Experimental Study on the Degradation of Acaricides on the Surface of Kumquat Cuimi by Nonthermal Air Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Instruments and Materials
2.2. Experimental Setup
2.3. Experimental Methods
2.3.1. Preparation of Contaminated Fruits and Treatment
2.3.2. Preparation of Contaminated Fruits and Treatment
- (1)
- Standard curve preparation process:
- (2)
- Chromatographic conditions:
- (3)
- Mass Spectrometry Conditions:
- (4)
- Pesticide degradation rate:
2.3.3. Data Processing
3. Results and Analysis
3.1. Mass Spectrum of Acaricide Solution before and after Plasma Treatment
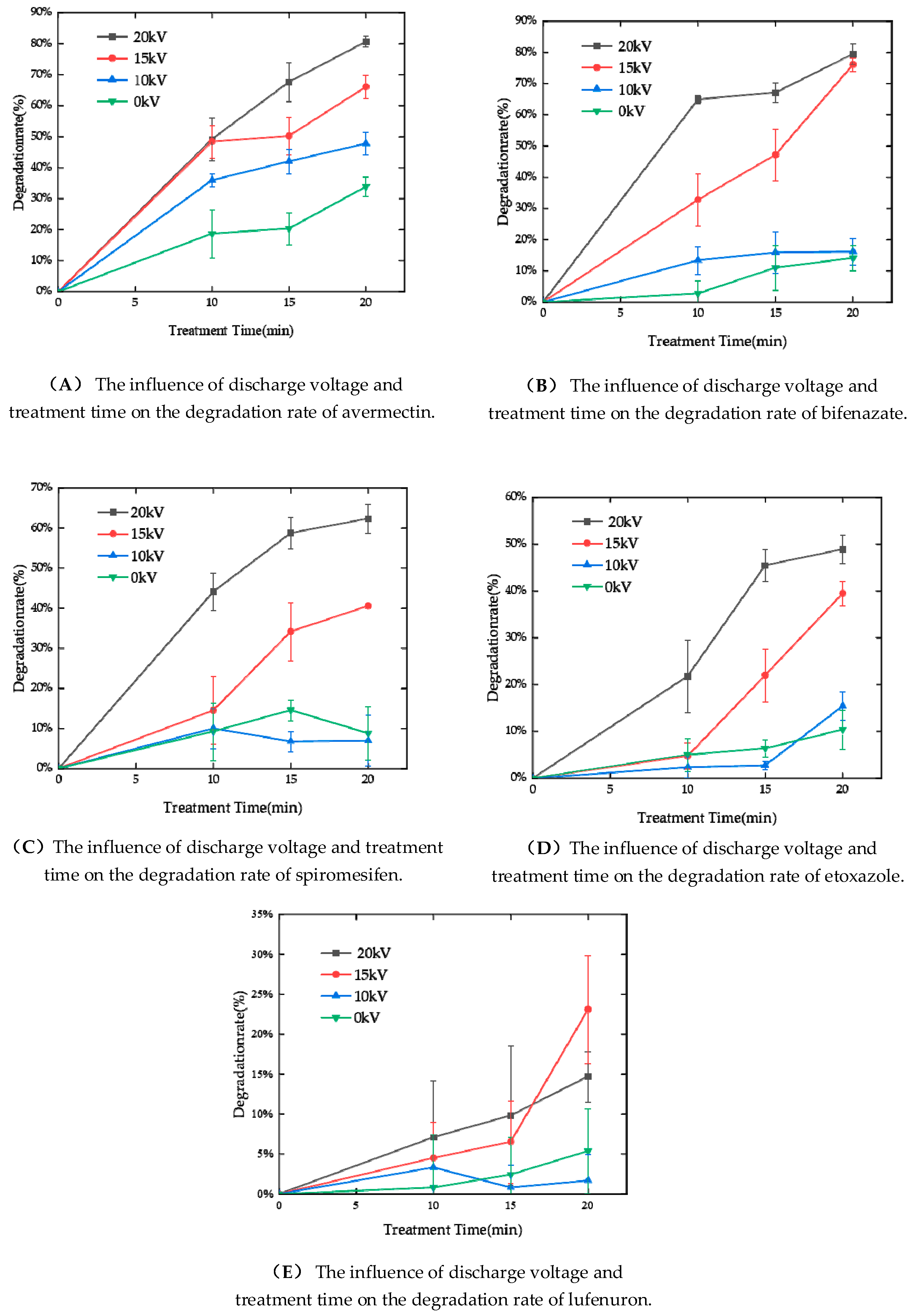
