Methicillin Resistance of Food-Borne Biofilm-Forming Staphylococci
Abstract
:1. Introduction
2. Materials and Methods
| Isolate Code | Species | Antibiotic Resistance Profile | Virulence Genes |
|---|---|---|---|
| 11 | S. aureus | FOX, AMP, MET, VANC, PEN, ERY | hlb |
| 15 | S. aureus | AMP, PEN, ERY | - |
| 32 | S. aureus | FOX, AMP, MET, PEN, SXT | hlb |
| 47 | S. aureus | VANC, PEN, ERY | - |
| 48 | S. aureus | PEN, ERY | hlb, sea |
| 49 | S. aureus | PEN | - |
| 50 | S. aureus | FOX, AMP, MET, VANC, PEN | hlb |
| 52 | S. aureus | VANC | - |
| 53 | S. aureus | FOX, AMP, MET, VANC, PEN, TET, ERY | hlb, sed |
| 68 | S. aureus | FOX, MET, VANC, PEN, TET, ERY | hlb, sec |
| 24 | S. pasteuri | PEN | sec |
| 37 | S. pasteuri | - | hlb |
| 40 | S. pasteuri | PEN, TET | - |
| 41 | S. pasteuri | ERY | hlb |
| 43 | S. pasteuri | FOX, MET, PEN, ERY | hlb |
| 6 | S. saprophyticus | PEN | - |
| 13 | S. saprophyticus | FOX, MET, PEN, VANC, TET, ERY | sec |
| 19 | S. saprophyticus | FOX, MET, VANC, PEN, ERY | sec, sed, sak |
| 26 | S. saprophyticus | FOX, MET, PEN, ERY | sec |
| 31 | S. saprophyticus | PEN, ERY | seb, hlb |
| 35 | S. saprophyticus | FOX, MET, PEN, ERY | sec |
| 59 | S. saprophyticus | FOX, MET, PEN, SXT | sec |
| 1 | S. warneri | PEN, ERY | sak |
| 18 | S. warneri | AMP, PEN, ERY | sec, sak |
| 9 | S. warneri | ERY | sec, sak |
| 10 | S. warneri | ERY | sak |
| 20 | S. warneri | PEN | sec |
| 22 | S. warneri | PEN | sec |
| 34 | S. warneri | AMP, PEN, GEN, ERY | sec |
| 21 | S. xylosus | PEN | sec, sed |
| 23 | S. xylosus | AMP, PEN, ERY | - |
| 28 | S. xylosus | FOX, MET, VAN, PEN | - |
| 30 | S. xylosus | PEN | - |
| 65 | S. xylosus | VANC, PEN, ERY | seb, sec, sed |
