Predicting Hospitalization, Organ Dysfunction, and Mortality in Post-Endoscopic Retrograde Cholangiopancreatography Acute Pancreatitis: Are SIRS and qSOFA Reliable Tools?
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Patients
2.2. Procedures and Devices
2.3. Patient Evaluation before and during the Procedure
2.4. Patient Post-ERCP Follow-Up
2.5. Definition of Post-ERCP Complications
2.6. Statistical Analysis and Report of Results
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandrasekhara, V.; Khashab, M.A.; Muthusamy, V.R.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Gurudu, S.R.; et al. Adverse events associated with ERCP. Gastrointest. Endosc. 2017, 85, 32–47. [Google Scholar] [CrossRef]
- Andriulli, A.; Loperfido, S.; Napolitano, G.; Niro, G.; Valvano, M.R.; Spirito, F.; Pilotto, A.; Forlano, R. Incidence rates of post-ERCP complications: A systematic survey of prospective studies. ACG 2007, 102, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.; Akshintala, V.S.; Afghani, E.; Elmunzer, B.J.; Kim, K.J.; Lennon, A.M.; Khashab, M.A.; Kalloo, A.N.; Singh, V.K. Incidence, severity, and mortality of post-ERCP pancreatitis: A systematic review by using randomized, controlled trials. Gastrointest. Endosc. 2015, 81, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Lehman, G.; Vennes, J.; Geenen, J.E.; Russell, R.C.; Meyers, W.C.; Liguory, C.; Nickl, N. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest. Endosc. 1991, 37, 383–393. [Google Scholar] [CrossRef]
- Masci, E.; Mariani, A.; Curioni, S.; Testoni, P.A. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: A meta-analysis. Endoscopy 2003, 35, 830–834. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. GUT 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Khashab, M.A.; Chithadi, K.V.; Acosta, R.D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Fonkalsrud, L.; Lightdale, J.R.; et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc. 2015, 81, 81–89. [Google Scholar] [CrossRef]
- Domagk, D.; Oppong, K.W.; Aabakken, L.; Czakó, L.; Gyökeres, T.; Manes, G.; Meier, P.; Poley, J.W.; Ponchon, T.; Tringali, A.; et al. Performance measures for ERCP and endoscopic ultrasound: A European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy 2018, 50, 1116–1127. [Google Scholar] [CrossRef]
- Doyle, D.J.; Goyal, A.; Garmon, E.H. American Society of Anesthesiologists Classification; StatPearls Publishing: Tampa, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441940 (accessed on 10 May 2023).
- Balan, G.G.; Arya, M.; Catinean, A.; Sandru, V.; Moscalu, M.; Constantinescu, G.; Trifan, A.; Stefanescu, G.; Sfarti, C.V. Anatomy of Major Duodenal Papilla Influences ERCP Outcomes and Complication Rates: A Single Center Prospective Study. J. Clin. Med. 2020, 9, 1637. [Google Scholar] [CrossRef]
- Testoni, P.A.; Mariani, A.; Aabakken, L.; Arvanitakis, M.; Bories, E.; Costamagna, G.; Devière, J.; Dinis-Ribeiro, M.; Dumonceau, J.M.; Giovannini, M.; et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2016, 48, 657–683. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference 2001. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef]
- Synger, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock. JAMA 2016, 315, 610–801. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef]
- Yokoe, M.; Hata, J.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Wakabayashi, G.; Kozaka, K.; Endo, I.; Deziel, D.J.; Miura, F.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepato-Biliary Pancreat. Sci. 2018, 25, 41–54. [Google Scholar] [CrossRef]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.H.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepato-Biliary Pancreat. Sci. 2018, 25, 17–30. [Google Scholar] [CrossRef]
