ethyl acetate extract (0.39 mg GAE/g DW), and the lowest was in hexane extract (0.19 mg GAE/g DW). Meanwhile, methanol extract contained the highest TFC (1.01 mg RE/g DW), followed by hexane extract (0.49 mg RE/g DW), and ethyl acetate extract (0.26 mg RE/g DW) (Figure 2).
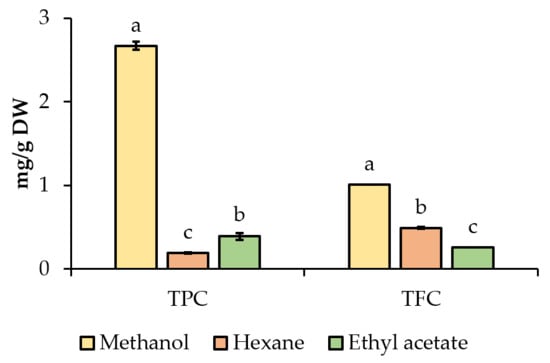
Figure 2.
Total phenolic (TPC) and flavonoid (TFC) contents of extracts from Arachis pintoi. Different letters (a, b, c) enclosed with columns within a parameter (TPC and TFC) are significantly different (p < 0.05).
3.7. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
In this experiment, based on comparing retention times, molecular weights, and similarities, GC-MS was applied to determine the allelochemical constituents present in methanol, hexane, and ethyl acetate extracts. The relative content of a compound in an extract sample was calculated by JEOL’s GC-MS Mass Center System Version 2.65a, and then expressed as peak area (% of total) (Table 5). Accordingly, the compound with the highest peak area can be considered as the most dominant in the tested extract.

Table 5.
Chemical constituents detected in extracts from Arachis pintoi by GC-MS analysis.
As shown in Table 5, there were 12 chemical constituents in methanol extract, in which some major compounds showed a high total area (%), including methyl palmitate (15.23%), acetamide, N-acetyl-N-5methyl (17.6%), palmitic acid (10.2%), n-Decanoic acid (10.2%), pyrrole (8.13%), and 5.10-Pentadecadien-1-ol. (Z.Z)-(8.13%). The other constituents ranged from 0.6% to 4.04%. In the hexane extract, a total of 15 chemical constituents were detected by GC-MS analysis. Some main compounds with high total peak area (%) were found, including methyl palmitate (24.02%), palmitic acid (20.03%), 9.12-octadecadienoic acid, methyl ester (16.12%), 1-hexyl-2-nitrocyclohexane (12.15%), 1H-imidazole. 1-acetyl- (2.61%), and γ-sitosterol (1.89%).
As shown in Table 5, a total of 14 chemical compounds were detected in the ethyl acetate extract. Some major compounds with a high total peak area (%) were found, specifically 1-Butanol and 2-methyl-. Acetate reached 22.37%, followed by Fosfosal (7.26%), 1-Hexyl-2-nitrocyclohexane (6.83%), palmitic acid (6.43%), and dihydroxyacetone (7.14%). The other constituents ranged from 0.65% to 1.71%, respectively. Overall, 35 compounds were detected from those extracts of A. pintoi powders. Among them, 14 substances potentially involved in the phytotoxic activity of A. pintoi were identified, including pyranones, benzofurans, phenolic acids, stearic acid, palmitic acid, fatty acids, and triterpenoids (Table 5).
