Abstract
The aim of this study was to evaluate the impact of exercise order in multicomponent training (MCT) on the maximum voluntary contraction (MVC) of older women. A total of 91 older women, ranging in age from 60 to 85 years, were randomly assigned to either Group A or Group B. Group A performed a warm up followed by aerobic training and resistance training, whereas Group B followed a warm up followed by resistance training and aerobic training. A control group (CG) did not engage in any exercise interventions. Statistical analysis was conducted using one-way ANOVA for between-group comparisons, and ANOVA was used for repeated measures. The results revealed that Group A demonstrated significant increases in MVC for knee extensors (KEs) between M1 and M3 (p < 0.001) and between M2 and M3 (p < 0.001). Similarly, Group A exhibited significant increases in MVC for knee flexors (KFs) between M1 and M3 (p = 0.001) and between M2 and M3 (p < 0.001). Both Group A and Group B demonstrated significant increases in MVC for elbow flexors (EFs) between M1 and M3 (p < 0.001). Furthermore, Group B showed a significant increase in hand grip strength (HGS) between M1 and M3 (p < 0.001). Overall, the findings suggest that initiating MCT with aerobic training followed by resistance training is the most effective approach for improving muscle strength in older women.
1. Introduction
The aging population poses a significant economic burden due to potential functional dependency and long-term care requirements [1]. Additionally, older individuals who have experienced adverse life events, disability, and physical ailments are at an increased risk of developing depressive symptoms [2]. The age-related disuse of the muscular system leads to functional impairments, heightened morbidity, and a loss of autonomy and independence [3]. Physical activity, functional abilities, and fitness have been identified as crucial factors in improving and maintaining the quality of life and autonomy of older individuals [4].
Training sessions incorporating both resistance and aerobic exercises have been shown to prevent the decline of motor and neuromuscular function [5,6]. Resistance exercise enhances muscle strength and mass, thereby minimizing the risk of sarcopenia and frailty in older adults [7,8,9,10,11,12,13,14,15,16]. On the other hand, aerobic exercise stimulates cardiorespiratory and muscle oxidative capacity [17]. Recognizing the potential benefits of each exercise type, the American College of Sports Medicine (ACSM) recommends multicomponent training (MCT) as the most suitable exercise approach for older individuals, combining aerobic, strength, flexibility, and balance exercises to provide multi-modal stimuli [18]. Given the versatility of MCT, different exercise order stimuli can be implemented within an exercise periodization. This approach, based on the principle of adaptation, is expected to yield various outcomes depending on the training and exercise types employed. Current evidence on MCT suggests improvements in multiple body systems among older individuals, including enhanced cardiorespiratory fitness, regulation of metabolic profiles, improved functional and cognitive performance, and increased quality of life. MCT has also demonstrated efficacy as a non-pharmacological strategy with respect to enhancing the activities of daily living (ADLs) and physical functional performance in older adults with dementia [19,20]. Furthermore, MCT is effective in mitigating the onset of sarcopenia and the progression toward physical frailty [16]. In an experimental and controlled study, Shiotsu et al. [21] found that performing aerobic exercise after high-intensity resistance exercise was more effective in reducing arterial stiffness in older men. In terms of strength adaptations, Cadore et al. [22] demonstrated that resistance training prior to aerobic exercise was the most effective exercise order for increasing muscle strength in older men. However, aside from the studies conducted by Cadore et al. [22] and Shiotsu et al. [21], we did not find additional literature addressing the impact of exercise order on various physical outcomes in older individuals.
This knowledge gap limits the understanding of the effects of different exercise orders within MCT on the physical adaptations of older individuals. Therefore, investigating the effects of different MCT exercise orders is highly relevant in applied practice as it provides valuable insights to coaches who can tailor their periodization based on individual responsiveness profiles. Given the current state of knowledge, this study aims to evaluate the effects of exercise order within a 32-week MCT program on physically active older women. We hypothesize that the order of aerobic and resistance training in MCT sessions may have differing impacts on muscle strength in older individuals. However, due to the scarcity of evidence on this topic, we cannot definitively predict the exact adaptations that different exercise orders within MCT may elicit.
2. Methods
2.1. Participants
This is a randomized controlled trial following the updated CONSORT guidelines for experimental research trials [23]. Ninety-one physically active older women, with ages ranging from 60 to 81, voluntarily participated in the present study. The minimum sample size per group was defined as 25 using the G*Power program, in which the researchers considered an effect size of 0.5 (large) based on Cohen’s d effect size cutoffs [24], alpha = 0.05, power = 0.8, number of groups = 3, and number of moments = 3. Participants were randomly assigned to one of three groups, Group A (aerobic training prior to resistance training), Group B (resistance training prior to aerobic training), or the control group (CG), which did not engage in any exercise during the intervention period. Prior to commencing the study, all participants were provided with detailed explanations of the research procedures and provided written informed consent. Participants were instructed to maintain their regular daily routines and activities. Table 1 provides an overview of the participants’ characteristics, with 29 participants in the CG, 30 participants in Group A, and 32 participants in Group B. No statistically significant differences were observed between groups in terms of the body mass index (BMI) and age. The average age of the participants was 69.62 years with a standard deviation of 5.16. Additionally, the mean BMI was 26.93 kg/m2 with a standard deviation of 4.07.

