Neuroprotective Effects of High-Intensity Interval Training through Neuroplastic Changes in a Restraint Stress-Induced Depression Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
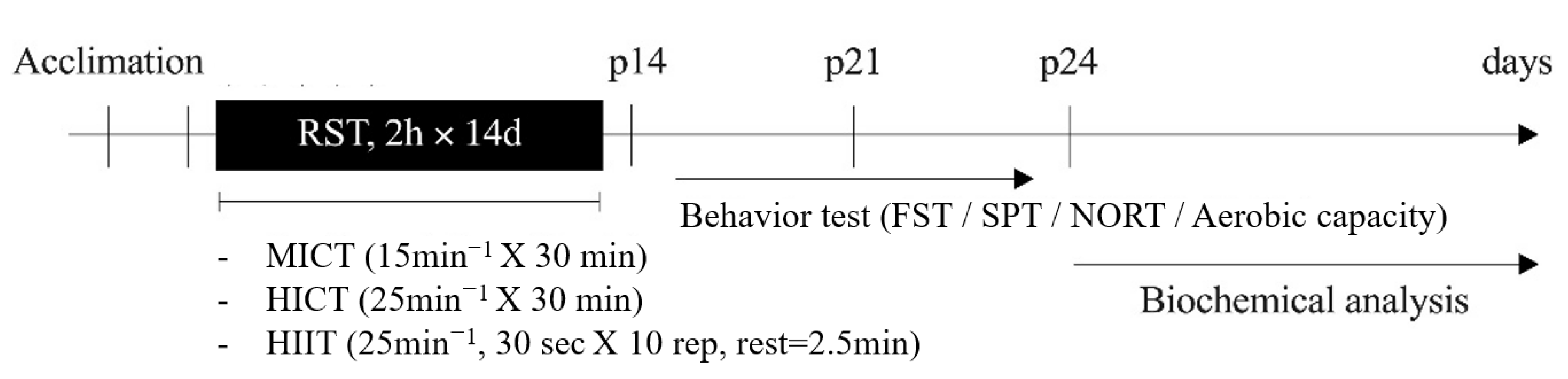
2.2. Animal Models of Depressive Disorders
2.3. Exercise Method
2.4. Forced Swim Test (FST)
2.5. Sucrose Preference Test (SPT)
2.6. Novel Object Recognition Test (NORT)
2.7. Aerobic Capacity
2.8. Administration and Detection of Bromodeoxyuridine (BrdU)
2.9. Tissue Preparation
2.10. Spectrophotometry
2.11. Western Blot
2.12. Immunohistochemistry
2.13. Statistical Analyses
3. Results
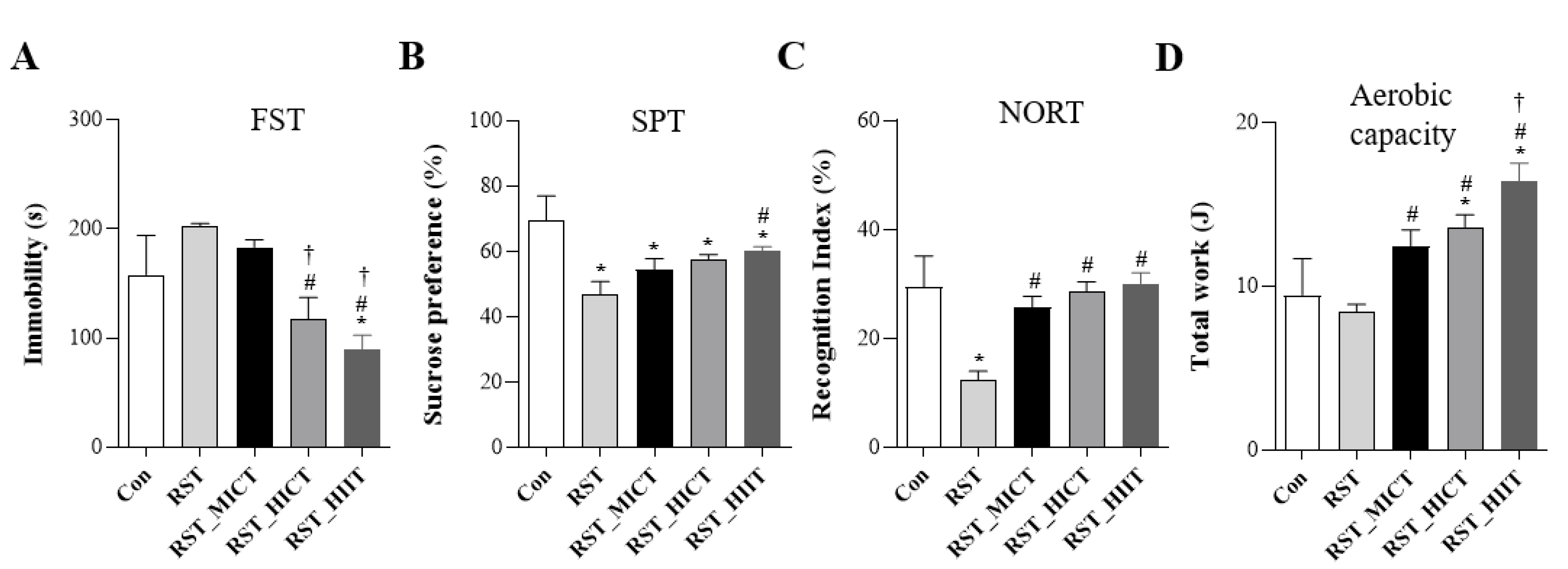
3.1. Changes in Behavioral Function with Treadmill Exercise Intensity
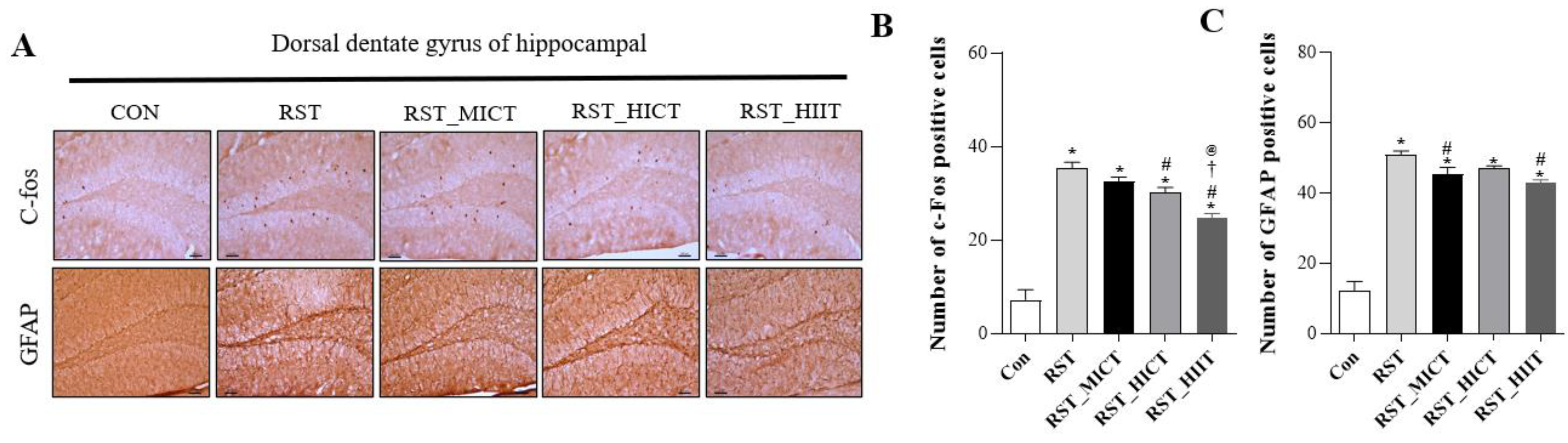
3.2. Changes in Neurogenesis-Related Variables with Treadmill Exercise Intensity
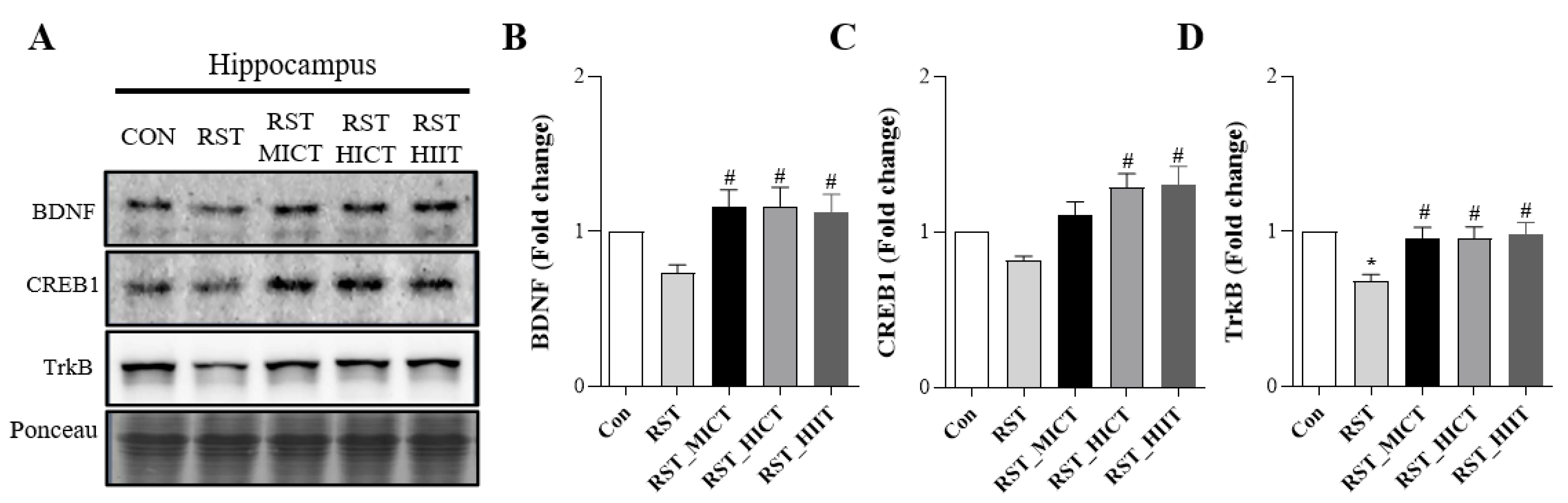
3.3. Changes in Neuroplasticity-Related Metrics with Treadmill Exercise Intensity
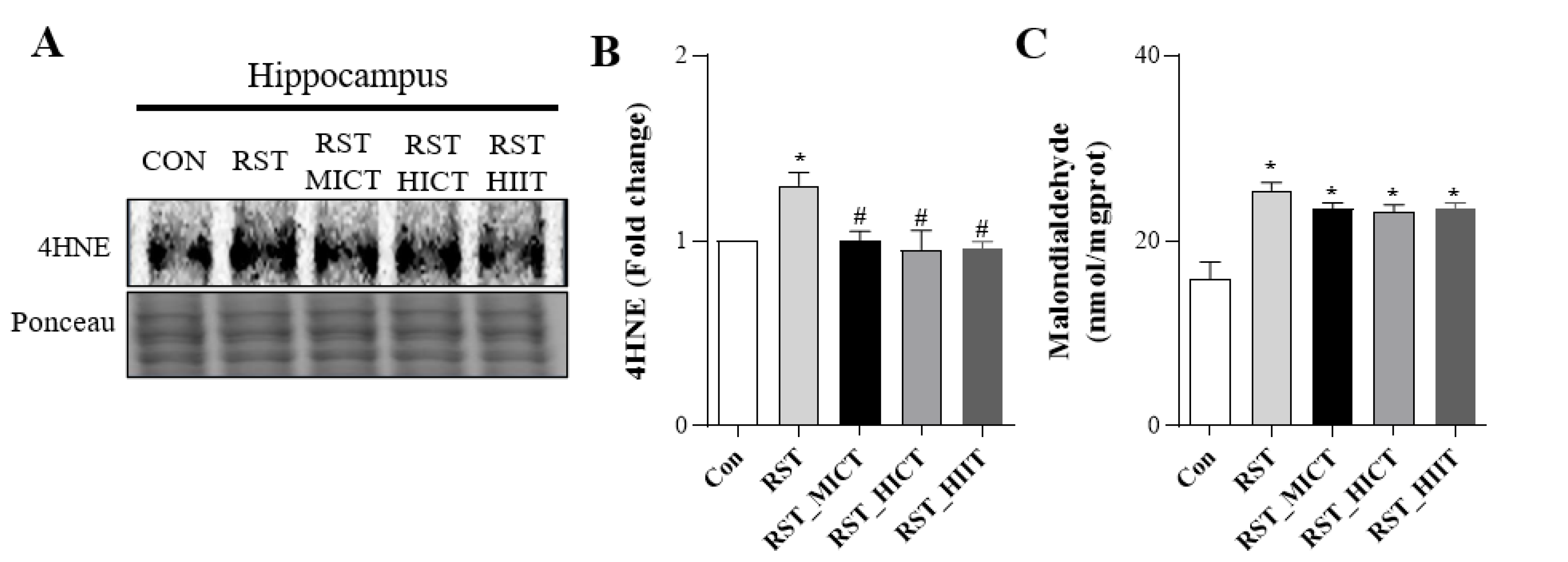
3.4. Changes in Oxidative Stress with Treadmill Intensity
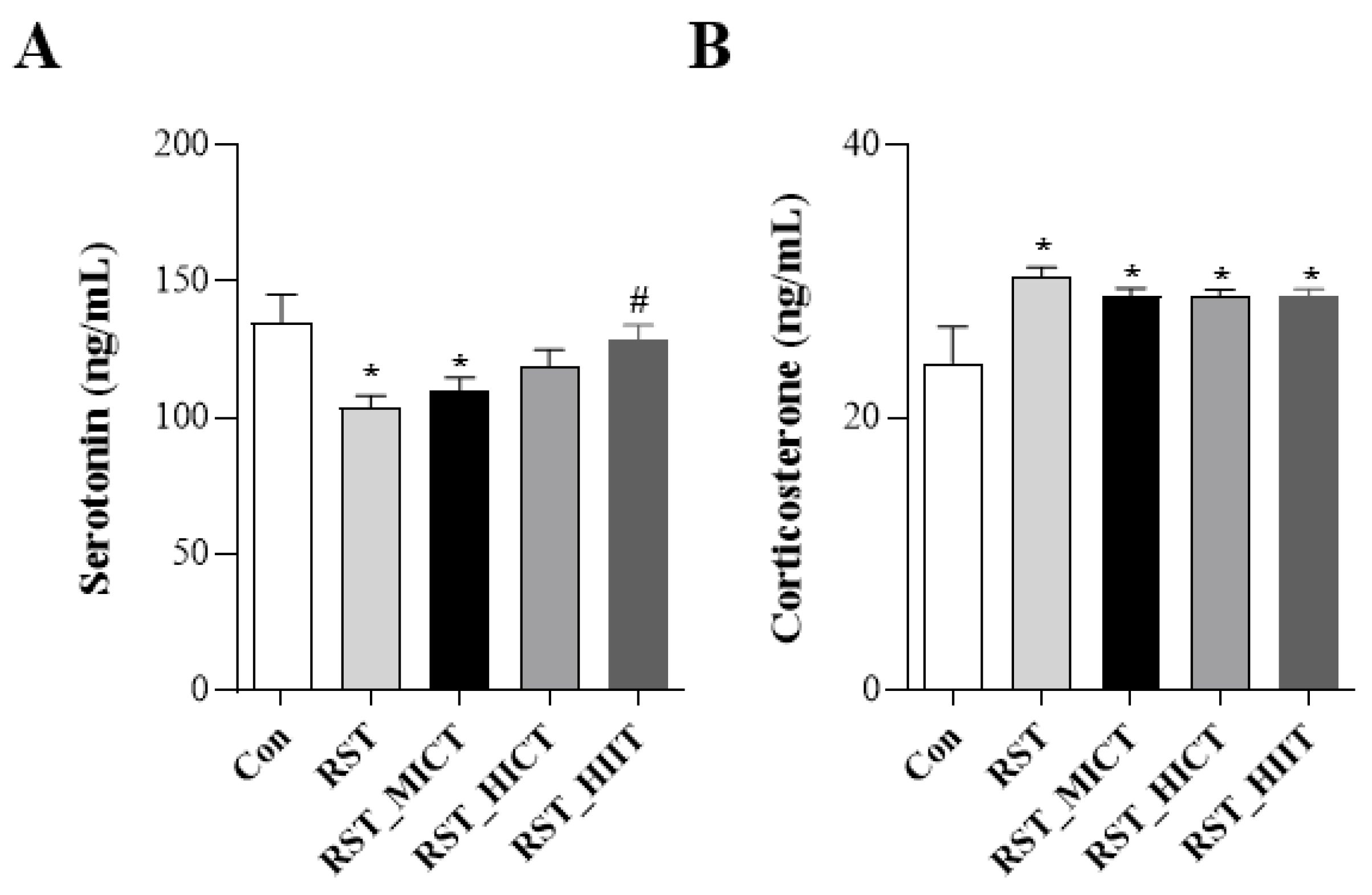
3.5. Changes in Stress Response Markers with Treadmill Exercise Intensity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 181. [Google Scholar] [CrossRef] [Green Version]
- Sher, L.; Grunebaum, M.F.; Burke, A.K.; Chaudhury, S.; Mann, J.J.; Oquendo, M.A. Depressed Multiple-Suicide-Attempters—A High-Risk Phenotype. Crisis 2017, 38, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cui, R. The Effects of Psychological Stress on Depression. Curr. Neuropharmacol. 2015, 13, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef]
- Blier, P. Neurobiology of depression and mechanism of action of depression treatments. J. Clin. Psychiatry 2016, 77, e319. [Google Scholar] [CrossRef]