4. Discussion
In recent years, there has been a surge in worldwide studies on exploiting allelochemicals from plants and fungi as sources of bioherbicides. Several plant-derived products have been developed commercially such as dicamba, mesotrine, biolaphes, cinmethylin, and artemisinin [39,40]. Some allelochemicals extracted from higher plants, such as leptospermone (Zeneca), benzoxazinones (BASF), cineole (Shell), and quinolinic acid (BASF), have been applied in agriculture with promising results [17,41]. To the best of our knowledge, there have been 19 bioherbicides to be developed and globally marketed from plant extracts, essential oils, and microorganisms [42,43]. In an attempt to explore allelopathic plants, over the past decade, our team has screened the allelopathic potential of several hundreds of higher plant species in Japan and Southeast Asia, and some strong allelopathic plants were selected and examined for their impacts on the emergence of weeds and pathogens [10,17]. Indeed, numerous studies have assessed the allelopathic potential of plants in artificial or controlled conditions (bioassays) against the tested plants; however, such studies sometimes may either overestimate the actual allelopathic effects of the plants or may not even inhabit the natural environments of the supposed allelopathic plants [44]. Therefore, selecting the tested plants for bioassays is one of the important factors to accurately determine the allelopathic impacts of the candidate plant species. To address this issue, in this study, we have presented the inhibitory effects of A. pintoi against the germination and growth of several tested plants including E. crus-galli, natural paddy weeds, and cultivated species (O. sativa, V. radiata, and L. sativa) under various environments (laboratory, greenhouse, and field). Notably, among the cultivated species used in this study, L. sativa was reported to be highly sensitive to allelochemicals even at low concentrations and to either stimulators or inhibitors used in allelopathic studies [17], while the major paddy weed (E. crus-galli) and common crops (O. sativa, V.radiata, Medicago sativa, etc.) are suitable as the tested plants for determining the inhibitory magnitude of the candidate plant species [27].
In laboratory bioassays and greenhouse experiments, the growth of E. crus-galli and tested plants were significantly suppressed and varied based on the doses applied and the tested plant species. Remarkably, the roots of all tested plants were the most suppressed by 72.9 to 89.3% (Table 1). Our results were in agreement with the report of Morikawa et al. [45], which indicated that the dry leaves of A. pintoi at 200 mg/L remarkably reduced the radicle and hypocotyl of L. sativa by using the sandwich method. At the lower doses, the tested plants were either negligibly inhibited or slightly promoted (Table 1, Table 2 and Table 3) because A. pintoi contains multi-nutritive values and mineral contents, which may lead to plant growth enhancement [46,47]. Our findings were also congruent with the previous results of Monteles et al. [48], who reported that A.pintoi extracts exerted the elongation of pepper (Capsicum annum), tomato (Solanum lycopersicum), and E. crus-galli (barnyard grass) at low concentrations [49].
In the field trials, the magnitude of E. crus-galli and natural weed biomass reduction were significant differences at doses of 100–200 g/m2, indicating that the suppressive ability of weeds was directly proportional to the dose of application. The results are in agreement with those of preceding reports, in which the suppressing activity of candidate plant powders has been indicated to be dose-dependent against tested plants [17,50]. Furthermore, candidate plants may selectively inhibit weeds without harmful effects on rice production [7,17]. Accordingly, we found that despite a significant reduction in the total dry weight of natural paddy weeds, A. pintoi did not induce any injury to rice plants; rather, it promoted rice yield by over 30% (Table 4). These results were in line with our previous studies wherein application of 1–2 tons/ha dry biomass of allelopathic plants, namely neem (A. indica), passion fruit (P. edulis), alfalfa (M. sativa), buckwheat (Fagopyrum esculentum), and kava (Piper methysticum), dramatically minimized the growth of major rice paddy weeds including monochoria (Monochoria vaginalis), India toothcup (Rotala indica), dirty dora (Cyperus difformis), and E. crus-galli, together with an increase in rice yield by over 20% [10,17,18]. Xuan et al. [17] explained that enhanced rice yield was caused by the release of potential nutrients, such as N, P, K, Ca, and Mg, during the application of plant-derived materials. On the other hand, weeds and rice plants compete for resources, such as light, space, and nutrition, etc., which can significantly reduce rice yield. Conversely, rice plants can thrive and reach their maximum yield potential when weed growth is limited, inhibited or absent. Practically, A. pintoi has been reported as a promising green manure with multiple benefits comprising soil coverage and nitrogen fixation [20,21,24,25]. In addition, other studies have demonstrated that the growth and yield components of corn (Zea mays) and tomato (Solanum lycopersicum) were significantly enhanced using a living mulch of A. pintoi [51,52,53] that concurrently reduced weed growth in some tropical upland crops as well as improving soil fertility and erosion [53]. Moreover, plant-derived allelochemicals and their interactions of plants–weeds–soils–microorganisms may actively regulate and contribute to promoting crop yields [11,54]. Thus, it is suggested that the allelochemicals and nutritional effects of A. pintoi may help elevate rice yield in the current study. On the other hand, when competitive weeds are inhibited by this candidate material, rice plants will have more opportunities to absorb multi-nutrients, thereby providing an advantage for their own growth. However, the allelochemical decomposition and nutrient contents of A. pintoi in paddy soils and their effects on rice production should be comprehensively investigated in future studies.