Table 1.
Description of maximum knee extensor flexor voluntary isometric contraction in each group for the three moments of the program.
The participants included in the study were selected based on specific criteria. Firstly, they were required to be over 60 years old to ensure the inclusion of older individuals. Additionally, participants had to demonstrate autonomy in their daily life activities, indicating a level of independence. Furthermore, individuals with chronic disabling conditions such as cardiovascular, respiratory, metabolic, or joint diseases were excluded from the study, as well as those taking medications that could potentially interfere with the experimental protocol.
To establish the exclusion criteria, certain factors were considered. Participants were excluded if they had an absence in more than 25% of the overall training sessions, as this would compromise their level of engagement and adherence to the intervention. Moreover, missing more than four sessions in a month was considered excessive and could impact the consistency of the participant’s involvement in the study. Lastly, individuals who failed to meet the required evaluation moments were also excluded from the analysis, ensuring data completeness and accuracy. Figure 1 exemplifies the participant’s recruitment and inclusion in the study.
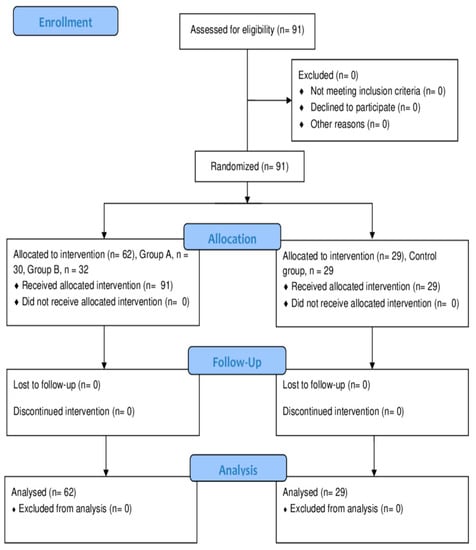
Figure 1.
CONSORT flow diagram for the study.
2.1.1. Multicomponent Training Program Description
The training sessions in this study had a duration of 50 to 60 min and consisted of five main components. Firstly, there was a warm-up phase lasting 5–8 min, involving slow walking and stretching exercises to prepare the participants for subsequent activities. The aerobic exercise component lasted 15–20 min and included walking, jogging, and dancing, aiming for a target intensity of 12–14 points on Borg’s perceived exertion scale [25]. The intervention protocol included stimuli for muscle resistance and strength components and comprised a circuit of 1 to 3 sets of exercises using elastic bands and free weights, targeting the major muscle groups. Rest periods of 40–60 s were allowed between sets. At the beginning of the trial, an adaptive phase was implemented to gradually introduce the participants to the exercise protocol, starting with 8 reps in a single set and progressing to 12–15 reps and 3 sets. The training program also included 5–8 min of static and dynamic balancing exercises utilizing sticks, balls, and balloons to enhance balance and stability. Finally, a 5 min cool-down period was implemented, involving breathing and stretching exercises, to gradually reduce the intensity of the session. All training sessions and assessments were conducted at the High School of Education (ESE) within the Instituto Politécnico de Bragança (IPB).
Both experimental groups (Group A and Group B) performed the same multicomponent training exercises. The only difference between the two groups was the order in which aerobic and resistance exercises were conducted. Group A followed the following sequence: warm up, aerobic training, resistance training, and cool-down phase. On the other hand, Group B performed the warm up, followed by resistance training, aerobic training, and cool-down phase. The control group (CG) did not participate in any exercise intervention throughout the study period. Figure 2 illustrates the study’s outline and provides a visual representation of the experimental design and the groups involved in the investigation. The participants were evaluated three times during the 32-week intervention period. The first assessment (M1) took place at the initiation of the training program. The second assessment (M2) was conducted after 16 weeks of training, serving as an intermediate evaluation. The final assessment (M3) was performed after the completion of 32 weeks of training. It is important to note that all groups underwent the same evaluation procedures at each assessment point, ensuring the consistency and comparability of measurements.
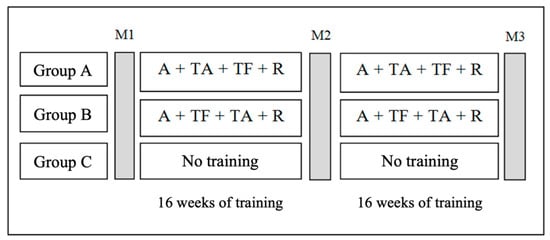
Figure 2.
Intervention outline. Notes: A = Warm up; TA = aerobic training; TF = resistance training; R = recovery; M1-M3; assessment moments.
2.1.2. Lateral Preference Assessment
The Dutch Handedness Questionnaire, developed by Van Strien [26], was utilized to assess the upper limb preference of participants during specific activities. The evaluators acted blindly during data collection to reduce the evaluation bias. The questionnaire consists of 16 questions with three possible answers: “left hand”, “right hand”, or “any of them”. The total score, ranging from 0 to 32, is obtained by summing the 16 answers. A score of 4 or less indicates “strongly left-handed”, a score of 28 or more indicates “strongly right-handed”, and a score between 5 and 27 indicates the “ambidextrous” group.