3.2. Plasma Degradation Effect of Five Acaricides on the Surface of Kumquats
4. Discussion
4.1. Chemical Bonds in Pesticide Molecules That Are Prone to Oxidation
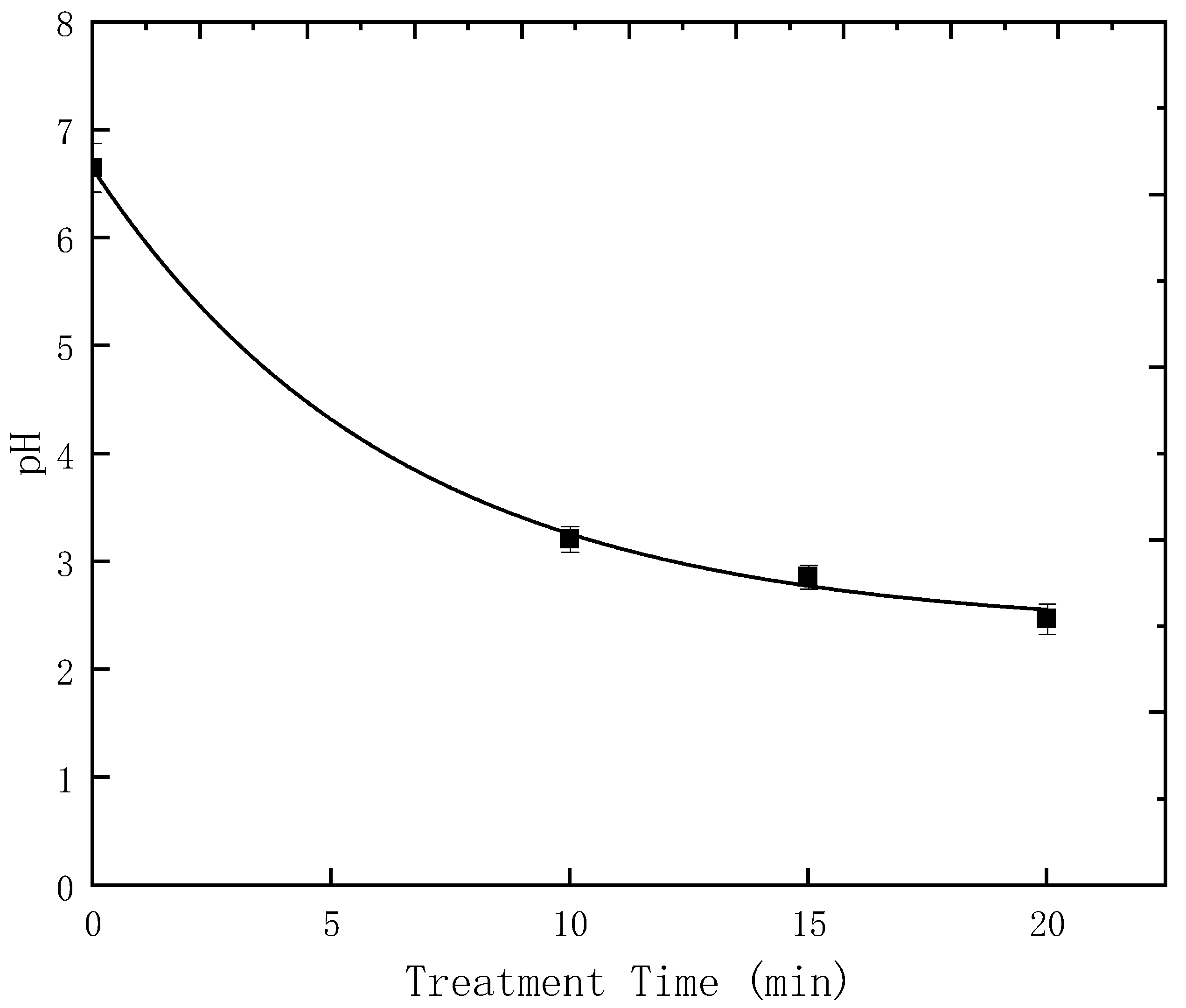
4.2. The Impact of pH on the Degradation of Pesticide Molecules
4.3. The Impact of XLogP3-AA on the Degradation of Pesticide Molecules
5. Conclusions
- The results show that plasma has a significant degradation effect on the acaricide residues on the surface of kumquat cuimi. The discharge voltage and treatment time have a significant impact on the degradation rate. The degradation efficiency varies for different acaricides and is closely related to their molecular structure, solution pH, and hydrophobicity.