- Rajkowska, G. Depression: What we can learn from postmortem studies. Neuroscientist 2003, 9, 273–284. [Google Scholar] [CrossRef]
- Nihonmatsu-Kikuchi, N.; Hayashi, Y.; Yu, X.J.; Tatebayashi, Y. Depression and Alzheimer’s disease: Novel postmortem brain studies reveal a possible common mechanism. J. Alzheimers Dis. 2013, 37, 611–621. [Google Scholar] [CrossRef]
- Tan, E.Y.L.; Köhler, S.; Hamel, R.E.G.; Muñoz-Sánchez, J.L.; Verhey, F.R.J.; Ramakers, I. Depressive Symptoms in Mild Cognitive Impairment and the Risk of Dementia: A Systematic Review and Comparative Meta-Analysis of Clinical and Community-Based Studies. J. Alzheimers Dis. 2019, 67, 1319–1329. [Google Scholar] [CrossRef]
- Peng, L.; Bonaguidi, M.A. Function and Dysfunction of Adult Hippocampal Neurogenesis in Regeneration and Disease. Am. J. Pathol. 2018, 188, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Rosenblat, J.D.; Kakar, R.; McIntyre, R.S. The Cognitive Effects of Antidepressants in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Neuropsychopharmacol. 2015, 19, pyv082. [Google Scholar] [CrossRef] [Green Version]
- Popova, N.K.; Ilchibaeva, T.V.; Naumenko, V.S. Neurotrophic Factors (BDNF and GDNF) and the Serotonergic System of the Brain. Biochemistry 2017, 82, 308–317. [Google Scholar] [CrossRef]
- Kondo, M.; Nakamura, Y.; Ishida, Y.; Shimada, S. The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol. Psychiatry 2015, 20, 1428–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogh, J.; Speyer, H.; Gluud, C.; Nordentoft, M. Exercise for patients with major depression: A protocol for a systematic review with meta-analysis and trial sequential analysis. Syst. Rev. 2015, 4, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivar, C.; Potter, M.C.; van Praag, H. All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top. Behav. Neurosci. 2013, 15, 189–210. [Google Scholar] [CrossRef] [Green Version]