To determine the dominant lower limb, participants were asked about their preferred limb for performing tasks such as kicking a ball [27]. The protocol for assessing the maximum voluntary contraction (MVC) involved measuring the muscle’s voluntary isometric contraction (MVIC) in the participant’s dominant lower limb for knee flexors (KFs) and knee extensors (KEs). The assessment lasted for 10 s, during which the participants were instructed to exert maximum strength against a fixed arm. A Globos dynamometer with a resolution to the hundredth was used and placed on leg extension and leg curl machines. Each participant performed three attempts with more than a 2 min interval between each attempt, and the best trial was recorded [28,29].
Following the recommendations of the American Society for Exercise Physiology [30], the MVC assessment for elbow flexors (EFs) was conducted. Participants stood with their elbows flexed at 90 degrees and their forearms in maximum supination. A dynamometer was attached with one end near the participant’s foot and the other end in their hand. Similarly to the MVC assessment for lower limbs, three attempts were performed with a 2 min interval between each attempt, and the best result was recorded [18].
Hand grip strength (HGS) was assessed using a Jamar manual dynamometer, following the guidelines of the American Society of Hand Therapists. Participants were seated in a chair with no arm support, and their dominant elbow was flexed at 90 degrees with a neutral forearm position. The dynamometer was individually adjusted according to the participant’s hand size. They were instructed to exert maximum strength without sudden movements and maintain muscle contraction for five seconds. Three measurements were taken with a one-minute rest interval between attempts, and the best result was recorded to minimize the impact of muscle fatigue [30,31].
The testing order of the assessments was randomized for each participant to mitigate order bias and minimize the potential influence of fatigue on the results.
2.2. Statistical Analysis
All descriptive and inferential analyses were conducted using IBM SPSS software, version 27.0 (IBM, Armonk, NY, USA). A confidence interval of 95% (p < 0.05) was considered for determining statistical significance. Continuous variables were presented as means ± standard deviations. Prior to analyses, we assessed data distribution using the Shapiro–Wilk test and examined asymmetry and kurtosis values. In cases where the assumption of sphericity was violated, the Greenhouse–Geisser correction was applied to the dataset. To compare between groups, a one-way ANOVA was employed, while a repeated measures ANOVA was used to carry out comparisons between assessment moments. The post hoc Bonferroni test was utilized for identifying significant pairwise comparisons. We defined delta (Δ) 1 as the difference between M1 and M2, and Δ2 was defined as the difference between M1 and M3. Multiple linear regression analysis was performed using the significant MVIC variable’s Δ while controlling for age as a confounding factor. The effect size was assessed using eta-squared (η2), which represents the ratio between the sum of squares of the model and the total sum of squares. We interpreted the effects based on the recommendations provided by Ferguson (2009): η2 > 0.640 for a high effect, η2 ≤ 0.640 for a moderate effect, η2 ≤ 0.250 for a small effect, and η2 ≤ 0 indicating no effect [24].
3. Results
The results in Table 1 showed that Group A demonstrated significant increases in knee extension (KE) maximum voluntary isometric contraction (MVIC) compared to the control group (CG) across all evaluation moments (M1, M2, and M3). Group B did not show statistically significant increases in KE MVIC. There was a decrease in KE MVIC in the CG between M1 and M2, as well as between M1 and M3. Group A exhibited a significant increase in KE MVIC between M1 and M3 and between M2 and M3. Group B did not show significant increases in KE MVIC at any time point.
Regarding knee flexion (KF) MVIC, Group A showed significantly higher values compared to the CG at all evaluation moments. Group B also demonstrated higher KF MVIC compared to the CG, but the difference was only significant at M3. There were no significant differences between groups in KF MVIC between M1 and M2. The CG exhibited a decrease in KF MVIC between M1 and M2, as well as between M1 and M3. Group A showed a significant increase in KF MVIC between M1 and M3, as well as between M2 and M3. Group B showed a significant increase in KF MVIC only between M1 and M3.
The results in Table 2 showed that, in terms of EF MVIC, Group A had significantly higher values compared to the CG at all evaluation moments. Group B also showed higher EF MVIC compared to the CG, but the difference was significant only at M1 to M3. There were no significant differences between groups in EF MVIC between M1 and M2. The CG exhibited a decrease in EF MVIC between M1 and M2, as well as between M1 and M3. Group A showed a significant increase in EF MVIC between M1 and M3, as well as between M2 and M3. Group B showed a significant increase in EF MVIC only between M1 and M3.

Table 2.
Description of elbow flexors maximum voluntary isometric contraction and hand grip strength in each group for the three moments of the program.
Handgrip strength exhibited statistically significant differences between groups at all evaluation moments (M1, M2, and M3), as well as in the overall comparison from M1 to M3. Group A demonstrated higher HGS than the control group (CG) at M1, M2, and M3. Group B had higher HGS than the CG at M2 and M3. There were no significant differences between groups in HGS from M1 to M2. However, when comparing the changes over time, the CG showed a decrease in HGS between M1 and M3, as well as between M2 and M3. Group A did not show significant differences between moments. Group B demonstrated a significant increase in HGS between M1 and M3.