- Elmunzer, B.J.; Scheiman, J.M.; Lehman, G.A.; Chak, A.; Mosler, P.; Higgins, P.D.; Hayward, R.A.; Romagnuolo, J.; Elta, G.H.; Sherman, S.; et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N. Engl. J. Med. 2012, 366, 1414–1422. [Google Scholar] [CrossRef]
- Freeman, M.L.; Nelson, D.B.; Sherman, S.; Haber, G.B.; Herman, M.E.; Dorsher, P.J.; Moore, J.P.; Fennerty, M.B.; Ryan, M.E.; Shaw, M.J.; et al. Complications of endoscopic biliary sphincterotomy. N. Engl. J. Med. 1996, 335, 909–919. [Google Scholar] [CrossRef]
- Easler, J.J.; Fogel, E.L. Prevention of post-ERCP pancreatitis: The search continues. Lancet Gastroenterol. Hepatol. 2021, 6, 336. [Google Scholar] [CrossRef]
- Buter, A.; Imrie, C.W.; Carter, C.R.; Evans, S.; McKay, C.J. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br. J. Surg. 2002, 89, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Lund, H.; Tønnesen, H.; Tønnesen, M.H.; Olsen, O. Long-term recurrence and death rates after acute pancreatitis. Scand. J. Gastroenterol. 2006, 41, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.A.; Tariq, A.; Tariq, U.; Kim, K.; Ponor, L.; Lennon, A.M.; Canto, M.I.; Gurakar, A.; Yu, Q.; Dunbar, K.; et al. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin. Gastroenterol. Hepatol. 2012, 10, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Kumbhari, V.; Sinha, A.; Reddy, A.; Afghani, E.; Cotsalas, D.; Patel, Y.A.; Storm, A.C.; Khashab, M.A.; Kalloo, A.N.; Singh, V.K. Algorithm for the management of ERCP-related perforations. Gastrointest. Endosc. 2016, 83, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Cader, R.; Akshintala, V.S.; Hutfless, S.M.; Zaheer, A.; Khan, V.N.; Khashab, M.A.; Lennon, A.M.; Kalloo, A.N.; Singh, V.K. Systemic inflammatory response syndrome between 24 and 48 h after ERCP predicts prolonged length of stay in patients with post-ERCP pancreatitis: A retrospective study. Pancreatology 2015, 15, 105–110. [Google Scholar] [CrossRef]
- Sessler, D.I.; Bloomstone, J.A.; Aronson, S.; Berry, C.; Gan, T.J.; Kellum, J.A.; Plumb, J.; Mythen, M.G.; Grocott, M.P.; Edwards, M.R.; et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br. J. Anaesth. 2019, 122, 563–574. [Google Scholar] [CrossRef]
- Churpek, M.M.; Snyder, A.; Han, X.; Sokol, S.; Pettit, N.; Howell, M.D.; Edelson, D.P. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am. J. Respir. Crit. Care Med. 2017, 195, 906–911. [Google Scholar] [CrossRef]
- Schwed, A.C.; Boggs, M.M.; Pham, X.B.; Watanabe, D.M.; Bermudez, M.C.; Kaji, A.H.; Kim, D.Y.; Plurad, D.S.; Saltzman, D.J.; de Virgilio, C. Association of admission laboratory values and the timing of endoscopic retrograde cholangiopancreatography with clinical outcomes in acute cholangitis. JAMA Surg. 2016, 151, 1039–1045. [Google Scholar] [CrossRef]
- Futier, E.; Lefrant, J.Y.; Guinot, P.G.; Godet, T.; Lorne, E.; Cuvillon, P.; Bertran, S.; Leone, M.; Pastene, B.; Piriou, V.; et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: A randomized clinical trial. JAMA 2017, 318, 1346–1357. [Google Scholar] [CrossRef]
- Hallac, A.; Puri, N.; Applebury, D.; Myers, K.; Dhumal, P.; Thatte, A.; Srikureja, W. The value of quick sepsis-related organ failure assessment scores in patients with acute pancreatitis who present to emergency departments: A three-year cohort study. Gastroenterol. Res. 2019, 12, 67. [Google Scholar] [CrossRef]
