Abstract
The potential of Calendula officinalis water extract against fungi Aspergillus niger and Penicillium sp. and the effect of extract addition on the quality of wheat bread were investigated. In vitro, the extract reduced the mycelial growth and biomass production of A. niger, but there was no inhibitory effect on Penicillium sp. Enriched bread showed significantly higher total phenolic content, by about 77% and 95% in the bread, in which 10% and 15% of the water was replaced with extract, respectively. The antioxidant potential against DPPH• was significantly higher (compared to the control) in both variants used in the experiment, and the level of antioxidant activity increased with the addition of extract. The enriched bread had good quality characteristics—lower baking losses and higher volume than the control. The moisture content and acidity of the crumb of the extract-enriched bread were also higher. The extract additive used did not affect the sensory properties of the bread.
1. Introduction
Bread and other bakery products are important diet components in many parts of the world. The baking mainly uses white wheat flour obtained after removing wheat (Triticum aestivum L.) bran and germ, which are sources of fibre, phytochemicals, and important nutrients. Because of this, the final product contains less dietary fibre and phenolic compounds. Foods enriched with natural antioxidants are being developed to recompense for this loss. Functional foods consumed as part of a regular diet provide biologically active ingredients that provide health benefits [1]. Unfortunately, bread is a perishable food that goes through physical, chemical, sensory, and microbiological changes during storage. One of the biggest problems in baking is the growth of toxigenic fungi, mainly from the genera Aspergillus and Penicillium [2]. The growth of fungi leads to quality changes in food, but it can also have negative health effects, as some of these microorganisms produce mycotoxins. They are a public health problem due to their widespread occurrence in the world’s food supply [3]. To avoid spoilage of food products by fungi, preservatives are used. However, they have some disadvantages, including altering the aftertaste of light flour products, delaying dough fermentation [4], and not being inert to the consumer. There are reports in the literature of the occurrence of urticaria and contact dermatitis after consuming products with sorbic acid, which is approved for preserving bread [5]. In addition, chemical preservatives are ineffective against some common fungal species in bread, such as Penicillium paneum and P. roqueforti [2].
The threat to food health safety posed by the presence of toxigenic fungi and the demand for natural products has prompted the search for new methods of preserving baked goods and eliminating harmful microorganisms and their metabolites. There is great interest in natural ingredients with multifunctional properties with potential uses in food production. Plant extracts have been widely studied as potential biopreservatives, as plants contain many important antimicrobial compounds, including phenolic compounds, glucosinolates, alkaloids, cyanogenic glycosides, and oxylipins [6,7].
In this context, Calendula officinalis (common marigold), a member of the Asteraceae family, is a valuable product with rich medicinal and functional properties. Many secondary metabolites are responsible for the biological activity of marigolds, such as flavonoids, triterpenoids, and polyphenols, found mainly in flower extract [8,9]. These compounds are responsible for antioxidant activity, exhibit neuroprotective effects, prevent degenerative diseases such as diabetes and cardiovascular diseases, and have anticancer, anti-inflammatory, and antimicrobial effects [10]. Extracts from Calendula officinalis petals have proven antimicrobial activity against many pathogens, including Pseudomonas aeruginosa, Staphylococcus aureus [11], Escherichia coli, Bacillus subtilis, Klebsiella aerogenes, Enterococcus faecalis, Candida albicans, C. glabrata, Aspergillus flavus, A. fumigatus, and A. niger [12].
Reports from other researchers indicate that, depending on the plant species, the best solvents for extracting bioactive compounds are acetone, ethanol, and methanol [13,14]. However, according to current trends, analytical procedures should strive to be minimal, as simple as possible to perform, low-cost, and environmentally friendly. Therefore, the study presented in this paper decided to use deionized water as a solvent to extract bioactive compounds from marigolds. Water extract preparation is a relatively easy and inexpensive process, which is important from the consumer’s point of view.
This study aimed to evaluate the fungistatic properties of the Calendula officinalis water extract against fungi Aspergillus niger and Penicillium sp. and to determine the effect of extract addition on the quality of wheat bread.
2. Materials and Methods
2.1. Fungal Pathogens
Pure cultures of Aspergillus niger and Penicillium sp., isolated from mouldy bread, were obtained from the Department of Analysis and Food Quality Assessment of the University of Life Sciences in Lublin, Poland. The cultures of fungi were kept fresh and viable by periodical transfers on YPD medium (10 g yeast extract, 20 g peptone, 20 g glucose, 20 g agar per 1 L of distilled water; pH 6.0 ± 0.2) under aseptic conditions throughout the study. Strains were stored at 4 °C for routine cultivation.
2.2. Plant Material
Dried petals of Calendula officinalis originating from organic farming (Dary Natury, Grodzisk, Poland) were purchased at a local pharmacy and stored at room temperature.
2.3. Preparation of Plant Water Extract
Calendula officinalis flower water extract was prepared using the method described by Gonelimali et al. [15], with some modifications. The extraction was done using deionised water with a sample-to-solvent ratio of 1:20 (w/v). The dried material was powdered in a laboratory mill (IKA A11 basic, IKA-Werke GmbH & Co. KG, Staufen, Germany) until it passed through a 0.35 mm sieve. Twenty g of powder was soaked in distilled water in a round bottom flask, heated for 30 min at 90 °C, and then incubated for 24 h at 37 °C and 150 rpm in a shaking incubator. The obtained extracts were centrifuged for 10 min at 10,000 rpm and filtered using Whatman No. 1 filter.
2.4. Qualitative Phytochemical Screening
The water extract of Calendula officinalis flowers was evaluated for qualitative determination of major phytochemicals, including alkaloids, saponins, tannins, flavonoids, glycosides, terpenoids, and reducing sugars according to the methodology described below [16,17,18]. Specific reagents were used for each chemical group to induce chemical reactions that developed distinct colours and/or precipitates characteristic of each class of substances.
Test for alkaloids: A few drops of sulfuric acid were added to the 3 mL of extract, and the formation of orange colour indicated the presence of flavonoids.