- Nishii, A.; Amemiya, S.; Kubota, N.; Nishijima, T.; Kita, I. Adaptive Changes in the Sensitivity of the Dorsal Raphe and Hypothalamic Paraventricular Nuclei to Acute Exercise, and Hippocampal Neurogenesis May Contribute to the Antidepressant Effect of Regular Treadmill Running in Rats. Front. Behav. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, M.; Yamamura, Y.; Liu, Y.F.; Min-Chul, L.; Matsui, T.; Shima, T.; Soya, M.; Takahashi, K.; Soya, S.; McEwen, B.S.; et al. Hormetic effects by exercise on hippocampal neurogenesis with glucocorticoid signaling. Brain Plast. 2015, 1, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- Borrega-Mouquinho, Y.; Sánchez-Gómez, J.; Fuentes-García, J.P.; Collado-Mateo, D.; Villafaina, S. Effects of High-Intensity Interval Training and Moderate-Intensity Training on Stress, Depression, Anxiety, and Resilience in Healthy Adults During Coronavirus Disease 2019 Confinement: A Randomized Controlled Trial. Front. Psychol. 2021, 12, 643069. [Google Scholar] [CrossRef]
- Astorino, T.A.; Edmunds, R.M.; Clark, A.; King, L.; Gallant, R.A.; Namm, S.; Fischer, A.; Wood, K.M. High-Intensity Interval Training Increases Cardiac Output and VO2max. Med. Sci. Sports Exerc. 2017, 49, 265–273. [Google Scholar] [CrossRef]
- Crozier, J.; Roig, M.; Eng, J.J.; MacKay-Lyons, M.; Fung, J.; Ploughman, M.; Bailey, D.M.; Sweet, S.N.; Giacomantonio, N.; Thiel, A.; et al. High-Intensity Interval Training After Stroke: An Opportunity to Promote Functional Recovery, Cardiovascular Health, and Neuroplasticity. Neurorehabilit. Neural Repair 2018, 32, 543–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Maldonado, A.; Rentería, I.; García-Suárez, P.C.; Moncada-Jiménez, J.; Freire-Royes, L.F. The Impact of High-Intensity Interval Training on Brain Derived Neurotrophic Factor in Brain: A Mini-Review. Front. Neurosci. 2018, 12, 839. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Koyama, Y.; Nakamura, Y.; Shimada, S. A novel 5HT3 receptor-IGF1 mechanism distinct from SSRI-induced antidepressant effects. Mol. Psychiatry 2018, 23, 833–842. [Google Scholar] [CrossRef]
