In Vivo Bone Progression in and around Lattice Implants Additively Manufactured with a New Titanium Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Implants
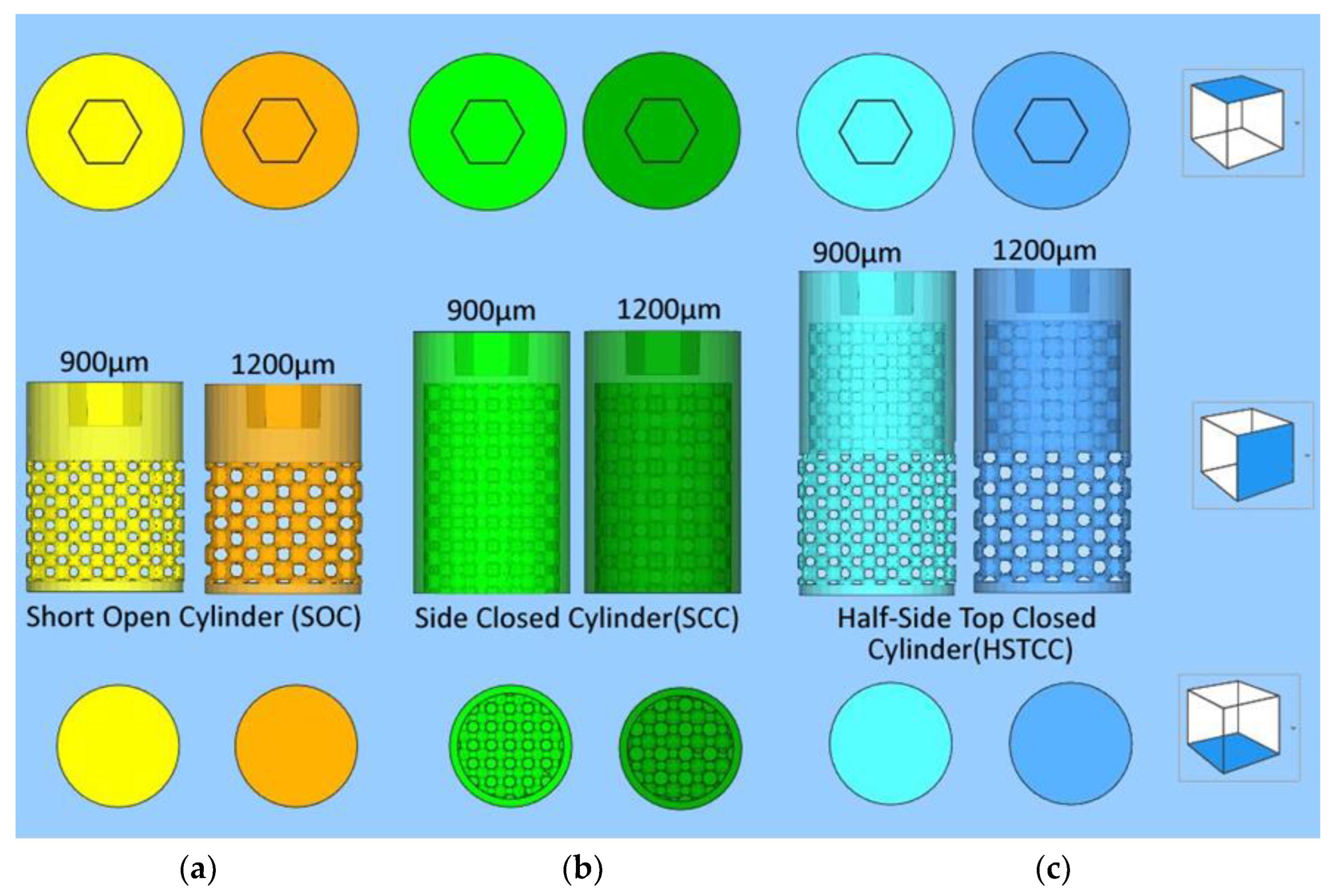
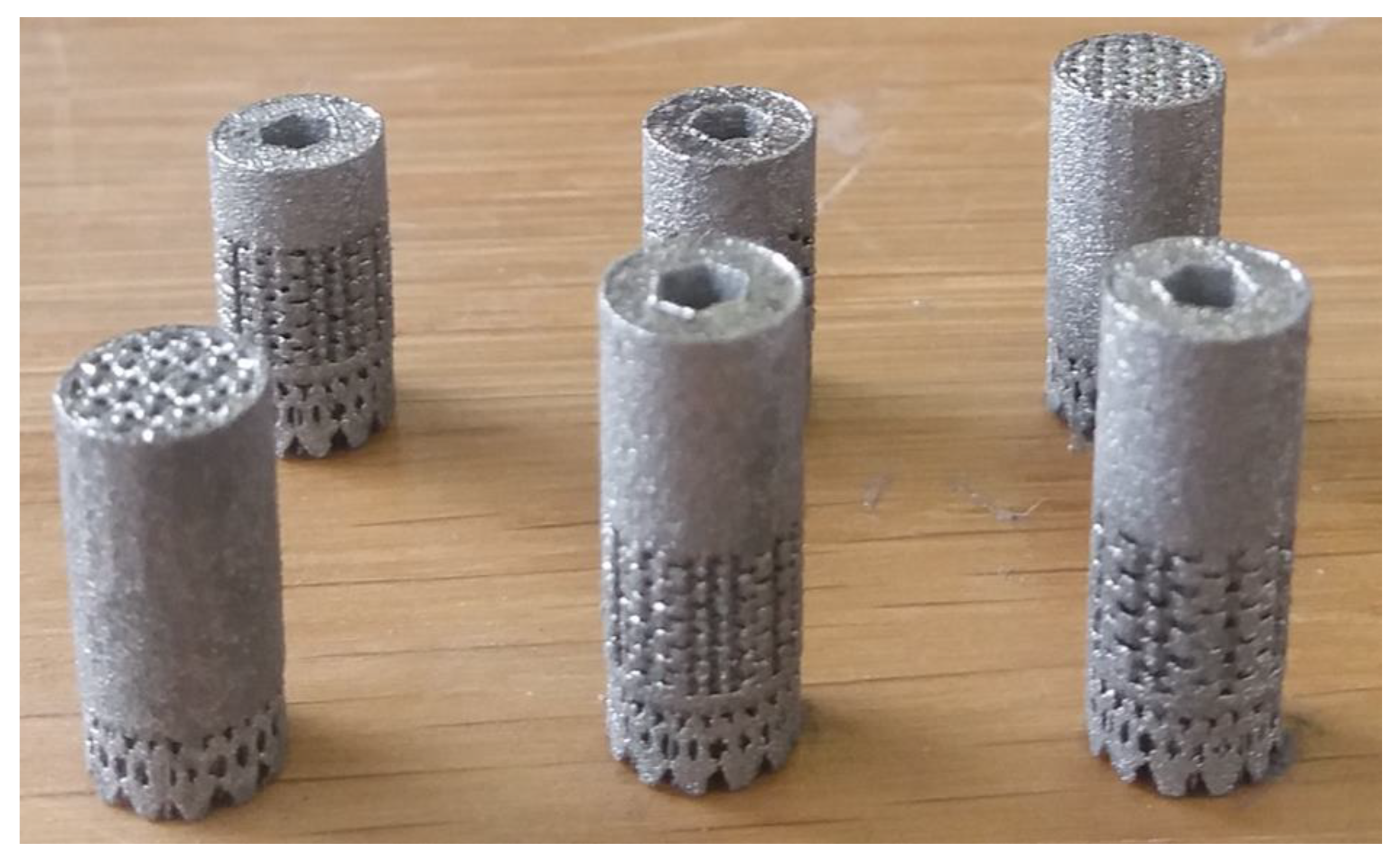
2.1.1. Designs with a Lattice Structure
- ➢
- General shape of the three designs: cylinders with a diameter of 6 mm and a top hexagonal hole conceived to accommodate a holder;
- ➢
- Differences between the three different designs:
- Short side-open cylinder: 5 mm of lattices for 8 mm in total height. The top and bottom of the cylinder are closed by dense surfaces. In this way, the bone penetration is peripherally limited (Figure 1a);
- Side-closed cylinder: 8 mm of lattice for 10 mm in total height The bottom of the cylinder is open. In this way, the bone penetration is bottom limited (Figure 1b).