Test for saponins: 3 mL of extract was shaken vigorously with 3 mL of distilled water in a test tube, and the mixture was warmed. The formation of stable foam was taken as an indication of the presence of saponins.
Test for tannins: 3 mL of the extract was mixed with 3 mL of water and heated in a water bath. Then ferric chloride was added to the mixture. The dark green colour indicated the presence of tannins.
Test for flavonoids: A few drops of lead acetate solution were added to the 3 mL extract. A yellow-colour precipitate indicated the presence of flavonoids.
Test for glycosides: To 2 mL of the extract, 3 mL of glacial acetic acid, one drop of 5% FeCl3 and concentrated H2SO4 were added. A reddish-brown colour appeared at the junction of the two liquid layers, and the top layer was blue-green, indicating the presence of glycosides.
Test for terpenoids: 3 mL of the extract was mixed with 2 mL of chloroform and 3 mL of concentrated sulfuric acid added carefully to form a layer. A reddish-brown colour indicated the presence of terpenoids.
Test for reducing sugars: To 0.5 mL of extract, 5 mL of Benedict’s reagent was added. Then the mixture was boiled for 5 min. The presence of a bluish-green precipitate indicated the presence of reducing sugars.
2.5. Phenolic Content of Calendula officinalis Water Extract
The total phenolic content (TPC) of the extract was determined using Folin-Ciocalteau Reagent (FCR) as described by Singleton and Rossi [19]. To 0.1 mL of the extract were added 3 mL of distilled water and 0.4 mL FCR. After 3 min, 1.5 mL of sodium carbonate (100 g/L) was added, and the contents were mixed and allowed to stand for 30 min. The absorbance of the resulting solutions was measured at 760 nm using a UV–VIS spectrophotometer (Cary 50 Scan, Varian, USA) against a reagent blank. The concentration of TPC was calculated from a standard curve constructed using gallic acid solutions following the method described above. The range of gallic acid standard concentration was between 0 and 1 g/L. The TPC in the extract was expressed as gallic acid equivalents (GAE) in mg/g of dry weight (DW). The experiment was performed in triplicate.
2.6. Antifungal Activity of Calendula officinalis Water Extract In Vitro
2.6.1. Direct Contact Assay
The effect of the Calendula officinalis water extract on mycelial growth was studied according to the methodology described by Gleń and Boligłowa [20]. Twenty millilitres of YPD medium supplemented with Calendula officinalis water extract (5 mg/mL) at 5%, 10%, and 15% (v/v) were poured into sterilized Petri dishes (inner diameter 90 mm). After the medium had solidified, it was inoculated centrally with a 10-day-old culture of fungi A. niger and Penicillium sp. discs (5 mm in diameter). Control was the Petri dishes with pure YPD medium inoculated similarly. The Petri dishes were incubated at 28 °C. The experiment was performed in triplicate.
The effect of Calendula officinalis water extract on the mycelial growth of the tested fungi after 3, 5, and 7 days was calculated from the formula:
where C and T are the average diameters (mm) of fungal mycelia in the control and the treatment, respectively.
Inhibitory activity % = (C − T)/C × 100%
2.6.2. Influence of Calendula officinalis Water Extract on Fungal Biomass Production
Preparation of Spore Suspension of Aspergillus niger and Penicillium sp.
Aspergillus niger and Penicillium sp. were kept on YPD medium plates at 28 °C. Spores of fungus were harvested from 10-day-old cultures in Ringer’s solution (peptone 1 g, NaCl 8 g per 1 L of distilled water; pH 6.9 ± 0.2) and loosened by 15 min shaking. The suspension was adjusted to 1 × 106 spores/mL.
Fungal Biomass Production
The biomass growth of tested fungi was carried out in 200 mL Erlenmeyer flasks per 100 mL of YPG medium (10 g yeast extract, 20 g peptone, 20 g glucose per 1 L of distilled water; pH 6.0 ± 0.2), in which part (5%, 10%, and 15%) was replaced with a previously prepared Calendula officinalis water extract, according to the methodology of Gleń and Boligłowa [20] with some modifications. One mL of the prepared spore suspension of the tested fungi was added to each flask. The control was the flask with pure YPG medium inoculated in the same way. The flasks were incubated at 28 °C for 7 days and at 150 rpm in a shaking incubator. After the incubation period, the contents of each flask were filtered through standard filter paper (each filter had been weighed). The harvested mycelium was dried at 80 °C until a constant weight was obtained. The fungal biomass of each treatment was weighed and compared with the control. The experiment was performed in triplicate.
The influence of Calendula officinalis water extract on fungal biomass production was calculated by using the formula:
where: Wbc is the weight of biomass control, and Wbt is the weight of biomass treatment.
Inhibitory activity % = ((Wbc − Wbt)/Wbc) × 100%
2.7. Effect of the Calendula officinalis Water Extract Addition on Wheat Bread Quality
2.7.1. Laboratory Baking
The control bread was prepared from wheat flour type 750 (150 g), water (90 mL), compressed baker’s yeast (4.5 g), saccharose (1.5 g), and salt (3 g). The study material was wheat bread made of dough, in which part of the water (10% and 15%) prescribed by the recipe was replaced with a previously prepared Calendula officinalis water extract (5 mg/mL). The ingredients were mixed, and the dough was formed for 50 min at 35 °C. Bread loaves were baked for 25 min at 210 °C, then immediately transferred to the laminar flow cabinet and cooled under sterile conditions. Bread loaves were weighted, transferred into plastic bags (one loaf per bag), sealed, and stored in room conditions (until the next day) for further analysis.
2.7.2. Bread Quality Characteristics
The prepared bread dough was weighed, and the dough yield (Yd) was calculated according to the formula:
where: Wd is the weight of dough (g), and Fw is the weight of used flour (g).
Yd (%) = (Wd × 100%)/Fw
The cooled bread (24 h after baking) was weighed, and the bread yield (Yb) was calculated according to the formula:
where: Wb is the weight of bread after cooling (g), Yd is the dough yield (%), and Wd is the weight of dough (g).