- Seo, J.S.; Park, J.Y.; Choi, J.; Kim, T.K.; Shin, J.H.; Lee, J.K.; Han, P.L. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J. Neurosci. 2012, 32, 9690–9699. [Google Scholar] [CrossRef] [Green Version]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Josefsson, T.; Lindwall, M.; Archer, T. Physical exercise intervention in depressive disorders: Meta-analysis and systematic review. Scand. J. Med. Sci. Sports 2014, 24, 259–272. [Google Scholar] [CrossRef]
- Ranjbar, E.; Memari, A.H.; Hafizi, S.; Shayestehfar, M.; Mirfazeli, F.S.; Eshghi, M.A. Depression and Exercise: A Clinical Review and Management Guideline. Asian J. Sports Med. 2015, 6, e24055. [Google Scholar] [CrossRef] [Green Version]
- Kirby, E.D.; Muroy, S.E.; Sun, W.G.; Covarrubias, D.; Leong, M.J.; Barchas, L.A.; Kaufer, D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. eLife 2013, 2, e00362. [Google Scholar] [CrossRef]
- Luo, L.; Li, C.; Deng, Y.; Wang, Y.; Meng, P.; Wang, Q. High-Intensity Interval Training on Neuroplasticity, Balance between Brain-Derived Neurotrophic Factor and Precursor Brain-Derived Neurotrophic Factor in Poststroke Depression Rats. J. Stroke Cerebrovasc. Dis. 2019, 28, 672–682. [Google Scholar] [CrossRef]
- Viana, R.B.; Gentil, P.; Naves, J.P.A.; Rebelo, A.C.S.; Santos, D.A.T.; Braga, M.A.O.; de Lira, C.A.B. Interval Training Improves Depressive Symptoms But Not Anxious Symptoms in Healthy Women. Front. Psychiatry 2019, 10, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorhees, J.L.; Tarr, A.J.; Wohleb, E.S.; Godbout, J.P.; Mo, X.; Sheridan, J.F.; Eubank, T.D.; Marsh, C.B. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS ONE 2013, 8, e58488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.; Zhou, Y.; Hu, Z.; Lou, J.; Song, W.; Li, J.; Liang, X.; Chen, C.; Wang, S.; Yang, B.; et al. 24-hour-restraint stress induces long-term depressive-like phenotypes in mice. Sci. Rep. 2016, 6, 32935. [Google Scholar] [CrossRef] [Green Version]
- Tsuchimine, S.; Matsuno, H.; O’Hashi, K.; Chiba, S.; Yoshimura, A.; Kunugi, H.; Sohya, K. Comparison of physiological and behavioral responses to chronic restraint stress between C57BL/6J and BALB/c mice. Biochem. Biophys. Res. Commun. 2020, 525, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Masrour, F.F.; Peeri, M.; Azarbayjani, M.A.; Hosseini, M.J. Voluntary Exercise During Adolescence Mitigated Negative the Effects of Maternal Separation Stress on the Depressive-Like Behaviors of Adult Male Rats: Role of NMDA Receptors. Neurochem. Res. 2018, 43, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Sohroforouzani, A.M.; Shakerian, S.; Ghanbarzadeh, M.; Alaei, H. Effect of forced treadmill exercise on stimulation of BDNF expression, depression symptoms, tactile memory and working memory in LPS-treated rats. Behav. Brain Res. 2022, 418, 113645. [Google Scholar] [CrossRef]
- Castrén, E.; Võikar, V.; Rantamäki, T. Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 2007, 7, 18–21. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Carlson, P.J.; Singh, J.B.; Zarate, C.A., Jr.; Drevets, W.C.; Manji, H.K. Neural circuitry and neuroplasticity in mood disorders: Insights for novel therapeutic targets. NeuroRx 2006, 3, 22–41. [Google Scholar] [CrossRef] [Green Version]
