A Well-Known Plant and New Therapeutic Strategies: Turmeric and Its Components in Oral Inflammatory Diseases Treatment
Abstract
1. Introduction
2. Historical Background
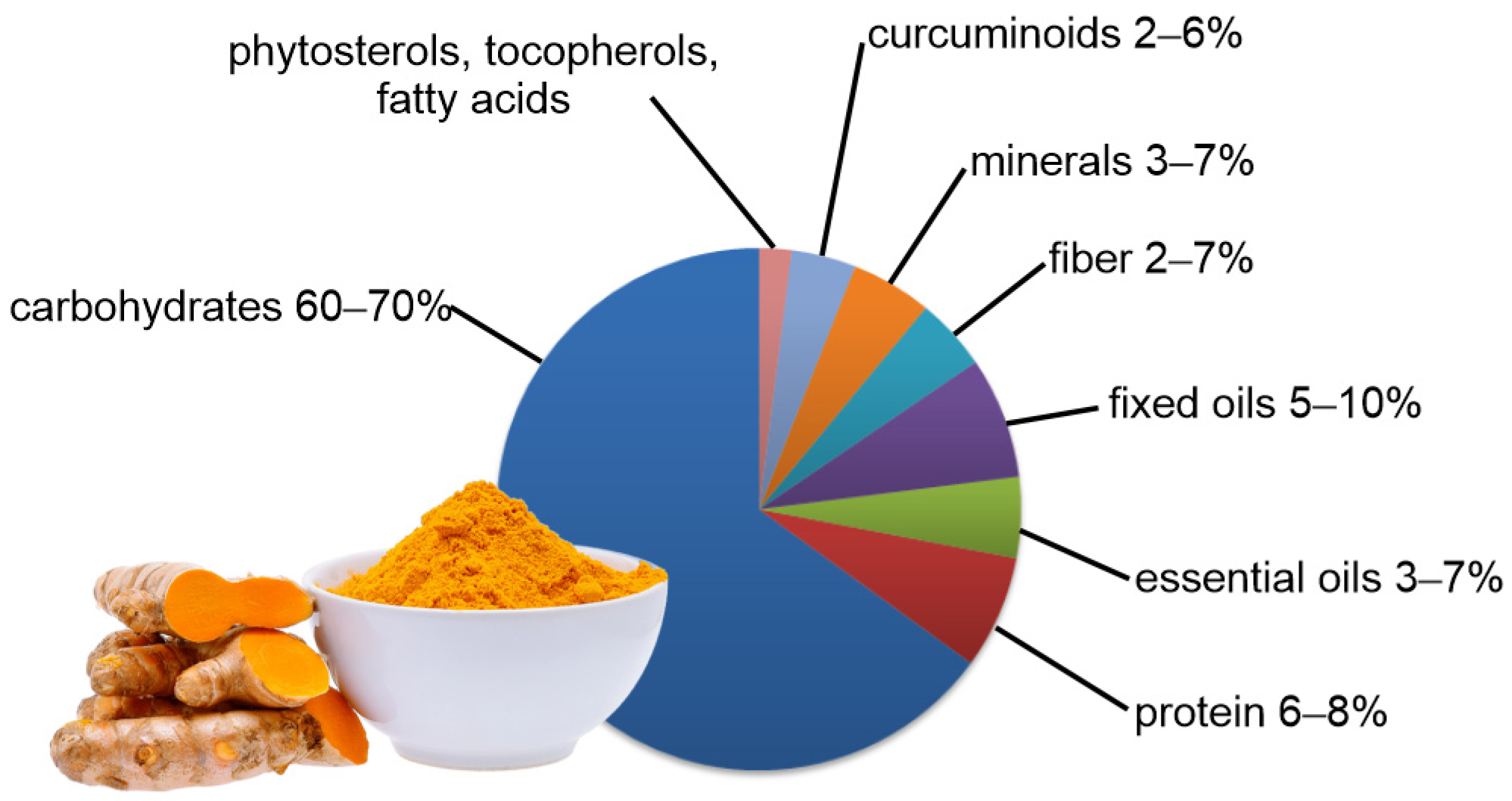
3. Active Ingredients of Turmeric
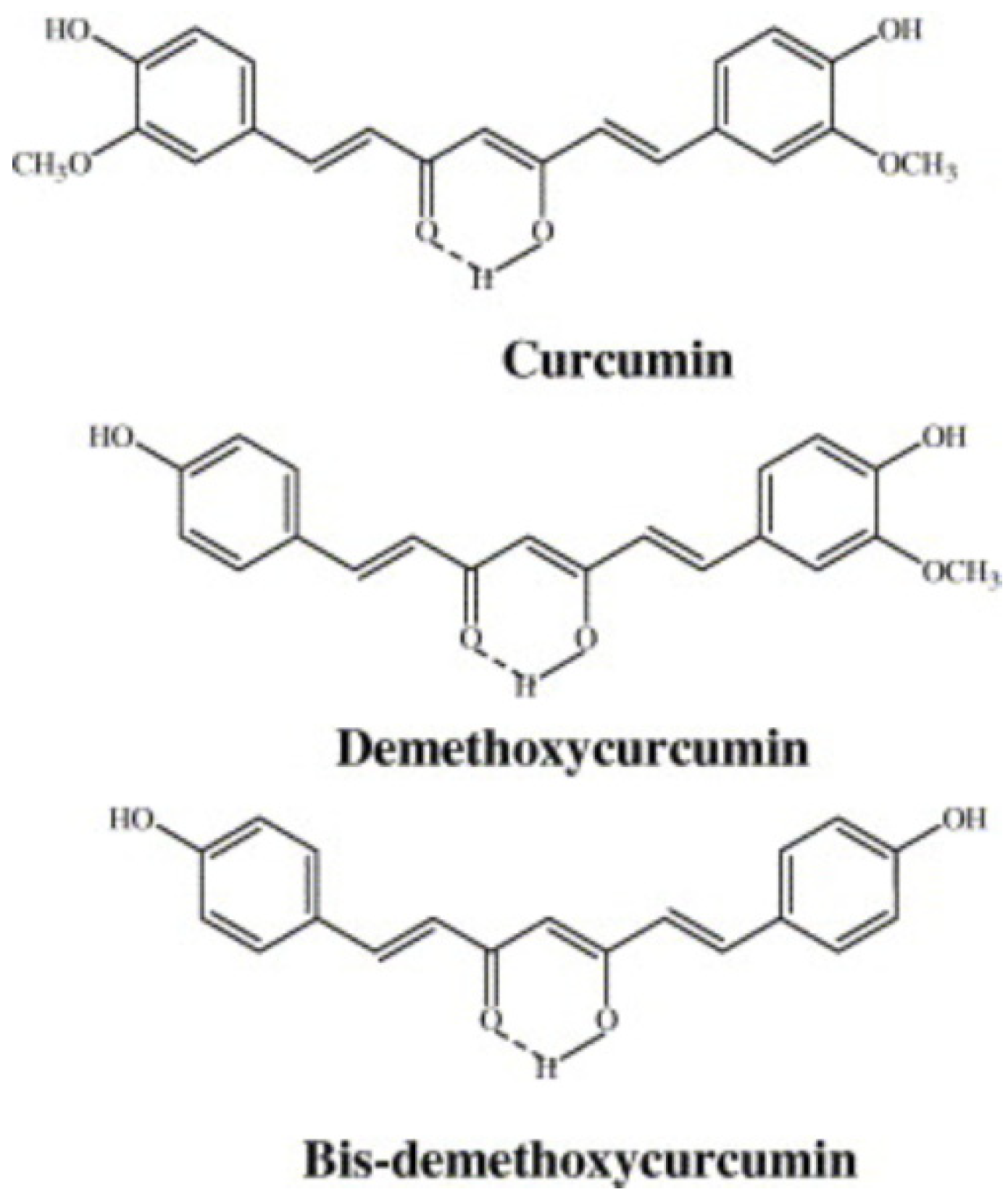
3.1. Curcuminoids
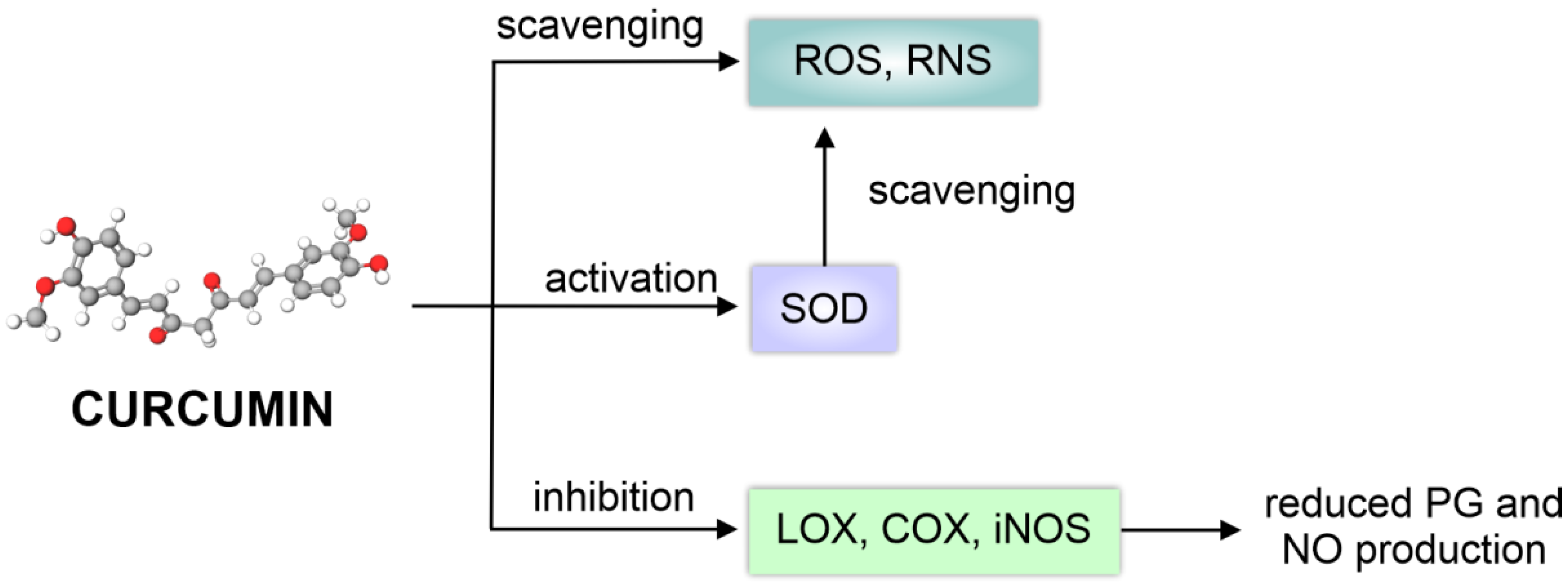
3.1.1. Biological Activity of Curcuminoids
3.1.2. Potential Problems Related to Curcumin Application
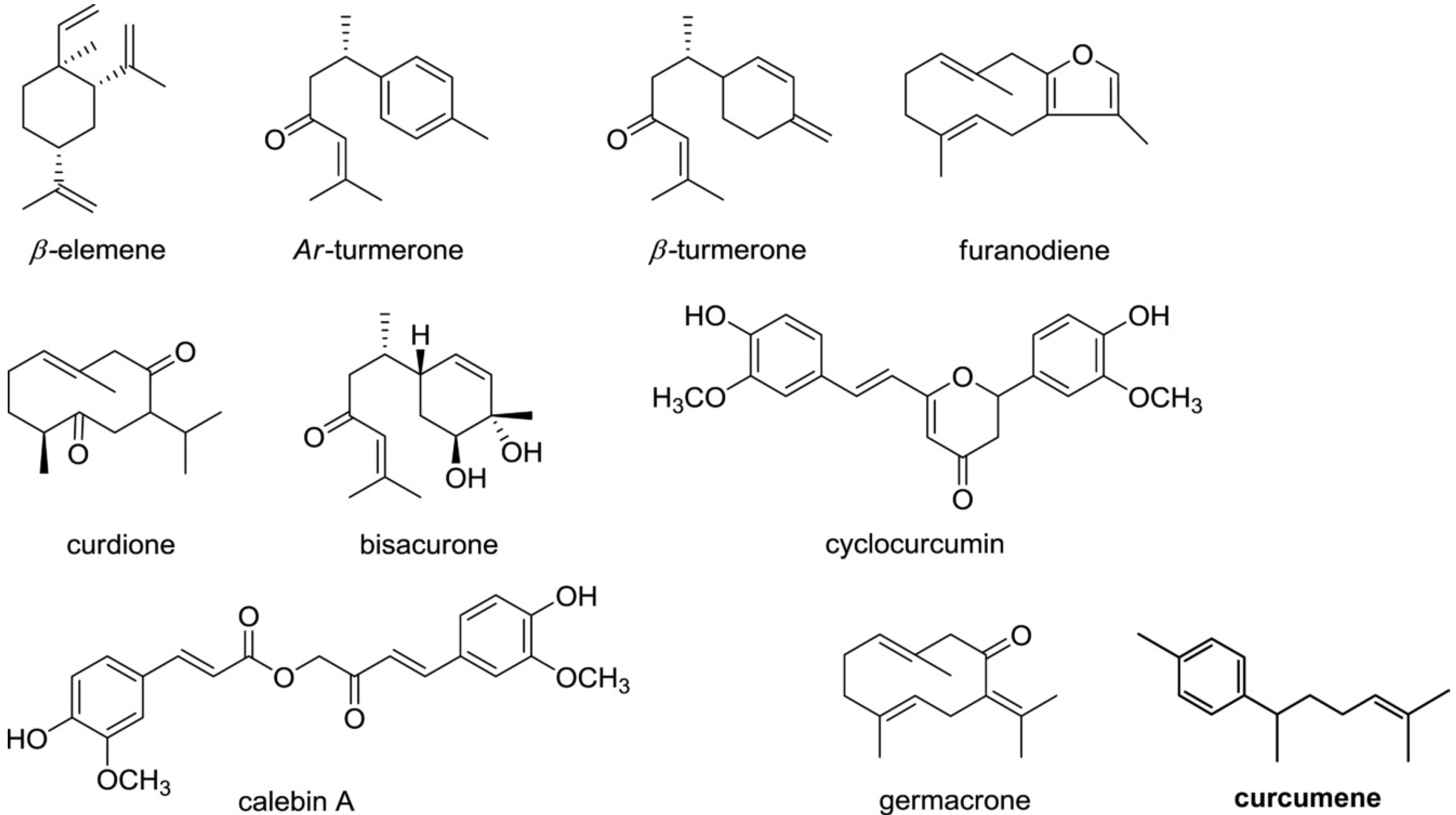
3.2. Biological Activity of Other Turmeric Constituents
4. Topical Applications of Curcuminoids
5. Topical Curcumin-Based Formulations in Oral Diseases
5.1. Periodontal Disease
5.2. Oral Mucositis and Mouth Ulcers
5.3. Gingivitis
5.4. Other Inflammatory Conditions
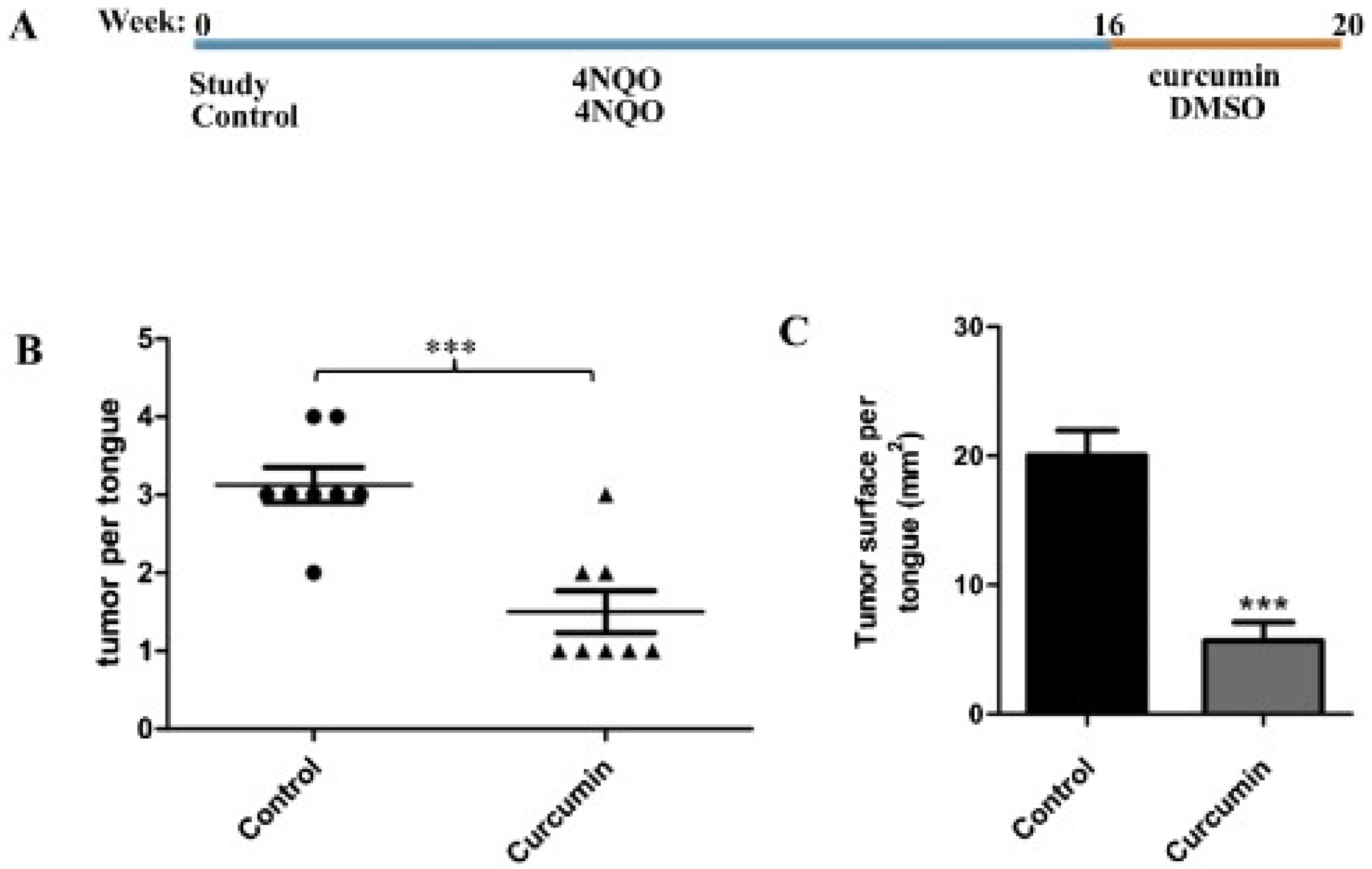
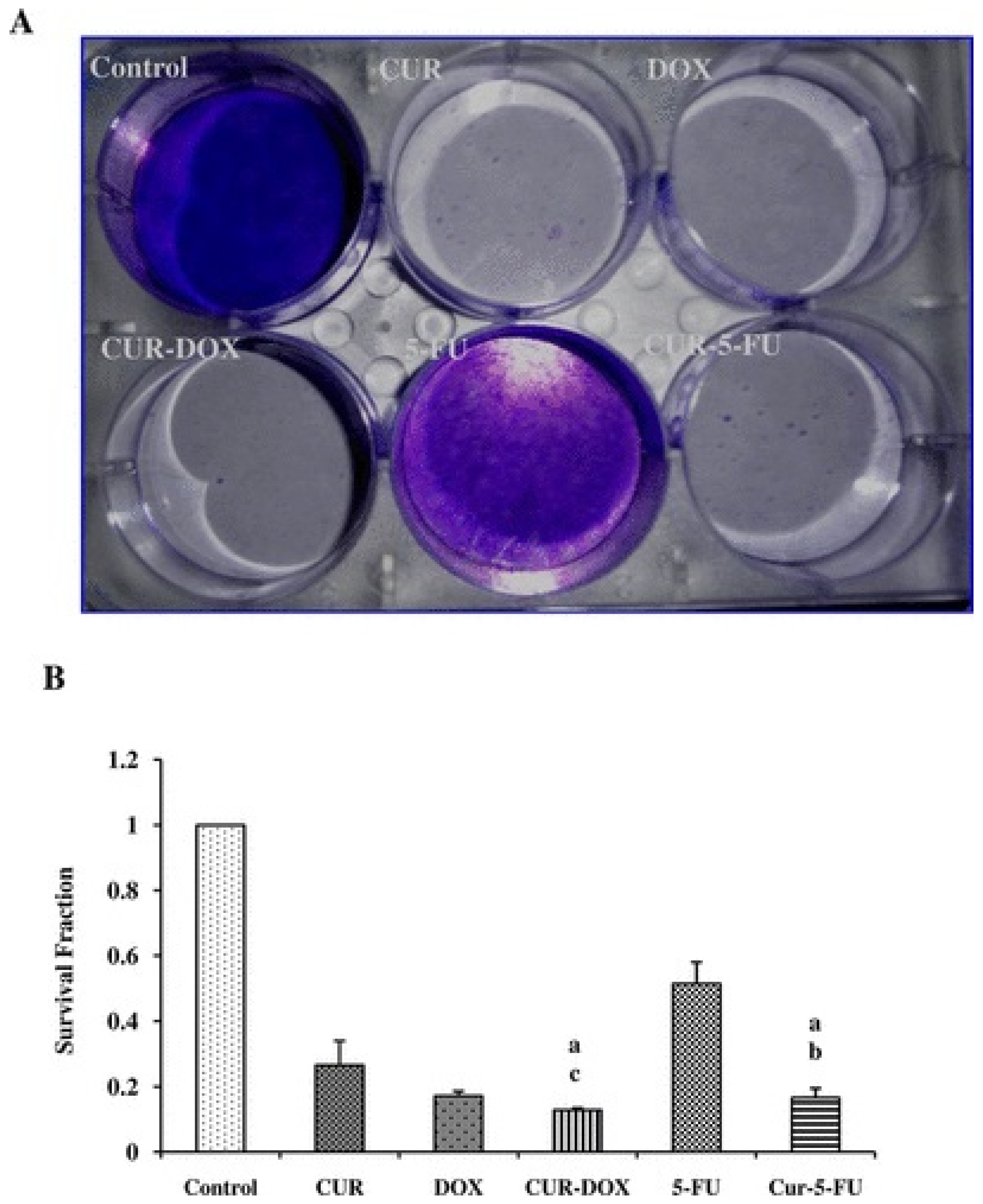
6. Curcumin in Oral Cancer
7. Conclusions and Future Directions
- the existing in vivo evidence of curcumin efficacy in oral diseases seems to be promising but is still insufficient;
- more extensive in vivo investigations, performed with the use of harmonized protocols, are required;