Yb (%) = (Wb × Yd)/Wd
The total baking loss (Bl) was calculated according to the formula:
where: Wd is the weight of dough (g), and Wb is the weight of bread after cooling (g).
Bl (%) = ((Wd − Wb))/Wd × 100%
Bread loaf volume was measured 24 h after baking using the millet (Cenchrus americanus L.) seed displacement method. Specific volume (SV) was calculated as the loaf volume divided by weight. The Polish standard determined bread acidity and crumb moisture (PN-A-74108: 1996). To determine acidity, 25 g of bread was weighed into flasks each and 250 mL of distilled water at about 60 °C was added, then closed tightly with a rubber stopper. The sample was shaken for 3 min, then allowed to stand for another 3 min and shaken again for 1 min. The suspension was filtered through cotton wool, and 50 mL of filtrate was taken from each sample into a conical flask. It was titrated with 0.1 M NaOH solution in the presence of a few drops of 1% phenolphthalein until the pink color of the solution appeared and persisted for 30 s. The result was calculated according to the formula:
where: x5—bread acidity [°], a—volume of 0.1 M NaOH solution used for titration [mL].
x5 = 2a
2.8. Phenolics Content and Antioxidant Potential of Bread
2.8.1. Extraction of Bioactive Compounds
The bread samples (1 g) were ground, placed in Falcon tubes, and shaken with 10 mL of a 4:1 (v/v) ethanol/water mixture for 120 min in a laboratory shaker. Then, the samples were centrifuged at 3000 rpm for 10 min. The supernatant was stored at −18 °C for further analysis.
2.8.2. Phenolics Content
The TPC of the bread extract was determined using FCR as described in Section 2.5. and the results are expressed as gallic acid equivalents in mg/g of bread.
2.8.3. DPPH (2-Diphenyl-1-Picrylhydrazyl) Radical Scavenging Activity
The DPPH assay was determined according to Shahidi et al. [21], with some modifications. A 0.15 mL of the prepared extract was mixed with 2.85 mL of a 0.1 mM solution of DPPH• in 75% methanol. The absorbance was measured after 30 min of reaction at 517 nm. The scavenging percentage was calculated using the formula:
where Asample is the absorbance of the mixture of sample and DPPH•; Acontrol is the absorbance of the control (DPPH• solution).
Scavenging activity % = (Acontrol − Asample)/Acontrol × 100%
2.8.4. ABTS (2,2′-Azobis(3-Etylobenzotiazolino-6-Sulfonianu)) Radical Scavenging Activity
The ABTS assay was determined according to Re et al. [22], with some modifications. The radical solution was prepared with ABTS and potassium persulfate, diluted in water to a final concentration of 2.45 mM, and left in the dark for 16 h to allow for radical development. The solution was diluted to reach the absorbance measures around 0.7 at 734 nM. Then, 2.85 mL of the ABTS•+ solution was mixed with 0.15 mL of each sample. The absorbance was measured after 30 min of the reaction at 734 nm. The scavenging percentage was calculated using the formula:
where Asample is the absorbance of the sample and ABTS•+; A control is the absorbance of the control (ABTS•+ solution).
Scavenging activity % = (Acontrol − Asample)/Acontrol × 100%
2.9. Microbiological Quality of Bread
The number of yeasts and moulds per 1 g of bread was determined using the plate technique on YGC medium (5 g yeast extract, 20 g glucose, 0.1 g chloramphenicol, 15 g agar per 1 L of distilled water; pH 6.6 ± 0.2). The assay was performed on the first day (after cooling) and after 7 days of storage in sterile Petri dishes at 28 °C. To isolate yeasts and moulds from bread, 10 g of crumbs were collected under sterile conditions under a laminar chamber into 300 mL conical flasks containing 90 mL of sterile Ringer’s solution. The flasks were shaken at 180 rpm for 10 min. Then serial dilutions were made, and 1 mL was transferred to sterile Petri dishes containing YGC medium with chloramphenicol. The flasks were incubated for 5 days at 28 °C. After incubation, the colonies were counted and expressed as colony-forming units per gram (cfu/g) of samples. The test was performed in triplicate for each sample.
2.10. Consumer Evaluation
Consumer evaluation of bread was conducted on 29 consumers (18–25 years old). The bread was cut into slices about 1.5 cm thick the day after baking and coded with a three-digit number. The external appearance, crust (appearance and connection with the crumb; colour; thickness), crumb (appearance; colour; porosity; elasticity), smell, and taste were assessed. The bread was evaluated using the 5-point quality scale method. Grade 5 meant very good quality, grade 4—good quality, grade 3—satisfactory quality, grade 2—unsatisfactory quality, and grade 1—bad quality. The evaluation was based on a table with assigned definitions to each scale of scores for individual bread characteristics. Each quality discriminant has assigned an appropriate weighting factor (external appearance 0.1; crust 0.1; crumb 0.2; smell 0.2; taste 0.4). Plain water was used for mouth rinsing before and after each sample test.
2.11. Statistical Analysis
The results were analyzed statistically using Statistica 13.3 (StatSoft, Cracow, Poland) and Excel 2019 (Microsoft, Washington, DC, USA). A one-way analysis of variance (ANOVA) was carried out to compare the results, and the significance of differences between group means was determined using Tukey’s post hoc test. All statistical hypotheses were verified at the significance level of p < 0.05.
3. Results and Discussion
3.1. Qualitative Phytochemical Screening
The results of qualitative phytochemical screening of the Calendula officinalis water extract are shown in Table 1. The preliminary phytochemical test results indicated the presence of alkaloids, saponins, flavonoids, glycosides and terpenoids in the tested extract.

Table 1.
Phytochemical screening of the Calendula officinalis flower water extract.