- Kozisek, M.E.; Middlemas, D.; Bylund, D.B. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol. Ther. 2008, 117, 30–51. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Yan, C.H.; Lu, C.Q.; Xu, J.; Huang, H.; Shen, X.M. Calmodulin activation is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Neurol. Res. 2009, 31, 707–713. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Hillman, C. The influence of exercise on cognitive abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef] [Green Version]
- Schuch, F.B.; Vancampfort, D.; Rosenbaum, S.; Richards, J.; Ward, P.B.; Stubbs, B. Exercise improves physical and psychological quality of life in people with depression: A meta-analysis including the evaluation of control group response. Psychiatry Res. 2016, 241, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Vaidya, V.A. Cyclic AMP response element binding protein and brain-derived neurotrophic factor: Molecules that modulate our mood? J. Biosci. 2006, 31, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Denham, J.; Marques, F.Z.; O’Brien, B.J.; Charchar, F.J. Exercise: Putting action into our epigenome. Sports Med. 2014, 44, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Baktir, M.A.; Srivatsan, M.; Salehi, A. Neuroprotective effects of physical activity on the brain: A closer look at trophic factor signaling. Front. Cell. Neurosci. 2014, 8, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duman, C.H.; Schlesinger, L.; Russell, D.S.; Duman, R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008, 1199, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.E.; Eng, L.F. Glial fibrillary acidic protein in chronic relapsing experimental allergic encephalomyelitis in SJL/J mice. J. Neurosci. Res. 1987, 18, 203–208. [Google Scholar] [CrossRef]
- Lee, T.H.; Jang, M.H.; Shin, M.C.; Lim, B.V.; Kim, Y.P.; Kim, H.; Choi, H.H.; Lee, K.S.; Kim, E.H.; Kim, C.J. Dependence of rat hippocampal c-Fos expression on intensity and duration of exercise. Life Sci. 2003, 72, 1421–1436. [Google Scholar] [CrossRef]