- Half-side-closed cylinder: 10.4 mm of lattice for 12.4 mm in total. The top and bottom of the cylinder are closed by dense surfaces. In this way, the bone penetration is half peripherally limited (Figure 1c);
- ➢
- Ø Shape of the elementary cells of the lattice: cubic octahedron (diagonal cell);
- ➢
- Ø Size of the elementary cells of the lattice: 900 µm (octahedron with diagonal cell size of 350 µm) or 1200 µm (octahedron with diagonal cell size of 450 µm);
- ➢
- Ø Number of implants studied: five labeled 1382, 1384, 1385, 1387, and 1386 as targeted in Figure 1.
2.1.2. Manufacturing in a New Titanium Alloy Using a Powder Bed Fusion-Laser Beam (PBF-LB) Process
- (1)
- 60 min in an ultrasound tank with 60 °C distilled water without any detergent;
- (2)
- 30 min soaking in Dentasept 3 H rapid for decontamination;
- (3)
- Rinsing by ultrasound;
- (4)
- Cleaning with benzalkonium chloride, chloramine T, E.D.T.A betatetrasodium, and isopropylic alcohol;
- (5)
- Drying at DPH21;
- (6)
- Sterilization by surgical autoclave.
2.2. Animal
2.2.1. Animal Model
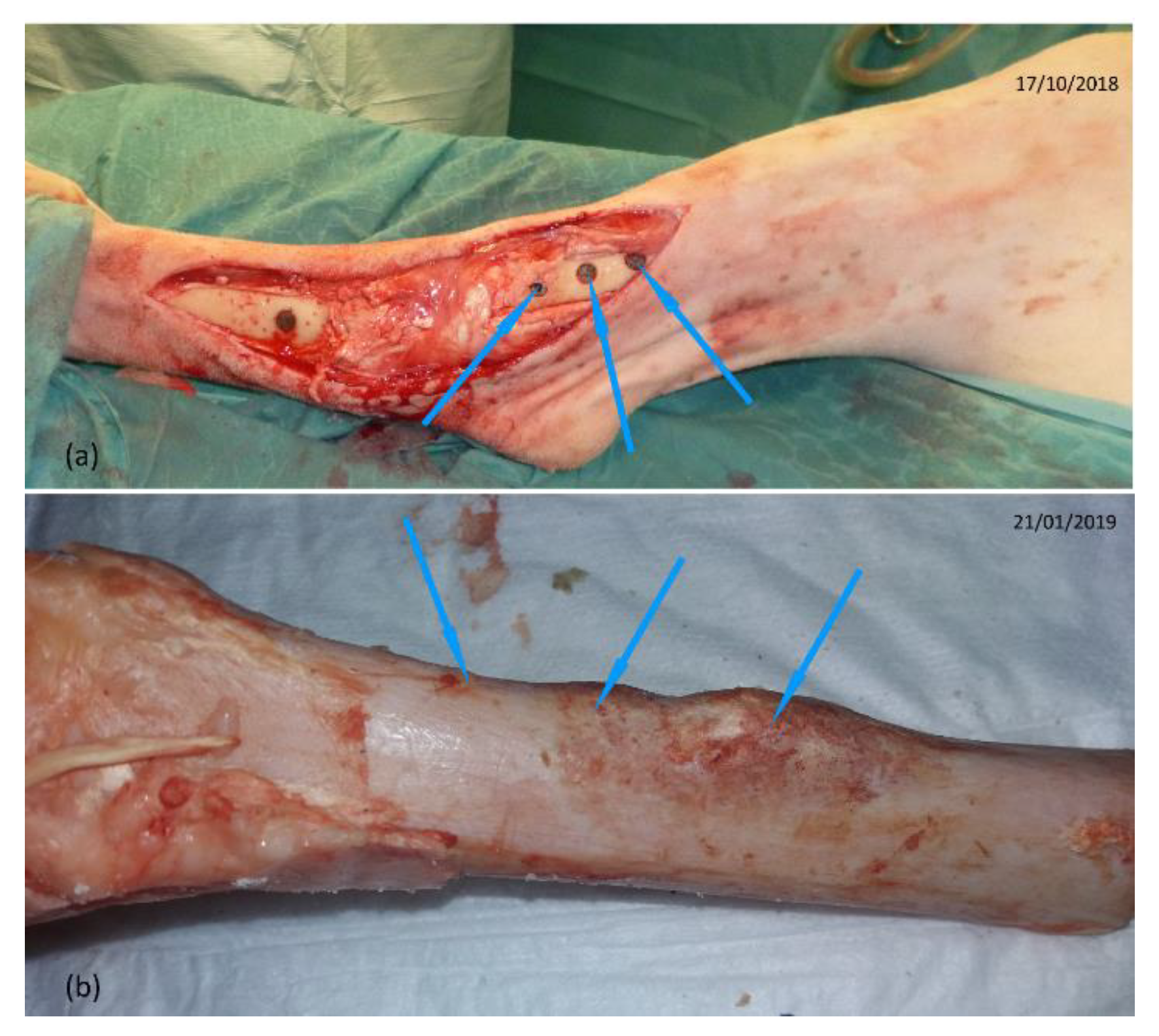
2.2.2. Surgical Steps of the Implant Placement
- (1)
- Pre-medication by intravenous injection of a mixture of ketamine (6 mg/kg) and diazepam (0.5 mg/kg);
- (2)
- Intubation with an endo-tracheal probe of 9 mm internal diameter;
- (3)
- Connection to respirator with maintenance of anesthesia by inhalation of 2.5% isoflurane in an air/oxygen mixture with 60% O2;
- (4)
- Analgesia: IV injection of morphine (0.1 mg/kg);
- (5)
- NSAIDs: IM injection of meloxicam (0.4 mg/kg);
- (6)
- Antibiotic: IM injection of 0.15 mL/kg of PeniDHS (combination of penicillin and streptomycin).