The results agree with the literature data [23,24] and indicate that marigold can be considered a potential raw material for the extraction of bioactive components. Calendula officinalis is an important medicinal plant with diverse phytochemicals and biological activities, such as antioxidant, anti-inflammatory, and antimicrobial. Among the phytochemicals in marigold petals, triterpenes, flavonoids, phenolic acids, quinones and coumarins, and carotenoids are reported to play the most important role [25]. These compounds are responsible for antimicrobial activity, and their mechanisms of action are diverse, including inhibition of nucleic acid synthesis, interference with cytoplasmic membrane function, and slowing of microbial metabolism [26].
3.2. Total Phenolic Content of Calendula officinalis Water Extract
The TPC determined in the Calendula officinalis water extract was 12.8 ± 0.007 mg GAE/g DM.
Using various solvents and extraction conditions, many researchers have attempted to extract phenolic compounds from Calendula officinalis. Sytar et al. [27] showed that among the tested methanolic extracts of leaves of the representative family Asteraceae, marigold leaf extracts have been shown to have the highest total content of these compounds (1.125 ± 0.153 mg/g DM). A study by Olennikov and Kashchenko [28] reported that the TPC in the ethanolic extract of marigold leaves ranged from 29.21 to 50.24 mg/g, depending on the variety. In a study by Piekut [29], the ethanolic extract of marigold flowers had a low TPC content of 6.19 mg/g DM. Petkova et al. [30] found that calendula infusion showed a TPC of 16.49 ± 0.20 mg/g DM, while the decoction had a higher content of these compounds (19.44 ± 0.04 mg/g DM). Water extracts of Calendula officinalis flowers and leaves were studied by Mubashar Sabir et al. [31], showing that the TPC was 72.91 ± 2.1 mg/g and 23.1 ± 0.8 mg/g, respectively. Krochmal-Marczak and Kiełtyka-Dadasiewicz [32] found a significantly lower polyphenols content ranging from 0.06 to 3.64 mg/100 g DW in an infusion of marigold flowers, depending on brewing time and temperature.
Comparing the obtained results with the above reports by other authors, it can be concluded that the TPC in the plant extract is affected by many factors. These include the type of solvents used, their ratio, extraction time and temperature, the chemical composition and physical properties of the plant materials, and even the part of the plant used (flowers, leaves). Data available in the literature state that the chemical compounds found in plants are also influenced by the region where the plant is grown, climatic conditions (e.g., rainy and dry seasons), growing season (beginning or end of flowering), and genetic factors [27,33]. Therefore, all these factors should be considered when comparing the TPC in an extract from a given plant. The repeatability of chemical composition is one of the main problems in using plant extracts as food preservative ingredients. Extracts of natural origin, or different batches of extracts produced by different manufacturers, can exhibit quantitative and qualitative variability.
3.3. Antifungal Activity of Calendula officinalis Water Extract In Vitro
3.3.1. Direct Contact Assay
The study found that adding Calendula officinalis water extract to the medium limited the mycelial growth of A. niger after 3, 5, and 7 days of incubation on Petri dishes (Figure 1). The greatest inhibition was found in a Petri dish with the addition of 15% (v/v) of water extract in the medium, and it ranged from 49.81% on the 7th day of cultivation to 62.98% on the 3rd day. On the other hand, the tested extract did not affect inhibiting the mycelial growth of Penicillum sp. (Figure 2).
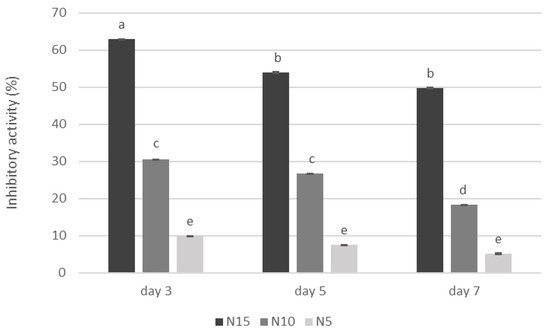
Figure 1.
Effect of adding Calendula officinalis water extract on the mycelial growth of Aspergillus niger. N15, N10, N5—addition of Calendula officinalis water extract to the medium in doses of 15%, 10%, and 5% (v/v), respectively; Values marked with the same letters do not differ significantly at p < 0.05 (Tukey’s post hoc test); mean value (n = 3) ± SD.
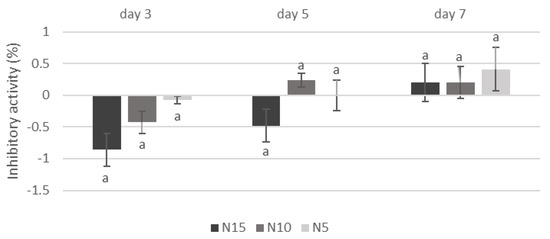
Figure 2.
Effect of adding Calendula officinalis water extract on the mycelial growth of Penicillium sp. N15, N10, N5—addition of Calendula officinalis water extract to the medium in doses of 15%, 10%, and 5% (v/v), respectively; Negative values denote stimulation of fungal biomass production; Values marked with the same letters do not differ significantly at p < 0.05 (Tukey’s post hoc test); mean value (n = 3) ± SD.
Numerous literature reports indicate the effectiveness of extracts prepared from various plant species, including marigolds, against pathogenic microorganisms [6,12,27,34,35]. Efstratiou et al. [12] found that methanolic and ethanolic extracts of Calendula officinalis petals showed excellent antifungal activity against 9 different species from genera Candida, Aspergillus, and Exophiala. Similar to the present study, the researchers proved that marigold extract reduced the growth of A. niger on Petri dishes [12]. Rigane et al. [36] also showed such activity for water-methanol extracts from calendula flowers and leaves. Ikeura et al. [37] screened the antifungal activity of extracts from 16 plants (dichloromethane and diethyl ether were the solvents) against Penicillium expansum and showed that marigold extract strongly reduced the growth of the pathogen by the steam contact method.
The antimicrobial properties of Calendula officinalis are due to the presence of numerous bioactive compounds; among them, phenolic compounds play an important role in the control of fungi [38]. The literature shows that their antimicrobial activity is based on different mechanisms due to the wide variation in the structure of these substances. Phenolic compounds can interact with the cytoplasmic membrane, cell wall, nucleic acids, and energy transport, altering or inhibiting their functions. They also denature enzymes or bind vitamins, minerals, or carbohydrates, making these compounds unavailable to microorganisms [39].
