Innovative Implantable Left Ventricular Assist Device—Performance under Various Resistances and Operating Frequency Conditions
Abstract
1. Introduction
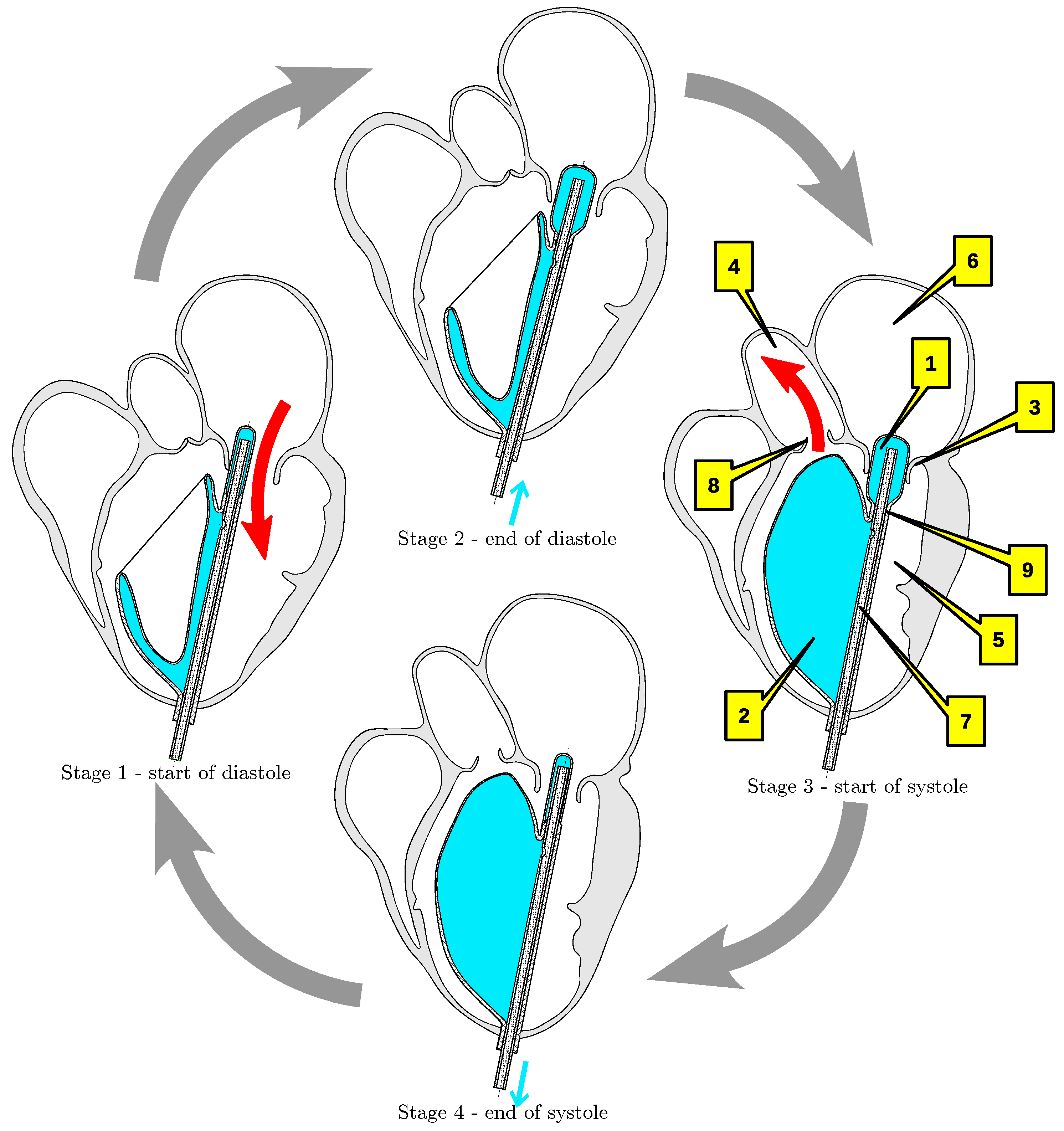
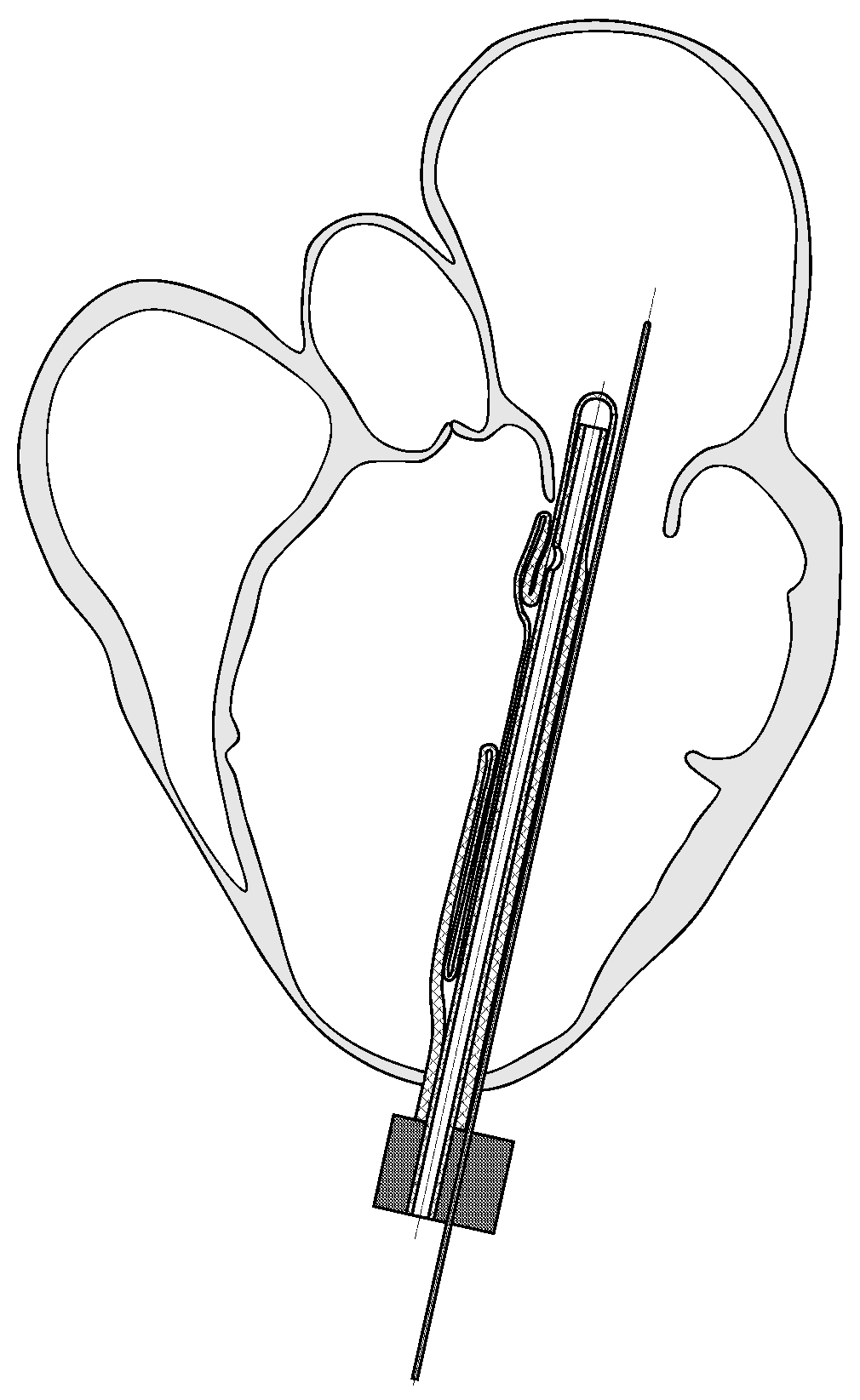
2. Implantable Left Ventricular Assist Device
3. Methods
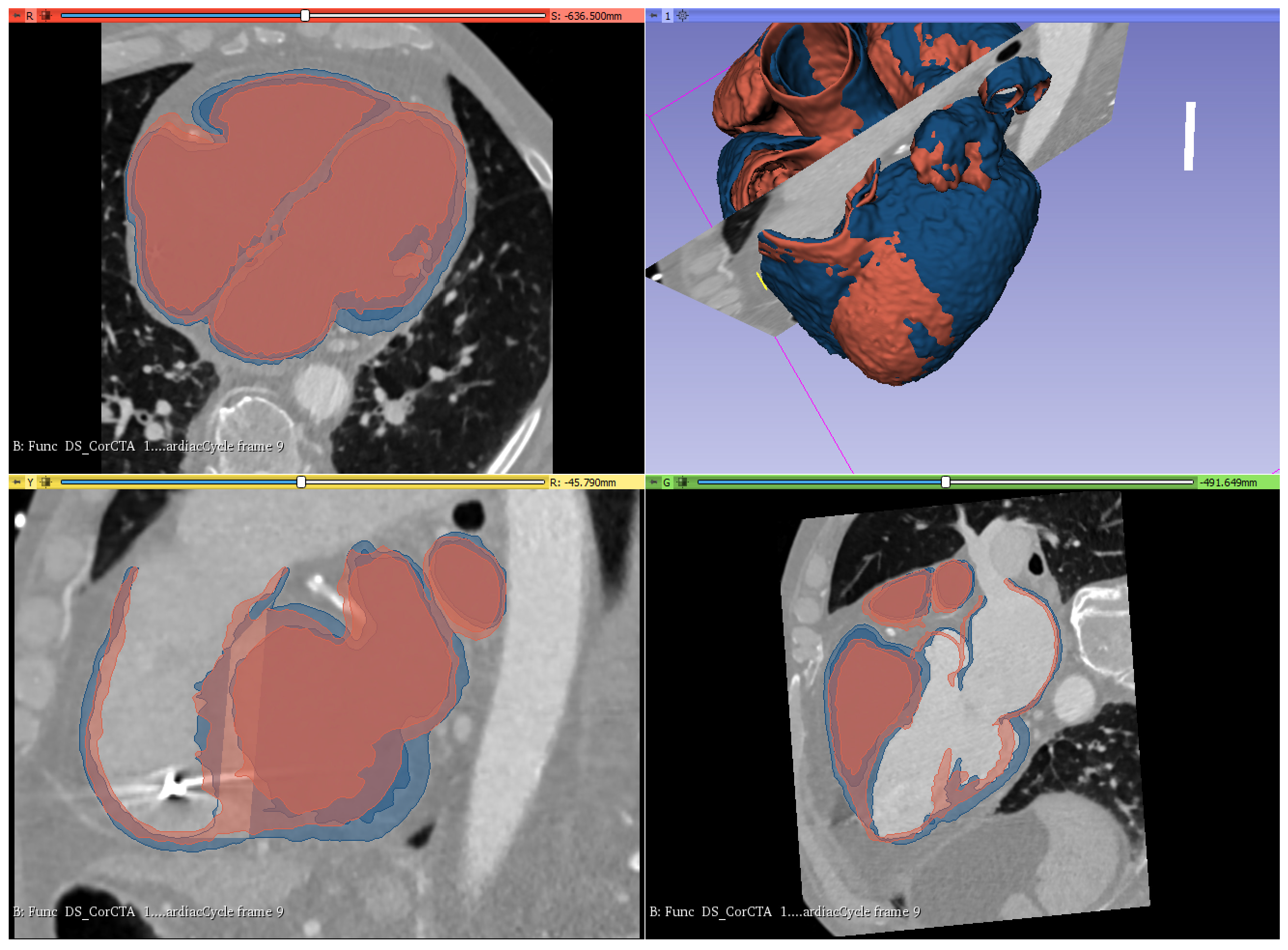
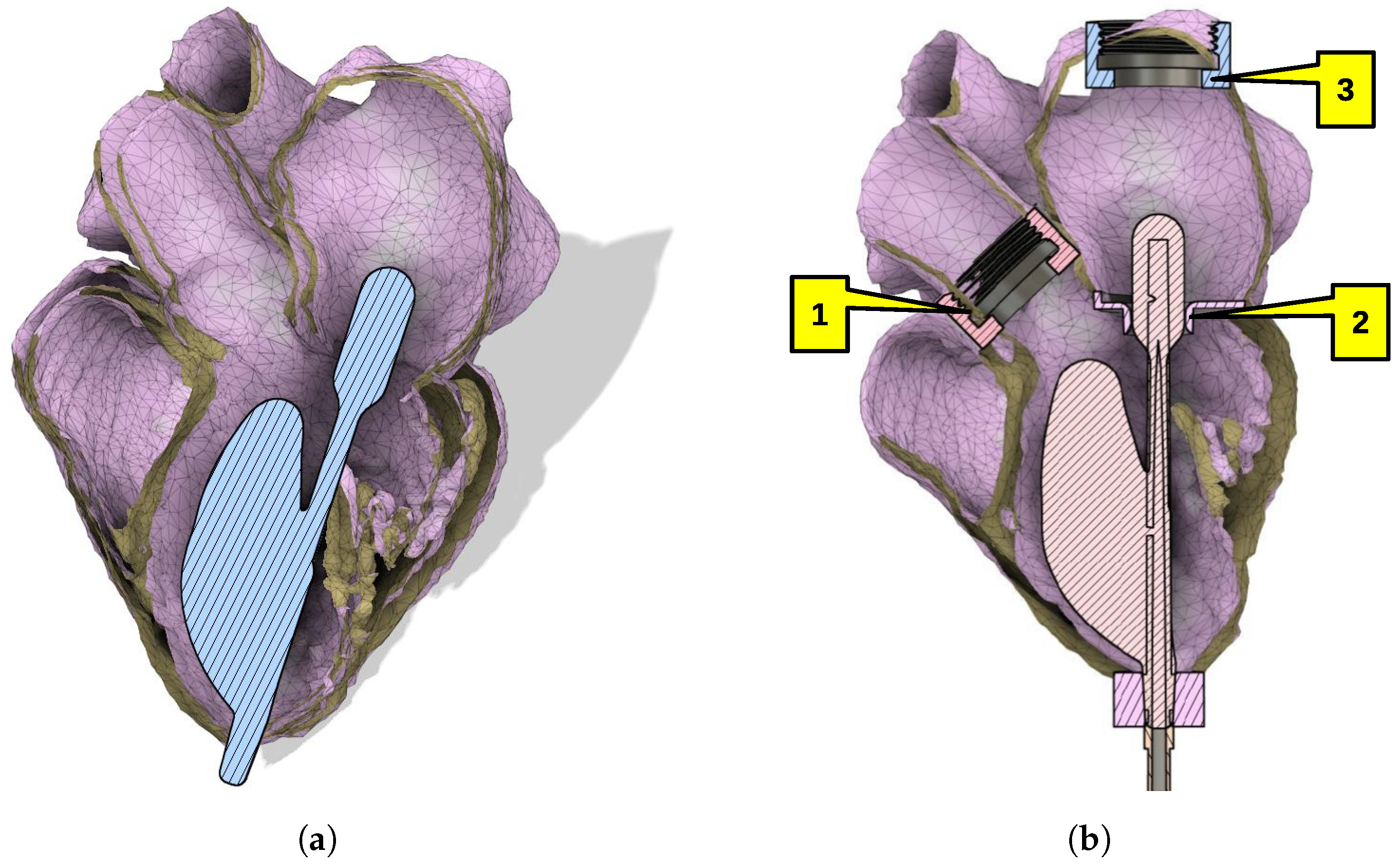
3.1. Geometry Reconstruction
- LVEF 40%—a heart muscle of very small size. It was verified that the developed ventricular balloon would cooperate in the left ventricle of this heart;
- LVEF 30%—small sized myocardium, which was the basis for the development of the ventricular balloon shape;
- LVEF 17%—heart muscle of very large size. It was verified that the developed balloon would cooperate in the left ventricle of this heart.
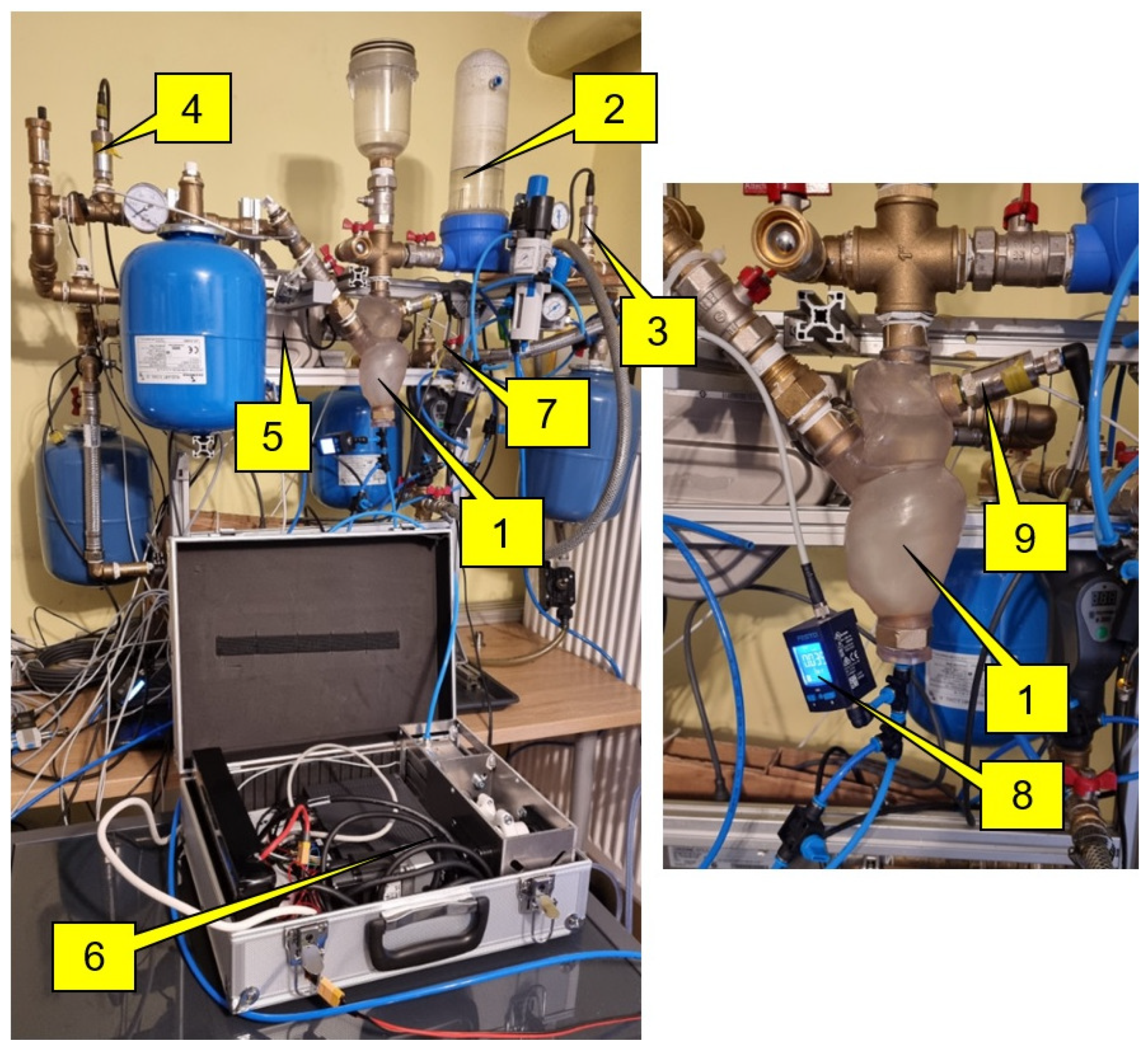
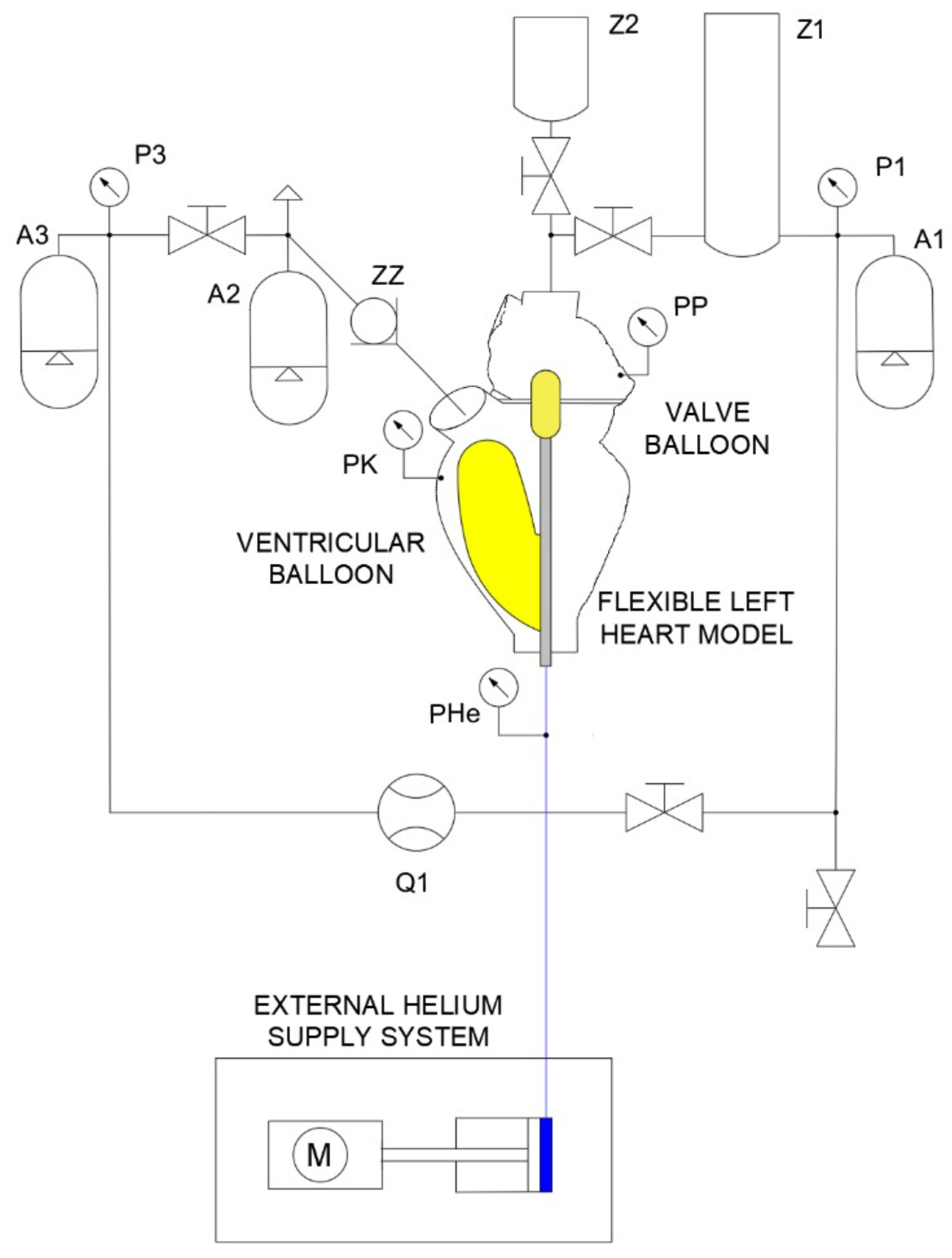
3.2. Experimental Stand