2.2.3. Surgical Steps of the Implant Removal
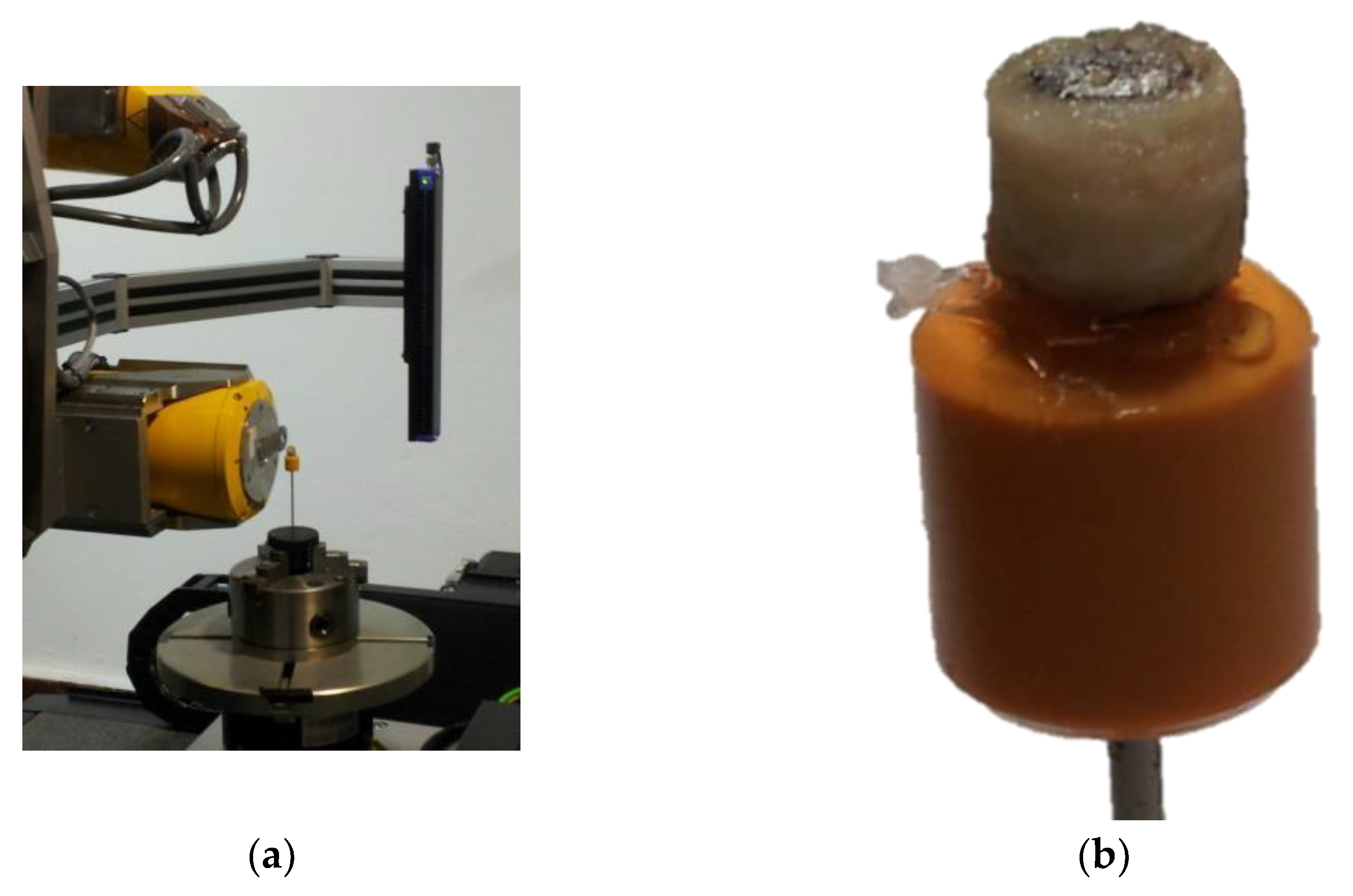
2.3. Characterization of the Implant Using an X-ray Computed Tomography (XCT) System
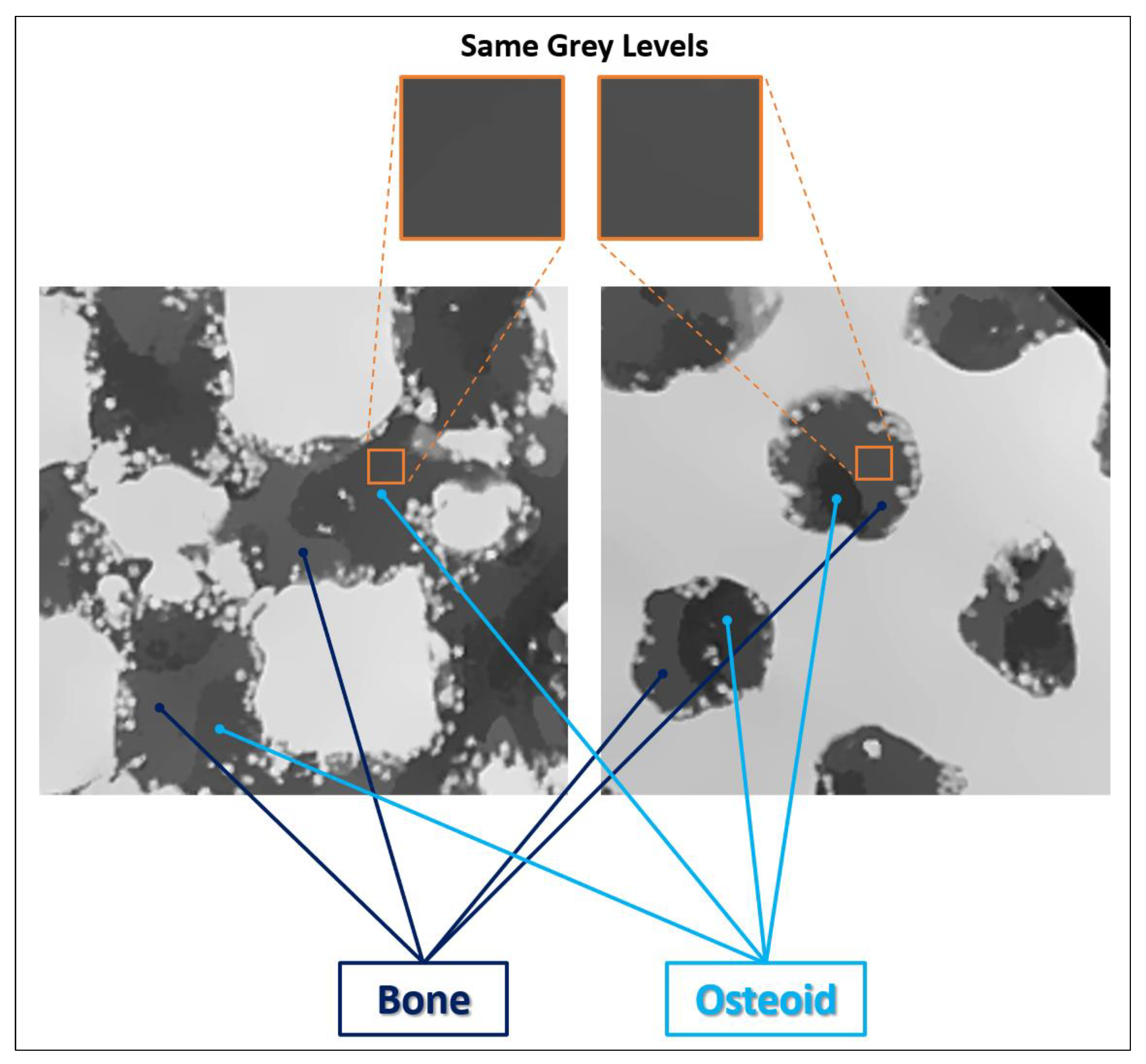
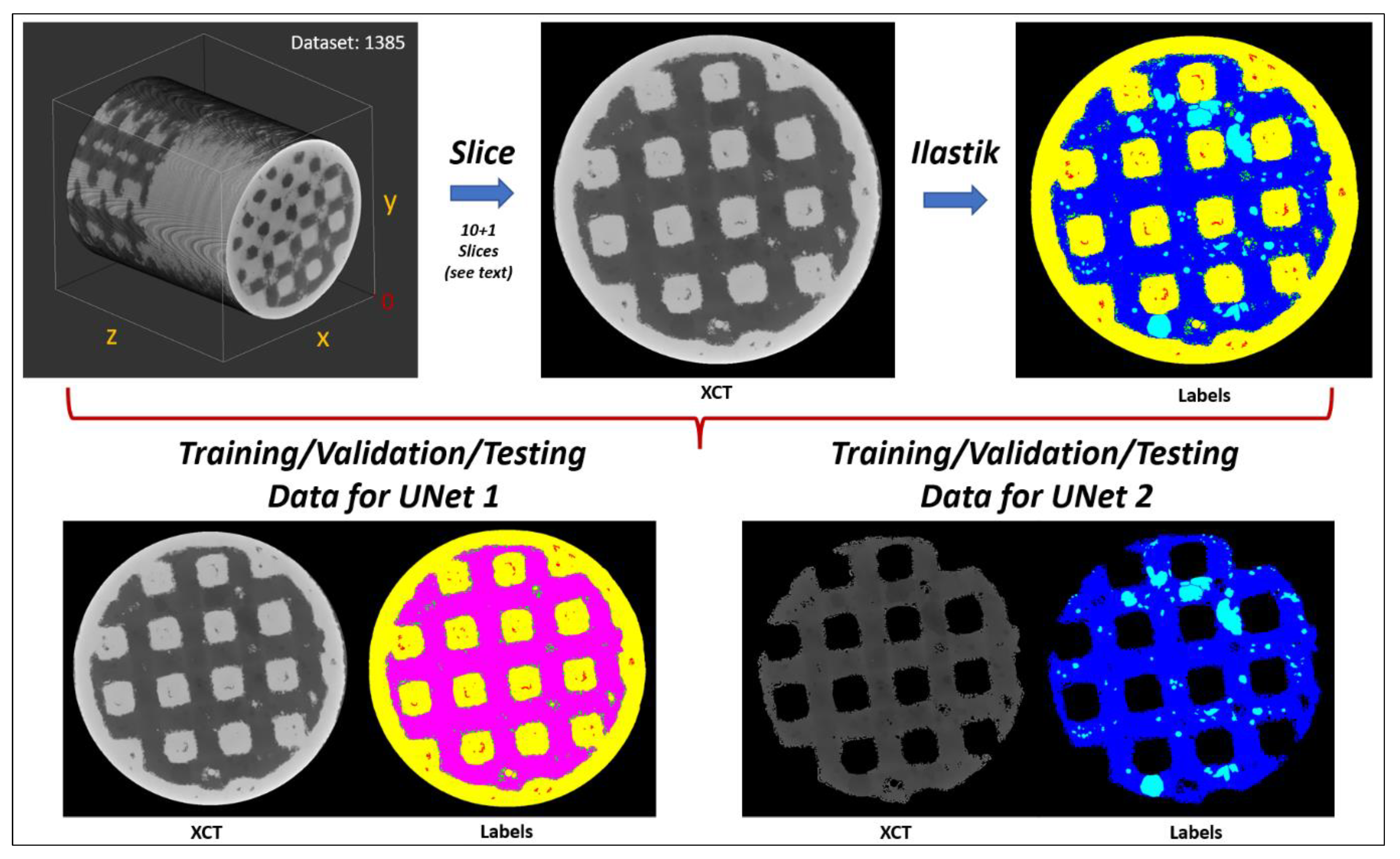
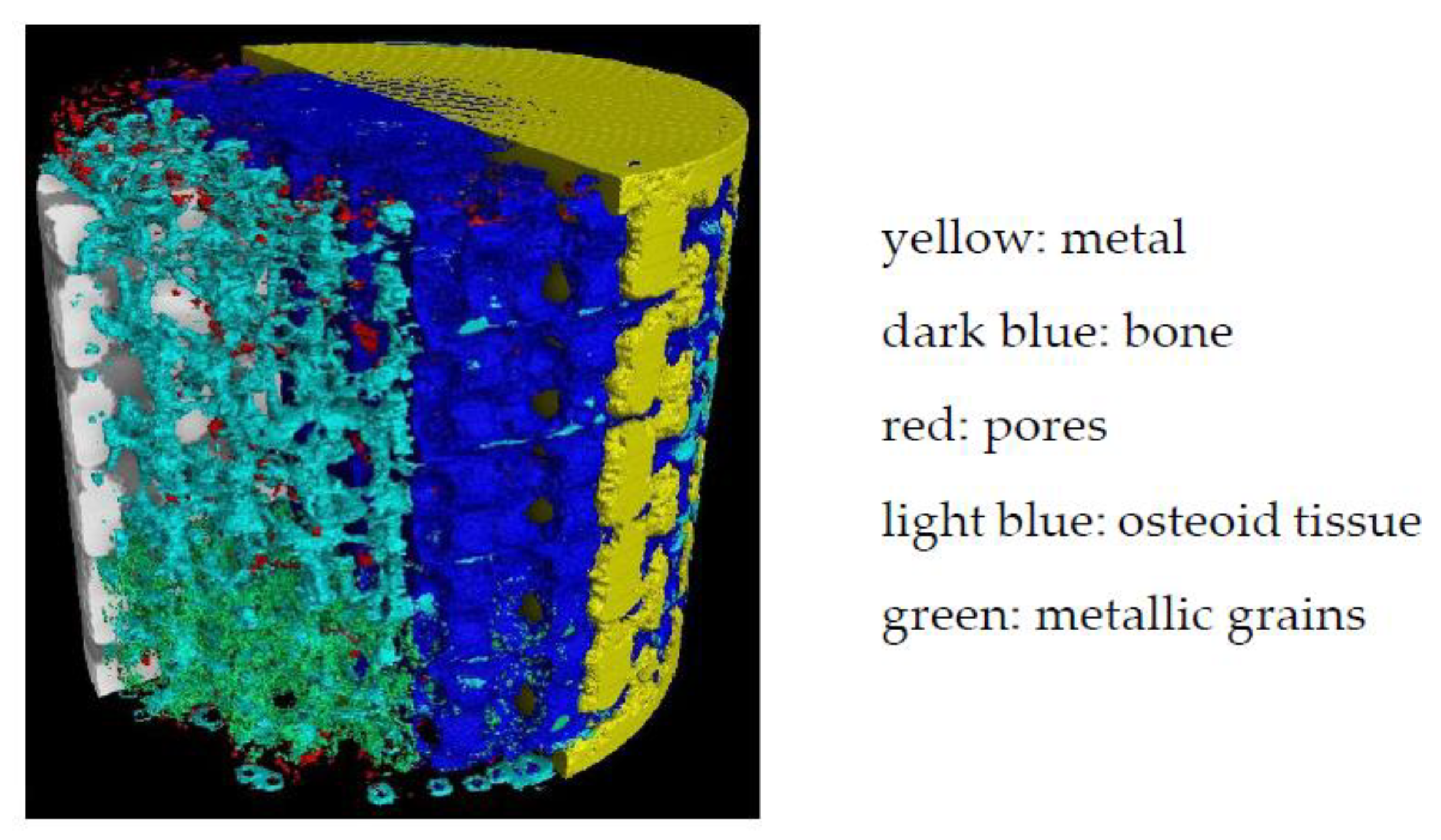
2.4. Analysis of the XCT Images Implementing a Machine Learning (ML) Segmentation
2.5. Evaluations of the Osseointegration Process
2.5.1. Bone–Implant Interface (BII) and Bone–Implant Contact (BIC)
2.5.2. Bone Progression in the Lattice Structure
3. Results and Discussion Related to the Evaluations of the Osseointegration Process
3.1. Visual Inspection before the Implant Removal
3.2. BII and BIC
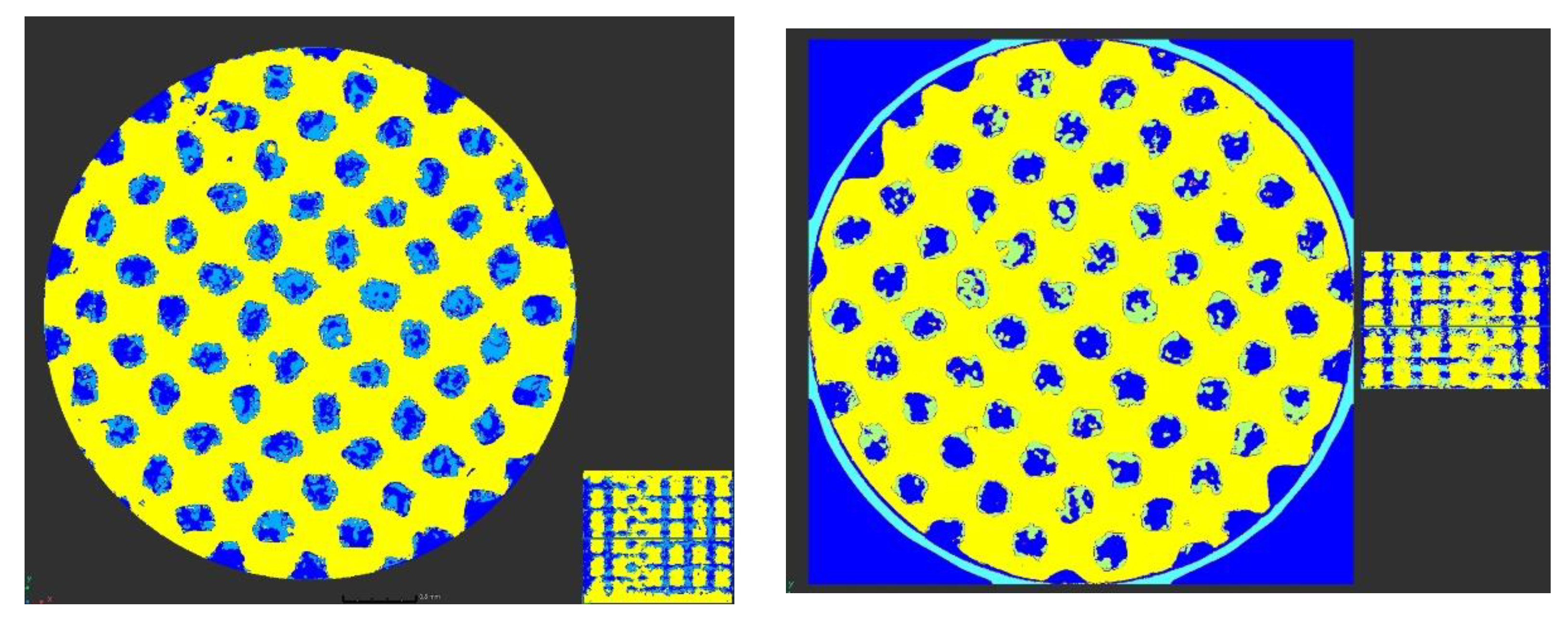
3.3. Bone Progression in Lattice Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Alloy | Modulus | Yield Strength | Ultimate Tensile Strength | Elongation | Fatigue Endurance |
|---|---|---|---|---|---|
| GPa | MPa | MPa | (%) | MPa (107 Cycles) | |
| SS 316L (UNS S31603) | 165 | 170 | 485 | 35 | 140 |
| Co-Cr-Mo (UNS R31537) | 210 | 480 | 780 | 12 | 400 |
