Monitoring of Surgically Treated Upper Arm Fracture by Implanted Antenna at 402 MHz
Abstract
:1. Introduction
2. The Monitoring Application
3. The Method
3.1. The Upper Arm Model
3.2. Fracture Monitoring
3.3. Biocompatibility
3.4. Operating Frequency and Biological Effect
4. Simulation and Discussion
5. Measurement and Validation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malasinghe, L.P.; Ramzan, N.; Dahal, K. Remote patient monitoring: Acomprehensive study. J. Ambient. Intell. Humaniz. Comput. 2019, 10, 57–76. [Google Scholar] [CrossRef] [Green Version]
- Kasban, H.; El-Bendary, M.A.M.; Salama, D.H. A comparative study of Medical imaging techniques. Int. J. Inf. Sci. Intell. Syst. 2015, 4, 37–58. [Google Scholar]
- Li, Y.; Duan, W.; Yang, L.; Zhao, X. Study on the Electromagnetic field distribution of an Implantable Antenna for Intelligence Health Monitoring System. IEEE Access 2019, 7, 28749–28756. [Google Scholar] [CrossRef]
- Damaj, A.W.; El Misilmani, H.M.; Abou Chahine, S. Implantable Antenna for Biomedical Applications: An Overview on Alternative Antenna Design Methods and Challenges. In Proceedings of the 2018 International Conference on High Performance Computing and Simulation, Orleans, France, 16–20 July 2018; pp. 31–37. [Google Scholar]
- Skrivervik, A.K. Implantable Antennas: The challenge of efficiency. In Proceedings of the 2013 7th European Conference on Antenna and Propagation EUCAP, Gothenburg, Sweden, 8–12 April 2013; pp. 3627–3631. [Google Scholar]
- Skrivervik, A.K.; Merli, F. Design Strategies for Implantable Antennas. In Proceedings of the 2011 Loughborough Antenna and Propagation Conference, Loughborough, UK, 4–15 November 2011; pp. 1–5. [Google Scholar]
- Liu, C.; Guo, Y.X.; Xiao, S. A Review of Implantable Antennas for Wireless Biomedical Devices. 2016. Available online: https://www.semanticscholar.org/paper/A-Review-of-Implantable-Antennas-for-Wireless-Liu-Guo/bc57aa622cf5902e61bb1893fff563554f71ccc6 (accessed on 1 March 2023).
- Shah, S.A.A.; Yoo, H. Scalp-Implantable Antenna systems for Intracranial pressure Monitoring. IEEE Trans. Antenna Propag. 2016, 66, 2170–2173. [Google Scholar] [CrossRef]
- Lucisano, J.Y.; Routh, T.L.; Lin, J.T.; Gough, D.A. Glucose Monitoring in Individuals with Diabetes Using a Long-Term Implanted Sensor/Telemetry System and Model. IEEE Trans. Biomed. Eng. 2017, 64, 1982–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.; Niloy, T.R.; Hasan, R.R.; Rahman, M.A. In-body antenna for Monitoring and Controlling Pacemaker. Adv. Sci. Technol. Eng. Syst. J. 2020, 5, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Fallahpour, M.; Zoughi, R. Antenna miniaturization techniques. A review of topology and material based methods. IEEE Antennas Propag. Mag. 2018, 60, 38–50. [Google Scholar] [CrossRef]
- Krzysztofik, W.J.; Cao, T.N. Metamaterials in Application to improve antenna parameters. Metamater. Metasurfaces 2018, 12, 63–85. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Ali, T.; Pai, M.M. Electromagnetic Metamaterials: A new paradigm of Antenna design. IEEE Access 2021, 9, 18722–18751. [Google Scholar] [CrossRef]
- Crean, T.E. Nallamothu SV Distal Humerus Fractures. Bookshelf ID: NBK531474. Medline. 2019. Available online: https://pubmed.ncbi.nlm.nih.gov/30285369/ (accessed on 1 March 2021).