Table 3 presents the results of the multiple regression analysis examining the significant variations (Δ) identified in the previous analysis while controlling for the “age” confounding factor. R2 statistics ranged from 15% (Δ1 Model, KE MVIC) to 44% (Δ2 Model, KE MVIC variable), indicating the proportion of variance explained by the models. The adjusted R2 effect sizes ranged from 12% to 42%, taking into account the number of predictors in each model. All reported models achieved statistical significance and were not influenced by the adjustment for “age”. Among the five significant models, Group A exhibited a larger slope in two variables (KF MVIC and EF MVIC), while Group B demonstrated a larger slope in two variables (KE Δ1 and HGS). The KE Δ2 model showed similar slopes for both Group A and Group B. These significant slopes indicated significant differences between Group A, Group B, and the control group in either M1 and M3 or M1 and M2, suggesting distinct patterns of change over time.

Table 3.
Multiple regression analysis results.
4. Discussion
The objective of this study was to examine the effects of a 32-week moderate-intensity continuous training (MCT) program on maximum voluntary isometric contraction (MVIC) in older women. Our main findings revealed that Group A exhibited greater improvements in most variables than Group B. Notably, in the present study, commencing the MCT program with aerobic exercises proved to be the most effective exercise intervention order for stimulating older women’s KE MVIC. Enhancing KE strength is closely associated with maintaining a high level of functionality throughout life [32]. This improved strength contributes efficiently to muscle contractions during activities of daily living (ADLs), such as climbing stairs, using public transportation, and navigating obstacles while walking, reducing fatigue and the likelihood of falls [33,34]. In contrast, the control group experienced significant and moderate effect size decreases in KE MVIC between the initial and second evaluation moments. These findings highlight the risks associated with prolonged periods of physical inactivity, including increased susceptibility to sarcopenia, frailty, and falls, which ultimately diminishes the quality of life for older individuals [35,36].
Furthermore, in terms of KF MVIC, Group A demonstrated moderate effect size increases in all three evaluation moments compared to the control group. Conversely, Group B did not exhibit significant improvements in KF MVIC over time (p > 0.05). Improving KF muscle strength is positively associated with walking speed [37] and static and dynamic balance [12] and inversely associated with the incidence of falls [38]. Moreover, evidence suggests a positive association between the agonist and antagonist muscles involved in knee movement for efficient walking and lower limb function during ADLs [38]. These findings underscore the importance of implementing interventions that are capable of improving both KF and KE strength in older individuals. In line with this, the MCT program performed by the older women in this MCT intervention stimulated improvements in both muscle groups, aligning with the perspective of synergistic muscle strength development. Conversely, the control group, as well as Group A and Group B, experienced significant and moderate effect size decreases in KF MVIC between the first and second evaluation moments. This decline is considered a negative factor for sarcopenia development and negatively impacts gait patterns, making it a noticeable characteristic in elderly individuals prone to falls [39,40]. These results emphasize the importance of maintaining continuous physical activity to promote optimal well-being in older individuals [41].
In relation to elbow flexor (EF) MVC, we observed that only Group A exhibited significant improvements across all three evaluation moments compared to the control group (CG) and Group B. Furthermore, we found that Group A demonstrated a reduced effect size increase in EF MVC from M3 to M1, while Group B showed a significantly reduced effect size increase compared to the CG but only from M3 to M2. Interestingly, Group A consistently exhibited significantly greater EF MVC than Group B at the first and second evaluation moments, indicating that initiating the MCT program with aerobic exercises was more effective in stimulating improvements in EF strength in older women.
Elbow flexor strength serves as a reliable indicator of the ability to efficiently perform multi-joint movements in daily activities, such as grasping and moving objects [42]. Despite not evaluating this specific variable, a possible adaptation recurring during strength gains is neural activation, which plays a role in the EF’s contractile capacity, contributing to coordination during daily tasks [42]. Unlike the findings for knee extensor (KE) and knee flexor (KF) MVIC, we observed smaller effect sizes in EF MVC after the intervention. This discrepancy may be partially explained by the fact that the MCT program did not provide a similar stimulus to the upper limbs as it did to the lower limbs. Cai et al. [42] suggest that MCT interventions should be designed to equalize or at least increase the stimulus for upper limb strength development and the efficient performance of activities of daily living [42]. In contrast, the CG experienced significant and moderate effect size decreases in EF MVC between the first and second evaluation moments. The loss of EF strength is considered a negative factor for the functionality of older individuals and is associated with overall strength decline [43]. Studies by Monteiro et al. [44] and Sobrinho et al. [45] have emphasized the importance of training EF strength via physical exercise to enable older individuals to effectively utilize their arms in daily tasks and maintain physical independence throughout life [44,45].
Regarding handgrip strength (HGS) results, both Group A and Group B exhibited significantly higher HGS compared to the CG at all evaluation moments, with Group A surpassing Group B in all measurements. However, only Group B demonstrated significant increases in HGS between moments, specifically from M3 to M1, with a reduced effect size. These specific study findings contradicted the main results, showing that initiating MCT using resistance exercises was the most efficient with respect to improving the older women’s HGS, although these changes were only observed from M2 to M3. HGS serves as an important physical capacity indicator for overall muscle strength and vitality [46,47]. It is also positively associated with cognitive function [48] and inversely associated with the risk of neurodegeneration in older individuals [49]. In contrast, the CG exhibited statistically significant and moderate effect size decrements in HGS between the first and third evaluation moments, highlighting yet another negative effect of not participating in the MCT program during the 32-week period. Reduced HGS in older individuals is associated with risks of sarcopenia, frailty, and early mortality [35]. Moreover, low HGS has been linked to cognitive impairment and neurological disorders [36,50].