In this study, we found that the field trials applying A. pintoi materials did not provide the same level of dry weight inhibition as a synthetic herbicide and hand-weeding treatments, but rice yield was significantly promoted (Table 4). Therefore, it may be advantageous, because the remaining weeds can be managed by lower doses of herbicides [7]. Moreover, this legume grows abundantly in the tropic and subtropics, and farmers can easily collect a large amount; however, a dose of 200 g/m2, which is equivalent to the amount of 2 tons/ha of dried plant materials, would require much time and fieldwork from farmers. There is a conspicuous question about whether adding doses of 100–200 g/m2 of dried allelopathic plant materials into paddy fields may affect the growth of crops and weeds. In fact, our research team has conducted numerous field experiments using different dried plant materials at similar doses, but few plant species exhibited weed inhibition ability. Although the decomposition of plant materials can enrich soil fertility and promote crop yields, some potent allelopathic plant species with limited biomass resources are not feasible given the current trend of the declining labor force in agricultural production in many rice-producing countries [7,11,17].
Our findings suggest that A. pintoi is a promising candidate plant that may contribute to developing effective bioherbicides for weed management in rice fields. Basically, the allelopathic responses depend on their released allelochemicals into the environment, which can be extracted by using suitable solvents to obtain high yields of potent phytotoxic substances (allelochemicals) [10,34]. In this study, we used different solvents to obtain MeOH, hexane, and EtOAc extracts to determine phytotoxic activity and detect major allelochemicals of A. pintoi. Among them, phenolics are the largest class, with a vast number of reported phenolic allelochemicals. MeOH and EtOAc extracts revealed the highest TPC, but their inhibitory effects on the germination rate, root length, and shoot height of E. crus-galli and L. sativa were lower than those of the hexane extract. In contrast, hexane extract had the lowest TPC, but showed the most potent suppressing effects against the growths of tested plants (Table 5 and Figure 2). These results imply that phenolic compounds might not be the major allelochemicals in A. pintoi. However, nonpolar, volatile, and low molecular weight compounds in hexane extract might be the main factors corresponding to the phytotoxic responses of A. pintoi. These compounds are appropriately identified by GC-MS. Accordingly, methyl palmitate is the most dominant compound detected in the hexane extract, which has been previously reported as an allelochemical with suppression against chlorophyll and antioxidant enzymes (superoxide and dismutase peroxidase) of weeds [55]. In addition, palmitic acid, the second most abundant compound detected in hexane extract, is a well-known weed inhibitor, which exhibited prevention on superoxide dismutase and chlorophylls a and b of weeds [56,57,58]. Other fatty acids identified in hexane extracts, including 9.12-octadecadienoic acid, methyl ester, and stearic acid have been reported as allelochemicals in previous studies [59,60,61]. Furthermore, some allelochemicals belonging to the group of triterpenoids were found, including stigmasterol, γ-sitosterol, and lupeol, which may also play a role in the phytotoxic response of A. pintoi. Therefore, the interactions between compounds may be more important than individual compounds in determining biological activity [34,62,63]. Thus, the concomitant appearance of fatty acids and triterpenoids may play a determinant role in the inhibitory effects of A. pintoi against weeds. However, the effects of these potential allelochemicals on rice growth and yield should be seriously and thoroughly clarified in future investigations.