- due to low toxicity, low cost and promising literature reports, curcumin is worth considering mostly as an adjuvant therapy in some oral conditions;
- unfavorable physicochemical features of curcumin (e.g., susceptibility to degradation, poor solubility in water) may limit its application;
- in the case of nanoformulations, frequently investigated as potential carriers for curcumin in order to minimize the impact of its unfavorable properties, safety profile should be carefully assessed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad Khan, M.S.; Ahmad, I. Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine: Advancements in Herbal Products as Novel Drug Leads; Academic Press: Cambridge, MA, USA, 2019; pp. 3–13. [Google Scholar]
- Sucher, N.J. The application of Chinese medicine to novel drug discovery. Expert Opin. Drug Discov. 2012, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Scheidt, C.; Otuki, M.; Santos, A.R. Biological activity of plant extracts: Novel analgesic drugs. Expert Opin. Emerg. Drugs 2005, 6, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Luo, Y.; Tian, M.; Zhang, S.-Y.; Lu, R.; Wang, J.-H.; Kasimu, R.; Li, X. Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014, 47, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Aravindaram, K.; Yang, N.-S. Anti-Inflammatory Plant Natural Products for Cancer Therapy. Planta Med. 2010, 76, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.K.; Kaushik, M.S.; Mishra, S.K.; Raj, P.; Singh, P.K.; Pandey, K.D. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: A review. 3 Biotech 2017, 7, 357. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2012, 15, 195–218. [Google Scholar] [CrossRef]
- Normando, A.G.C.; de Menêses, A.G.; de Toledo, I.P.; Borges, G.Á.; de Lima, C.L.; dos Reis, P.E.D.; Guerra, E.N.S. Effects of turmeric and curcumin on oral mucositis: A systematic review. Phyther. Res. 2019, 33, 1318–1329. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontology 2000 2016, 72, 153–175. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Feller, L.; Altini, M.; Lemmer, J. Inflammation in the context of oral cancer. Oral Oncol. 2013, 49, 887–892. [Google Scholar] [CrossRef]
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 5 October 2021).