2.1. Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC)
2.2. Assessment of Biofilm Formation Capacity
2.2.1. Polystyrene Surface (P24 Microplates)
2.2.2. Stainless Steel Coupons
2.2.3. Glass Test Tubes
2.3. Evaluation of Methicillin Resistance of Biofilm Produced on Polystyrene Surface (P24 Microplates)
2.4. Interpretation of Results and Data Analysis
3. Results
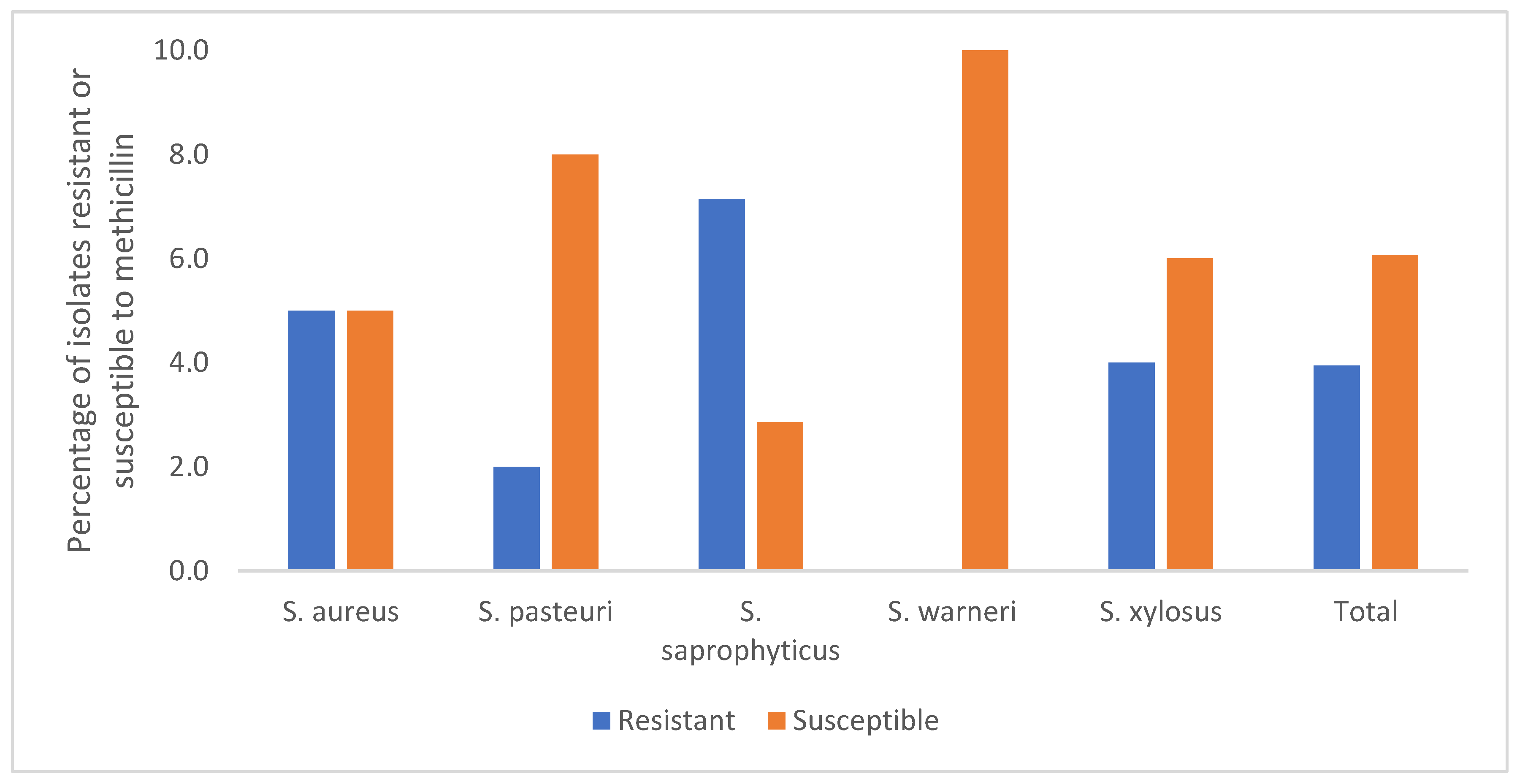
3.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Methicillin
| Strain Code | Species | MIC (µg/mL) | MBC (µg/mL) | MBC/MIC |
|---|---|---|---|---|
| 11 | S. aureus | 8 | 8 | 1 |
| 15 | S. aureus | 2 | 4 | 2 |
| 32 | S. aureus | 16 | 32 | 2 |
| 47 | S. aureus | 2 | 4 | 2 |
| 48 | S. aureus | 4 | 4 | 1 |
| 49 | S. aureus | 4 | 8 | 2 |
| 50 | S. aureus | 32 | >32 | - |
| 52 | S. aureus | 4 | 8 | 2 |
| 53 | S. aureus | >32 | >32 | - |
| 68 | S. aureus | >32 | >32 | - |
| 41 | S. pasteuri | 4 | 4 | 1 |
| 24 | S. pasteuri | 4 | 8 | 2 |
| 37 | S. pasteuri | 4 | 4 | 1 |
| 40 | S. pasteuri | 4 | 4 | 1 |
| 43 | S. pasteuri | >32 | >32 | - |
| 6 | S. saprophyticus | 2 | 4 | 2 |
| 13 | S. saprophyticus | 8 | 8 | 1 |
| 19 | S. saprophyticus | >32 | >32 | - |
| 26 | S. saprophyticus | 8 | 16 | 2 |
| 31 | S. saprophyticus | 4 | 16 | 4 |
| 35 | S. saprophyticus | 8 | 16 | 2 |
| 59 | S. saprophyticus | 32 | >32 | - |
| 1 | S. warneri | 4 | 8 | 2 |
| 18 | S. warneri | 2 | 4 | 2 |
| 9 | S. warneri | 4 | 8 | 2 |
| 10 | S. warneri | 2 | 4 | 2 |
| 20 | S. warneri | 2 | 2 | 1 |
| 22 | S. warneri | 2 | 4 | 2 |
| 34 | S. warneri | 2 | 4 | 2 |
| 21 | S. xylosus | 4 | 8 | 2 |
| 23 | S. xylosus | 1 | 2 | 2 |
| 28 | S. xylosus | 32 | >32 | - |
| 30 | S. xylosus | 4 | 4 | 1 |
| 65 | S. xylosus | 32 | >32 | - |
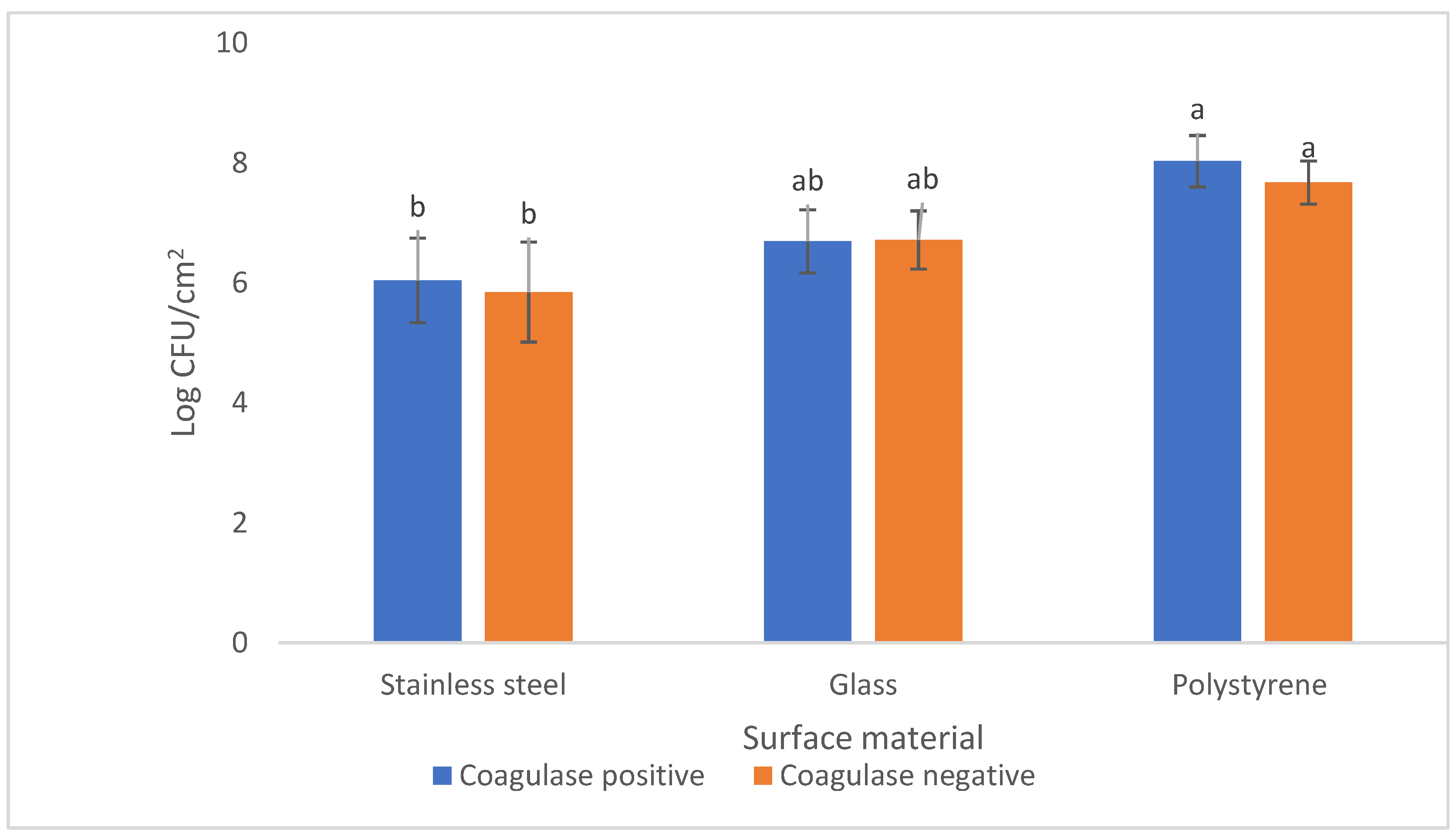