- Construction of a hydraulic system consisting of a flexible left heart, flow reservoir replacing the pulmonary veins, compensation vessels simulating the flexibility of the human circulatory system, flexible and rigid hydraulic lines, liquid and gas pressure sensors, liquid temperature sensors, liquid flow meter.
- Placement and positioning of a balloon set in the left ventricle.
- Filling the hydraulic system with liquid (artificial blood).
- Before testing the system with the heart model, the balloon set was inflated with helium to an appropriate value (75 mm Hg). Furthermore, the piston rod of the compressor cylinder was retracted to its maximum.
- Starting the external helium supply system from an ODROID computer.
- Performing experimental tests under different operating frequencies and for different inflation and deflation times of the ventricular and valve balloons. The pressure in the hydraulic system was varied by increasing the flow resistance by means of a hydraulic valve setting, allowing for the simulation of different operating states through the fluid pressure in the system.
- Performing tests for the most typical human heart frequencies, namely: 60, 80, and 100 cycles per minute.
- Analysis of data from artificial blood pressure sensors located at several locations in the heart and hydraulic system installation, gas (helium) pressure, and artificial blood flow rate.
4. Results and Discussion
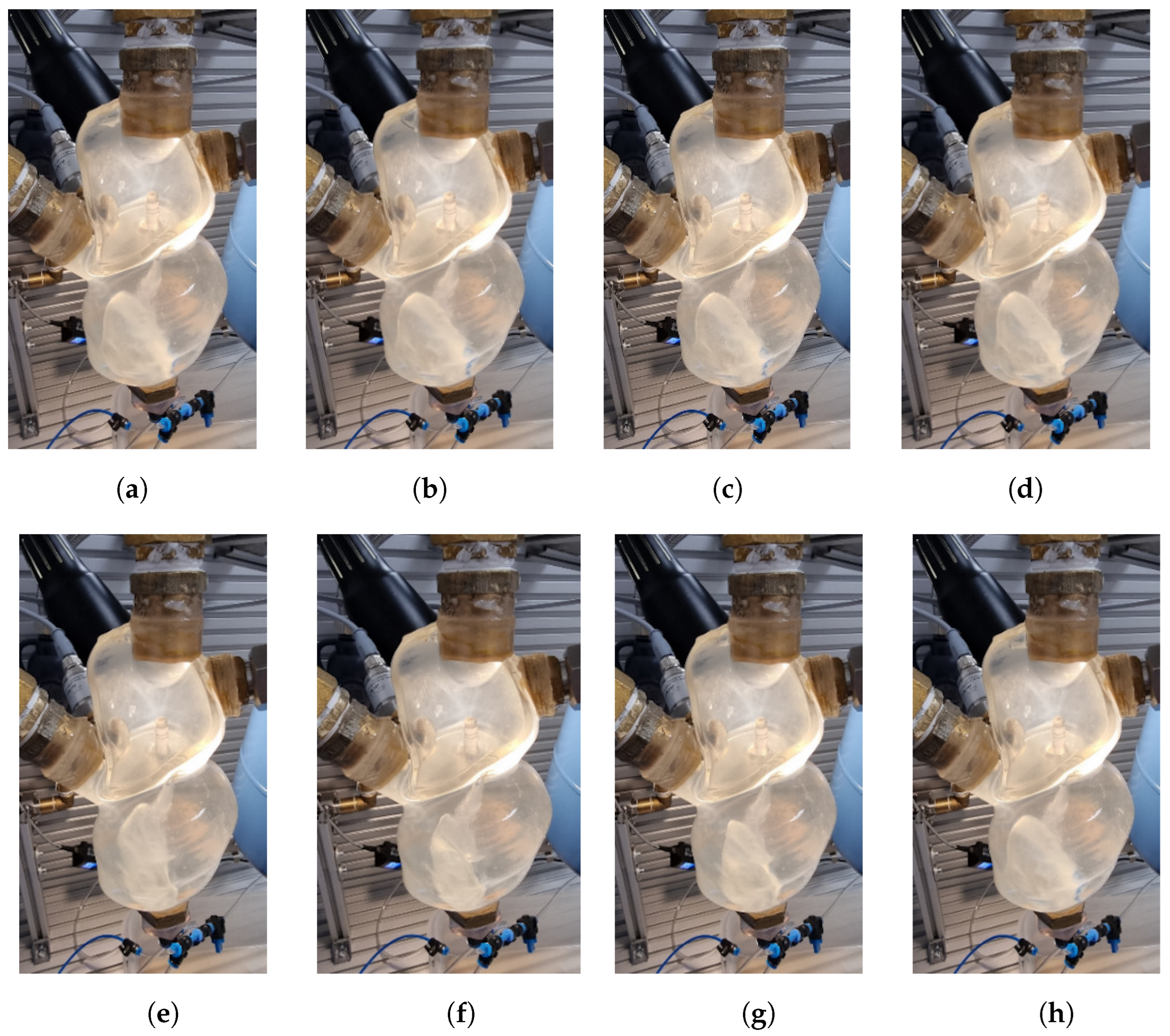
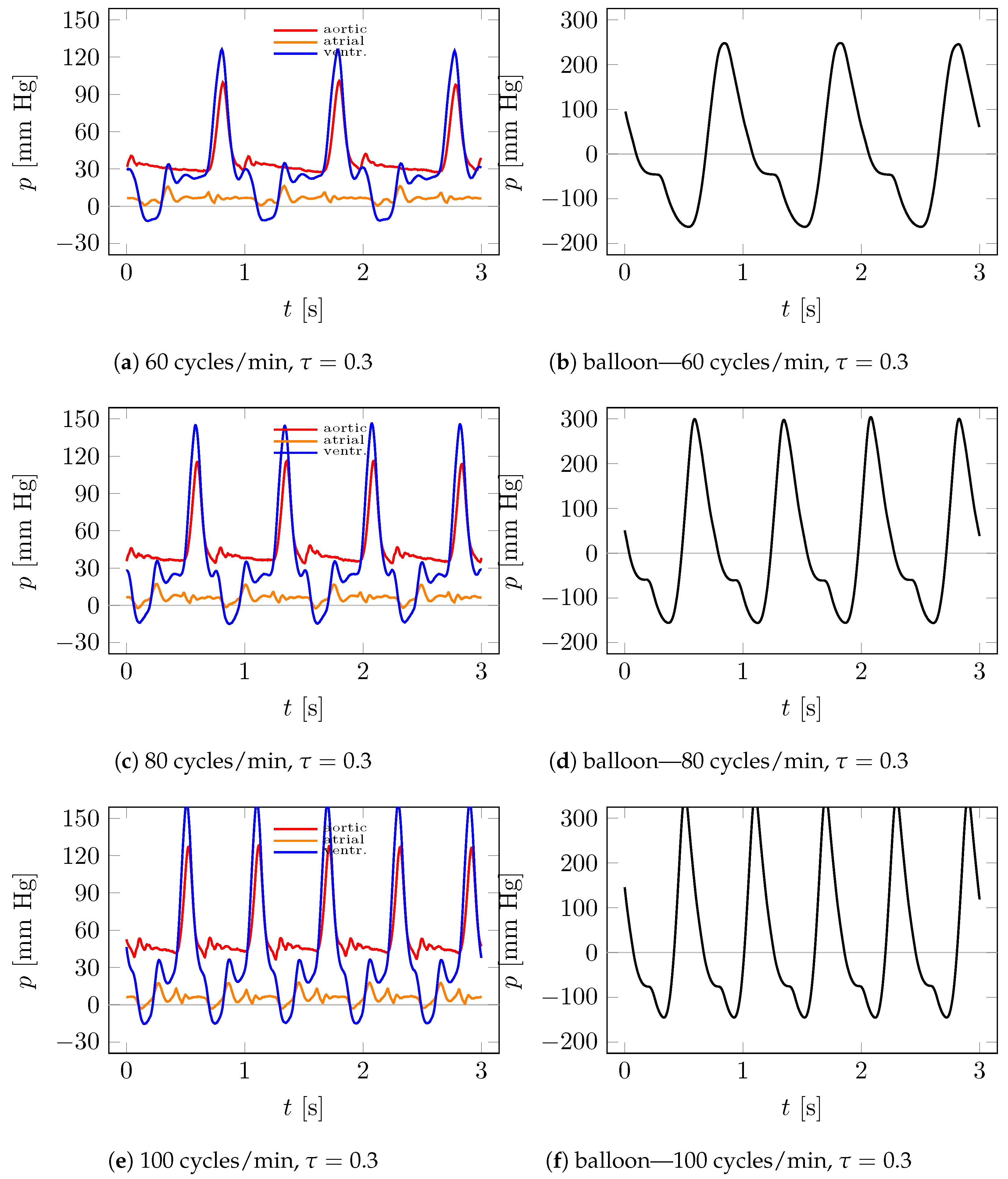
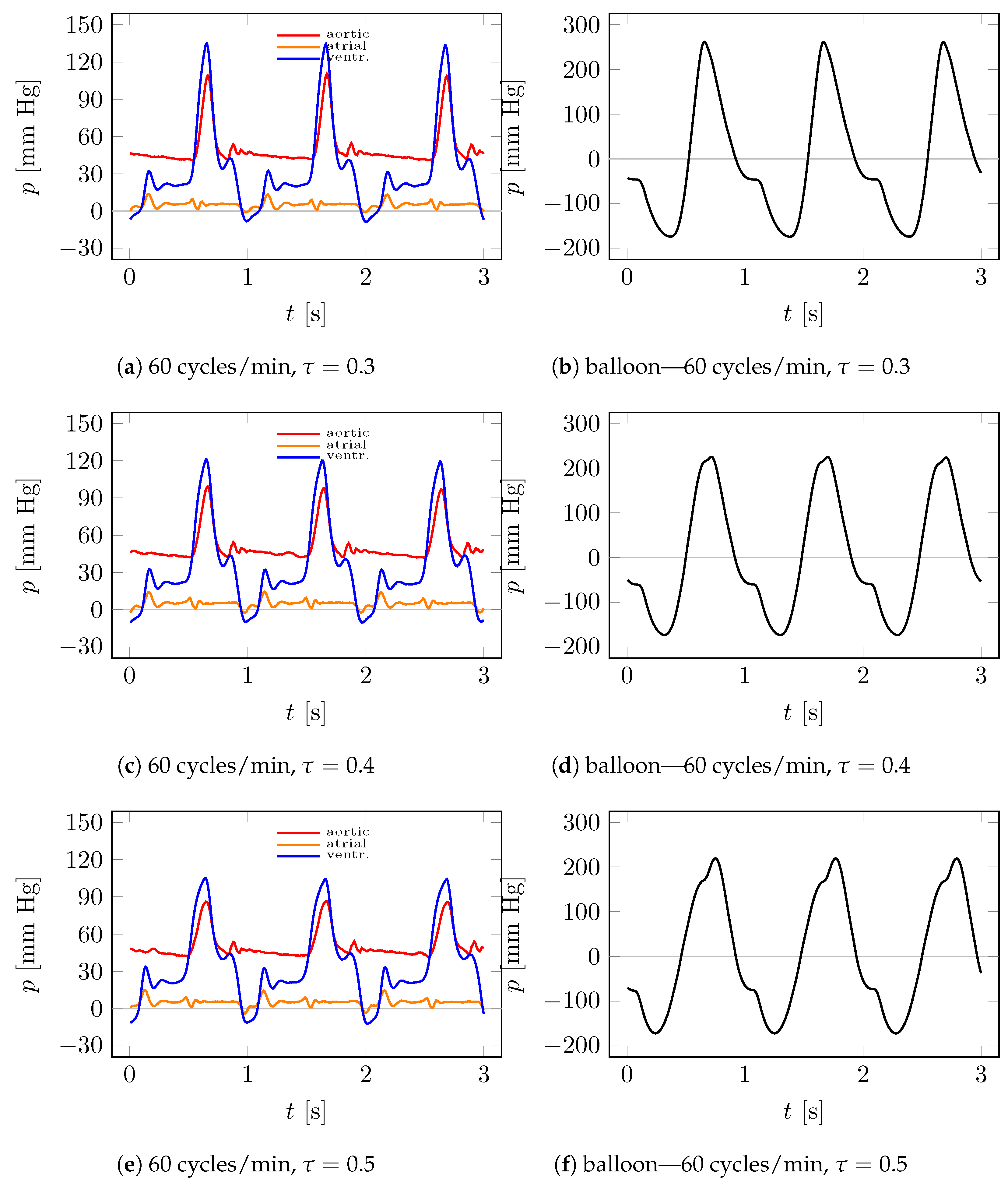
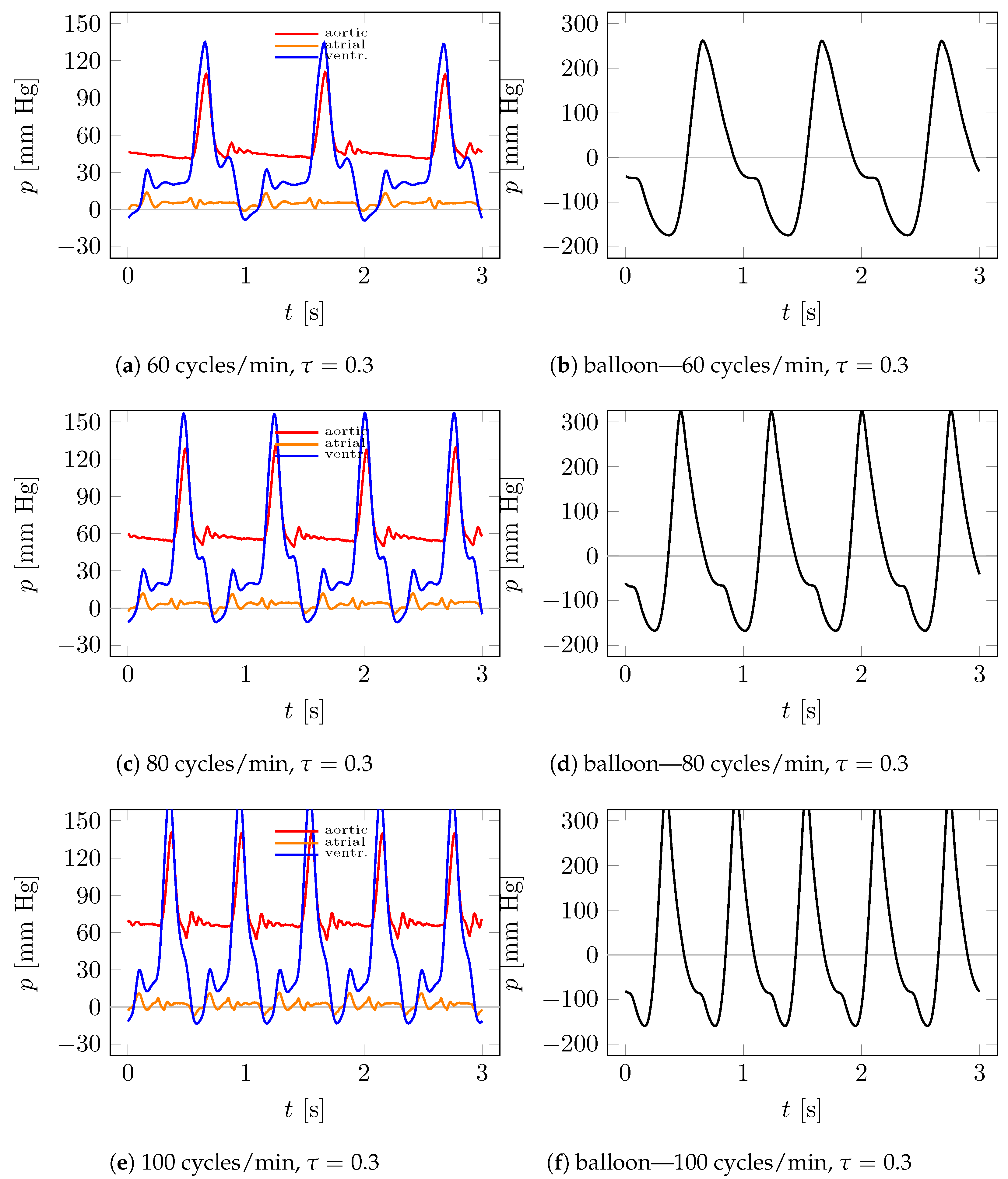
4.1. Visualization
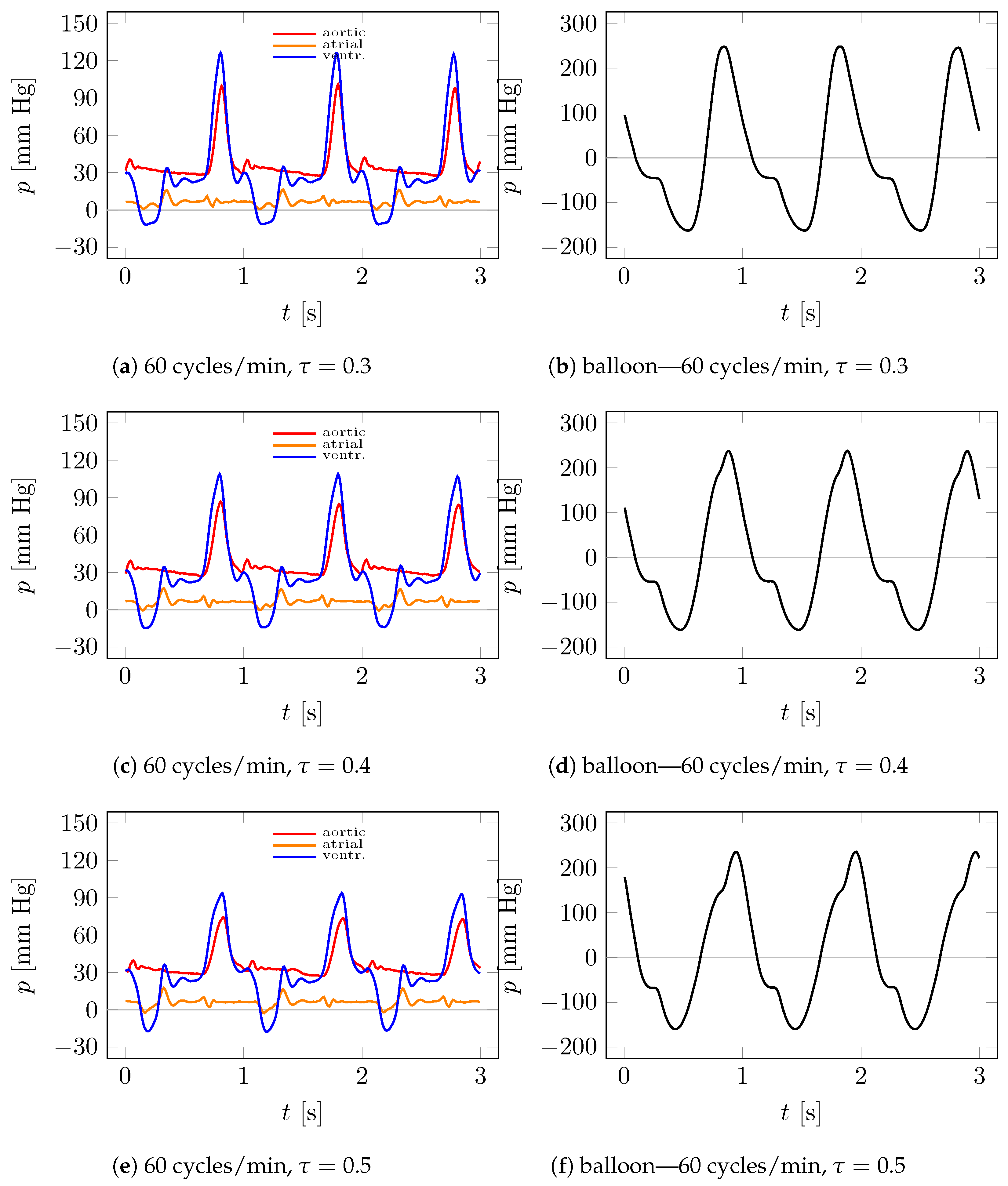
4.2. Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update: A report from the American heart association. Circulation 2017, 135, 146–603. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xi, Y.; Wang, H.; Sun, A.; Deng, X.; Chen, Z.; Fan, Y. A new way to evaluate thrombotic risk in failure heart and ventricular assist devices. Med. Nov. Technol. Devices 2022, 16, 100135. [Google Scholar] [CrossRef]
- Yancy, C.W.; Januzzi, J.L.; Allen, L.A.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Jessup, M.; Lindenfeld, J.; Maddox, T.M.; et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M., III; Long, J.W.; et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.; Colombo, P.C. Left ventricular assist devices: A rapidly evolving alternative to transplant. J. Am. Coll. Cardiol. 2015, 65, 2542–2555. [Google Scholar] [CrossRef]
- Quader, M.; Toldo, S.; Chen, Q.; Hundley, G.; Kasirajan, V. Heart transplantation from donation after circulatory death donors: Present and future. J. Card. Surg. 2020, 35, 875–885. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States. A policy statement from the American heart association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Berk, Z.B.K.; Zhang, J.; Chen, Z.; Tran, D.; Griffith, B.P.; Wu, Z.J. Evaluation of in vitro hemolysis and platelet activation of a newly developed maglev LVAD and two clinically used LVADs with human blood. Artif. Organs 2019, 43, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Garbade, J.; Bittner, H.B.; Barten, M.J.; Mohr, F.-W. Current trends in implantable left ventricular assist devices. Cardiol. Res. Pract. 2011, 2011, 290561. [Google Scholar] [CrossRef]
- Kafagy, D.H.; Dwyer, T.W.; McKenna, K.L.; Mulles, J.P.; Chopski, S.G.; Moskowitz, W.B.; Throckmorton, A.L. Design of axial blood pumps for patients with dysfunctional fontan physiology: Computational studies and performance testing. Artif. Organs 2015, 39, 34–42. [Google Scholar] [CrossRef]
- Ward, S.T.; Liang, Q.; Pagani, F.D.; Zhang, M.; Kormos, R.L.; Aaronson, K.D.; Althouse, A.D.; Nallamothu, B.K.; Likosky, D.S. A roadmap for evaluating the use and value of durable ventricular assist device therapy. J. Heart Lung Transplant. 