- Plasma is a high-energy physical state that can degrade acaricide residues through non-elastic collisions of high-energy particles and oxidation of active groups. However, this study indicates that a higher discharge voltage and longer treatment time do not necessarily result in better degradation efficiency. This is because the active species generated in the plasma can decompose the pesticide molecules into smaller ones, leading to an increase in the concentration of small molecules in the solution, which in turn inhibits the degradation of large molecules. This study provides insights into the practical application of nonthermal plasma for the degradation of pesticide residues on fruit and vegetable surfaces.
- We aim to determine the effective control and optimization of nonthermal plasma parameters for different pesticide residues.
- Taking a micro-scale approach, we aim to explore the dynamic process of pesticide degradation by plasma-active species.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Talon, M. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Urbaneja, A.; Grout, T.G.; Gravena, S.; Wu, F.; Cen, Y.; Stansly, P.A. Citrus Pests in a Global World//The Genus Citrus; Woodhead Publishing: Delhi, India, 2020; pp. 333–348. [Google Scholar]
- Yoon, Y.J.; Kim, E.S.; Hwang, Y.S.; Choi, C.Y. Avermectin: Biochemical and molecular basis of its biosynthesis and regulation. Appl. Microbiol. Biotechnol. 2004, 63, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Sasama, Y.; Takahashi, N.; Ikemi, N. Cyflumetofen, a novel acaricide—Its mode of action and selectivity. Pest Manag. Sci. 2013, 69, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Qiao, C.; Wang, C.; Luo, J.; Guo, L.; Pang, T.; Xie, H. Simultaneous determination of spirodiclofen, spiromesifen, and spirotetramat and their relevant metabolites in edible fungi using ultra-performance liquid chromatography/tandem mass spectrometry. Sci. Rep. 2021, 11, 1547. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Q.; Yang, R.; Li, C.; Fan, S.; Cai, M.; Zhou, X.; Zhang, Z. Quantitative assessment of selective degradation behavior of etoxazole in different classes of organisms by compound-specific isotope analysis. Ecotoxicol. Environ. Saf. 2023, 252, 114632. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-de-Cabezón, F.J.; Pérez-Moreno, I.; Zalom, F.G.; Marco, V. Effects of lufenuron on Lobesia botrana (Lepidoptera: Tortricidae) egg, larval, and adult stages. J. Econ. Entomol. 2006, 99, 427–431. [Google Scholar] [CrossRef]
- Eslami, Z.; Mahdavi, V.; Tajdar-Oranj, B. Probabilistic health risk assessment based on Monte Carlo simulation for pesticide residues in date fruits of Iran. Environ. Sci. Pollut. Res. 2021, 28, 42037–42050. [Google Scholar] [CrossRef]
- Yang, L.; Wang, G.; Fan, J. Research progress on standards, detection, and degradation techniques for pesticide residues in agricultural products. Chin. Agric. Sci. Bull. 2005, 21, 111. [Google Scholar]
- Li, L.; Jiang, S.; Liu, F. Removal methods of pesticide residues in vegetables. Pesticide 2005, 44, 347–351. [Google Scholar]
- Goskonda, S.; James, G.; Junk, T. Sonochemical degradation of aromatic organic pollutants. Waste Manag. 2002, 22, 351–356. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Q.; Zhao, X.; Yin, H. Research progress on biodegradation of pesticide residues. Chin. Agric. Sci. Bull. 2021, 37, 117–124. [Google Scholar]