- Hosseinpoor, A.; Itani, L.; Petersen, P. Socio-economic inequality in oral healthcare coverage: Results from the World Health Survey. J. Dent. Res. 2012, 91, 275–281. [Google Scholar] [CrossRef]
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The biological efficacy of natural products against acute and chronic inflammatory diseases in the oral region. Medicines 2018, 5, 122. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2011; pp. 263–288. ISBN 9781439807163. [Google Scholar]
- Ravindran, P.N.; Babu, K.N.; Sivaraman, K. (Eds.) Turmeric: The Genus Curcuma, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780429124013. [Google Scholar]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phyther. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef]
- Jesuthasan, A.S.; Uluwaduge, D.I. Ethnobotanics used in folk medicine of Tamil culture in Sri Lanka: A scientific review. J. Integr. Med. 2017, 15, 19–26. [Google Scholar] [CrossRef]
- Scott, A.; Power, R.C.; Altmann-Wendling, V.; Artzy, M.; Martin, M.A.S.; Eisenmann, S.; Hagan, R.; Salazar-Garciá, D.C.; Salmon, Y.; Yegorovi, D.; et al. Exotic foods reveal contact between South Asia and the near East during the second millennium BCE. Proc. Natl. Acad. Sci. USA 2021, 118, e2014956117. [Google Scholar] [CrossRef]
- Nair, K.P. Turmeric: Origin and History. In Turmeric (Curcuma longa L.) and Ginger (Zingiber officinale Rosc.)—World’s Invaluable Medicinal Spices; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 1–6. [Google Scholar]
- Jayaprakasha, G.K.; Jaganmohan Rao, L.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Miłobȩdzka, J.; Kostanecki, S.V.; Lampe, V. Zur Kenntnis des Curcumins. Berichte Dtsch. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Horbańczuk, M.; Tzvetkov, N.T.; Mocan, A.; Carradori, S.; Maggi, F.; Marchewka, J.; Sut, S.; Dall’Acqua, S.; Gan, R.Y.; et al. Curcumin: Total-Scale Analysis of the Scientific Literature. Molecules 2019, 24, 1393. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Ampasavate, C.; Sung, B.; Aggarwal, B.B.; Limtrakul, P. Demethoxycurcumin suppresses migration and invasion of MDA-MB-231 human breast cancer cell line. Eur. J. Pharmacol. 2010, 627, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases. Eur. Cardiol. Rev. 2019, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.; Jha, A.; Youssef, D.; Rupasinghe, H. Curcumin and Its Carbocyclic Analogs: Structure-Activity in Relation to Antioxidant and Selected Biological Properties. Molecules 2013, 18, 5389–5404. [Google Scholar] [CrossRef]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled Trial. J. Diet. Suppl. 2015, 13, 209–220. [Google Scholar] [CrossRef]
- Momeni, H.R.; Eskandari, N. Curcumin protects the testis against cadmium-induced histopathological damages and oxidative stress in mice. Hum. Exp. Toxicol. 2020, 39, 653–661. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, Q.; Xiang, L.; Dong, X.; Li, H.; Ni, J.; Wan, L.; Cai, G.; Chen, G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther. Clin. Risk Manag. 2017, 13, 1099. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Maa, F.; Liu, F.; Ding, L.; You, M.; Yue, H.; Zhou, Y.; Hou, Y. Anti-inflammatory effects of curcumin are associated with down regulating microRNA-155 in LPS-treated macrophages and mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. Today 2017, 22, 1582–1592. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Investig. 2017, 35, 1–22. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-Based Media in Nose-to-Brain Drug Delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier and Neurotherapeutics. NeuroRx 2005, 2, 1–2. [Google Scholar] [CrossRef]
- Cui, M.; Ono, M.; Kimura, H.; Liu, B.; Saji, H. Synthesis and structure-affinity relationships of novel dibenzylideneacetone derivatives as probes for ß-Amyloid Plaques. J. Med. Chem. 2011, 54, 2225–2240. [Google Scholar] [CrossRef]
- Chongzhao, R.; Xiaoyin, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-β deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef]
- Tu, P.; Fu, H.; Cui, M. Compounds for imaging amyloid-β deposits in an Alzheimer’s brain: A patent review. Expert Opin. Ther. Pat. 2015, 25, 413–423. [Google Scholar] [CrossRef]
- Dai, Q.; Zhou, D.; Xu, L.; Song, X. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des. Devel. Ther. 2018, 12, 4095–4105. [Google Scholar] [CrossRef] [PubMed]
- Mazieiro, R.; Frizon, R.R.; Barbalho, S.M.; Goulart, R.D.A. Is Curcumin a Possibility to Treat Inflammatory Bowel Diseases? J. Med. Food 2018, 21, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Trigo-gutierrez, J.K.; Vega-chacón, Y.; Soares, A.B.; Mima, E.G.D.O. Antimicrobial activity of curcumin in nanoformulations: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef]
- Kulac, M.; Aktas, C.; Tulubas, F.; Uygur, R.; Kanter, M.; Erboga, M.; Ceber, M.; Topcu, B.; Ozen, O.A. The effects of topical treatment with curcumin on burn wound healing in rats. J. Mol. Histol. 2013, 44, 83–90. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Bernabé-Pineda, M.; Ramírez-Silva, M.T.; Romero-Romo, M.; González-Vergara, E.; Rojas-Hernández, A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 1091–1097. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 316, 359–380. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhao, J.; Ding, Y.; Li, L. A cost-effective method to prepare curcumin nanosuspensions with enhanced oral bioavailability. J. Colloid Interface Sci. 2017, 485, 91–98. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Grill, A.E.; Koniar, B.; Panyam, J. Co-delivery of natural metabolic inhibitors in a self-microemulsifying drug delivery system for improved oral bioavailability of curcumin. Drug Deliv. Transl. Res. 2014, 4, 344–352. [Google Scholar] [CrossRef]
- Yen, F.L.; Wu, T.H.; Tzeng, C.W.; Lin, L.T.; Lin, C.C. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J. Agric. Food Chem. 2010, 58, 7376–7382. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Hu, L.; Jia, Y.; Niu, F.; Jia, Z.; Yang, X.; Jiao, K. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J. Agric. Food Chem. 2012, 60, 7137–7141. [Google Scholar] [CrossRef]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef]
- Hagl, S.; Kocher, A.; Schiborr, C.; Kolesova, N.; Frank, J.; Eckert, G.P. Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice—Impact on bioavailability. Neurochem. Int. 2015, 89, 234–242. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Gupta, S.; Rai, S.; Shrivatava, A.; Tripathi, S.; Singh, S.; Khopade, A.J.; Kesharwani, P. Curcumin loaded poly (amidoamine) dendrimer-plamitic acid core-shell nanoparticles as anti-stress therapeutics. Drug Dev. Ind. Pharm. 2020, 46, 412–426. [Google Scholar] [CrossRef]
- Wu, X.; Xu, J.; Huang, X.; Wen, C. Self-microemulsifying drug delivery system improves curcumin dissolution and bioavailability. Drug Dev. Ind. Pharm. 2011, 37, 15–23. [Google Scholar] [CrossRef]
- Jaisamut, P.; Wiwattanawongsa, K.; Graidist, P.; Sangsen, Y.; Wiwattanapatapee, R. Enhanced Oral Bioavailability of Curcumin Using a Supersaturatable Self-Microemulsifying System Incorporating a Hydrophilic Polymer; In Vitro and In Vivo Investigations. AAPS PharmSciTech 2018, 19, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Wairkar, S. Recent advances in cyclosporine drug delivery: Challenges and opportunities. Drug Deliv. Transl. Res. 2019, 9, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Pharmazie 2002, 57, 820–824. [Google Scholar] [PubMed]
- Liu, Y.; Cai, Y.; Ying, D.; Fu, Y.; Xiong, Y.; Le, X. Ovalbumin as a carrier to significantly enhance the aqueous solubility and photostability of curcumin: Interaction and binding mechanism study. Int. J. Biol. Macromol. 2018, 116, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Kumar, S.; Raut, J.; Singh, M.; Kaur, S.; Sharma, G.; Roldan, T.L.; Trehan, S.; Holloway, J.; Wahler, G.; et al. Systematic development and characterization of novel, high drug-loaded, photostable, curcumin solid lipid nanoparticle hydrogel for wound healing. Antioxidants 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Dodangeh, M.; Grabchev, I.; Gharanjig, K.; Staneva, D.; Tang, R.C.; Sheridan, M. Modified PAMAM dendrimers as a matrix for the photostabilization of curcumin. New J. Chem. 2020, 44, 17112–17121. [Google Scholar] [CrossRef]