| Ti Grade 2 | 103 | 340 | 430 | 20 | 300 |
| Ti-6Al-4V (ASTM F136) | 95 | 795 | 860 | 10 | 420 |
| ZTP10 (Heat-treated) | 110 | 910 | 1135 | 10 | 840 |
| ZTM14N (As-built) * this study | 55 | 743 | 780 | 13 | 420 |
| ZTM14N (Heat-treated) * | 38 | 651 | 574 | 6 | 530 |
References
- Abbas, M.M.; Rasheed, M. Solid State Reaction Synthesis and Characterization of Cu doped TiO2 Nanomaterials. J. Phys. Conf. Ser. 2020, 1795, 012059. [Google Scholar] [CrossRef]
- Alshalal, I.; Al-Zuhairi, I.H.-M.; Awad Abtan, A.; Rasheed, M.; Khalil Asmail, M. Characterization of wear and fatigue behavior of aluminum piston alloy using alumina nanoparticles. J. Mech. Behav. Biomed. Mater. 2023, 32, 20220280. [Google Scholar] [CrossRef]
- Khorasani, M.; Ghasemi, A.-H.; Leary, M.; Downing, D.; Gibson, I.; Sharabian, E.G.; Kozhuthala, V.-J.; Brandt, M.; Bateman, S.; Rolfe, B. Benchmark models for conduction and keyhole modes in laser-based powder bed fusion of Inconel 718. Opt. Laser Technol. 2023, 164, 109509. [Google Scholar] [CrossRef]