- Meng, X.; Guo, Y.; Wang, Y.; Fan, S.; Wang, K.; Han, W. A Systematic Review of Photolysis and Hydrolysis Degradation Modes, Degradation Mechanisms, and Identification Methods of Pesticides. J. Chem. 2022, 2022, 9552466. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X.; Li, C. Evaluation of seven chemical pesticides by mixed microbial culture (PCS-1): Degradation ability, microbial community, and Medicago sativa phytotoxicity. J. Hazard. Mater. 2020, 389, 121834. [Google Scholar] [CrossRef]
- Zhang, J.; Han, R.; Su, Y.; Liu, J.; Ren, J. Research progress on microbial degradation of pesticide residues. In Public Plant Protection and Green Control; Wu, K., Ed.; China Agriculture Science and Technology Press: Beijing, China, 2010; pp. 640–645. [Google Scholar]
- Zheng, Y.; Wu, S.; Dang, J.; Wang, S.; Liu, Z.; Fang, J.; Zhang, J. Reduction of phoxim pesticide residues from grapes by atmospheric pressure non-thermal air plasma activated water. J. Hazard. Mater. 2019, 377, 98–105. [Google Scholar] [CrossRef]
- Gerrity, D.; Stanford, B.D.; Trenholm, R.A.; Snyder, S.A. An evaluation of a pilot-scale nonthermal plasma advanced oxidation process for trace organic compound degradation. Water Res. 2010, 44, 493–504. [Google Scholar] [CrossRef]
- Massines, F.; Ghérardi, N.; Naudé, N.; Ségur, P. Recent advances in the understanding of homogeneous dielectric barrier discharges. Eur. Phys. J.-Appl. Phys. 2009, 47, 22805. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, M.; Jin, X. Application of glow discharge plasma for wastewater treatment. Electrochim. Acta 2012, 83, 501–512. [Google Scholar] [CrossRef]
- Novák, I.; Pollak, V.; Chodak, I. Study of surface properties of polyolefins modified by corona discharge plasma. Plasma Process. Polym. 2006, 3, 355–364. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold atmospheric plasma: Charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Altaf, N.U.H.; Naz, M.Y.; Shukrullah, S.; Ghamkhar, M.; Irfan, M.; Rahman, S.; Mahnashi, M.H. Non-Thermal Plasma Reduction of Ag+ Ions into Silver Nanoparticles in Open Atmosphere under Statistically Optimized Conditions for Biological and Photocatalytic Applications. Materials 2022, 15, 3826. [Google Scholar] [CrossRef]
- Sato, M.; Tokutake, T.; Ohshima, T.; Sugiarto, A.T. Aqueous phenol decomposition by pulsed discharges on the water surface. IEEE Trans. Ind. Appl. 2008, 44, 1397–1402. [Google Scholar] [CrossRef]
- Lukes, P.; Locke, B.R. Plasmachemical oxidation processes in a hybrid gas–liquid electrical discharge reactor. J. Phys. D Appl. Phys. 2005, 38, 4074. [Google Scholar] [CrossRef]
- Tomizawa, S.; Tezuka, M. Kinetics and mechanism of the organic degradation in aqueous solution irradiated with gaseous plasma. Plasma Chem. Plasma Process. 2007, 27, 486–495. [Google Scholar] [CrossRef]
- Jufang, Z.; Jierong, C.; Xiaoyong, L.I. Remove of phenolic compounds in water by low-temperature plasma: A review of current research. J. Water Resour. Prot. 2009, 1, 613. [Google Scholar]
- Shang, K.; Li, W.; Wang, X.; Lu, N.; Jiang, N.; Li, J.; Wu, Y. Degradation of p-nitrophenol by DBD plasma/Fe2+/persulfate oxidation process. Sep. Purif. Technol. 2019, 218, 106–112. [Google Scholar] [CrossRef]