This study has several limitations that should be considered when interpreting the results. Firstly, the absence of dietary control prevented us from conducting a more comprehensive analysis of the effects of the intervention. Additionally, the lack of control over participants’ physical activity levels limits our understanding of specific training effects. Additionally, caution is needed regarding the generalization of these results due to the reduced participant number in each group (not exceedingly more than 32 per group). On the other hand, the present study exhibits important strengths. The findings provide valuable insights with respect to the optimal order of MCT exercises for physically active older women. These results suggest that independent older women may benefit from incorporating this exercise program with respect to improving or maintaining muscle strength, promoting autonomy and functional independence as they age. Interestingly, the current evidence about physical exercise physiology indicates that, when coaches aim to maximize muscle strength gains in individuals, the better exercise session order is strength exercises followed by aerobics because it preconizes better muscle work production in a training session [51]. However, this study proved the contrary when aerobics followed by resistance/strength training was the best combination. Researchers can consider this study’s finding as a new research gap, where they could deepen their understanding of this phenomenon.
Thus, for future research, we recommend conducting randomized controlled trials using larger samples, which can reduce the results’ bias and improve the evidence’s reliability with respect to exercise order preparation and older women’s muscle strength outcomes. In addition, future research must include strict control over physical activity levels and dietary behavior. Furthermore, it would be valuable to investigate the effects of different MCT intensities combined with varying exercise orders. In addition, we suggest exploring biochemical and molecular analyses to gain deeper insights into the physiological adaptations associated with MCT interventions organized within different orders. Overall, while this study contributes to the understanding of the benefits of MCT for older women, further research is needed to address the aforementioned limitations and expand the knowledge in this area.
5. Practical Applications
The findings of this study have important implications for both researchers and practitioners in the field of exercise science, specifically in the context of designing effective exercise programs for physically active older women. Based on the results of this study, it is recommended that exercise programs for independent older women incorporate a multicomponent training (MCT) approach. Specifically, the order of exercises should begin with aerobic training followed by resistance training. This exercise order has been shown to be more effective in improving or maintaining muscle strength, which is crucial for maintaining autonomy and functional independence in aging individuals. Practitioners working with older women should consider individual differences in terms of physical fitness levels, exercise preferences, and health conditions when designing MCT programs. By tailoring the exercise program to each individual’s needs and capabilities, practitioners can optimize benefits and ensure a safe and enjoyable exercise experience. The study’s findings underscore the importance of focusing on knee extensor (KE) and knee flexor (KF) strength training. Improving KE and KF strength is associated with enhanced functionality, mobility, and reduced fall risk in daily activities. Thus, practitioners should include exercises that specifically target these muscle groups to maximize the functional benefits for older women. Future research should explore the effects of the different intensities of MCT and varying exercise orders. This will provide valuable insights into the optimal training parameters for improving muscle strength and functional capacity in older women. Researchers and practitioners can use this information to refine exercise prescription guidelines and develop evidence-based interventions. While this study did not control dietary factors, future research should consider the impact of nutrition on the outcomes of MCT interventions. Researchers should explore the potential synergistic effects of exercise and dietary interventions in improving muscle strength and functional outcomes in older women. The study highlights the negative consequences of prolonged periods of physical inactivity, such as decreases in muscle strength and increased risks of sarcopenia, fragility, and falls. This emphasizes the importance of promoting continuous physical activity engagement among older women in order to maintain their quality of life, functional independence, and overall well-being.
6. Conclusions
This study provides valuable insights into the effects of a 32-week multicomponent training (MCT) program on the muscle strength of physically active older women. The findings shed light on the benefits of incorporating specific exercise orders and highlight the importance of targeted muscle groups in maintaining functional independence and quality of life during aging. The results indicate that starting the MCT program with aerobic exercises followed by resistance training (Group A) yielded greater improvements in knee flexor (KF) and knee extensor (KE) muscle strength compared to the reverse exercise order (group B). Group A demonstrated significant improvements in most variables related to knee flexor and extensor strength, while Group B showed improvements in KF strength from M2 to M3 and in handgrip strength (HGS) from M1 to M3. These findings suggest that the exercise order can significantly influence the effectiveness of the intervention in improving muscle strength in older women. Furthermore, the study emphasizes the importance of KE and KF strength in maintaining functionality and mobility and reducing the risk of falls in daily activities. Improving KE and KF strength using MCT interventions has been shown to positively impact the activities of daily living, such as climbing stairs, using public transportation, and maneuvering around obstacles, thus enhancing overall functional capacity.
The results also highlight the need for continuous physical activity engagement in older women in order to prevent declines in muscle strength. The control group (CG) demonstrated significant decreases in KE and elbow flexor (EF) strength between certain time points, indicating the negative consequences of physical inactivity over time. These findings underscore the importance of promoting and maintaining physical activity levels among older women to mitigate the risk of sarcopenia, fragility, and compromised quality of life.