In another concern, a limitation of GC-MS operation can be mentioned that some peaks might not be effectively detected and reported due to physicochemical properties, structural features, and contaminations. Furthermore, the identification of detected compounds in this study was based on GC-MS results compared to the library, which needs further confirmation. Particularly, other advanced spectroscopic techniques, such as LC-MS/MS and LC-HRMS, are highly recommended to be applied in forthcoming studies to comprehensively explore the phytochemical profiles of A. pintoi as well as the main components responsible for its allelopathic activity. Additionally, NMR and X-ray crystallography analyses should be conducted to confirm the structure of the candidate compounds.
5. Conclusions
In summary, our study showed that A. pintoi exhibits strong phytotoxic effects against the growth of the tested plants and weeds and stimulated rice yield. Among the different extracts, the hexane extract demonstrated the highest suppression against the growth of E. crus-galli and tested plants, followed by EtOAc and MeOH extracts. Using GC-MS analyses, 14 new constituents were identified including phenolic acids, stearic acid, palmitic acid, fatty acids, pyranones, and benzofurans, which may be responsible for the herbicidal activity and play a vital role in the phytotoxic response of this legume. The findings suggest that A. pintoi is a promising source for developing natural herbicides to control paddy weeds in rice fields. However, future isolation and purification should be conducted to determine their actual active compounds which mainly act as natural herbicides in this legume.
Author Contributions
Conceptualization, T.D.K., K.H.T., N.V.V. and T.T.T.H.; methodology, P.T.T., T.D.K., T.T.T.H. and N.T.N.; software, P.T.T., L.H.A., L.T.K.L., T.D.K. and T.D.X.; data curation, P.T.T. and L.H.A.; writing—original draft preparation, P.T.T., L.H.A., L.T.K.L.; N.T.Q., T.D.X., T.D.K. and C.C.N.; writing—review and editing, T.D.K., N.V.V., L.T.K.L., K.H.T., T.D.X., C.C.N., T.T.T.H. and V.X.D.; supervision, T.D.K. and N.V.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Anaya, A.L. Allelopathic organisms and molecules: Promising bio-regulators for the control of plant diseases, weeds, and other pests. In Allelochemicals: Biological Control of Plant Pathogens and Diseases; Inderjit, Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 31–79. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R. Phytotoxic interference of Ageratum conyzoides with wheat (Triticum aestivum). J. Agron. Crop. Sci. 2003, 189, 341–346. [Google Scholar] [CrossRef]
- Khanh, T.D.; Linh, L.H.; Linh, T.H.; Quan, N.T.; Cuong, D.M.; Hien, V.T.T.; Ham, L.H.; Xuan, T.D. Integration of Allelopathy to Control Weeds in Rice. In Herbicides—Current Research and Case Studies in Use; Andrew, P., Jessica, K., Eds.; IntechOpen: London, UK, 2013; p. 664. [Google Scholar]
- Mandava, N.B. CRC Handbook of natural herbicides: Methods, volume I theory, practice and detection. In CRC Series in Naturally Occurring Pesticides; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2018. [Google Scholar]
- Chauhan, S.B. Biology and Management of Problematic Crop Weed Species; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Khanh, T.D.; Chung, I.M.; Tawata, S.; Xuan, T.D. Weed suppression by Passiflora edulis and its potential allelochemicals. Weed Res. 2006, 46, 296–303. [Google Scholar] [CrossRef]
- ILS-Institute of Legislation Study, Vietnam. Some Issues on Plant Protection and Quarantine; Institute for Legislative Studies: Hanoi, Vietnam, 2013. [Google Scholar]
- Thanh, P.L.; Tran, T.A. Highly Hazardous Pesticides in Vietnam: A Situational Analysis. Report, 1-38. An Giang University/University of Economics Ho Chi Minh: International Pollutants Elimination Network-IPEB for a Toxics-Free Future, 2020. Available online: https://ipen.org/documents/highly-hazardous-pesticides-vietnam-situational-analysis (accessed on 5 January 2023).