- Doucet, J.P.; Squinto, S.P.; Bazan, N.G. Fos-jun and the primary genomic response in the nervous system. Possible physiological role and pathophysiological significance. Mol. Neurobiol. 1990, 4, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Dragunow, M.; Robertson, H.A.; Robertson, G.S. Amygdala kindling and c-fos protein(s). Exp. Neurol. 1988, 102, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Bergami, M.; Santi, S.; Formaggio, E.; Cagnoli, C.; Verderio, C.; Blum, R.; Berninger, B.; Matteoli, M.; Canossa, M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J. Cell Biol. 2008, 183, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [Green Version]
- Moradi-Kor, N.; Dadkhah, M.; Ghanbari, A.; Rashidipour, H.; Bandegi, A.R.; Barati, M.; Kokhaei, P.; Rashidy-Pour, A. Protective Effects of Spirulina platensis, Voluntary Exercise and Environmental Interventions Against Adolescent Stress-Induced Anxiety and Depressive-Like Symptoms, Oxidative Stress and Alterations of BDNF and 5HT-3 Receptors of the Prefrontal Cortex in Female Rats. Neuropsychiatr. Dis. Treat. 2020, 16, 1777–1794. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vasconcelos-Moreno, M.P.; Borowsky, C.; Zimmermann, A.B.; Wollenhaupt-Aguiar, B.; Ferrari, P.; de Almeida Fleck, M.P. The effects of exercise on oxidative stress (TBARS) and BDNF in severely depressed inpatients. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 605–613. [Google Scholar] [CrossRef]
- Silva, L.A.D.; Tortelli, L.; Motta, J.; Menguer, L.; Mariano, S.; Tasca, G.; Silveira, G.B.; Pinho, R.A.; Silveira, P.C.L. Effects of aquatic exercise on mental health, functional autonomy and oxidative stress in depressed elderly individuals: A randomized clinical trial. Clinics 2019, 74, e322. [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, J.W. The Effect of Regular Exercise on Neurotransmitter Concentration in Elderly Woman. Korea Sport Res. 2006, 17, 143–152. [Google Scholar]