3.3.2. Influence of Calendula officinalis Water Extract on Fungal Biomass Production
There was a significant inhibition of A. niger biomass production after 7 days of incubation when 15% and 10% Calendula officinalis water extract were added to the culture medium. The reduction in pathogen biomass was 15.50% and 9.17%, respectively. On the other hand, as in the previous experiment on Petri dishes, the study extract did not significantly affect the inhibition of Penicillium sp. biomass production.
Studies on the inhibitory effect of plant extract on the growth of pathogenic fungi biomass have been reported by some researchers. Bajwa et al. [40] evaluated the potential of water extracts of Parthenium hysterophorus, a plant in the Asteraceae family, against three pathogenic fungi: Drechslera tetramera, Phoma glomerata, and A. niger. The lower extract concentrations proposed in the experiment caused a decrease in fungal biomass production, and this response was species-specific. In contrast, increasing extract concentrations increased fungal biomass production at all harvest intervals. Gleń and Boligłowa [20] showed that water extracts of walnut (Juglans regia) leaves, birch (Betula verrucosa Ehrh.) bark, and nettle (Utrica dioica L.) herb strongly reduced the biomass production of Fusarium culmorum (29.60–53.70%) and Botrytis cinerea (17.90–53.80%), while in some variants of the experiment tested extracts showed a stimulating effect on the biomass growth of Alternaria alternata, Phoma exiqua, and Sclerotina sclerotiorum. El–Mohamedy and Abdallah [41] tested the antifungal activity of Moringa oleifera Lam. seed oil and extract against Fusarium oxysporum, F. solani, Alternaria solani, A. alternate, Rhizoctonia solani, Sclerotium rolfsii and Macrophomina phaseolina, and the researchers showed that study oil and extract had an inhibitory effect on the growth of pathogen biomass.
The results presented in the paper and reports by other researchers indicate that a particular plant extract can reduce the biomass production of one pathogen and have no effect or even stimulate the growth of another. Therefore, testing a broad spectrum of product-specific microorganisms is appropriate when designing new foods with plant extracts. This will make it possible to create foods of high quality and high safety for the consumer.
3.4. Bread Quality Characteristics
Due to the low fungistatic activity obtained with the 5% addition of Calendula officinalis water extract in the culture medium, it was decided to use only the higher addition in bread production and replace the water recommended by the recipe with 10% and 15% extract. The evaluation of the control and bread with the addition of extract quality parameters is shown in Table 2. The appearance of the prepared bread is shown in Figure 3.

Table 2.
Evaluation of bread quality parameters.
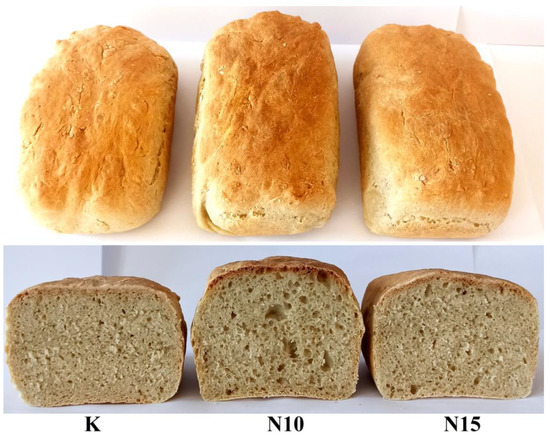
Figure 3.
The appearance of control bread (K) and with the addition of Calendula officinalis water extract, 10% (N10) and 15% (N15), respectively.
The addition of Calendula officinalis water extract used in the study did not affect bread yields, and the results were similar to the control. The experiments found that bread with the extract showed significantly lower baking losses compared to the control. The total baking loss depends on numerous factors, including the size, shape, volume, and type of bread, dough management and baking method, and even the type of oven used [42]. Alotaibi et al. [43] report that the lower baking losses in bread with the addition of marigold may be due to the presence of a large amount of lutein, which binds to water and thus reduces moisture loss. Accordingly, it was found that the enriched bread had a significantly higher (about 18%) moisture content compared to the control. The bread with extract also had significantly higher volume than the control, by about 23% and 26% in the bread, in which 15% and 10% of the water was replaced with Calendula officinalis extract, respectively. The specific bread volume is considered one of the main characteristics defining consumer acceptability [43], so the results obtained in this paper are significant. In contrast to the present study, Alotaibi et al. [43] found that enriching bread with dried marigold flower powder reduced volume. In the experiments presented here, it was also noted that the acidity of the bread increased as the addition of extract increased.
Dough expansion depends on dough rheological characteristics, primarily due to the gluten and starch components. Adding bioactive ingredients may physically modify the development of bread dough [44] since antioxidants can form complexes with proteins and/or polysaccharides. Several studies have shown an adverse effect of the powdered plant material addition on bread volume [45]. This can be explained by the presence of fibre, which interacts with proteins and weakens the gluten network of the dough, resulting in a reduction in the amount of CO2 retained in the dough during fermentation [46]. Jakubczyk et al. [47] report that Calendula officinalis has a high total fibre content (62.33 ± 9.17 g/100 g), but most of the fraction is insoluble (57.54 ± 8.32 g/100 g). In general, insoluble fibres reduce the technological quality of baked goods, while soluble fibres have a positive effect [48], so using water extract was reasonable in the present study.