- Khanh, T.D.; Chung, I.M.; Xuan, T.D.; Tawata, S. The exploitation of crop allelopathy in sustainable agricultural production. J. Agron. Crop. Sci. 2005, 191, 172–184. [Google Scholar] [CrossRef]
- Khanh, T.D.; Chung, I.M.; Tawata, S.; Xuan, T.D. Allelopathy for weed management in sustainable agriculture. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2, 034. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Marone, P.G. Status of the biopesticide market and prospects for new bioherbicides. Pest Manag. Sci. 2023. first published. [Google Scholar] [CrossRef]
- Khanh, T.D.; Anh, L.H.; Nghia, L.T.; Huu Trung, K.; Bich Hien, P.; Minh Trung, D.; Dang Xuan, T. Allelopathic Responses of Rice Seedlings under Some Different Stresses. Plants 2018, 7, 40. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the role of allelochemicals in plant defences. In Advances in Botanical Research, How Plants Communicate with Their Biotic Environment; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, p. 384. [Google Scholar]
- Putnam, A.R.; Tang, C.S. The Science of Allelopathy; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Xuan, T.D.; Shinkichi, T.; Khanh, T.D.; Chung, I.M. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: An overview. Crop Prot. 2005, 24, 197–206. [Google Scholar] [CrossRef]
- Khanh, T.D.; Elzaawely, A.A.; Chung, I.M.; Ahn, J.K.; Tawata, S.; Xuan, T.D. Role of allelochemicals for weed management in rice. Allelopathy. J. 2007, 19, 85–96. [Google Scholar]
- Chou, C.H. Allelopathy and sustainable agriculture. In Allelopathy: Organisms, Processes and Application; ACS Symposium Series No. 582; Inderjit, Dakshini, K.M.M., Einhellig, F.A., Eds.; American Chemistry Society: Washington, DC, USA, 1995; pp. 211–223. [Google Scholar]
- Hoang, V.; Loan, N.; Tam, B.; Thiem, T. Effects of Fertilization Ratios on the Growth of Pinto Peanut (Arachis Pintoi) under Drought Stress Conditions. Vietnam. J. Agric. Sci. 2019, 1, 249–260. [Google Scholar]
- Carvalho, M.A.; Quesenberry, K.H. Morphological characterization of the USA Arachis pintoi Krap. and Greg. collection. Plant Syst. Evol. 2009, 277, 1–11. [Google Scholar] [CrossRef]
- de Sousa-Machado, I.B.; Felippe, T.; Garcia, R.; Pacheco, G.; Moreira, D.; Mansur, E. Total phenolics, resveratrol content and antioxidant activity of seeds and calluses of pinto peanut (Arachis pintoi Krapow.& W.C.Greg.). Plant Cell Tissue Organ Cult. 2018, 134, 491–502. [Google Scholar]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Solis, R.; Pezo, M.; Arévalo, L.; Lao, C.; Alegre, J.; Pérez, K. Evaluation of leguminous species as cover crops associated with sacha inchi. Pesqui. Agropecuária Trop. 2019, 49, e58011. [Google Scholar] [CrossRef]
- Sumiahadi, A.; Chozin, M.A.; Guntoro, D. Effectiveness of Arachis pintoi Karp. & Greg. As Biomulch to Control Weeds on Maize Cultivation. Int. J. Innov. Approach Agric. Res. 2019, 3, 680–689. [Google Scholar]
- Karimmojeni, H.; Rezaei, M.; Tseng, T.-M.; Mastinu, A. Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae 2022, 8, 169. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tsuzuki, E.; Tawata, S.; Khanh, T.D. Method to determine allelopathic potential of crop plants for weed control. Allelopath. J. 2004, 13, 149–164. [Google Scholar]
- Fujii, Y.; Parvez, S.S.; Parvez, M.M.; Ohmae, Y.; Iida, O. Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol. Manag. 2003, 3, 233–241. [Google Scholar] [CrossRef]