2018, 37, 146–150. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.B.; Böhm, M. Management of end stage heart failure. Heart 2007, 93, 626–631. [Google Scholar] [CrossRef]
- Chaudhry, S.P.; Stewart, G.C. Advanced heart failure: Prevalence, natural history, and prognosis. Heart Fail. Clin. 2016, 12, 323–333. [Google Scholar] [CrossRef]
- Farrar, D.J.; Hill, J.D.; Gray, L.A., Jr.; Pennington, D.G.; McBride, L.R.; Pierce, W.S.; Pae, W.E.; Glenville, B.; Ross, D.; Galbraith, T.A.; et al. Heterotopic prosthetic ventricles as a bridge to cardiac transplantation. N. Engl. J. Med. 1988, 318, 333–340. [Google Scholar] [CrossRef]
- Sawa, Y. Current status of third-generation implantable left ventricular assist devices in Japan, Duraheart and HearWare. Surg. Today 2015, 45, 672–681. [Google Scholar] [CrossRef]
- Hrobowski, T.; Lanfear, D.E. Ventricular assist devices: Is destination therapy a viable alternative in the non-transplant candidate? Curr. Heart Fail. Rep. 2013, 10, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.abiomed.com/products-and-services/impella (accessed on 28 May 2023).
- Abrams, D.; Combes, A.; Brodie, D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J. Am. Coll. Cardiol. 2014, 63, 2769–2778. [Google Scholar] [CrossRef]
- Takayama, H.; Truby, L.; Koekort, M.; Uriel, N.; Colombo, P.; Mancini, D.M.; Jorde, U.P.; Naka, Y. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J. Heart Lung Transplant. 2013, 32, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Sarna, J.; Kustosz, R.; Major, R.; Lackner, J.M.; Major, B. Polish artificial heart—New coatings, technology, diagnostics. Bull. Pol. Acad. Sci. Tech. Sci. 2010, 58, 329–335. [Google Scholar] [CrossRef]
- Available online: https://www.levitronix.com/ (accessed on 28 May 2023).