- Huang, X.; Ding, S.; Lang, L.; Gong, S. Compressive response of selective laser-melted lattice structures with different strut sizes based on theoretical, numerical and experimental approaches. Rapid Prototyp. J. 2023, 29, 209–217. [Google Scholar] [CrossRef]
- Yue, M.; Li, M.; An, N.; Yang, K.; Wang, J.; Zhou, J. Modeling SEBM process of tantalum lattices. Rapid Prototyp. J. 2023, 29, 232–245. [Google Scholar] [CrossRef]
- Obaton, A.-F.; Fain, J.; Djemaï, M.; Meinel, D.; Léonard, F.; Mahé, E.; Lécuelle, B.; Fouchet, J.-J.; Bruno, G. In vivo XCT bone characterization of lattice structured implants fabricated by additive manufacturing: A case report. Heliyon 2017, 3, e00374. [Google Scholar] [CrossRef]
- Djemai, A.; Fouchet, J.-J. Process for Manufacturing a Titanium Niobium Zirconium (tnz) Beta-Alloy with a Very Low Modulus of Elasticity for Biomedical Applications and Method for Productive Same by Additive Manufacturing. WO2017137671A1, 17 August 2017. [Google Scholar]
- ASTM WK84537; New Specification for Additive Manufacturing—Finished Part Properties—Titanium-27Niobium-21Zirconium via Laser Beam Powder Bed Fusion for Medical Implant Applications. ASTM: West Conshohocken, PA, USA, 2022.