- Launonen, A.P.; Sumrein, B.O.; Reito, A.; Lepola, V.; Paloneva, J.; Jonsson, K.B.; Wolf, O.; Strom, P.; Berg, H.E.; Felländer-Tsai, L.; et al. Operative versus non-operative treatment of proximal humeral fractures: A multicentered normalized controlled trial. PLoS Med. 2019. [Google Scholar] [CrossRef] [Green Version]
- Zeinelabedeen, W.; Uyguroglu, R. Characterization of human body channel for oblique incident case of plane wave in the 0.1–10 GHz frequency band for special health care monitoring application. In Proceedings of the SIU 2020, the 28th IEEE Conference on Signal Processing and Communications Applications, Gaziantep, Turkey, 5–7 October 2020; pp. 1–4. [Google Scholar]
- Bocan, K.N.; Mickle, M.H.; Sejdić, E. Tissue Variability and Antennas for Power Transfer to wireless implantable Medical Devices. IEEE J. Transl. Eng. Health Med. 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Jung, Y.H.; Qiu, Y.; Lee, S.; Shih, T.Y.; Xu, Y.; Xu, R.; Lee, J.; Schendel, A.A.; Lin, W.; Williams, J.C.; et al. A Compact Parylene-Coated Wireless Flexible Antenna For Implantable Electronics. IEEE Antennas Wirel. Propag. Lett. 2016, 5, 1382–1385. [Google Scholar] [CrossRef]
- Patil, K.S.; Rufus, E. A review on antennas for biomedical implants used for IoT based health care. Sens. Rev. 2020, 40, 273–280. [Google Scholar] [CrossRef]
- Merli, F.; Bolomey, L.; Zurcher, J.F.; Corradini, G.; Meurville, E.; Skrivervik, A.K. Design, realization, and measurements of a miniature antenna for implantable wireless communication systems. IEEE Trans. Antennas Propag. 2011, 59, 3544–3555. [Google Scholar]
- Mahe, Y.; Chousseaud, A.; Brunet, M.; Froppier, B. New flexible medical compact antenna: Design and analysis. Int. J. Antenna Propag. 2012, 2012, 837230. [Google Scholar] [CrossRef] [Green Version]
- Psathas, K.A.; Kiourti, A.; Nikita, K.S. A novel conformal antenna for ingestible capsule endoscopy in the Med-Radio band. In Progress in Electromagnetic Research Symposium Proceeding; PIERS: Stockholm, Sweden, 2013; pp. 1899–1902. [Google Scholar]
- Xu, L.J.; Guo, Y.X.; Wu, W. Bandwidth enhancement of an implantable antenna. IEEE Antenna Wirel. Propag. Lett. 2014, 14, 1510–1513. [Google Scholar] [CrossRef]
- Alrawashdeh, R.S.; Huang, Y.; Kod, M.; Sajak, A.A.B. A broadband flexible loop antenna with complementary split ring resonator. IEEE Antennas Wirel. Propag. Lett. 2015, 14, 1506–1509. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Gao, Y.M.; Du, M. Propagation characteristics of electromagnetic wave on multiple tissue interfaces in wireless deep implant communication. IET Microw. Antenna Propag. 2018, 12, 2034–2040. [Google Scholar] [CrossRef]
- Zeinelabedeen, W.; Uyguroglu, R. A study on health care monitoring of femoral shaft fracture healing by using implanted antenna for wireless in-toout body channel communication. J. Electromagn. Waves Appl. 2022, 36, 722–742. [Google Scholar] [CrossRef]
- Islam, M.N.; Yuce, M.R. Review of Medical Implant Communication System (MICS) Band and Network. ICT Express 2016, 2, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Pflug, H.W.; Visser, H.J.; Kiyani, N.F.; Dolmans, G.; Philips, K.; Kanda, K.; Hamaminato, M.; Masui, S. Radio Channel Characterization for 400 MHz Implanted Devices. In Proceedings of the 2014 IEEE Wireless Communication and Networking Conference WCNC Track 1 (PHY and Fundamentals), Istanbul, Turkey, 6–9 April 2014; pp. 293–298. [Google Scholar]
- Hasgall, P.A.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.C.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Version 4.0. 15 May 2018. Available online: https://itis.swiss/database (accessed on 14 March 2021). [CrossRef]
- Omar, A.A.; Bashayreh, Q.M.; Al-Shamali, A.M. Investigation of the effect of obliquely incident plane wave on a human head at 900 and 1800 MHZ. Int. J. RF Microw. Comput. Aided Eng. 2010, 20, 133–140. [Google Scholar] [CrossRef]
- C95.1-2019; IEEE Standard for Safety Levels with Respect to Human Exposure to Electric, Magnetic, and Electromagnetic Fields, 0 Hz to 300 GHz. The Institute of Electrical and Electronics Engineers: New York, NY, USA, 2019.
- Khan, A.Q.; Riaz, M.; Bilal, A. Various Types of Antennas with Respect to their Applications: A Review. Int. J. Multidiscip. Sci. Eng. 2016, 7, 1–8. [Google Scholar]
- Nunes, K. Repairing Major Bone Breaks with Open Reduction Internal Fixation Surgery, Medically Reviewed by William Morrison, M.D. 16 January 2019. Available online: https://www.healthline.com/health/orif-surgery (accessed on 10 October 2021).