- Urashima, K.; Chang, J.S. Removal of volatile organic compounds from air streams and industrial flue gases by non-thermal plasma technology. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 602–614. [Google Scholar] [CrossRef]
- Kan, C.W.; Chan, K.; Yuen CW, M.; Miao, M.H. Surface properties of low-temperature plasma treated wool fabrics. J. Mater. Process. Technol. 1998, 83, 180–184. [Google Scholar] [CrossRef]
- Foest, R.; Schmidt, M.; Becker, K. Microplasmas, an emerging field of low-temperature plasma science and technology. Int. J. Mass Spectrom. 2006, 248, 87–102. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, J.H.; Sun, D.W. Effects of dielectric barrier discharge cold plasma treatments on degradation of anilazine fungicide and quality of tomato (Lycopersicon esculentum Mill) juice. Int. J. Food Sci. Technol. 2021, 56, 69–75. [Google Scholar] [CrossRef]
- Cong, L.; Huang, M.; Zhang, J.; Yan, W. Effect of dielectric barrier discharge plasma on the degradation of malathion and chlorpyrifos on lettuce. J. Sci. Food Agric. 2021, 101, 424–432. [Google Scholar] [CrossRef]
- Mahbubeh Mousavi, S.; Imani, S.; Dorranian, D.; Larijani, K.; Shojaee, M. Effect of cold plasma on degradation of organophosphorus pesticides used on some agricultural products. J. Plant Prot. Res. 2017, 57, 25–35. [Google Scholar] [CrossRef]
- Feng, X.; Ma, X.; Liu, H.; Xie, J.; He, C.; Fan, R. Argon plasma effects on maize: Pesticide degradation and quality changes. J. Sci. Food Agric. 2019, 99, 5491–5498. [Google Scholar] [CrossRef]
- Mahdavi, V.; Eslami, Z.; Golmohammadi, G.; Tajdar-oranj, B.; Behbahan, A.K.; Khaneghah, A.M. Simultaneous determination of multiple pesticide residues in Iranian saffron: A probabilistic health risk assessment. J. Food Compos. Anal. 2021, 100, 103915. [Google Scholar] [CrossRef]
- Misra, N.N.; Zuizina, D.; Cullen, P.J.; Keener, K.M. Characterization of a novel atmospheric air cold plasma system for treatment of packaged biomaterials. Trans. Am. Soc. Agric. Biol. Eng. 2013, 56, 1011–1016. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6434889 (Abamectin), CID 176879 (Bifenazate), CID 9907412 (Spiromesifen), CID 153974 (Etoxazole) and CID 71777 (Lufenuron) (2023). Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 22 May 2023).
- Thirumdas, R.; Annapure, U.S. Enzyme Inactivation in Model Systems and Food Matrixes by Cold Plasma; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Li, S.; Cui, J.; Jiang, Y.; Fang, H. Study on the degradation of imidacloprid pesticide wastewater by low-temperature plasma. High Volt. Eng. 2011, 37, 2517–2522. [Google Scholar]
- Lv, Y.; Zou, L.; Li, H.; Chen, Z.; Wang, X.; Sun, Y.; Fang, L.; Zhao, T.; Zhang, Y. Investigation of non-thermal atmospheric plasma for the degradation of avermectin solution. Plasma Sci. Technol. 2021, 23, 5. [Google Scholar] [CrossRef]
- Odukkathil, G.; Vasudevan, N. Toxicity and bioremediation of pesticides in agricultural soil. Rev. Environ. Sci. Biotechnol. 2013, 12, 421–444. [Google Scholar] [CrossRef]
- Wang, J.; Xing, C.; Xia, J.; Chen, H.; Zhang, J.; Yan, W. Degradation of carbendazim in aqueous solution by dielectric barrier discharge cold plasma: Identification and toxicity of degradation products. Food Chem. 2023, 403, 134329. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 25429 (Carbendazim) and CID 6115 (Aniline) (2023). Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 6 June 2023).