3.2. Evaluation of Biofilm-Forming Ability on Tree Types of Surfaces
| Isolate Code | Species | Biofilm-Forming Ability (log CFU/cm−2) | ||
|---|---|---|---|---|
| Stainless Steel | Glass | Polystyrene | ||
| 11 | S. aureus | 4.98 ± 0.01 | 6.99 ± 0.04 | 8.32 ± 0.02 |
| 15 | S. aureus | 6.86 ± 0.01 | 6.30 ± 0.02 | 8.47 ± 0.53 |
| 32 | S. aureus | 5.85 ± 0.06 | 6.36 ± 0.09 | 7.56 ± 0.11 |
| 42 | S. aureus | 6.26 ± 0.07 | 6.56 ± 0.07 | 8.05 ± 0.19 |
| 47 | S. aureus | 4.58 ± 0.02 | 6.30 ± 0.01 | 8.00 ± 0.01 |
| 49 | S. aureus | 6.24 ± 0.07 | 7.63 ± 0.04 | 7.04 ± 0.13 |
| 53 | S. aureus | 6.25 ± 0.01 | 7.66 ± 0.01 | 8.20 ± 0.47 |
| 48 | S. aureus | 5.53 ± 0.01 | 6.83 ± 0.01 | 7.83 ± 0.07 |
| 1 | S. warneri | 4.30 ± 0.04 | 6.41 ± 0.03 | 7.72 ± 0.04 |
| 18 | S. warneri | 6.49 ± 0.09 | 7.67 ± 0.03 | 8.04 ± 0.02 |
| 6 | S. saprophyticus | 6.32 ± 0.01 | 6.72 ± 0.10 | 7.66 ± 0.03 |
| 19 | S. saprophyticus | 5.32 ± 0.02 | 6.10 ± 0.01 | 7.68 ± 0.03 |
| 35 | S. saprophyticus | 4.85 ± 0.06 | 6.53 ± 0.01 | 7.60 ± 0.09 |
| 21 | S. xylosus | 6.28 ± 0.01 | 7.11 ± 0.01 | 7.40 ± 0.16 |
| 28 | S. xylosus | 4.85 ± 0.06 | 6.33 ± 0.02 | 7.37 ± 0.05 |
| 30 | S. xylosus | 5.41 ± 0.03 | 6.72 ± 0.03 | 8.13 ± 0.05 |
| 40 | S. pasteuri | 6.28 ± 0.01 | 7.13 ± 0.01 | 6.85 ± 0.11 |
| 43 | S. pasteuri | 6.97 ± 0.01 | 7.46 ± 0.01 | 7.97 ± 0.12 |
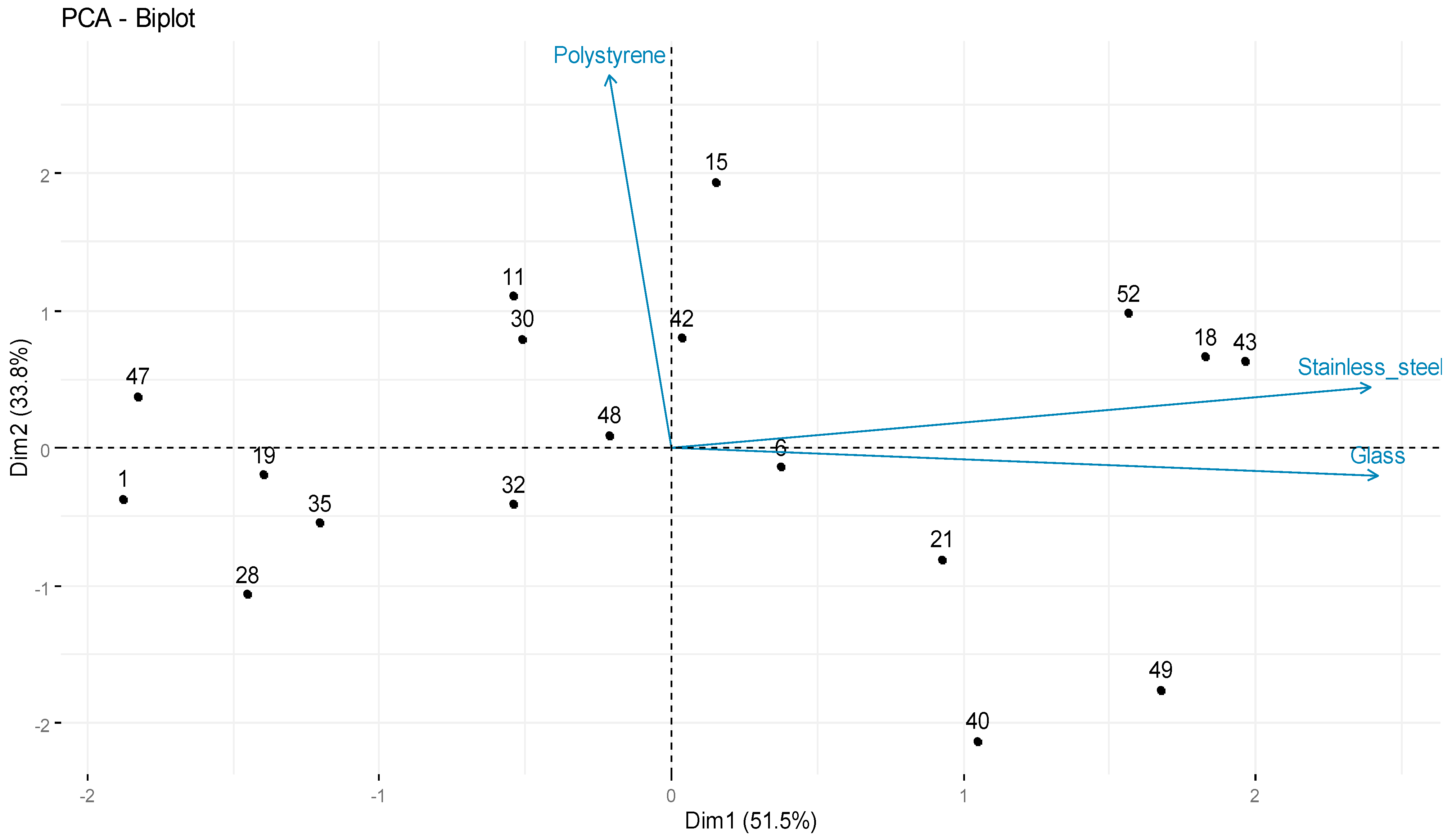
| Glass | Polystyrene | |
|---|---|---|
| Stainless steel | r = 0.54 | r = 0.04 |
| p-value = 0.02 | p-value = 0.86 | |
| Polystyrene | r = −0.09 | |
| p-value = 0.72 |
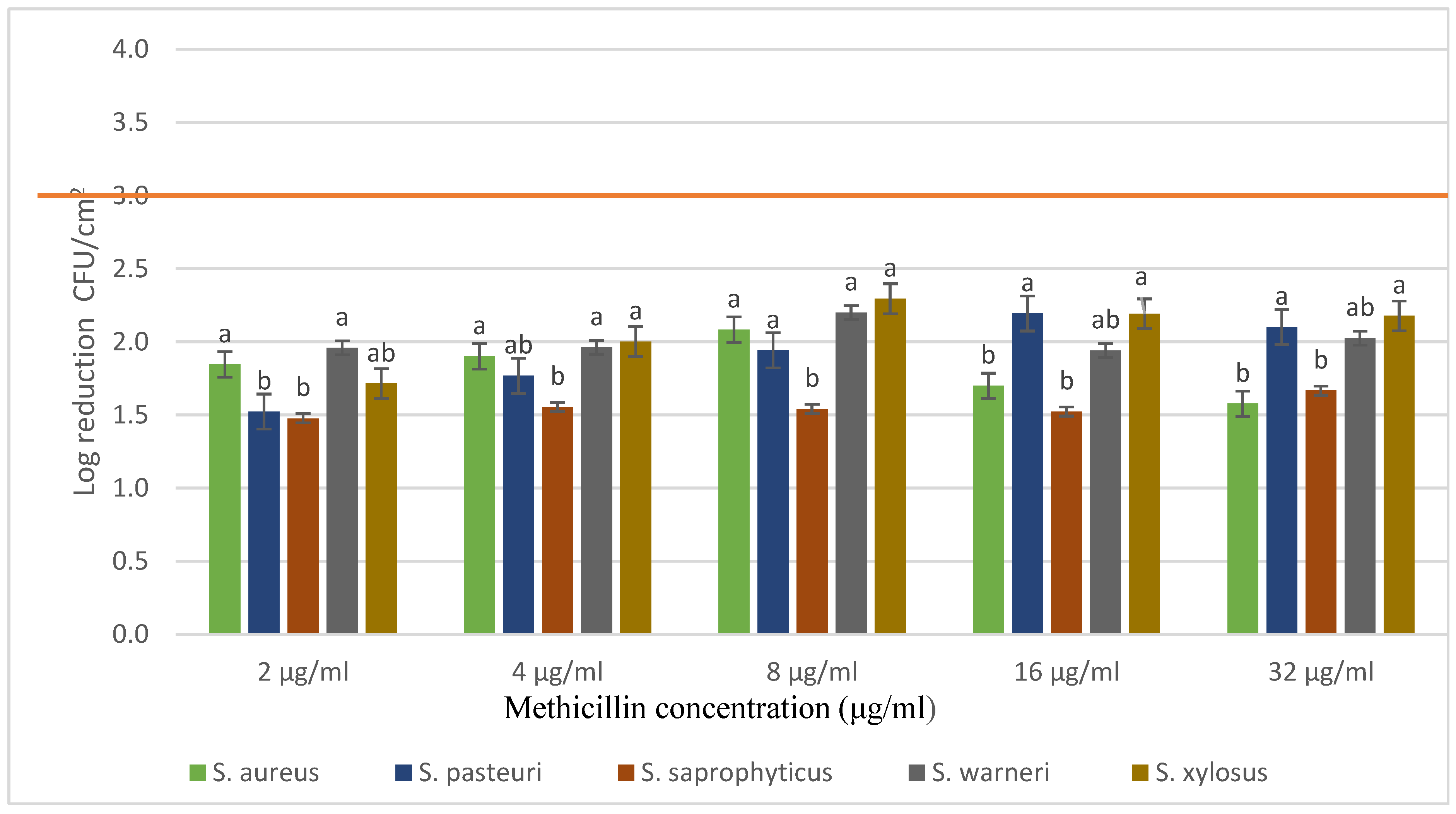