- Thang, P.T.; Vien, N.V.; Trung, K.H.; Khanh, T.D. Allelopathic Potential of Boehmeria nivea (L.) Gaudich. against the Growth of Barnyardgrass (Echinochloa crus-galli) in Laboratory and Nethouse Conditions. Vietnam. J. Agric. Sci. 2019, 17, 891–900. [Google Scholar]
- Thang, P.T.; Vien, N.V.; Nhung, N.T.; Linh, N.H.T.; Khanh, T.D. Evaluation of the Allelopathic Potential of Love Grass (Chrysopogon aciculatus (Retz.) Trin.) Among the Different Screening Conditions. Vietnam. J. Agric. Sci. 2022, 20, 1361–1373. [Google Scholar]
- Khanh, T.D.; Hong, N.H.; Xuan, T.D.; Chung, I.M. Paddy weed control by medicinal and leguminous plants from Southeast Asia. Crop Prot. 2005, 24, 421–431. [Google Scholar] [CrossRef]
- Khanh, T.D.; Cong, L.C.; Xuan, T.D.; Lee, S.J.; Kong, D.S.; Chung, I.M. Weed-Suppressing Potential of Dodder (Cuscuta hygrophilae) and its Phytotoxic Constituents. Weed Sci. 2008, 56, 119–127. [Google Scholar] [CrossRef]
- Hong, N.H.; Xuan, T.D.; Eji, T.; Khanh, T.D. Paddy weed control by higher plants from Southeast Asia. Crop Prot. 2004, 23, 255–261. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Tran, H.-D.; Thuy, N.T.D.; Trang, L.T.; Huong, C.T.; Andriana, Y.; Tuyen, P.T. Antioxidant, α-amylase and α-glucosidase inhibitory activities and potential constituents of Canarium tramdenum Bark. Molecules 2019, 24, 605. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; DaSilva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Minh, T.N.; Xuan, T.D.; Tran, H.-D.; Van, T.M.; Andriana, Y.; Khanh, T.D.; Quan, N.V.; Ahmad, A. Isolation and Purification of Bioactive Compounds from the Stem Bark of Jatropha podagrica. Molecules 2019, 24, 889. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Tran, H.-D.; Dieu Thuy, N.T. Inhibitory Activities of Momilactones A, B, E, and 7-Ketostigmasterol Isolated from Rice Husk on Paddy and Invasive Weeds. Plants 2019, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.K.; Batish, D.; Singh, H.P. Allelopathy and its implications in agroecosystems. J. Crop Prod. 1998, 1, 169–202. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Khanh, T.D.; Xuan, T.D.; Chung, I.M. Rice allelopathy and possibility for weed management. Ann. Appl. Biol. 2007, 151, 325–339. [Google Scholar] [CrossRef]
- Morikawa, C.I.O.; Miyaura, R.; Figueroa, M.L.T.; Salgado, E.L.R.; Fujii, Y. Screening of 170 Peruvian plant species for allelopathic activity by using the Sandwich method. Weed Biol. Manag. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Khamseekhiew, B.; Liang, J.B.; Wong, C.C.; Jalan, Z.A. Ruminal and intestinal digestibility of some tropical legume forages. Asian-Australas. J. Anim. Sci. 2001, 14, 321–325. [Google Scholar] [CrossRef]
- Rao, I.M.; Kerridge, P.C. Mineral nutrition of forage Arachis. In Biology and Agronomy of Forage Arachis; Kerridge, P.C., Hardy, B., Eds.; Centro Internacional de Agricultura Tropical: Valle del Cauca, Colombia, 1993; pp. 71–83. [Google Scholar]
- Monteles, F.; Melo, T.A.; Lima Filho, F.; Sousa, R.; Silva, M.; Serra, I. Efeito alelopático dos extratos aquosos de amendoim forrageiro (Arachis pintoi) e da erva-de-touro (Tridax procumbens) sobre a germinação de sementes de tomate e pimentão. Cad. De Agroecol. 2011, 6, 1–5. [Google Scholar]