- Boyer, R.; Collings, E.W.; Welsch, G. Materials Properties Handbook: Titanium Alloys; ASM International: Almere, The Netherlands, 1994. [Google Scholar]
- Lee, D.-G.; Mi, X.; Eom, T.K.; Lee, Y. Bio-Compatible Properties of Ti–Nb–Zr Titanium Alloy with Extra Low Modulus. J. Biomater. Tissue Eng. 2016, 6, 798–801. [Google Scholar] [CrossRef]
- Kurtz, M.; Wessinger, A.; Mace, A.; Moreno-Reyes, A.; Gilbert, J. Corrosion Resistance of Additively Manufactured Titanium Alloys in Physiological and Inflammatory-Simulating Environments: Ti-6Al-4v Versus Ti-29Nb-21zr. Mater. Eng. 2022. [Google Scholar] [CrossRef]
- Kurtz, M.; Wessinger, A.; Mace, A.; Moreno-Reyes, A.; Gilbert, J. Additively manufactured Ti-29Nb-21Zr shows improved oxide polarization resistance versus Ti-6Al-4V in inflammatory stimulating solution. J. Biomed. Mater. Res. 2023, 1–16. [Google Scholar]
- Walker, P.R.; LeBlanc, J.; Sikorska, M. Effects of aluminum and other cations on the structure of brain and liver chromatin. Biochemistry 1989, 28, 3911–3915. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Ushida, T.; Tateishi, T.; Okazaki, Y.; Asao, S. Effect of Ti, Al, and V ions on the relative growth rate of fibroblasts (L929) and osteoblasts (MC3T3-E1) cells. Biomed. Mater. Eng. 1996, 6, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation, Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2015; Volume 9351, pp. 234–241. [Google Scholar] [CrossRef]
- Buades, A.B.; Coll, J.-M.; Morel, A. Non-Local Algorithm for Image Denoising. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’05), San Diego, CA, USA, 20–25 June 2005; Volume 2, pp. 60–65. [Google Scholar]
- Tsamos, A.; Evsevleev, S.; Fioresi, R.; Faglioni, F.; Bruno, G. Synthetic data generation for automatic segmentation of X-ray computed tomography reconstructions of complex microstructures. J. Imaging 2023, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Neural Network Libraries. An Open-Source Software to Make Research, Development and Implementation of Neural Network More Efficient. Sony Corp. Available online: https://nnabla.org/ (accessed on 9 January 2022).
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization, ICLR 2015. Available online: https://www.semanticscholar.org/paper/a6cb366736791bcccc5c8639de5a8f9636bf87e8 (accessed on 9 January 2022).
- Nishikawa, T.; Masuno, K.; Mori, M.; Tajine, Y.; Kakudo, K.; Tanaka, A. Calcification at the interface between titanium implants and bone observation with confocal laser scanning microscopy. J. Oral. Implantol. 2006, 32, 211–217. [Google Scholar] [CrossRef]
- Goto, T. Ostéointégration and dentals implants. Clin. Calcium 2014, 24, 265–271. Available online: https://pubmed.ncbi.nlm.nih.gov/24473360/ (accessed on 10 May 2023).
- Sundell, G.; Dahlin, C.; Andersson, M.; Thuvander, M. The bone implant interface of dentals implants in humans on the atomic scale. Acta Biomater. 2017, 48, 445–450. [Google Scholar] [CrossRef]
- Jain, R.; Kapour, D. The dynamic interface: A review. J. Int. Soc. Prev. Commun. Dent 2015, 5, 354–358. [Google Scholar] [CrossRef]
- Bernhardt, R.; Kublisch, E.; Schultz, M.-C.; Eckelt, U.; Stadlinger, B. Comparison of bone-implant contact and bone-implant volume between 2D-histological sections and 3D-SRµCT slices. Eur. Cell Mater. 2012, 23, 237–247. [Google Scholar] [CrossRef]
- Lyu, H.-Z.; Lee, J.H. Correlation between two-dimensional micro-CT and histomorphometry for assessment of the implant osseointegration in rabbit tibia model. Biomater. Res. 2011, 25, 11. [Google Scholar] [CrossRef]
- Hong, J.-M.; Kim, H.-G.; Luke Yeo, I.-S. Comparison of three-dimensional digital analysis and the two-dimensional histomorphometric analysis of the bone-implant interface. PLoS ONE 2022, 17, e276269. [Google Scholar] [CrossRef]
- Lian, Z.; Guan, H.; Ivanovski, S.; Loo, Y.-C.; Johnson, N.-W.; Zhang, H. Effect of bone to implant percentage on bone remodeling surrounding a dental implant. Int. J. Oral Maxillofac. Surg. 2010, 39, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2020/561 of the European Parliament and of the Council of 23 April 2020 Amending Regulation (EU) 2017/745 on Medical Devices, as Regards the Dates of Application of Certain of Its Provisions (Text with EEA Relevance), Document 32020R0561. Available online: http://data.europa.eu/eli/reg/2020/561/oj (accessed on 6 May 2023).