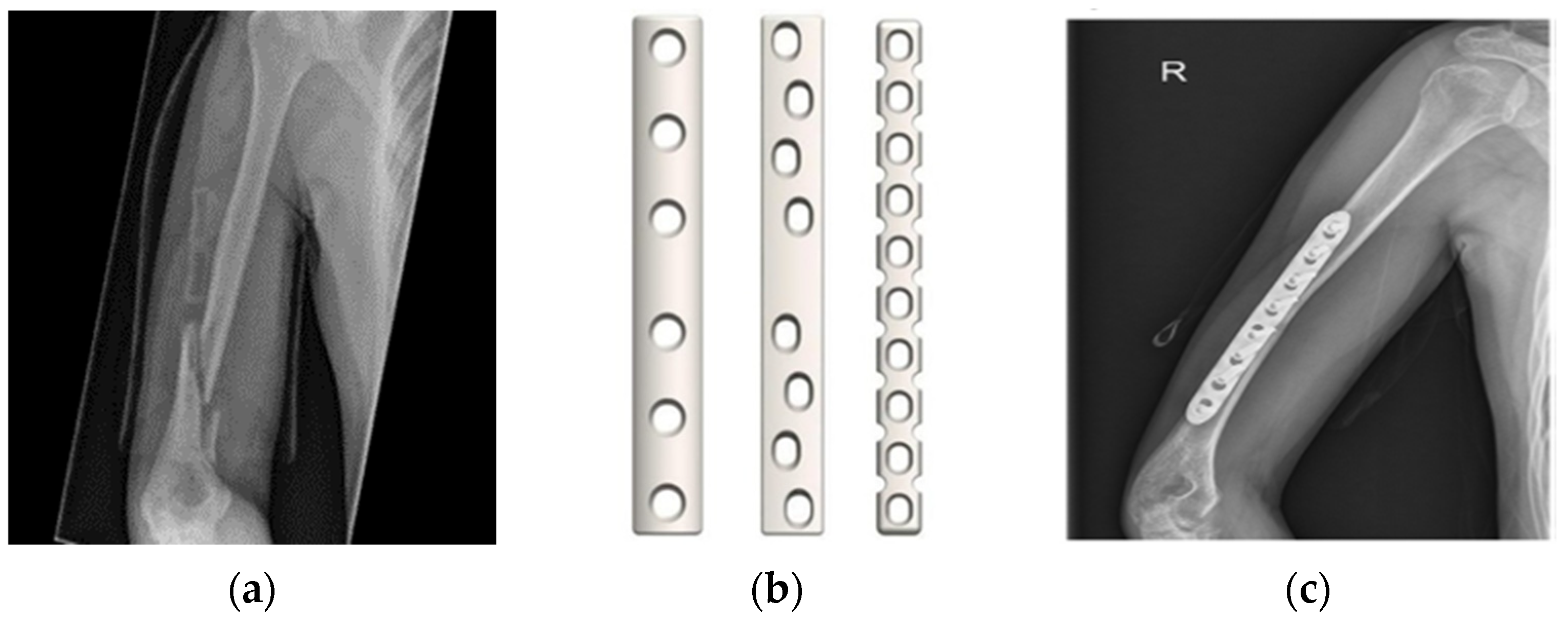
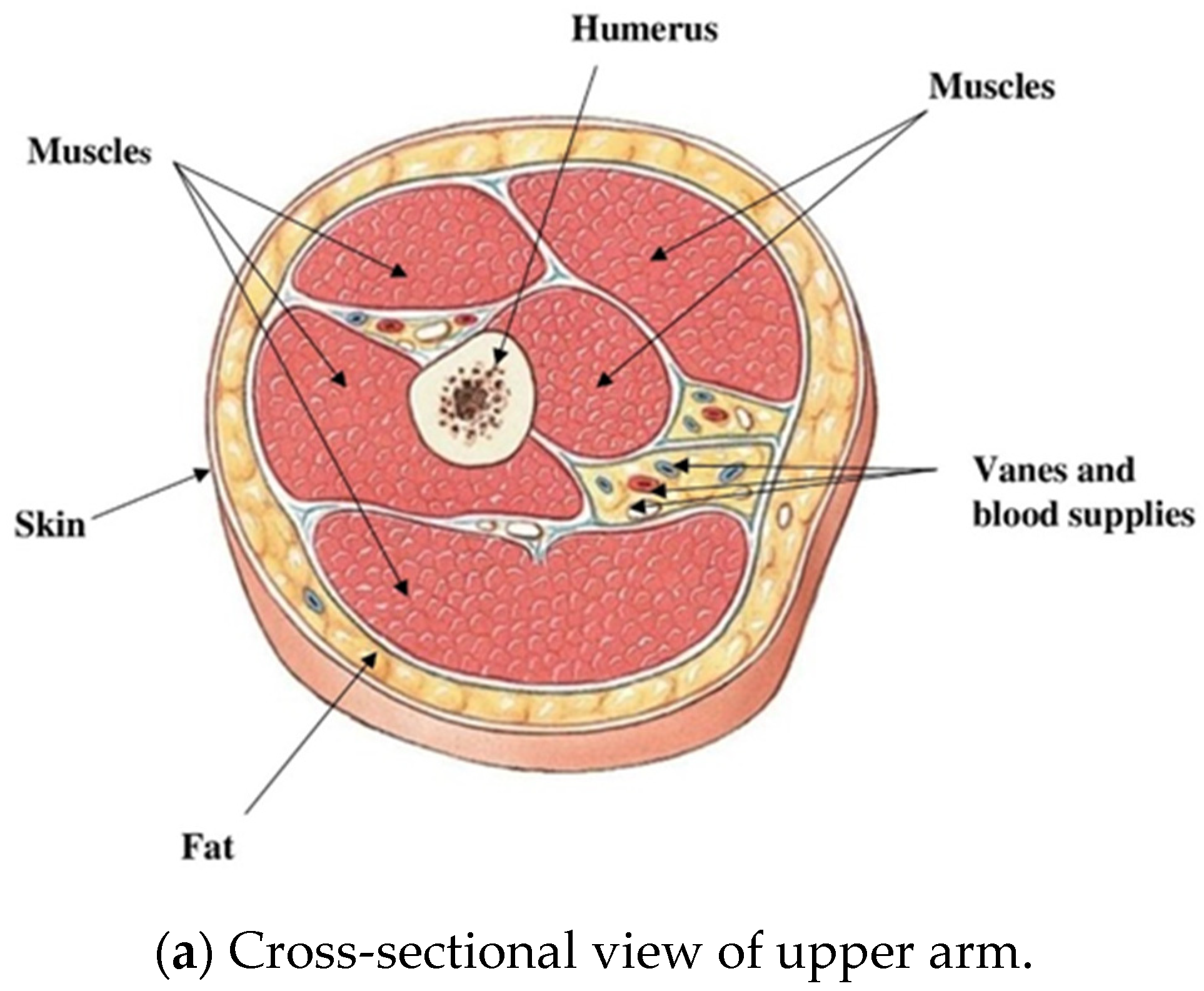

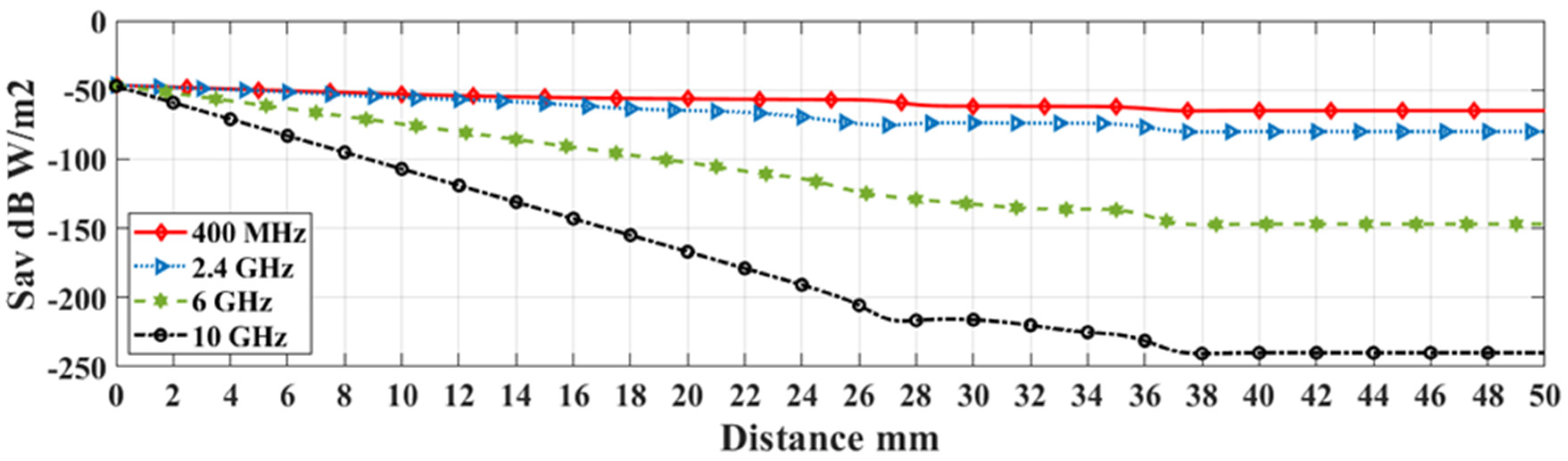














| Tissue Thickness | Dimension in mm |
|---|---|
| Bone | 12.5 |
| Muscle | 27.5 |
| Fat | 8.5 |
| Skin | 1.5 |
| Length of model | 350 |
| Fracture thickness | 2 |
| Split thickness | 3 |
| Fixation plate length | 100 |
| Fixation plate width | 8 |
| Fixation plate thickness | 3 |
| Reference | Antenna Type | Operating Frequency | Size in mm3 | Substrate | Gain |
|---|---|---|---|---|---|
| [18] | Microstrip | 2.4 GHz | 482 | Polymide | −18.8 dB |
| [20] | Multilayer helical | 401–406 MHz | 301 | Rogers TMM10 | −28.8 dB |
| [21] | Microstrip and meandered | 434 MHz | 119 | FR4 | −33 dB |
| [22] | Microstrip | 402 MHz | 240 | Rogers RT/Duroid 5882 | −29.64 dB |
| [23] | Asymmetric dipole fed | 402 MHz | 264 | Rogers 3010 | −37 dB |
| [24] | Asymmetric dipole | 401–406 MHz | 75 | Polymide | −25 dB |