- Pecora, T.M.G.; Cianciolo, S.; Catalfo, A.; De Guidi, G.; Ruozi, B.; Cristiano, M.C.; Paolino, D.; Graziano, A.C.E.; Fresta, M.; Pignatello, R. Preparation, characterization and photostability assessment of curcumin microencapsulated within methacrylic copolymers. J. Drug Deliv. Sci. Technol. 2016, 33, 88–97. [Google Scholar] [CrossRef]
- Kumari, A.; Guliani, A.; Shukla, A.K.; Kumar, S.; Acharya, A. Green surfactant based synthesis of curcumin loaded poly lactic-co-glycolic acid nanoparticles with enhanced solubility, photo-stability and anti-biofilm activity. J. Drug Deliv. Sci. Technol. 2020, 59, 101884. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Buccarello, L.; Dragotto, J.; Mohammadi, A.; Corbo, M.; Feligioni, M. Obstacles against the Marketing of Curcumin as a Drug. Int. J. Mol. Sci. 2020, 21, 6619. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phyther. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Zaboli, M.; Barani, M.; Baino, F.; Bilal, M.; Sanchooli, E. Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies. Nanomaterials 2021, 11, 817. [Google Scholar] [CrossRef]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing Curcumin Oral Bioavailability Through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Jiang, L.; Kwok, H.F.; Lee, J.K.M.; Chan, K.M.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Turmeric ethanolic extract possesses stronger inhibitory activities on colon tumour growth than curcumin—The importance of turmerones. J. Funct. Foods 2016, 22, 565–577. [Google Scholar] [CrossRef]
- Antony, B.; Merina, B.; Iyer, V.; Judy, N.; Lennertz, K.; Joyal, S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95®CG (BiocurcumaxTM), A Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm. Sci. 2008, 70, 445. [Google Scholar] [CrossRef]
- Lantz, R.C.; Chen, G.J.; Solyom, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine 2005, 12, 445–452. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Chan, B.C.L.; Hon, P.M.; Lee, M.Y.H.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem. Toxicol. 2010, 48, 2011–2020. [Google Scholar] [CrossRef]
- Yang, S.; Liu, J.; Jiao, J.; Jiao, L. Ar-Turmerone Exerts Anti-proliferative and Anti-inflammatory Activities in HaCaT Keratinocytes by Inactivating Hedgehog Pathway. Inflammation 2020, 43, 478–486. [Google Scholar] [CrossRef]
- Li, Y.L.; Du, Z.Y.; Li, P.H.; Yan, L.; Zhou, W.; Tang, Y.D.; Liu, G.R.; Fang, Y.X.; Zhang, K.; Dong, C.Z.; et al. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018, 64, 319–325. [Google Scholar] [CrossRef]
- Saga, Y.; Hatakenaka, Y.; Matsumoto, M.; Yoshioka, Y.; Matsumura, S.; Zaima, N.; Konishi, Y. Neuroprotective effects of aromatic turmerone on activity deprivation-induced apoptosis in cerebellar granule neurons. Neuroreport 2020, 31, 1302–1307. [Google Scholar] [CrossRef]
- Hori, Y.; Tsutsumi, R.; Nasu, K.; Boateng, A.; Ashikari, Y.; Sugiura, M.; Nakajima, M.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H.; et al. Aromatic-Turmerone Analogs Protect Dopaminergic Neurons in Midbrain Slice Cultures through Their Neuroprotective Activities. Cells 2021, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, O.D.; Jham, G.N.; Barcelos, R.C.; Mendonça, F.A.; Ghiviriga, I. Isolation and Identification of the Principal Fungitoxic Component of Turmeric Essential Oil. J. Essent. Oil Res. 2011, 19, 387–391. [Google Scholar] [CrossRef]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K.; et al. Curcuminoids and Sesquiterpenoids in Turmeric (Curcuma longa L.) Suppress an Increase in Blood Glucose Level in Type 2 Diabetic KK-Ay Mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef]
- Shen Wu, X.; Xie, T.; Lin, J.; Zhu Fan, H.; Jiao Huang-Fu, H.; Feng Ni, L.; Fang Yan, H. An investigation of the ability of elemene to pass through the blood-brain barrier and its effect on brain carcinomas. J. Pharm. Pharmacol. 2010, 61, 1653–1656. [Google Scholar] [CrossRef]
- Lekshmi, P.C.; Arimboor, R.; Raghu, K.G.; Nirmala Menon, A. Turmerin, the antioxidant protein from turmeric (Curcuma longa) exhibits antihyperglycaemic effects. Nat. Prod. Res. 2012, 26, 1654–1658. [Google Scholar] [CrossRef]
- Panahi, Y.; Fazlolahzadeh, O.; Atkin, S.L.; Majeed, M.; Butler, A.E.; Johnston, T.P.; Sahebkar, A. Evidence of curcumin and curcumin analogue effects in skin diseases: A narrative review. J. Cell. Physiol. 2019, 234, 1165–1178. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Hung, C.-F.; Chiu, H.-C.; Wang, J.-J.; Chan, T.-F. Efficacy and irritancy of enhancers on the in-vitro and in-vivo percutaneous absorption of curcumin. J. Pharm. Pharmacol. 2003, 55, 593–601. [Google Scholar] [CrossRef]
- Waghule, T.; Gorantla, S.; Rapalli, V.K.; Shah, P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Emerging Trends in Topical Delivery of Curcumin Through Lipid Nanocarriers: Effectiveness in Skin Disorders. AAPS PharmSciTech 2020, 21, 284. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Çakan, D.; Aydın, S.; Demir, G.; Başak, K. The effect of curcumin on healing in an animal nasal septal perforation model. Laryngoscope 2019, 129, E349–E354. [Google Scholar] [CrossRef] [PubMed]
- Lüer, S.; Troller, R.; Aebi, C. Antibacterial and Antiinflammatory Kinetics of Curcumin as a Potential Antimucositis Agent in Cancer Patients. Nutr. Cancer 2012, 64, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, M.; Qi, X.; Lin, G.; Cui, F.; Li, F.; Wu, X. Intranasal delivery of nanomicelle curcumin promotes corneal epithelial wound healing in streptozotocin-induced diabetic mice. Sci. Rep. 2016, 6, 29753. [Google Scholar] [CrossRef]
- Gong, C.Y.; Wu, Q.J.; Wang, Y.J.; Zhang, D.D.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Coco, R.; Memvanga, P.B.; Ucakar, B.; Des Rieux, A.; Vandermeulen, G.; Préat, V. Combined effect of PLGA and curcumin on wound healing activity. J. Control. Release 2013, 171, 208–215. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, H.; Cheng, S.; Zhai, G.; Shen, C. Development of curcumin loaded nanostructured lipid carrier based thermosensitive in situ gel for dermal delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 356–362. [Google Scholar] [CrossRef]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.G.; Schilling, A.F. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 2017, 9, e368. [Google Scholar] [CrossRef]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical Curcumin Nanocarriers are Neuroprotective in Eye Disease. Sci. Rep. 2018, 8, 11066. [Google Scholar] [CrossRef]
- Pradhan, N.; Guha, R.; Chowdhury, S.; Nandi, S.; Konar, A.; Hazra, S. Curcumin nanoparticles inhibit corneal neovascularization. J. Mol. Med. 2015, 93, 1095–1106. [Google Scholar] [CrossRef]
- Aboali, F.A.; Habib, D.A.; Elbedaiwy, H.M.; Farid, R.M. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: In-vitro and in-vivo assessment. Int. J. Pharm. 2020, 589, 119835. [Google Scholar] [CrossRef]
- Fernandes, L.D.S.; Amorim, Y.M.; da Silva, E.L.; Silva, S.C.; Santos, A.J.A.; Peixoto, F.N.; Pires, L.M.N.; Sakamoto, R.Y.; Pinto, F.D.C.H.; Scarpa, M.V.C.; et al. Formulation, stability study and preclinical evaluation of a vaginal cream containing curcumin in a rat model of vulvovaginal candidiasis. Mycoses 2018, 61, 723–730. [Google Scholar] [CrossRef]
- Vitali, D.; Bagri, P.; Wessels, J.M.; Arora, M.; Ganugula, R.; Parikh, A.; Mandur, T.; Felker, A.; Garg, S.; Kumar, M.N.V.R.; et al. Curcumin Can Decrease Tissue Inflammation and the Severity of HSV-2 Infection in the Female Reproductive Mucosa. Int. J. Mol. Sci. 2020, 21, 337. [Google Scholar] [CrossRef]
- Azuine, M.A.; Bhide, S.V. Protective single/combined treatment with betel leaf and turmeric against methyl (acetoxymethyl) nitrosamine-induced hamster oral carcinogenesis. Int. J. Cancer 1992, 51, 412–415. [Google Scholar] [CrossRef]
- Periodontal Disease|Oral Health Conditions|Division of Oral Health|CDC. Available online: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html (accessed on 9 December 2021).