- Phan, K.L.; Phong, N.H.T.; Nguyen, L.V.; Ho, L.T. Allelopathy and allelochemical quantitative analysis in Pito peanut (Arachis pintoi). J. Viet. Sci. Tech. 2021, 63, 41–46. [Google Scholar]
- Anh, L.H.; Quan, V.N.; Nghia, L.T.; Xuan, T.D. Phenolic allelochemicals: Achievements, limitations, and prospective approaches in weed management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar]
- Bangi, J.C.; Cosico, W. Corn yield and soil properties in Cotabato as influenced by the living mulch Arachis pintoi. Philipp. J. Crop Sci. 2007, 32, 58–68. [Google Scholar]
- Chozin, A.M.; Kartika, J.G.; Baharudin, D.R. The use of Arachis pintoi as biomulch in tomato cultivation. J. Hort. Indones. 2014, 4, 168–174. [Google Scholar]
- Chozin, M.A.; Nuryana, F.I.; Guntoro, D.; Sumiahadi, A.; Badriyah, R.N.; Wibowo, A.P. Potency of Arachis pintoi Krap.& Greg.as biomulch in the tropical upland agriculture. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 196, p. 012011. [Google Scholar]
- Kong, C.-H.; Xuan, T.D.; Khanh, T.D.; Tran, H.-D.; Trung, N.T. Allelochemicals and Signaling Chemicals in Plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef]
- Anwar, T.; Qureshi, H.; Mahnashi, M.H.; Kabir, F.; Parveen, N.; Ahmed, D.; Afzal, U.; Batool, S.; Awais, M.; Alyami, S.A.; et al. Bioherbicidal ability and weed management of allelopathic methyl esters from Lantana camara. Saudi J. Biol. Sci. 2021, 28, 4365–4374. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.; Tirillini, B.; Properzi, A.; Rol, C.; Venanzoni, R. Identification and bioactivity of the growth inhibitors in Tuber spp. methanolic extracts. Plant Biosyst. 2015, 149, 1000–1009. [Google Scholar] [CrossRef]
- Falquet, B.; Gfeller, A.; Pourcelot, M.; Tschuy, F.; Wirth, J. Weed suppression by common buckwheat: A review. Environ. Control. Biol. 2015, 53, 1–6. [Google Scholar] [CrossRef]
- Shen, S.; Ma, G.; Xu, G.; Li, D.; Jin, G.; Yang, S.; Clements, D.R.; Chen, A.; Wen, L.; Zhang, F.; et al. Allelochemicals identified from sweet potato (Ipomoea batatas) and their allelopathic effects on invasive alien plants. Front. Plant Sci. 2022, 13, 823947. [Google Scholar] [CrossRef]
- Kpoviessia, D.S.S.; Gdaguidia, F.A.; Gbenoua, J.D.; Accrombessia, G.C.; Haddadb, M.; Moudachiroua, M.; Quetin-Leclercqb, J. Allelopathic effects on cowpea (Vigna unguiculata (L.) Walp) plant and cytotoxic activities of sterols and triterpene isolated from Justicia anselliana (NEES) T. Anders. Electron. J. Nat. Sub. 2006, 1, 12–19. [Google Scholar]
- Gumilar, R.A.; Wijayanto, N.; Wulandari, A.S. Effect of Azadirachta excelsa and Melia azedarach extracts on soybean germination. Nus. Biosci. 2017, 9, 346–351. [Google Scholar] [CrossRef]
- Pooja, V.; Blaise, D.; Annie, J.S.; Manikandan, A. Allelopathic potential and allelochemicals indifferent intercrops for weed management inrainfed cotton. Curr. Sci. 2021, 120, 1035–1039. [Google Scholar]
- Anh, L.H.; Quan, N.V.; Lam, V.Q.; Iuchi, Y.; Takami, A.; Teschke, R.; Xuan, T.D. Antioxidant, anti-tyrosinase, anti-α-amylase, and cytotoxic potentials of the invasive weed Andropogon virginicus. Plants 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Anh, L.H.; Xuan, T.D.; Thuy, N.T.D.; Quan, N.V.; Trang, L.T. Antioxidant and α-amylase inhibitory activities and phytocompounds of Clausena indica Fruits. Medicines 2020, 7, 10. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).