- Sang, W.; Cui, J.; Feng, Y.; Mei, L.; Zhang, Q.; Li, D.; Zhang, W. Degradation of aniline in aqueous solution by dielectric barrier discharge plasma: Mechanism and degradation pathways. Chemosphere 2019, 223, 416–424. [Google Scholar] [CrossRef]

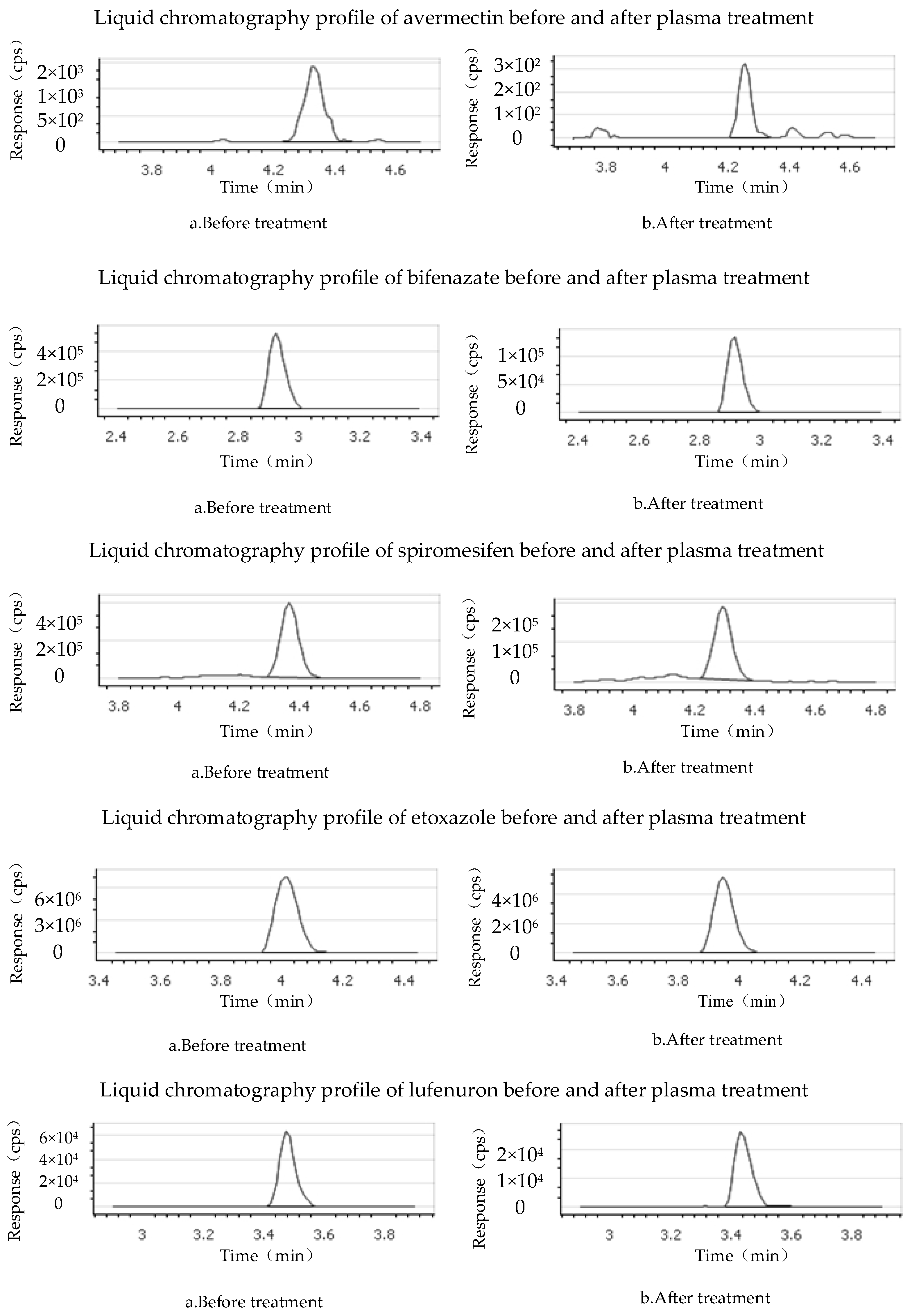
| Time (min) | Flow Rate (mL/min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|---|
| 0 | 0.3 | 80 | 20 |
| 2 | 0.3 | 20 | 80 |
| 6 | 0.3 | 5 | 95 |
| 7.5 | 0.3 | 5 | 95 |
| 7.6 | 0.3 | 80 | 20 |
| 10 | 0.3 | 80 | 20 |
| Acaricide | Parent Ion (m/z) | Product Ion (m/z) | Residence Time (S) | Cone Voltage (V) | Collision Energy (eV) | Quantitative Ion Pair * |
|---|---|---|---|---|---|---|
| Avermectin | 895.5 | 751.5 | 0.05 | 50 | 53 | * |
| Avermectin | 895.5 | 449 | 0.05 | 50 | 59 | |
| Bifenazate | 301.1 | 198.05 | 0.05 | 50 | 9 | * |
| Bifenazate | 301.1 | 170.1 | 0.05 | 50 | 21 | |
| Spiromesifen | 411.2 | 71.1 | 0.05 | 50 | 30 | * |
| Spiromesifen | 411.2 | 313.1 | 0.05 | 50 | 15 | |
| Etoxazole | 360.2 | 141 | 0.05 | 50 | 42 | * |
| Etoxazole | 360.2 | 304 | 0.05 | 50 | 25 | |
| Lufenuron | 511 | 158 | 0.05 | 50 | 24 | * |
| Lufenuron | 511 | 141 | 0.05 | 50 | 67 |
| Name | Molecular Weight | Molecular Formula | Chemical Structure Depiction | XLogP3-AA |
|---|---|---|---|---|
| Avermectin | 873.1 | C48H72O14 | 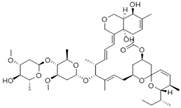 | 3.8 |