3.3. Evaluation of Methicillin Resistance of Biofilms Produced on Polystyrene Surface (P24 Microplates)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neopane, P.; Nepal, H.P.; Shrestha, R.; Uehara, O.; Abiko, Y. In Vitro Biofilm Formation by Staphylococcus aureus Isolated from Wounds of Hospital-Admitted Patients and Their Association with Antimicrobial Resistance. Int. J. Gen. Med. 2018, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, S.; Duncun Mosioma, N.; Karutha Pandian, S. Coral-Associated Bacteria as a Promising Antibiofilm Agent against Methicillin-Resistant and -Susceptible Staphylococcus aureus Biofilms. Evid. Based Complement. Altern. Med. 2012, 2012, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L.; Vasil, M.L.; Otto, M. Polysaccharide Intercellular Adhesin in Biofilm: Structural and Regulatory Aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Gajewska, J.; Chajecka-Wierzchowska, W. Biofilm Formation Ability and Presence of Adhesion Genes among Coagulase-Negative and Coagulase-Positive Staphylococci Isolates from Raw Cow’s Milk. Pathogens 2020, 9, 654. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, A.; Rego, F.; Brito, L.; Frank, J.F. Evaluation of Methods to Assess the Biofilm-Forming Ability of Listeria monocytogenes. J. Food Prot. 2012, 75, 1411–1417. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Rita Costa, A.; Desvaux, M.; di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2006, 22, 1B.1.1–1B.1.18. [Google Scholar] [CrossRef] [Green Version]
- Chung, P.Y.; Toh, Y.S. Anti-Biofilm Agents: Recent Breakthrough against Multi-Drug Resistant Staphylococcus aureus. Pathog. Dis. 2014, 70, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Salamandane, A.; Silva, A.C.; Brito, L.; Malfeito-Ferreira, M. Microbiological Assessment of Street Foods at the Point of Sale in Maputo (Mozambique). Food Qual. Saf. 2021, 5, fyaa030. [Google Scholar] [CrossRef]