3.5. Phenolics Content and Antioxidant Potential of Bread
The effect of adding Calendula officinalis water extract on the TPC and antioxidant activity of wheat bread is presented in Table 3. As expected, marigold extract positively affected fortified bread’s phenolic content and antioxidant status. Compared to the control, the TPC was significantly higher by about 77% and 95% in the bread, in which 10% and 15% of the water was replaced with Calendula officinalis extract, respectively. In addition, the experiment observed significantly higher free radical scavenging activity for ABTS•+ than DPPH•, which may be related to their solubility in water and organic solvents. The same relation was found in other studies for marigold flower extract [23,30]. In the present study, the antioxidant potential against DPPH• was significantly higher in both enriched bread than bread without extract. In this case, the level of antioxidant activity increased with the addition of marigold water extract. In contrast, the antiradical activity against ABTS•+ was higher only in bread, in which 15% of the water was replaced by Calendula officinalis extract.

Table 3.
Phenolics content and antioxidant activity of wheat bread with Calendula officinalis water extract.
Food fortification is of increasing interest to consumers. Many products are enriched to improve their phenolic levels and antioxidant potential, thereby increasing their health-promoting properties. This is particularly important for bread derived from white wheat flour. Edible flowers are a good source of compounds with antioxidant activity [10], and Calendula officinalis has been extensively studied in this aspect [23,27,30]. Measuring the antioxidant capacity can provide a wealth of information on the oxidation resistance, the quantitative contribution of antioxidants, or the antioxidant effects that may occur in the body during consumption [49]. The radical-scavenging properties of natural compounds are often associated with their ability to quench free radicals. Many literature reports confirm that adding plant extracts increases bread’s polyphenol content and antioxidant activity [50,51], and our results indicate the potential of Calendula officinalis in food fortification.
3.6. Microbiological Quality of Bread
After 7 days of bread storage in Petri dishes at 28 °C, there was no visual evidence of microbial spoilage. However, after the isolation of yeasts and moulds from the bread, their presence was found in all samples, but their amount was within acceptable standards for bread (Table 4). The highest number of yeast and mould was found in the control, both on the baking day (1.50 × 102 cfu/g) and after 7 days of storage (2.30 × 102 cfu/g). Similar numbers of these microorganisms (2.20 × 102 cfu/g) were found after storage in bread with 15% of the prepared extract. Conversely, the lowest number of yeast and mould on a baking day (1.23 × 102 cfu/g) as well as after 7 days of storage (1.90 × 102 cfu/g) was observed in bread in which 10% of the water was replaced with the Calendula officinalis extract.

Table 4.
Number of yeasts and moulds in bread.
One of the difficult challenges facing the bakery industry is to reduce microbial spoilage, especially fungi, thereby extending shelf life and guaranteeing product safety. It has been proven that some plant extracts can inhibit the growth of pathogenic microorganisms, so enriching bread is reasonable. Pinilla et al. [52] found that bread fortified with garlic extract was more microbiologically stable compared to the control, and the addition of the extract inhibited the growth of the fungi A. flavus, Penicillium herquei, and F. graminearum in the bread. A study by Doudi et al. [53] proved that cinnamon (Cinnamomum verum) extract improves the shelf life of bread, delays spoilage, and reduces the growth of Aspergillus sp. fungi. Numerous works by other researchers have prompted the search for natural bread additives to extend the shelf life of the finished product. Several reports in the literature about the effectiveness of marigold extract against fungi of the Aspergillus and Penicillium genera [12,36,37] pose a threat in baking. The results presented in this paper prove that the appropriate addition of Calendula officinalis water extract can be successfully applied to bread and positively affect the microbiological quality of the product.
3.7. Consumer Evaluation
The average consumer ratings of the bread from the individual parameters were similar in each sample and were not statistically significantly different from each other (Table 5). The control and enriched bread received similar overall ratings and were of good quality, according to consumers. The control bread received an overall score of 3.96, the bread in which 10% of the water was replaced with prepared Calendula officinalis water extract received 4.02, and in the case of 15% replacement of water with extract 3.94. The results show that the addition of Calendula officinalis water extract used in the experiment had no significant effect on the organoleptic properties of the bread.

Table 5.
Sensory evaluation of bread prepared with Calendula officinalis water extract.
The attractiveness of bread consists of many different characteristics, but the first to attract the consumer’s attention is external appearance and colour. Edible flowers or extracts from them can be an interesting alternative to artificial colours used in food technology [54]. The obtained Calendula officinalis extract was brown, but the amount of additive used was low enough that it did not significantly change the colour of the finished product. The addition of plant extracts rich in phenolic compounds, which aim to increase the antioxidant capacity of wheat bread, can also affect certain rheological properties of the dough, such as gumminess, strength, elasticity, adhesiveness, and chewiness, among others [1]. As a result, the sensory properties of the final product are also modified, which is not always acceptable to consumers. For example, in a study by Czaja et al. [51], according to consumers, bread enriched with onion extract had a less acceptable smell and taste than the control bread. In the development of functional foods, a key role is played by finding ingredients that allow specific beneficial effects to be achieved without modifying the sensory properties of the enriched product. Therefore, the results obtained in the present study are significant and encourage further research into using Calendula officinalis water extract for bread fortification.
4. Conclusions
The demand for natural, chemical-free, “clean label” products induces the search for new methods to extend food shelf life and eliminate harmful microorganisms from food products. Plants extracts are an active area for research due to their rich phytochemical composition. Calendula officinalis is widely used in cosmetics, while the present study demonstrated the potential of this plant in food fortification. The studied marigold extract showed antifungal properties against A. niger, and the enriched bread had satisfactory quality characteristics, exhibited antiradical activity, and had unchanged sensory properties. The results encourage further research into using Calendula officinalis in food technology.