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the united states: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Cho, Y.D.; Kim, W.J.; Ryoo, H.M.; Kim, H.G.; Kim, K.H.; Ku, Y.; Seol, Y.J. Current advances of epigenetics in periodontology from ENCODE project: A review and future perspectives. Clin. Epigenet. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a Classification System for Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Gottumukkala, S.N.V.S.; Sudarshan, S.; Mantena, S.R. Comparative evaluation of the efficacy of two controlled release devices: Chlorhexidine chips and indigenous curcumin based collagen as local drug delivery systems. Contemp. Clin. Dent. 2014, 5, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Izui, S.; Sekine, S.; Maeda, K.; Kuboniwa, M.; Takada, A.; Amano, A.; Nagata, H. Antibacterial Activity of Curcumin Against Periodontopathic Bacteria. J. Periodontol. 2016, 87, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Guru, S.R.; Adithya Reddy, K.; Rao, R.J.; Padmanabhan, S.; Guru, R.; Srinivasa, T.S. Comparative evaluation of 2% turmeric extract with nanocarrier and 1% chlorhexidine gel as an adjunct to scaling and root planing in patients with chronic periodontitis: A pilot randomized controlled clinical trial. J. Indian Soc. Periodontol. 2020, 24, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Grover, V.; Malhotra, R.; Gupta, M. Evaluation of curcumin gel as adjunct to scaling & root planing in management of periodontitis– Randomized clinical & biochemical investigation. Infect. Disord.-Drug Targets 2018, 18, 171–178. [Google Scholar] [CrossRef]
- Pérez-Pacheco, C.G.; Fernandes, N.A.R.; Primo, F.L.; Tedesco, A.C.; Bellile, E.; Retamal-Valdes, B.; Feres, M.; Guimarães-Stabili, M.R.; Rossa, C. Local application of curcumin-loaded nanoparticles as an adjunct to scaling and root planing in periodontitis: Randomized, placebo-controlled, double-blind split-mouth clinical trial. Clin. Oral Investig. 2021, 25, 3217–3227. [Google Scholar] [CrossRef]
- Perez-Pacheco, C.G.; Fernandes, N.A.R.; Camilli, A.C.; Ferrarezi, D.P.; Silva, A.F.; Zunareli, M.C.; Amantino, C.F.; Primo, F.L.; Guimarães-Stabilli, M.R.; Junior, C.R. Local administration of curcumin-loaded nanoparticles enhances periodontal repair in vivo. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 311–321. [Google Scholar] [CrossRef]
- Shirmohammadi, A.; Maleki Dizaj, S.; Sharifi, S.; Fattahi, S.; Negahdari, R.; Ghavimi, M.A.; Memar, M.Y. Promising Antimicrobial Action of Sustained Released Curcumin-Loaded Silica Nanoparticles against Clinically Isolated Porphyromonas gingivalis. Diseases 2023, 11, 48. [Google Scholar] [CrossRef]
- Zambrano, L.M.G.; Brandao, D.A.; Rocha, F.R.G.; Marsiglio, R.P.; Longo, I.B.; Primo, F.L.; Tedesco, A.C.; Guimaraes-Stabili, M.R.; Rossa, C. Local administration of curcumin-loaded nanoparticles effectively inhibits inflammation and bone resorption associated with experimental periodontal disease. Sci. Rep. 2018, 8, 6652. [Google Scholar] [CrossRef]
- Nasra, M.M.A.; Khiri, H.M.; Hazzah, H.A.; Abdallah, O.Y. Formulation, in-vitro characterization and clinical evaluation of curcumin in-situ gel for treatment of periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef]
- Mohammad, C.A.; Ali, K.M.; Al-Rawi, R.A.; Gul, S.S. Effects of Curcumin and Tetracycline Gel on Experimental Induced Periodontitis as an Anti-Inflammatory, Osteogenesis Promoter and Enhanced Bone Density through Altered Iron Levels: Histopathological Study. Antibiotics 2022, 11, 521. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Zhang, J.; De Souza Rastelli, A.N.; Yang, J.; Deng, D. Anti-Inflammatory Efficacy of Curcumin as an Adjunct to Non-Surgical Periodontal Treatment: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 808460. [Google Scholar] [CrossRef]
- Wendorff-Tobolla, L.M.; Wolgin, M.; Wagner, G.; Klerings, I.; Dvornyk, A.; Kielbassa, A.M. A Systematic Review and Meta-Analysis on the Efficacy of Locally Delivered Adjunctive Curcumin (Curcuma longa L.) in the Treatment of Periodontitis. Biomedicines 2023, 11, 481. [Google Scholar] [CrossRef]
- Duncan, M.; Grant, G. Oral and intestinal mucositis—Causes and possible treatments. Aliment. Pharmacol. Ther. 2003, 18, 853–874. [Google Scholar] [CrossRef]
- Scully, C.; Sonis, S.; Diz, P. Oral mucositis. Oral Dis. 2006, 12, 229–241. [Google Scholar] [CrossRef]
- Jones, J.A.; Avritscher, E.B.C.; Cooksley, C.D.; Michelet, M.; Bekele, B.N.; Elting, L.S. Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support. Care Cancer 2006, 14, 505–515. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Specht, L. Oral complications in the head and neck radiation patient: Introduction and scope of the problem. Support. Care Cancer 2002, 10, 36–39. [Google Scholar] [CrossRef]
- Ruban David, P.; Timple Shree, K. Effectiveness of Turmeric Mouthwash and Sodium Bicarbonate Mouthwash to Reduce Oral Mucositis among Patient Undergoing Radiation Therapy. Sch. Int. J. Tradit. Complement. Med. 2019, 600077, 2617–3891. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, G.; Wei, Z. Prophylactic and Therapeutic Effects of Curcumin on Treatment-Induced Oral Mucositis in Patients with Head and Neck Cancer: A Meta-Analysis of Randomized Controlled Trials. Nutr. Cancer 2021, 73, 740–749. [Google Scholar] [CrossRef]
- Mansourian, A.; Amanlou, M.; Shirazian, S.; Moosavian Jahromi, Z.; Amirian, A. The effect of “Curcuma longa” topical gel on radiation -induced oral mucositis in patients with head and neck cancer. Int. J. Radiat. Res. 2015, 13, 269–274. [Google Scholar] [CrossRef]
- Rao, S.; Dinkar, C.; Vaishnav, L.K.; Rao, P.; Rai, M.P.; Fayad, R.; Baliga, M.S. The Indian spice turmeric delays and mitigates radiation-induced oral mucositis in patients undergoing treatment for head and neck cancer: An investigational study. Integr. Cancer Ther. 2014, 13, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Arun, P.; Sagayaraj, A.; Azeem Mohiyuddin, S.M.; Santosh, D. Role of turmeric extract in minimising mucositis in patients receiving radiotherapy for head and neck squamous cell cancer: A randomised, placebo-controlled trial. J. Laryngol. Otol. 2020, 134, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Delavarian, Z.; Pakfetrat, A.; Ghazi, A.; Jaafari, M.R.; Homaei Shandiz, F.; Dalirsani, Z.; Mohammadpour, A.H.; Rahimi, H.R. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec. Care Dent. 2019, 39, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Meidan, I.; Sellam, G.; Simaan, S.; Zeevi, I.; Waldman, E.; Weintraub, M.; Revel-Vilk, S. Topical Curcumin for the Prevention of Oral Mucositis in Pediatric Patients: Case Series. Altern. Ther. Health Med. 2013, 19, 21–24. [Google Scholar] [PubMed]