Author Contributions
Conceptualization, A.M.M. and S.R.; methodology, A.M.M. and S.R.; software, S.R.; validation, P.F. and J.E.T.; formal analysis, S.R. and T.M.B. and F.R.; investigation, A.M.M.; resources, A.M.M.; data curation, S.R. and A.M.M.; writing—original draft preparation, A.M.M. and S.R.; writing—review and editing, S.M., S.R., J.E.T., S.E., T.M.B., P.F. and F.R.; visualization, A.M.M.; supervision, A.M.M. and P.F.; project administration, A.M.M.; funding acquisition, P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funds via the Portuguese Foundation for Science and Technology. I.P.: grant number UIDB/04748/2020 and grant number UID/04045/2020.
Institutional Review Board Statement
The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and received approval from the Scientific Board of the Higher Institute of Educational Sciences of the Douro (Approval Number: 2.576, granted on 21 September 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The first author can provide data upon reasonable request.
Acknowledgments
We extend our sincere appreciation to all participants for their invaluable contributions to this research study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scott, A.J.; Ellison, M.; Sinclair, D.A. The Economic Value of Targeting Aging. Nat. Aging 2021, 1, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Komalasari, R.; Mpofu, E.; Prybutok, G.; Ingman, S. Daily Living Subjective Cognitive Decline Indicators in Older Adults with Depressive Symptoms: A Scoping Review and Categorization Using Classification of Functioning, Disability, and Health (ICF). Healthcare 2022, 10, 1508. [Google Scholar] [CrossRef]
- Anton, M.M.; Spirduso, W.W.; Tanaka, H. Age-Related Declines in Anaerobic Muscular Performance: Weightlifting and Powerlifting. Med. Sci. Sports Exerc. 2004, 36, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Liffiton, J.A.; Horton, S.; Baker, J.; Weir, P.L. Successful Aging: How Does Physical Activity Influence Engagement with Life? Eur. Rev. Aging Phys. Act. 2012, 9, 103–108. [Google Scholar] [CrossRef]
- Baker, M.K.; Atlantis, E.; Fiatarone Singh, M.A. Multi-Modal Exercise Programs for Older Adults. Age Ageing 2007, 36, 375–381. [Google Scholar] [CrossRef]
- Häkkinen, K.; Newton, R.U.; Gordon, S.E.; McCormick, M.; Volek, J.S.; Nindl, B.C.; Gotshalk, L.A.; Campbell, W.W.; Evans, W.J.; Häkkinen, A.; et al. Changes in Muscle Morphology, Electromyographic Activity, and Force Production Characteristics during Progressive Strength Training in Young and Older Men. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, B415–B423. [Google Scholar] [CrossRef] [PubMed]
- Romero-Arenas, S.; Martínez-Pascual, M.; Alcaraz, P.E. Impact of Resistance Circuit Training on Neuromuscular, Cardiorespiratory and Body Composition Adaptations in the Elderly. Aging Dis. 2013, 4, 256–263. [Google Scholar] [CrossRef]
- Pereira, A.; Izquierdo, M.; Silva, A.J.; Costa, A.M.; Bastos, E.; González-Badillo, J.J.; Marques, M.C. Effects of High-Speed Power Training on Functional Capacity and Muscle Performance in Older Women. Exp. Gerontol. 2012, 47, 250–255. [Google Scholar] [CrossRef]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Nozawa, T.; Sekiguchi, A.; Nouchi, H.; Kawashima, R. Beneficial Effects of Short-Term Combination Exercise Training on Diverse Cognitive Functions in Healthy Older People: Study Protocol for a Randomized Controlled Trial. Trials 2012, 13, 200. [Google Scholar] [CrossRef]
- Martins, W.R.; de Oliveira, R.J.; Carvalho, R.S.; de Oliveira Damasceno, V.; da Silva, V.Z.M.; Silva, M.S. Elastic Resistance Training to Increase Muscle Strength in Elderly: A Systematic Review with Meta-Analysis. Arch. Gerontol. Geriatr. 2013, 57, 8–15. [Google Scholar] [CrossRef]
- Freiberger, E.; Häberle, L.; Spirduso, W.W.; Zijlstra, G.A.R. Long-Term Effects of Three Multicomponent Exercise Interventions on Physical Performance and Fall-Related Psychological Outcomes in Community-Dwelling Older Adults: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2012, 60, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wan, Q.; Zhou, W.; Feng, X.; Shang, S. Factors Associated with Balance Function in Patients with Knee Osteoarthritis: An Integrative Review. Int. J. Nurs. Sci. 2017, 4, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-W.; Jung, S.-W.; Kim, S.-W.; Lee, J.-M.; Jung, H.C.; Song, J.-K. Effects of 16 Weeks of Resistance Training on Muscle Quality and Muscle Growth Factors in Older Adult Women with Sarcopenia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6762. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Domingos, C.; Monteiro, D.; Morouço, P. A Review on Aging, Sarcopenia, Falls, and Resistance Training in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 874. [Google Scholar] [CrossRef]