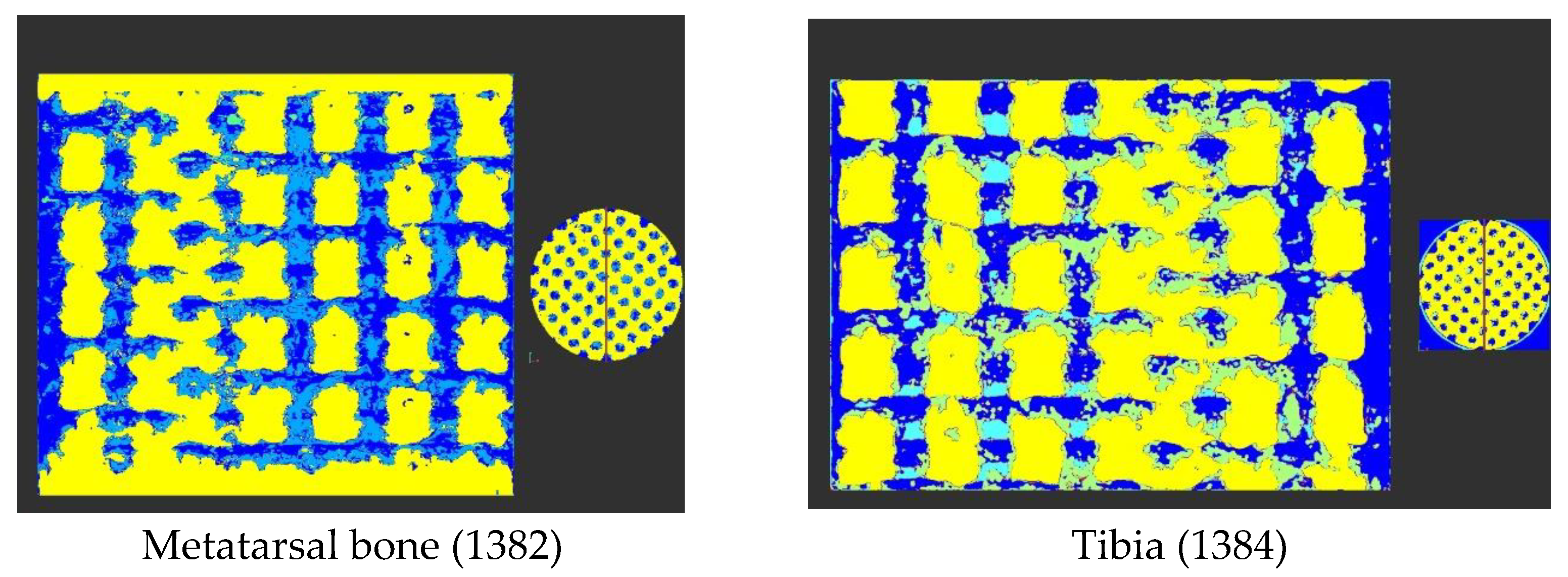
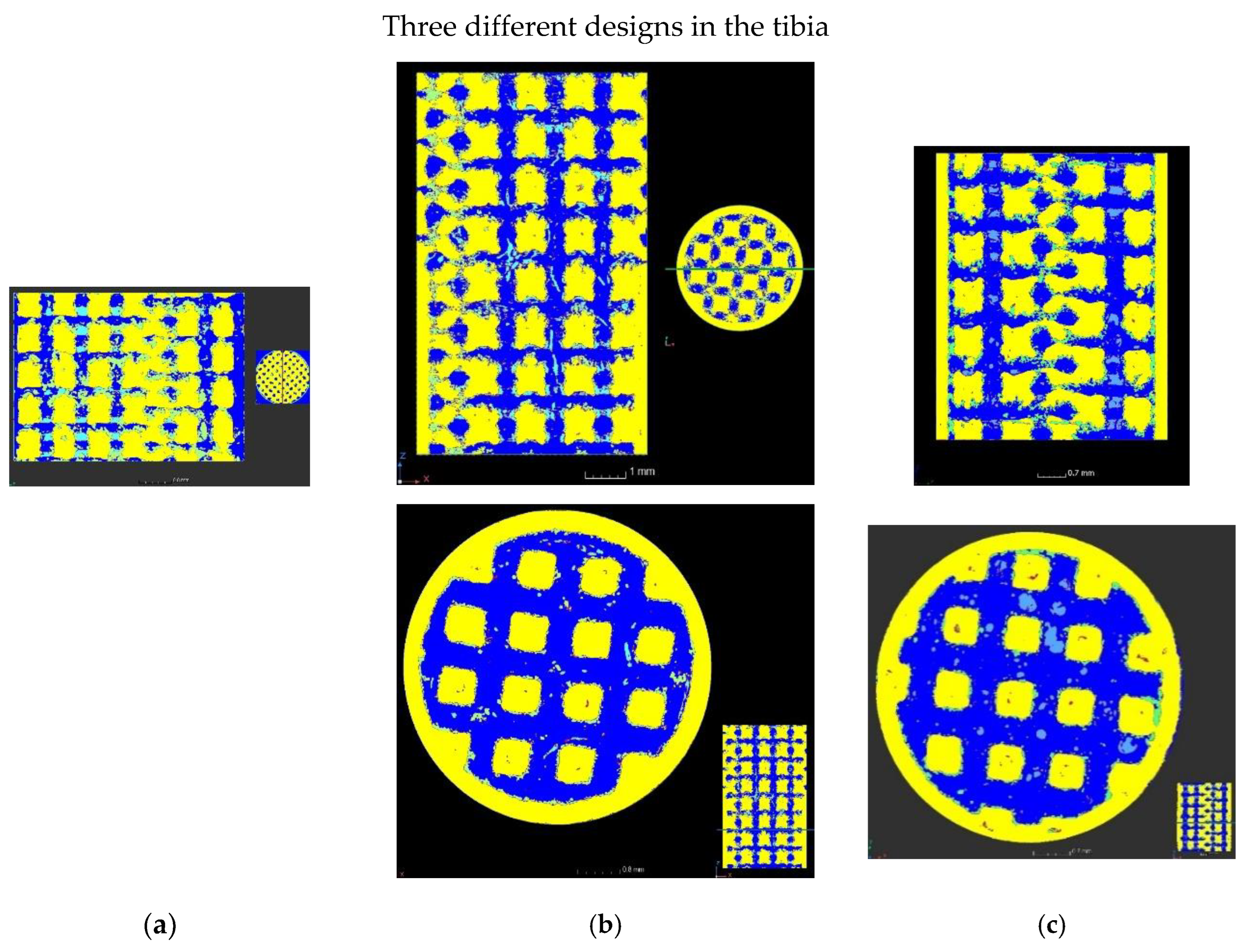
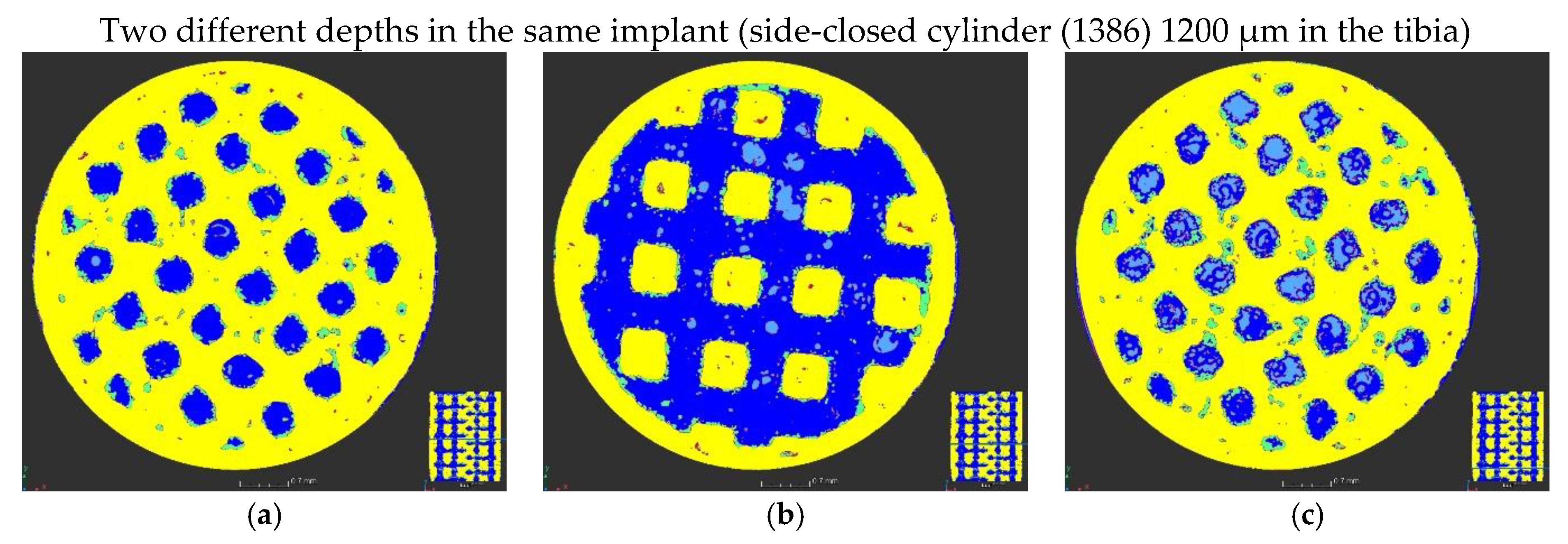
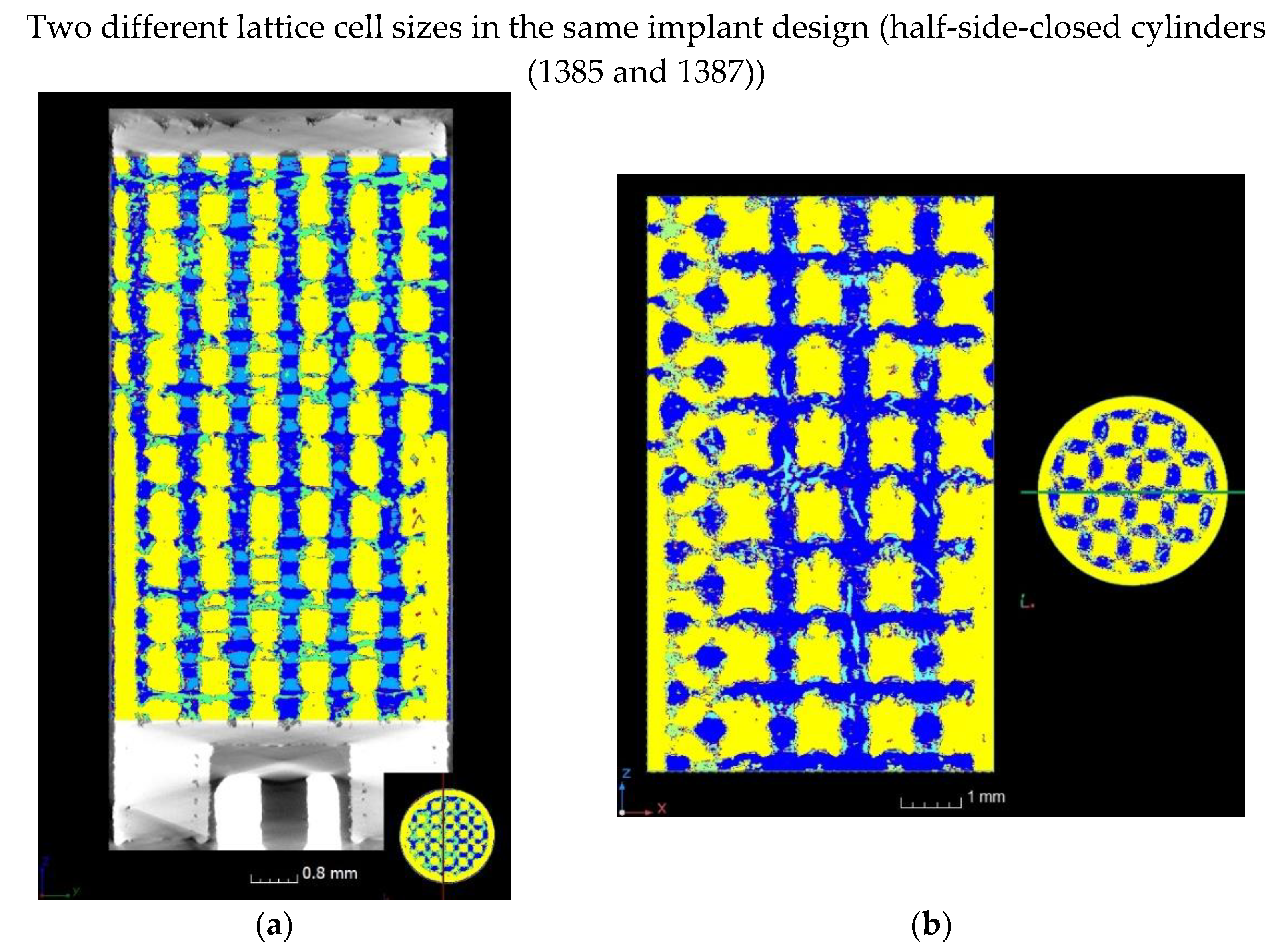
| Accelerating Voltage (kV) | Current (µA) | Number of Projection | Exposure Time per Projection (ms) | Number of Projection Averaged | Scan Duration (h) | Voxel Size (µm) |
|---|---|---|---|---|---|---|
| 120 | 120 | 3142 | 1000 | 2 | 2 | 6.8 |
| Angular Position around the Cylinder | 0° | 45° | 90° | 135° | Total |
|---|---|---|---|---|---|
| Number of pixels in the selected image area | 148 | 151 | 150 | 154 | 603 |
| Number of pixels for which bone and metal have no contact | 1 | 5 | 17 | 5 | 28 |
| % of pixels free of bone or metal | 0.6 | 3.3 | 11.3 | 3.2 | 4.6 |
| BIC (%) | 99.4 | 96.7 | 88.7 | 96.8 | 95.4 |
| Angular Position around the Cylinder | 0° | 45° | 90° | 135° | Total |
|---|---|---|---|---|---|
| Number of pixels in the selected image area | 38 | 300 | 254 | 61 | 653 |
| Number of pixels for which bone and metal have no contact | 0 | 0 | 47 | 0 | 47 |
| % of pixels free of bone or metal | 0 | 0 | 18.5 | 0 | 7.2 |