- Salamandane, A.; Oliveira, J.; Coelho, M.; Ramos, B.; Cunha, M.V.; Malfeito-Ferreira, M.; Brito, L. Enterotoxin- and Antibiotic-Resistance-Encoding Genes Are Present in Both Coagulase-Positive and Coagulase-Negative Foodborne Staphylococcus Strains. Appl. Microbiol. 2022, 2, 367–380. [Google Scholar] [CrossRef]
- ISO 6888-1 ISO 6888-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Technique Using Baird-Parker Agar Medium—Amendmen. International Standard: Geneva, Switzerland, 1999.
- ISO 6888-2 ISO 6888-2; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive and Other Species)—Staphylococci (Staphylococcus aureus Part 2: Technique Using Rabbit Plasma Fibrinogen Agar Medium. International Standard: Geneva, Switzerland, 2008.
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.; Bertolotti, L.; Brito, L.; Civera, T. Biofilm Formation and Disinfectant Susceptibility of Persistent and Non-Persistent Listeria monocytogenes Isolates from Gorgonzola Cheese Processing Plants. Foodborne Pathog. Dis. 2016, 13, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Raad, I.; Darouiche, R.; Hachem, R.; Sacilowski, M.; Bodey, G.P. Antibiotics and Prevention of Microbial Colonization of Catheters. Antimicrob. Agents Chemother. 1995, 39, 2397–2400. [Google Scholar] [CrossRef] [Green Version]
- Kumari, H.; Chakraborti, T.; Singh, M.; Chakrawarti, M.K.; Mukhopadhyay, K. Prevalence and Antibiogram of Coagulase Negative Staphylococci in Bioaerosols from Different Indoors of a University in India. BMC Microbiol. 2020, 20, 211. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, E.-S.; Song, K.-J.; Kim, B.-M.; Ham, J.-S.; Oh, M.-H. Development of Desiccation-Tolerant Probiotic Biofilms Inhibitory for Growth of Foodborne Pathogens on Stainless Steel Surfaces. Foods 2022, 11, 831. [Google Scholar] [CrossRef]