- Noor, H.; Reid, J.; Slee, A. Resistance Exercise and Nutritional Interventions for Augmenting Sarcopenia Outcomes in Chronic Kidney Disease: A Narrative Review. J. Cachexia Sarcopenia Muscle 2021, 12, 1621–1640. [Google Scholar] [CrossRef]
- Kumar, P.; Umakanth, S.; Girish, N. A Review of the Components of Exercise Prescription for Sarcopenic Older Adults. Eur. Geriatr. Med. 2022, 13, 1245–1280. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- Carvalho, M.J.; Marques, E.; Mota, J. Training and Detraining Effects on Functional Fitness after a Multicomponent Training in Older Women. Gerontology 2009, 55, 41–48. [Google Scholar] [CrossRef]
- Bouaziz, W.; Lang, P.O.; Schmitt, E.; Kaltenbach, G.; Geny, B.; Vogel, T. Health Benefits of Multicomponent Training Programmes in Seniors: A Systematic Review. Int. J. Clin. Pract. 2016, 70, 520–536. [Google Scholar] [CrossRef]
- Shiotsu, Y.; Watanabe, Y.; Tujii, S.; Yanagita, M. Effect of Exercise Order of Combined Aerobic and Resistance Training on Arterial Stiffness in Older Men. Exp. Gerontol. 2018, 111, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Izquierdo, M.; Pinto, S.S.; Alberton, C.L.; Pinto, R.S.; Baroni, B.M.; Vaz, M.A.; Lanferdini, F.J.; Radaelli, R.; González-Izal, M.; et al. Neuromuscular Adaptations to Concurrent Training in the Elderly: Effects of Intrasession Exercise Sequence. Age 2013, 35, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Williams, N. The Borg Rating of Perceived Exertion (RPE) Scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef]
- Van Strien, J. The Dutch Handedness Questionnaire. J. Clin. Exp. Neuropsychol. 2003. [Google Scholar] [CrossRef]
- Coren, S.; Porac, C.; Duncan, P. Lateral Preference Behaviors in Preschool Children and Young Adults. Child. Dev. 1981, 52, 443–450. [Google Scholar] [CrossRef]
- Goncalves, M.M.; Marson, R.A.; Fortes, M.d.S.R.; Neves, E.B.; da Silva Novaes, J. The Relationship between Total Muscle Strength and Anthropometric Indicators in Brazilian Army Military. Rev. Bras. De. Obesidade Nutr. E Emagrecimento 2017, 11, 322–329. [Google Scholar]
- Kellis, E.; Katis, A. Quantification of Functional Knee Flexor to Extensor Moment Ratio Using Isokinetics and Electromyography. J. Athl. Train. 2007, 42, 477–485. [Google Scholar]
- Kuzala, E.A.; Vargo, M.C. The Relationship between Elbow Position and Grip Strength. Am. J. Occup. Ther. 1992, 46, 509–512. [Google Scholar] [CrossRef]
- Caldwell, L.S.; Chaffin, D.B.; Dukes-Dobos, F.N.; Kroemer, K.H.; Laubach, L.L.; Snook, S.H.; Wasserman, D.E. A Proposed Standard Procedure for Static Muscle Strength Testing. Am. Ind. Hyg. Assoc. J. 1974, 35, 201–206. [Google Scholar] [CrossRef]
- Abdalla, P.P.; Dos Santos Carvalho, A.; Dos Santos, A.P.; Venturini, A.C.R.; Alves, T.C.; Mota, J.; de Sousa Oliveira, A.; Ramos, N.C.; Marini, J.A.G.; Machado, D.R.L. Cut-off Points of Knee Extension Strength Allometrically Adjusted to Identify Sarcopenia Risk in Older Adults: A Cross-Sectional Study. Arch. Gerontol. Geriatr. 2020, 89, 104100. [Google Scholar] [CrossRef] [PubMed]
- Casaña, J.; Calatayud, J.; Silvestre, A.; Sánchez-Frutos, J.; Andersen, L.L.; Jakobsen, M.D.; Ezzatvar, Y.; Alakhdar, Y. Knee Extensor Muscle Strength Is More Important Than Postural Balance for Stair-Climbing Ability in Elderly Patients with Severe Knee Osteoarthritis. Int. J. Environ. Res. Public Health 2021, 18, 3637. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Hoshino, M.; Ohyama, S.; Hori, Y.; Yabu, A.; Kobayashi, A.; Tsujio, T.; Kotake, S.; Nakamura, H. Relationship of Back Muscle and Knee Extensors with the Compensatory Mechanism of Sagittal Alignment in a Community-Dwelling Elderly Population. Sci. Rep. 2021, 11, 2179. [Google Scholar] [CrossRef]