- Available online: https://www.cardiovascular.abbott/ (accessed on 28 May 2023).
- Available online: http://www.cardiacassist.com (accessed on 28 May 2023).
- Available online: https://global.medtronic.com/ (accessed on 28 May 2023).
- Stewart, G.C.; Givertz, M.M. Mechanical circulatory support for advanced heart failure: Patients and technology in evolution. Circulation 2012, 125, 1304–1315. [Google Scholar] [CrossRef]
- Chaudhry, S.P.; DeVore, A.D.; Vidula, H.; Nassif, M.; Mudy, K.; Birati, E.Y.; Gong, T.; Atluri, P.; Pham, D.; Sun, B.; et al. Left ventricular assist devices: A primer for the general cardiologist. J. Am. Heart Assoc. 2022, 11, e027251. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Trumble, D.R. Cardiac assist devices: Early concepts, current technologies, and future innovations. Bioengineering 2019, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Miller, M.A.; Baldwin, J.T.; Young, J.B. Sixth INTERMACS annual report: A 10,000-patient database. J. Heart Lung Transplant. 2014, 33, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Teuteberg, J.J.; Clevel, J.C., Jr.; Cowger, J.; Higgins, R.S.; Goldstein, D.J.; Keebler, M.; Kirklin, J.K.; Myers, S.L.; Salerno, C.T.; Stehlik, J.; et al. The Society of Thoracic Surgeons Intermacs 2019 annual report: The changing landscape of devices and indications. Ann. Thorac. Surg. 2020, 109, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.P.; Niu, J.; Winkelmayer, W.C.; Cheema, F.H.; Nair, A.P.; Morgan, J.A.; Fedson, S.E.; Deswal, A.; Navaneethan, S.D. Implantable ventricular assist device use and outcomes in people with end-stage renal disease. J. Am. Heart Assoc. 2018, 7, e008664. [Google Scholar] [CrossRef]
- Tesch, K.; Kaczorowska, K. The discrete-continuous, global optimisation of an axial flow blood pump, Flow. Turbul. Combust. 2019, 104, 777–793. [Google Scholar] [CrossRef]
- Torner, B.; Konnigk, L.; Abroug, N.; Wurm, H. Turbulence and turbulent flow structures in a ventricular assist device—A numerical study using the large-eddy simulation. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3431. [Google Scholar] [CrossRef]
- Pleşoianu, F.A.; Pleşoianu, C.E.; Bararu Bojan, I.; Bojan, A.; Ţăruş, A.; Tinică, G. Concept, design, and early prototyping of a low-cost, minimally invasive, fully implantable left ventricular assist device. Bioengineering 2022, 9, 201. [Google Scholar] [CrossRef]
- Shah, S.P.; Mehra, M.R. Durable left ventricular assist device therapy in advanced heart failure: Patient selection and clinical outcomes. Indian Heart J. 2016, 68, S45–S51. [Google Scholar] [CrossRef]
- Aaronson, K.D.; Slaughter, M.S.; Miller, L.W.; McGee, E.C.; Cotts, W.G.; Acker, M.A.; Jessup, M.L.; Gregoric, I.D.; Loyalka, P.; Frazier, O.H.; et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012, 125, 3191–3200. [Google Scholar] [CrossRef]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial left ventricular assist device for advanced heart failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef]
- Shi, J.; Yu, X.; Liu, Z. A review of new-onset ventricular arrhythmia after left ventricular assist device implantation. Cardiology 2022, 147, 315–327. [Google Scholar] [CrossRef]
- Vollkron, M.; Voitl, P.; Ta, J.; Wieselthaler, G.; Schima, H. Suction events during left ventricular support and ventricular arrhythmias. J. Heart Lung Transplant. 2007, 26, 819–825. [Google Scholar] [CrossRef]
- Kafagy, D.H.; Dwyer, T.W.; McKenna, K.L.; Mulles, J.P.; Chopski, S.G.; Moskowitz, W.B.; Throckmorton, A.L. Mechanical circulatory support devices for acute right ventricular failure. Circulation 2017, 136, 314–326. [Google Scholar] [CrossRef]
- Kassis, H.; Cherukuri, K.; Agarwal, R.; Kanwar, M.; Elapavaluru, S.; Sokos, G.G.; Moraca, R.J.; Bailey, S.H.; Murali, S.; Benza, R.L.; et al. Significance of residual mitral regurgitation after continuous flow left ventricular assist device implantation. JACC Heart Fail. 2017, 5, 81–88. [Google Scholar] [CrossRef]
- Kormos, R.L.; Teuteberg, J.J.; Pagani, F.D.; Russell, S.D.; John, R.; Miller, L.W.; Massey, T.; Milano, C.A.; Moazami, N.; Sundareswaran, K.S.; et al. Right ventricular failure in patients with the Heart-Mate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 1316–1324. [Google Scholar] [CrossRef]
- Han, J.J.; Acker, M.A.; Atluri, P. Left ventricular assist devices: Synergistic model between technology and medicine. Circulation 2018, 138, 2841–2851. [Google Scholar] [CrossRef]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- PCT Application, Implantable Left Ventricular Assist Device and System for Ventricular-assist for Use in Patients with End-Stage Heart Failure PCT/PL2021/050004. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021162564 (accessed on 28 May 2023).
- Tesch, K.; Jasinski, R.; Dabrowski, L.; Rogowski, J. Experimental investigation of the performance of an innovative implantable left ventricular assist device—Proof of concept. Appl. Sci. 2023, 13, 973. [Google Scholar] [CrossRef]
- Available online: https://www.slicer.org/ (accessed on 28 May 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasinski, R.; Tesch, K.; Dabrowski, L.; Rogowski, J. Innovative Implantable Left Ventricular Assist Device—Performance under Various Resistances and Operating Frequency Conditions. Appl. Sci. 2023, 13, 7785. https://doi.org/10.3390/app13137785
Jasinski R, Tesch K, Dabrowski L, Rogowski J. Innovative Implantable Left Ventricular Assist Device—Performance under Various Resistances and Operating Frequency Conditions. Applied Sciences. 2023; 13(13):7785. https://doi.org/10.3390/app13137785
Chicago/Turabian StyleJasinski, Ryszard, Krzysztof Tesch, Leszek Dabrowski, and Jan Rogowski. 2023. "Innovative Implantable Left Ventricular Assist Device—Performance under Various Resistances and Operating Frequency Conditions" Applied Sciences 13, no. 13: 7785. https://doi.org/10.3390/app13137785
APA StyleJasinski, R., Tesch, K., Dabrowski, L., & Rogowski, J. (2023). Innovative Implantable Left Ventricular Assist Device—Performance under Various Resistances and Operating Frequency Conditions. Applied Sciences, 13(13), 7785. https://doi.org/10.3390/app13137785