- Koseki, H.; Yonekura, A.; Shida, T.; Yoda, I.; Horiuchi, H.; Morinaga, Y.; Yanagihara, K.; Sakoda, H.; Osaki, M.; Tomita, M. Early Staphylococcal Biofilm Formation on Solid Orthopaedic Implant Materials: In Vitro Study. PLoS ONE 2014, 9, e107588. [Google Scholar] [CrossRef]
- Wagner, C.; Aytac, S.; Hänsch, G.M. Biofilm Growth on Implants: Bacteria Prefer Plasma Coats. Int. J. Artif. Organs 2011, 34, 811–817. [Google Scholar] [CrossRef]
- Frade, J.P.; Arthington-Skaggs, B.A. Effect of Serum and Surface Characteristics on Candida albicans Biofilm Formation. Mycoses 2011, 54, e154–e162. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Grijpma, D.W.; van der Mei, H.C.; Busscher, H.J.; Kuijer, R. Microbial Biofilm Growth versus Tissue Integration on Biomaterials with Different Wettabilities and a Polymer-Brush Coating. J. Biomed. Mater. Res. A 2010, 94, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Giaouris, E.; Heir, E.; Hébraud, M.; Chorianopoulos, N.; Langsrud, S.; Møretrø, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.J. Attachment and Biofilm Formation by Foodborne Bacteria in Meat Processing Environments: Causes, Implications, Role of Bacterial Interactions and Control by Alternative Novel Methods. Meat Sci. 2014, 97, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.F. Microbial Attachment to Food and Food Contact Surfaces. Adv. Food Nutr. Res. 2001, 43, 319–370. [Google Scholar] [CrossRef] [PubMed]