- Ramsey, K.A.; Rojer, A.G.M.; D’Andrea, L.; Otten, R.H.J.; Heymans, M.W.; Trappenburg, M.C.; Verlaan, S.; Whittaker, A.C.; Meskers, C.G.M.; Maier, A.B. The Association of Objectively Measured Physical Activity and Sedentary Behavior with Skeletal Muscle Strength and Muscle Power in Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2021, 67, 101266. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Tully, M.; Jacob, L.; Blackburn, N.; Adlakha, D.; Caserotti, P.; Soysal, P.; Veronese, N.; Sánchez, G.F.L.; Vancampfort, D.; et al. The Association Between Sedentary Behavior and Sarcopenia Among Adults Aged ≥65 Years in Low- and Middle-Income Countries. Int. J. Environ. Res. Public Health 2020, 17, 1708. [Google Scholar] [CrossRef] [PubMed]
- Aartolahti, E.; Lönnroos, E.; Hartikainen, S.; Häkkinen, A. Long-Term Strength and Balance Training in Prevention of Decline in Muscle Strength and Mobility in Older Adults. Aging Clin. Exp. Res. 2020, 32, 59–66. [Google Scholar] [CrossRef]
- de Moura, T.G.; Nagata, C.d.A.; Garcia, P.A. The Influence of Isokinetic Peak Torque and Muscular Power on the Functional Performance of Active and Inactive Community-Dwelling Elderly: A Cross-Sectional Study. Braz. J. Phys. Ther. 2020, 24, 256–263. [Google Scholar] [CrossRef]
- Boyer, K.A.; Andriacchi, T.P. The Nature of Age-Related Differences in Knee Function during Walking: Implication for the Development of Knee Osteoarthritis. PLoS ONE 2016, 11, e0167352. [Google Scholar] [CrossRef]
- Kristensen, M.T.; Hulsbæk, S.; Faber, L.L.; Kronborg, L. Knee Extension Strength Measures Indicating Probable Sarcopenia Is Associated with Health-Related Outcomes and a Strong Predictor of 1-Year Mortality in Patients Following Hip Fracture Surgery. Geriatrics 2021, 6, 8. [Google Scholar] [CrossRef]
- Barrachina-Igual, J.; Pablos, A.; Pérez-Ros, P.; Flor-Rufino, C.; Martínez-Arnau, F.M. Frailty Status Improvement after 5-Month Multicomponent Program PROMUFRA in Community-Dwelling Older People: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 4077. [Google Scholar] [CrossRef]
- Cai, N.M.; Dewald, J.P.A.; Gurari, N. Accuracy of Older Adults in Judging Self-Generated Elbow Torques during Multi-Joint Isometric Tasks. Sci. Rep. 2020, 10, 13011. [Google Scholar] [CrossRef] [PubMed]
- Ostolin, T.L.V.D.P.; Gonze, B.d.B.; de Oliveira Vieira, W.; de Oliveira, A.L.S.; Nascimento, M.B.; Arantes, R.L.; Romiti, M.; Sperandio, E.F.; Dourado, V.Z. Association between the Handgrip Strength and the Isokinetic Muscle Function of the Elbow and the Knee in Asymptomatic Adults. SAGE Open Med. 2021, 9, 2050312121993294. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.M.; Bartolomeu, R.F.; Forte, P.; Carvalho, J. The Effects of Three Different Types of Training in Functional Fitness and Body Composition in Older Women. J. Sport Health Res. 2019, 11, 289–304. [Google Scholar]
- Sobrinho, A.C.d.S.; de Almeida, M.L.; Rodrigues, G.d.S.; Bertani, R.F.; Lima, J.G.R.; Bueno Junior, C.R. Stretching and Multicomponent Training to Functional Capacities of Older Women: A Randomized Study. Int. J. Environ. Res. Public Health 2021, 19, 27. [Google Scholar] [CrossRef]
- Chan, J.; Lu, Y.-C.; Yao, M.M.-S.; Kosik, R.O. Correlation between Hand Grip Strength and Regional Muscle Mass in Older Asian Adults: An Observational Study. BMC Geriatr. 2022, 22, 206. [Google Scholar] [CrossRef]
- Sui, S.X.; Holloway-Kew, K.L.; Hyde, N.K.; Williams, L.J.; Tembo, M.C.; Mohebbi, M.; Gojanovic, M.; Leach, S.; Pasco, J.A. Handgrip Strength and Muscle Quality in Australian Women: Cross-Sectional Data from the Geelong Osteoporosis Study. J. Cachexia Sarcopenia Muscle 2020, 11, 690–697. [Google Scholar] [CrossRef]
- Jin, Y.-L.; Xu, L.; Jiang, C.-Q.; Zhang, W.-S.; Pan, J.; Zhu, F.; Zhu, T.; Thomas, G.N.; Lam, T.-H. Association of Hand Grip Strength with Mild Cognitive Impairment in Middle-Aged and Older People in Guangzhou Biobank Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 6464. [Google Scholar] [CrossRef]
- Jacob, M.E.; O’Donnell, A.; Samra, J.; Gonzales, M.M.; Satizabal, C.; Pase, M.P.; Murabito, J.M.; Beiser, A.; Seshadri, S. Grip Strength, Gait Speed and Plasma Markers of Neurodegeneration in Asymptomatic Middle-Aged and Older Adults. J. Frailty Aging 2022, 11, 291–298. [Google Scholar] [CrossRef]
- Kitamura, A.; Seino, S.; Abe, T.; Nofuji, Y.; Yokoyama, Y.; Amano, H.; Nishi, M.; Taniguchi, Y.; Narita, M.; Fujiwara, Y.; et al. Sarcopenia: Prevalence, Associated Factors, and the Risk of Mortality and Disability in Japanese Older Adults. J. Cachexia Sarcopenia Muscle 2021, 12, 30–38. [Google Scholar] [CrossRef]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Exercise Physiology: Nutrition, Energy, and Human Performance; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).