Author Contributions
Conceptualization, I.P.-K. and U.P.; methodology, I.P.-K. and U.P.; software, I.P.-K.; validation I.P.-K.; formal analysis, I.P.-K.; investigation, I.P.-K.; data curation, I.P.-K.; writing—original draft preparation, I.P.-K.; writing—review and editing, U.P.; supervision, U.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Czajkowska–González, Y.A.; Alvarez–Parrilla, E.; del Rocío Martínez–Ruiz, N.; Vázquez–Flores, A.A.; Gaytán–Martínez, M.; de la Rosa, L.A. Addition of Phenolic Compounds to Bread: Antioxidant Benefits and Impact on Food Structure and Sensory Characteristics. Food Prod. Process. Nutr. 2021, 3, 25. [Google Scholar] [CrossRef]
- Garcia, M.V.; Bernardi, A.O.; Copetti, M.V. The Fungal Problem in Bread Production: Insights of Causes, Consequences, and Control Methods. Curr. Opin. Food Sci. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A.; Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; et al. Mycotoxins: Factors Influencing Production and Control Strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Fik, M. Czerstwienie Pieczywa i Sposoby Przedłużania Jego Świeżości. ŻYWNOŚĆ. Nauk. Technol. Jakość 2004, 2, 5–22. [Google Scholar]
- Abdulmumeen, H.A.; Risikat, A.N.; Sururah, A.R. Food: Its Preservatives, Additives and Applications. Int. J. Chem. Biochem. Sci. 2012, 1, 36–47. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers as Sources of Phenolic Compounds with Bioactive Potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Hasan, S.; Zzaman, W.; Rana, M.R.; Ahmed, S.; Roy, M.; Sayem, A.; Matin, A.; Raposo, A.; et al. A Comprehensive Review on Bio-Preservation of Bread: An Approach to Adopt Wholesome Strategies. Foods 2022, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A Systematic Review of Calendula officinalis Extract for Wound Healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef]
- Martin, D.; Navarro Del Hierro, J.; Villanueva Bermejo, D.; Fernández-Ruiz, R.; Fornari, T.; Reglero, G. Bioaccessibility and Antioxidant Activity of Calendula officinalis Supercritical Extract as Affected by in Vitro Codigestion with Olive Oil. J. Agric. Food Chem. 2016, 64, 8828–8837. [Google Scholar] [CrossRef]
- Prabawati, N.B.; Oktavirina, V.; Palma, M.; Setyaningsih, W. Edible Flowers: Antioxidant Compounds and Their Functional Properties. Horticulturae 2021, 7, 66. [Google Scholar] [CrossRef]
- Hamad, M.N.; Mohammed, H.; Merdaw, M. Antibacterial Activity of Calendula officinalis Flowers in vitro. Ibn Al-Haitham J. Pure Appl. Sci. 2011, 24, 3. [Google Scholar] [CrossRef]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial Activity of Calendula officinalis Petal Extracts against Fungi, as Well as Gram-Negative and Gram-Positive Clinical Pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Truong, H.B.; Nguyen, H.C. Optimization of Extraction of Phenolic Compounds from Ocimum basilicum Leaves and Evaluation of Their Antioxidant Activity. Pharm. Chem. J. 2020, 54, 162–169. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Kannan, V.; Fahad, S.M.; Arumugam, C.S.; Vinothkumar, D.; Ramesh Babu, N.G. Phytochemical Screening of Bauhinia purpurea L.: An Important Medicinal Plant. Int. Res. J. Pharm. 2015, 6, 802–804. [Google Scholar] [CrossRef]
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial TTextiles: A Review. Plants 2022, 11, 2011. [Google Scholar] [CrossRef]
- Shaikh, J.R.; Patil, M. Qualitative Tests for Preliminary Phytochemical Screening: An Overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Gleń, K.; Boligłowa, E. Ocena Aktywności Fungistatycznej Wyciągów Roślinnych w Testach in Vitro. J. Res. Appl. Agric. Eng. 2012, 57, 104–109. [Google Scholar]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant Activity of White and Black Sesame Seeds and Their Hull Fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Ak, G.; Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Cakir, O.; Bensari, S.; Yilmaz, M.A.; Gallo, M.; Montesano, D. A Comparative Bio-Evaluation and Chemical Profiles of Calendula officinalis L. Extracts Prepared via Different Extraction Techniques. Appl. Sci. 2020, 10, 5920. [Google Scholar] [CrossRef]
- Ghaima, K.K.; Rasheed, S.F.; Ahmed, E.F. Antibiofilm, Antibacterial and Antioxidant Activities of Water Extract of Calendula officinalis Flowers. Int. J. Biol. Pharm. Res. 2013, 4, 465–470. [Google Scholar]
- Escher, G.B.; Borges, L.D.C.C.; Santos, J.S.; Cruz, T.M.; Marques, M.B.; Do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’ana, A.S.; Wen, M.; et al. From the Field to the Pot: Phytochemical and Functional Analyses of Calendula officinalis L. Flower for Incorporation in an Organic Yogurt. Antioxidants 2019, 8, 559. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Kordowska-Wiater, M.; Sosnowska, B.; Pytka, M. Oddziaływanie Ekstraktów Roślinnych Na Drobnoustroje; University of Life Sciences in Lublin: Lublin, Poland, 2020; ISBN 9788372593252. [Google Scholar]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative Analysis of Bioactive Phenolic Compounds Composition from 26 Medicinal Plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Componential Profile and Amylase Inhibiting Activity of Phenolic Compounds from Calendula officinalis L. Leaves. Sci. World J. 2014, 2014, 654193. [Google Scholar] [CrossRef]
- Piekut, J. Ocena Wybranych Ekstraktów Roślin Przyprawowych Pod Względem Ich Właściwości Przeciwdrobnoustrojowych Oraz Zawartości Fenolokwasów. Zesz. Probl. Postępów Nauk Rol. 2018, 588, 103–111. [Google Scholar] [CrossRef]