- Macia, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial Susceptibility Testing in Biofilm-Growing Bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Correia, E.; Pereira, J.E.; González-Machado, C.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Exploring the Biofilm Formation Capacity in S. pseudintermedius and Coagulase-Negative Staphylococci Species. Pathogens 2022, 11, 689. [Google Scholar] [CrossRef]
- Kranjec, C.; Morales Angeles, D.; Torrissen Mårli, M.; Fernández, L.; García, P.; Kjos, M.; Diep, D.B. Staphylococcal Biofilms: Challenges and Novel Therapeutic Perspectives. Antibiotics 2021, 10, 131. [Google Scholar] [CrossRef]
- Miragaia, M. Factors Contributing to the Evolution of Meca-Mediated β-Lactam Resistance in Staphylococci: Update and New Insights from Whole Genome Sequencing (WGS). Front. Microbiol. 2018, 9, 2723. [Google Scholar] [CrossRef] [Green Version]
- Shalaby, M.A.W.; Dokla, E.M.E.; Serya, R.A.T.; Abouzid, K.A.M. Penicillin Binding Protein 2a: An Overview and a Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2020, 199, 112312. [Google Scholar] [CrossRef]
- Zheng, Z.; Stewart, P.S. Penetration of Rifampin through Staphylococcus epidermidis Biofilms. Antimicrob. Agents Chemother. 2002, 46, 900–903. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Martínez, J.M.; Ballesta, S.; Pascual, Á. Activity and Penetration of Fosfomycin, Ciprofloxacin, Amoxicillin/Clavulanic Acid and Co-Trimoxazole in Escherichia Coli and Pseudomonas aeruginosa Biofilms. Int. J. Antimicrob. Agents 2007, 30, 366–368. [Google Scholar] [CrossRef]