- Patil, K.; Guledgud, M.V.; Kulkarni, P.K.; Deepika, K. Tayal Srishti Use of Curcumin Mouthrinse in Radio-Chemotherapy Induced Oral Mucositis Patients: A Pilot Study. J. Clin. Diagn. Res. 2015, 9, ZC59–ZC62. [Google Scholar]
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Daneshvar, R.; Saghafi, F. Efficacy of curcumin for amelioration of radiotherapy-induced oral mucositis: A preliminary randomized controlled clinical trial. BMC Cancer 2023, 23, 354. [Google Scholar] [CrossRef]
- Fardad, F.; Ghasemi, K.; Ansarinejad, N.; Khodakarim, N.; Nasiripour, S.; Farasatinasab, M. A comparative study to assess the effectiveness of curcumin, mucosamin, and chlorhexidine in chemotherapy-induced oral mucositis. Explore 2023, 19, 65–70. [Google Scholar] [CrossRef]
- Lau, C.B.; Smith, G.P. Recurrent aphthous stomatitis: A comprehensive review and recommendations on therapeutic options. Dermatol. Ther. 2022, 35, e15500. [Google Scholar] [CrossRef]
- Bakhshi, M.; Mahboubi, A.; Jaafari, M.R.; Ebrahimi, F.; Tofangchiha, M.; Alizadeh, A. Comparative efficacy of 1% curcumin nanomicelle gel and 2% curcumin gel for treatment of recurrent aphthous stomatitis: A double-blind randomized clinical trial. J. Evid. Based. Dent. Pract. 2022, 22, 101708. [Google Scholar] [CrossRef]
- Kia, S.J.; Mansourian, A.; Basirat, M.; Akhavan, M.; Mohtasham-Amiri, Z.; Moosavi, M.S. New concentration of curcumin orabase in recurrent aphthous stomatitis: A randomized, controlled clinical trial. J. Herb. Med. 2020, 22, 100336. [Google Scholar] [CrossRef]
- Al-Maweri, S.A. Efficacy of curcumin for management of oral submucous fibrosis: A systematic review of randomized clinical trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 300–308. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wu, V.; Malak, H.; Ahamed, A.P.; Lo, A.; Abraham, Y.; Miller, C. Effect of Turmeric Concentrations on the Rate of Growth of Oral Bacteria—An In-Vitro Study. Dent. J. 2021, 9, 26. [Google Scholar] [CrossRef]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.A.; Hazzah, W.A.; El-Massik, M.A.; Abdallah, O.Y. Gelucire-Based Nanoparticles for Curcumin Targeting to Oral Mucosa: Preparation, Characterization, and Antimicrobial Activity Assessment. J. Pharm. Sci. 2015, 104, 3913–3924. [Google Scholar] [CrossRef]
- Esposito, D.; Conte, C.; d’Angelo, I.; Miro, A.; Ungaro, F.; Quaglia, F. Mucoadhesive zein/beta-cyclodextrin nanoparticles for the buccal delivery of curcumin. Int. J. Pharm. 2020, 586, 119587. [Google Scholar] [CrossRef]
- de Cássia Dias Viana Andrade, R.; Pereira Rosa, L.; Pinto de Oliveira Santos, G.; Cristina da Silva, F. Comparative Study about the Ecacy of Low Level Laser Therapy and Curcumin Antimicrobial Photodynamic Therapy as a Coadjuvant Treatment of Oral Mucositis in Oncologic Patients:Antimicrobial, Analgesic and Degree Alteration Effect. Support. Care Cancer, 2021; preprints. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Bonifácio, B.V.; Baub, T.M.; Gremião, M.P.D.; Chorilli, M. In-situ gelling liquid crystal mucoadhesive vehicle for curcumin buccal administration and its potential application in the treatment of oral candidiasis. J. Biomed. Nanotechnol. 2019, 16, 1334–1344. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Sakamoto, T.; Zhengguang, L.; Yasui, H.; Hamada, H.; Kubo, H.; Nakajima, M. Curcumin inhibits epithelial-mesenchymal transition in oral cancer cells via c-Met blockade. Oncol. Lett. 2020, 19, 4177–4182. [Google Scholar] [CrossRef]
- Maulina, T.; Hadikrishna, I.; Hardianto, A.; Sjamsudin, E.; Pontjo, B.; Yusuf, H.Y. The therapeutic activity of curcumin through its anti-cancer potential on oral squamous cell carcinoma: A study on Sprague Dawley rat. SAGE Open Med. 2019, 7, 2050312119875982. [Google Scholar] [CrossRef]
- Lai, K.C.; Chueh, F.S.; Hsiao, Y.T.; Cheng, Z.Y.; Lien, J.C.; Liu, K.C.; Peng, S.F.; Chung, J.G. Gefitinib and curcumin-loaded nanoparticles enhance cell apoptosis in human oral cancer SAS cells in vitro and inhibit SAS cell xenografted tumor in vivo. Toxicol. Appl. Pharmacol. 2019, 382, 114734. [Google Scholar] [CrossRef]
- Stenberg, W.V. Periodontal Problems in Children and Adolescents. In Pediatric Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 371–378.e1. [Google Scholar]
- Gorrel, C.; Andersson, S.; Verhaert, L. Periodontal disease. In Veterinary Dentistry for the General Practitioner; Saunders, W.B., Ed.; 2013; pp. 97–119. ISBN 978-0-7020-4943-9. [Google Scholar]
- Malekzadeh, M.; Kia, S.J.; Mashaei, L.; Moosavi, M.S. Oral nano-curcumin on gingival inflammation in patients with gingivitis and mild periodontitis. Clin. Exp. Dent. Res. 2021, 7, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Pulikkotil, S.J.; Nath, S. Effects of curcumin on crevicular levels of IL-1β and CCL28 in experimental gingivitis. Aust. Dent. J. 2015, 60, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pathak, A.; Pal, M.; Sareen, S.; Goel, K. Comparative evaluation of topical application of turmeric gel and 0.2% chlorhexidine gluconate gel in prevention of gingivitis. Natl. J. Maxillofac. Surg. 2015, 6, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.M.; Behal, R.; Gilda, S.S. Comparative evaluation of 0.1% turmeric mouthwash with 0.2% chlorhexidine gluconate in prevention of plaque and gingivitis: A clinical and microbiological study. J. Indian Soc. Periodontol. 2012, 16, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Huang, P.; Chen, M.W. Curcumin attenuates cyclooxygenase-2 expression via inhibition of the NF-κB pathway in lipopolysaccharide-stimulated human gingival fibroblasts. Cell Biol. Int. 2013, 37, 443–448. [Google Scholar] [CrossRef]
- Grant, M.M.; Scott, A.E.; Matthews, J.B.; Griffiths, H.R.; Chapple, I.L.C. Pre-conditioning of gingival epithelial cells with sub-apoptotic concentrations of curcumin prevents pro-inflammatory cytokine release. J. Periodontal Res. 2023, 58, 634–645. [Google Scholar] [CrossRef]
- Roopashree, M.R.; Gondhalekar, R.V.; Shashikanth, M.C.; George, J.; Thippeswamy, S.H.; Shukla, A. Pathogenesis of oral lichen planus—A review. J. Oral Pathol. Med. 2010, 39, 729–734. [Google Scholar] [CrossRef]
- Kia, S.J.; Shirazian, S.; Mansourian, A.; Khodadadi Fard, L.; Ashnagar, S. Comparative Efficacy of Topical Curcumin and Triamcinolone for Oral Lichen Planus: A Randomized, Controlled Clinical Trial. J. Dent. 2015, 12, 789–796. [Google Scholar]
- Nosratzehi, T.; Arbabi-Kalati, F.; Hamishehkar, H.; Bagheri, S. Comparison of the Effects of Curcumin Mucoadhesive Paste and Local Corticosteroid on the Treatment of Erosive Oral Lichen Planus Lesions. J. Natl. Med. Assoc. 2018, 110, 92–97. [Google Scholar] [CrossRef]
- Amirchaghmaghi, M.; Pakfetrat, A.; Delavarian, Z.; Ghalavani, H.; Ghazi, A. Evaluation of the efficacy of curcumin in the treatment of oral lichen planus: A randomized controlled trial. J. Clin. Diagnostic Res. 2016, 10, ZC134–ZC137. [Google Scholar] [CrossRef]