- Petkova, D.; Mihaylova, D.; Denev, P.; Krastanov, A. Antioxidant Activity of Some Edible Flowers Water Extracts from Bulgaria. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2020, 77, 54. [Google Scholar] [CrossRef] [PubMed]
- Mubashar Sabir, S.; Khan, M.F.; Rocha, J.B.T.; Boligon, A.A.; Athayde, M.L. Phenolic Profile, Antioxidant Activities and Genotoxic Evaluations of Calendula officinalis. J. Food Biochem. 2015, 39, 316–324. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Kiełtyka-Dadasiewicz, A. Wpływ Temperatury Wody i Czasu Parzenia Na Właściwości Antyoksydacyjne Naparów z Nagietka Lekarskiego (Calendula officinalis L.). Herbalism 2018, 4, 43–51. [Google Scholar] [CrossRef]
- Avci, A.B.; Inan, M. Comparing of Cultivated Annual and Perennial Calendula officinalis L. Species. J. Agric. Nat. 2021, 4, 579–585. [Google Scholar] [CrossRef]
- Ali, E.M.; Abd El-Moaty, H. Antifungal Activity of Achillea santolina L. and Calendula officinalis L. Essential Oils and Their Constituents against Fungal Infection of Liver as Complication of Cyclophosphamide Therapy. J. Essent. Oil-Bear. Plants 2017, 20, 1030–1043. [Google Scholar] [CrossRef]
- Chaleshtori, S.H.; Kachoie, M.A.; Pirbalouti, A.G. Phytochemical Analysis and Antibacterial Effects of Calendula offcinalis Essential Oil. Biosci. Biotechnol. Res. Commun. 2016, 9, 517–522. [Google Scholar] [CrossRef]
- Rigane, G.; Ben Younes, S.; Ghazghazi, H.; Ben Salem, R. Investigation into the Biological Activities and Chemical Composition of Calendula officinalis L. Growing in Tunisia. Int. Food Res. J. 2013, 20, 3001–3007. [Google Scholar]
- Ikeura, H.; Somsak, N.K.F.; Kanlayanara, S.H.Y. Application of Selected Plant Extracts to Inhibit Growth of Penicillium expansum on Apple Fruits. Plant Pathol. J. 2011, 10, 79–84. [Google Scholar] [CrossRef]
- El-Shahir, A.A.; El-Wakil, D.A.; Latef, A.A.H.A.; Youssef, N.H. Bioactive Compounds and Antifungal Activity of Leaves and Fruits Methanolic Extracts of Ziziphus Spina-christi L. Plants 2022, 11, 746. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Smole Možina, S.; Katalinić, V.; Boban, N.; Generalić Mekinić, I. Interactions of Resveratrol with Other Phenolics and Activity against Food-Borne Pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, R.; Khalid, A.; Shahid Cheem, T. Antifungal Activity of Allelopathic Plant Extracts III: Growth Response of Some Pathogenic Fungi to Aqueous Extract of Parthenium hysterophorus. Plant Pathol. J. 2003, 2, 145–156. [Google Scholar] [CrossRef]
- El–Mohamedy, R.S.R.; Abdallah, A.M. Antifungal Activity of Moringa Oleifera Oil and Seed Extract against Some Plant Pathogenic Fungi. Middle East J. Agric. Res. 2014, 3, 42–249. [Google Scholar]
- Wirkijowska, A.; Zarzycki, P.; Sobota, A.; Nawrocka, A.; Blicharz-Kania, A.; Andrejko, D. The Possibility of Using By-Products from the Flaxseed Industry for Functional Bread Production. LWT 2020, 118, 108860. [Google Scholar] [CrossRef]
- Alotaibi, H.N.; Anderson, A.K.; Sidhu, J.S. Influence of Lutein Content of Marigold Flowers on Functional Properties of Baked Pan Bread. Ann. Agric. Sci. 2021, 66, 162–168. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Quek, S.; Perera, C.O. Physicochemical Properties of Bread Dough and Finished Bread with Added Pectin Fiber and Phenolic Antioxidants. J. Food Sci. 2011, 76, H97–H107. [Google Scholar] [CrossRef]
- Wójcik, M.; Różyło, R.; Cacak-, G. Textural and Sensory Properties of Wheat Bread Fortified with Nettle (Urtica dioica L.) Produced by the Scalded Flour Method. J. Food Process. Preserv. 2021, 45, e15851. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Kalisz, S.; Sujka, K. Effect of the Addition of Dried Dandelion Roots (Taraxacum officinale F. H. Wigg.) on Wheat Dough and Bread Properties. Molecules 2021, 26, 7564. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Koprowska, K.; Gottschling, A.; Janda-Milczarek, K. Edible Flowers as a Source of Dietary Fibre (Total, Insoluble and Soluble) as a Potential Athlete’s Dietary Supplement. Nutrients 2022, 14, 2470. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Bustos, M.C.; Vignola, M.B.; Paesani, C.; Salinas, C.N.; Pérez, G.T. A Study on Fibre Addition to Gluten Free Bread: Its Effects on Bread Quality and in Vitro Digestibility. J. Food Sci. Technol. 2017, 54, 244. [Google Scholar] [CrossRef]
- Zielińska, E.; Pankiewicz, U. Nutritional, Physiochemical, and Antioxidative Characteristics of Shortcake Biscuits Enriched with Tenebrio Molitor Flour. Molecules 2020, 25, 5629. [Google Scholar] [CrossRef]
- Baiano, A.; Viggiani, I.; Terracone, C.; Romaniello, R.; Del Nobile, M.A. Physical and Sensory Properties of Bread Enriched with Phenolic Aqueous Extracts from Vegetable Wastes. Czech J. Food Sci. 2015, 33, 247–253. [Google Scholar] [CrossRef]
- Czaja, A.; Czubaszek, A.; Wyspiańska, D.; Sokół-Łętowska, A.; Kucharska, A.Z. Quality of Wheat Bread Enriched with Onion Extract and Polyphenols Content and Antioxidant Activity Changes during Bread Storage. Int. J. Food Sci. Technol. 2020, 55, 1725–1734. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Thys, R.C.S.; Brandelli, A. Antifungal Properties of Phosphatidylcholine-Oleic Acid Liposomes Encapsulating Garlic against Environmental Fungal in Wheat Bread. Int. J. Food Microbiol. 2019, 293, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Doudi, M.; Setorki, M.; Rezayatmand, Z. Effects of Aqueous Extract of Cinnamomum verum on Growth of Bread Spoilage Fungi. Int. J. Med. Res. Health Sci. 2016, 5, 162–171. [Google Scholar]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible Flowers: Bioactive Profile and Its Potential to Be Used in Food Development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).