| Tissue | Relative Permittivity εr | Conductivity σ s/m |
|---|---|---|
| Bone | 13.1 | 0.0917 |
| Muscle | 5.71 | 0.797 |
| Fat | 11.6 | 0.0808 |
| Skin | 46.1 | 0.689 |
| Blood | 64.2 | 1.35 |
| Tissue | Bone | Muscle | Fat | Skin |
|---|---|---|---|---|
| (kg/m3) | 1840 | 1060 | 920 | 1010 |
| Antenna Parameter | Dimension in mm | Antenna Parameter | Dimension in mm |
|---|---|---|---|
| Radiator length | 111 | Substrate length | 116 |
| Radiator width | 1 | Substrate width | 5 |
| Radiator thickness | 0.035 | Substrate thickness | 0.5 |
| Gap | 1 | Coating thickness | 0.1 |
| Trace width | 1 |
| Humerus Status/Monitoring Parameter | Normal | Transverse Fractured | 30° Oblique Fractured | 60° Oblique Fractured | |
|---|---|---|---|---|---|
| Return loss dB | −38.15 | −15.8 | −16.5 | −15.5 | |
| Φ = 90° | Transmitted power density µW/m2 | 1602 | 1417.6 | 1417 | 1354.4 |
| % Reduction | 11.57% | 11.54% | 15.45% | ||
| Φ = −90° | Transmitted power density µW/m2 | 1166 | 1027.5 | 1026.7 | 982.4 |
| % Reduction | 11.87% | 11.95% | 15.75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeinelabedeen, W.; Uyguroglu, R. Monitoring of Surgically Treated Upper Arm Fracture by Implanted Antenna at 402 MHz. Appl. Sci. 2023, 13, 7786. https://doi.org/10.3390/app13137786
Zeinelabedeen W, Uyguroglu R. Monitoring of Surgically Treated Upper Arm Fracture by Implanted Antenna at 402 MHz. Applied Sciences. 2023; 13(13):7786. https://doi.org/10.3390/app13137786
Chicago/Turabian StyleZeinelabedeen, Wael, and Rasime Uyguroglu. 2023. "Monitoring of Surgically Treated Upper Arm Fracture by Implanted Antenna at 402 MHz" Applied Sciences 13, no. 13: 7786. https://doi.org/10.3390/app13137786
APA StyleZeinelabedeen, W., & Uyguroglu, R. (2023). Monitoring of Surgically Treated Upper Arm Fracture by Implanted Antenna at 402 MHz. Applied Sciences, 13(13), 7786. https://doi.org/10.3390/app13137786