- White, C.M.; Chamberlin, K.; Eisenberg, E. Curcumin, a turmeric extract, for oral lichen planus: A systematic review. Oral Dis. 2019, 25, 720–725. [Google Scholar] [CrossRef]
- Shih, Y.H.; Wang, T.H.; Shieh, T.M.; Tseng, Y.H. Oral submucous fibrosis: A review on etiopathogenesis, diagnosis, and therapy. Int. J. Mol. Sci. 2019, 20, 2940. [Google Scholar] [CrossRef]
- Hazarey, V.K.; Sakrikar, A.R.; Ganvir, S.M. Efficacy of curcumin in the treatment for oral submucous fibrosis—A randomized clinical trial. J. Oral Maxillofac. Pathol. 2015, 19, 145–152. [Google Scholar] [CrossRef]
- Yadav, M.; Aravinda, K.; Saxena, V.S.; Srinivas, K.; Ratnakar, P.; Gupta, J.; Sachdev, A.S.; Shivhare, P. Comparison of curcumin with intralesional steroid injections in Oral Submucous Fibrosis—A randomized, open-label interventional study. J. Oral Biol. Craniofacial Res. 2014, 4, 169–173. [Google Scholar] [CrossRef]
- Massano, J.; Regateiro, F.S.; Januário, G.; Ferreira, A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 102, 67–76. [Google Scholar] [CrossRef]
- Markopoulos, A.K. Current Aspects on Oral Squamous Cell Carcinoma. Open Dent. J. 2012, 6, 126–130. [Google Scholar] [CrossRef]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phyther. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef]
- Machado, F.C.; Adum de Matos, R.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Bioorg. Med. Chem. 2019, 27, 1882–1890. [Google Scholar] [CrossRef]
- Şueki, F.; Ruhi, M.K.; Gülsoy, M. The effect of curcumin in antitumor photodynamic therapy: In vitro experiments with Caco-2 and PC-3 cancer lines. Photodiagn. Photodyn. Ther. 2019, 27, 95–99. [Google Scholar] [CrossRef]
- Rocha, M.P.; Ruela, A.L.M.; Rosa, L.P.; Santos, G.P.O.; Rosa, F.C.S. Antimicrobial photodynamic therapy in dentistry using an oil-in-water microemulsion with curcumin as a mouthwash. Photodiagn. Photodyn. Ther. 2020, 32, 101962. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, H.; Sun, H.; Li, J.; Bai, Y. Antifungal effect of photodynamic therapy mediated by curcumin on Candida albicans biofilms in vitro. Photodiagn. Photodyn. Ther. 2019, 27, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.; Nikfarjam, F.; Butting, M.; Meissner, M.; König, A.; Bosca, A.R.; Kaufmann, R.; Heidemann, D.; Bernd, A.; Kippenberger, S.; et al. Photodynamic treatment of oral squamous cell carcinoma cells with low curcumin concentrations. J. Cancer 2017, 8, 1271–1283. [Google Scholar] [CrossRef]
- Lee, A.Y.L.; Fan, C.C.; Chen, Y.A.; Cheng, C.W.; Sung, Y.J.; Hsu, C.P.; Kao, T.Y. Curcumin Inhibits Invasiveness and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Through Reducing Matrix Metalloproteinase 2, 9 and Modulating p53-E-Cadherin Pathway. Integr. Cancer Ther. 2015, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Liu, L.; Luo, E.; Hu, J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch. Oral Biol. 2018, 92, 32–37. [Google Scholar] [CrossRef]
- Sivanantham, B.; Sethuraman, S.; Krishnan, U.M. Combinatorial Effects of Curcumin with an Anti-Neoplastic Agent on Head and Neck Squamous Cell Carcinoma Through the Regulation of EGFR-ERK1/2 and Apoptotic Signaling Pathways. ACS Comb. Sci. 2016, 18, 22–35. [Google Scholar] [CrossRef]

| Name, Dose (Manufacturer) | Active Ingredient (Dose) | Dosage Form | Application |
|---|---|---|---|
| Curenext® (Abbott India Ltd., Mumbai, India) | Curcuma longa extract (10 mg/g) | oral gel | gingivitis prevention |
| SNEC-G (Arbro Pharmaceuticals Pvt. Ltd., New Delhi, India) | curcumin (5 mg/g) | self-nanoemulsifying gel | mouth ulcers, gingivitis, periodontitis, bad breath |
| Colgate® Naturals (Colgate-Palmolive, Taguig, Philippines) | turmeric extract 1 | toothpaste | gum health improvement, plaque and bacteria removal |
| Curcuzenica (Herbzenica Lifesciences Pvt. Ltd., Bhubaneswar, India) | soluble Curcuma longa extract (1% w/w) | oral gel | aphthous ulcers, gingivitis, glossitis, oral mucositis, oral submucous fibrosis, mucositis and ulceration related to cancer treatment |
| Condition | Dosage Form | The Most Important Findings | Reference |
|---|---|---|---|
| Periodontal disease | collagen sponges |
| [117] |
| Periodontal disease | nanoparticles |
| [120] |
| Periodontal disease | gel |
| [121] |
| Periodontal disease | PLGA and PLA nanoparticles |
| [122] |
| Periodontal disease (periodontal repair) | nanoparticles |
| [123] |
| Periodontal disease | silica-based nanoparticles |
| [124] |
| Periodontal disease | PLGA nanoparticles (injection) |
| [125] |
| Periodontal disease | in situ-forming gel |
| [126] |
| Periodontal disease | topical gel |
| [127] |
| Oral mucositis related to chemotherapy | mouthwash |
| [141] |
| Oral mucositis related to chemo- and radiotherapy | oral rinse |
| [142] |
| Oral mucositis related to cancer treatment | mouthwash, oral curcuminoids |
| [143] |
| Oral mucositis related to chemotherapy | gel |
| [144] |
| Recurrent aphthous stomatitis | 1% nanomicelle gel, 2% plain gel |
| [146] |
| Recurrent aphthous stomatitis | curcumin-loaded Orabase® paste |
| [147] |
| In vitro antibacterial study | mouthwash |
| [149] |
| In vitro antimicrobial study | solid lipid nanoparticles |
| [150] |
| Oral mucositis related to cancer treatment | oral spray |
| [152] |
| Oral candidiasis | in situ gelling liquid crystal system |
| [153] |
| Squamous cell carcinoma | gefitinib and curcumin-loaded nanoparticles (peritoneal injection) |
| [155] |
| Gingivitis | gel with standardized turmeric rhizome extract |
| [160] |
| Gingivitis | gel |
| [161] |
| Gingivitis | mouthwash |
| [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtyłko, M.; Kunstman, P.; Bartylak, H.; Raszewski, Ł.; Osmałek, T.; Froelich, A. A Well-Known Plant and New Therapeutic Strategies: Turmeric and Its Components in Oral Inflammatory Diseases Treatment. Appl. Sci. 2023, 13, 7809. https://doi.org/10.3390/app13137809
Wojtyłko M, Kunstman P, Bartylak H, Raszewski Ł, Osmałek T, Froelich A. A Well-Known Plant and New Therapeutic Strategies: Turmeric and Its Components in Oral Inflammatory Diseases Treatment. Applied Sciences. 2023; 13(13):7809. https://doi.org/10.3390/app13137809
Chicago/Turabian StyleWojtyłko, Monika, Paweł Kunstman, Hanna Bartylak, Łukasz Raszewski, Tomasz Osmałek, and Anna Froelich. 2023. "A Well-Known Plant and New Therapeutic Strategies: Turmeric and Its Components in Oral Inflammatory Diseases Treatment" Applied Sciences 13, no. 13: 7809. https://doi.org/10.3390/app13137809
APA StyleWojtyłko, M., Kunstman, P., Bartylak, H., Raszewski, Ł., Osmałek, T., & Froelich, A. (2023). A Well-Known Plant and New Therapeutic Strategies: Turmeric and Its Components in Oral Inflammatory Diseases Treatment. Applied Sciences, 13(13), 7809. https://doi.org/10.3390/app13137809