- Walters, M.C.; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of Antibiotic Penetration, Oxygen Limitation, and Low Metabolic Activity to Tolerance of Pseudomonas aeruginosa Biofilms to Ciprofloxacin and Tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella Pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Maya, I.D.; Carlton, D.; Estrada, E.; Allon, M. Treatment of Dialysis Catheter–Related Staphylococcus aureus Bacteremia With an Antibiotic Lock: A Quality Improvement Report. Am. J. Kidney Dis. 2007, 50, 289–295. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID Guideline for the Diagnosis and Treatment of Biofilm Infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [Green Version]
- Bonneville, L.; Ortiz, S.; Maia, V.; Brito, L.; Martínez-Suárez, J.V. Strain and Growth Conditions May Regulate Resistance of Listeria Monocytogenes Biofilms to Benzalkonium Chloride. Appl. Sci. 2020, 10, 988. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamandane, A.; Correia, J.; Muetanene, B.A.; dos Santos, M.; Malfeito-Ferreira, M.; Brito, L. Methicillin Resistance of Food-Borne Biofilm-Forming Staphylococci. Appl. Sci. 2023, 13, 7725. https://doi.org/10.3390/app13137725
Salamandane A, Correia J, Muetanene BA, dos Santos M, Malfeito-Ferreira M, Brito L. Methicillin Resistance of Food-Borne Biofilm-Forming Staphylococci. Applied Sciences. 2023; 13(13):7725. https://doi.org/10.3390/app13137725
Chicago/Turabian StyleSalamandane, Acácio, Jussara Correia, Belo Afonso Muetanene, Madalena dos Santos, Manuel Malfeito-Ferreira, and Luísa Brito. 2023. "Methicillin Resistance of Food-Borne Biofilm-Forming Staphylococci" Applied Sciences 13, no. 13: 7725. https://doi.org/10.3390/app13137725
APA StyleSalamandane, A., Correia, J., Muetanene, B. A., dos Santos, M., Malfeito-Ferreira, M., & Brito, L. (2023). Methicillin Resistance of Food-Borne Biofilm-Forming Staphylococci. Applied Sciences, 13(13), 7725. https://doi.org/10.3390/app13137725