- Strüder, H.K.; Weicker, H. Physiology and pathophysiology of the serotonergic system and its implications on mental and physical performance. Part II. Int. J. Sports Med. 2001, 22, 482–497. [Google Scholar] [CrossRef] [PubMed]
| MICT | HICT | HIIT | |
|---|---|---|---|
| Exercise protocol | 15 m/min × 30 min | 25 m/min × 18 min | 25 m/min, 30 s × 10 rep (rest = 2.5 min) |
| Intensity | Moderate (Sub-LT) | High (Supra-LT) | High (Supra-LT) |
| Training volume (Daily running distance) | 450 m | 450 m | 125 m |
| Weekly exercise duration (Warm-up/main exercise/cool-down) | 200 min | 140 min | 200 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, D.-J.; Um, H.-S.; Choi, D.-H.; Cho, J.-Y. Neuroprotective Effects of High-Intensity Interval Training through Neuroplastic Changes in a Restraint Stress-Induced Depression Model. Appl. Sci. 2023, 13, 7680. https://doi.org/10.3390/app13137680
Hwang D-J, Um H-S, Choi D-H, Cho J-Y. Neuroprotective Effects of High-Intensity Interval Training through Neuroplastic Changes in a Restraint Stress-Induced Depression Model. Applied Sciences. 2023; 13(13):7680. https://doi.org/10.3390/app13137680
Chicago/Turabian StyleHwang, Dong-Joo, Hyun-Seob Um, Dong-Hun Choi, and Joon-Yong Cho. 2023. "Neuroprotective Effects of High-Intensity Interval Training through Neuroplastic Changes in a Restraint Stress-Induced Depression Model" Applied Sciences 13, no. 13: 7680. https://doi.org/10.3390/app13137680
APA StyleHwang, D.-J., Um, H.-S., Choi, D.-H., & Cho, J.-Y. (2023). Neuroprotective Effects of High-Intensity Interval Training through Neuroplastic Changes in a Restraint Stress-Induced Depression Model. Applied Sciences, 13(13), 7680. https://doi.org/10.3390/app13137680