| Bifenazate | 300.35 | C17H20N2O3 | 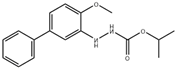 | 4.2 |
| Spiromesifen | 411.3 | C21H24Cl2O4 | 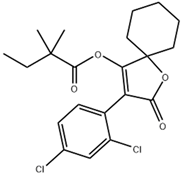 | 5.9 |
| Etoxazole | 359.4 | C21H23F2NO2 | 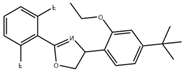 | 5.4 |
| Lufenuron | 511.1 | C17H8Cl2F8N2O3 |  | 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, S.; Chen, S.; Wang, X.; Zang, Y.; Wang, Z.; Wei, J. Experimental Study on the Degradation of Acaricides on the Surface of Kumquat Cuimi by Nonthermal Air Plasma. Appl. Sci. 2023, 13, 7560. https://doi.org/10.3390/app13137560
Qin S, Chen S, Wang X, Zang Y, Wang Z, Wei J. Experimental Study on the Degradation of Acaricides on the Surface of Kumquat Cuimi by Nonthermal Air Plasma. Applied Sciences. 2023; 13(13):7560. https://doi.org/10.3390/app13137560
Chicago/Turabian StyleQin, Si, Shuo Chen, Xiaonan Wang, Yuanfu Zang, Zifeng Wang, and Jie Wei. 2023. "Experimental Study on the Degradation of Acaricides on the Surface of Kumquat Cuimi by Nonthermal Air Plasma" Applied Sciences 13, no. 13: 7560. https://doi.org/10.3390/app13137560
APA StyleQin, S., Chen, S., Wang, X., Zang, Y., Wang, Z., & Wei, J. (2023). Experimental Study on the Degradation of Acaricides on the Surface of Kumquat Cuimi by Nonthermal Air Plasma. Applied Sciences, 13(13), 7560. https://doi.org/10.3390/app13137560