| BIC (%) | 100 | 100 | 81.5 | 100 | 92.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obaton, A.-F.; Fain, J.; Meinel, D.; Tsamos, A.; Léonard, F.; Lécuelle, B.; Djemaï, M. In Vivo Bone Progression in and around Lattice Implants Additively Manufactured with a New Titanium Alloy. Appl. Sci. 2023, 13, 7282. https://doi.org/10.3390/app13127282
Obaton A-F, Fain J, Meinel D, Tsamos A, Léonard F, Lécuelle B, Djemaï M. In Vivo Bone Progression in and around Lattice Implants Additively Manufactured with a New Titanium Alloy. Applied Sciences. 2023; 13(12):7282. https://doi.org/10.3390/app13127282
Chicago/Turabian StyleObaton, Anne-Françoise, Jacques Fain, Dietmar Meinel, Athanasios Tsamos, Fabien Léonard, Benoît Lécuelle, and Madjid Djemaï. 2023. "In Vivo Bone Progression in and around Lattice Implants Additively Manufactured with a New Titanium Alloy" Applied Sciences 13, no. 12: 7282. https://doi.org/10.3390/app13127282
APA StyleObaton, A.-F., Fain, J., Meinel, D., Tsamos, A., Léonard, F., Lécuelle, B., & Djemaï, M. (2023). In Vivo Bone Progression in and around Lattice Implants Additively Manufactured with a New Titanium Alloy. Applied Sciences, 13(12), 7282. https://doi.org/10.3390/app13127282