- Wang, Z.; Ahmed, S.; Shelat, V.G. Acute Cholangitis in Abdominal Sepsis; Springer: Cham, Switzerland, 2018; pp. 65–81. [Google Scholar]
- Windsor., J.A.; Johnson, C.D.; Petrov, M.S.; Layer, P.; Garg, P.K.; Papachristou, G.I. Classifying the severity of acute pancreatitis: Towards a way forward. Pancreatology 2015, 15, 101–104. [Google Scholar] [CrossRef]
- Vege, S.S.; DiMagno, M.J.; Forsmark, C.E.; Martel, M.; Barkun, A.N. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology 2018, 154, 1103–1139. [Google Scholar] [CrossRef]
- James, P.D.; Kaplan, G.G.; Myers, R.P.; Hubbard, J.; Shaheen, A.A.; Tinmouth, J.; Yong, E.; Love, J.; Heitman, S.J. Decreasing mortality from acute biliary diseases that require endoscopic retrograde cholangiopancreatography: A nationwide cohort study. Clin. Gastroenterol. Hepatol. 2014, 12, 1151–1159. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Kilgore, M.L.; Wilcox, C.M.; Eloubeidi, M.A. Relationship among hospital ERCP volume, length of stay, and technical outcomes. Gastrointest. Endosc. 2006, 64, 338–347. [Google Scholar] [CrossRef]
- Sultan, S.; Baillie, J. What are the predictors of post-ERCP pancreatitis, and how useful are they. JOP 2002, 3, 188–194. [Google Scholar]
- Jang, S.E.; Park, S.W.; Lee, B.S.; Shin, C.M.; Lee, S.H.; Kim, J.W.; Jeong, S.H.; Kim, N.; Lee, D.H.; Park, J.K.; et al. Management for CBD stone-related mild to moderate acute cholangitis: Urgent versus elective ERCP. Dig. Dis. Sci. 2013, 58, 2082–2087. [Google Scholar] [CrossRef]
- Adas, G.; Kemik, A.; Adas, M.; Koc, B.; Gurbuz, E.; Akcakaya, A.; Karahan, S. Metabolic and inflammatory responses after ERCP. Int. J. Biomed. Sci. 2013, 9, 237. [Google Scholar]
- Kim, P.K.; Deutschman, C.S. Inflammatory responses and mediators. Surg. Clin. N. Am. 2000, 80, 885–894. [Google Scholar] [CrossRef]

| Characteristics | All Group (N = 403) | Without PEP (n = 368) | With PEP (n = 35) | p-Value |
|---|---|---|---|---|
| Age, years, median (quartile) | 66 (55–75) | 66 (54–75) | 68 (59–79) | 0.215 |
| Gender, female/male, n (%) | 237/166 (58.8/41.2) | 218/150 (59.2/40.8) | 19/16 (54.3/45.7) | 0.569 |
| Environment, urban/rural, n (%) | 226/177 (56.1/43.9) | 210/158 (45.7/54.3) | 16/19 (57.1/42.9) | 0.196 |
| Personal history of ERCP procedures | ||||
| Personal history of ERCP procedures, n (%) | 81 (20.1) | 79 (21.5) | 2 (2.5) | 0.011 |
| Presence of in situ biliary stents, n (%) | 33 (8.2) | 33 (8.97) | 0 (0) | 0.012 |
| Type of indication | ||||
| urgent/elective, n (%) | 119/284 (29.5/70.5) | 110/258 (29.9/70.1) | 9/26 (25.7/74.3) | 0.597 |
| curative/palliative, n (%) | 355/48 (88.1/11.9) | 328/40 (89.1/10.9) | 27/8 (77.1/22.9) | 0.036 |
| Anatomy of the major papilla | (N = 322) | (n = 289) | (n = 33) | |
| anatomic, n (%) | 168 (52.2) | 150 (51.9) | 18 (54.5) | 0.082 |
| Tip I, n (%) | 36 (11.2) | 31 (10.7) | 5 (15.2) | 0.025 |
| Tip II, n (%) | 61 (18.9) | 59 (20.4) | 2 (6.1) | 0.001 |
| Tip III, n (%) | 32 (9.9) | 31 (10.7) | 1 (3) | 0.001 |
| Tip IV, n (%) | 25 (7.8) | 18 (6.2) | 7 (21.2) | 0.001 |
| Duodenal diverticula, n (%) | ||||
| Absent | 352 (87.3) | 320 (87) | 32 (91.4) | 0.425 |
| Tip 1 | 8 (2) | 8 (2.2) | 0 (0) | 0.067 |
| Tip 2 | 32 (7.9) | 30 (8.2) | 2 (5.7) | 0.092 |
| Tip 3 | 11 (2.7) | 10 (2.7) | 1 (2.9) | 0.846 |
| ASA classification, n (%) | ||||
| 1 | 236 (58.6) | 217 (59) | 19 (54.3) | 0.461 |
| 2 | 138 (34.2) | 127 (34.5) | 11 (31.4) | 0.537 |
| 3 | 21 (5.2) | 16 (4.3) | 5 (14.3) | 0.064 |
| 4 | 8 (2) | 8 (2.2) | 0 (0) | 0.077 |
| Cannulation, easy/difficult, n (%) | 285/118 (70.7/29.3) | 271/97 (73.6/26.4) | 14/21 (40/60) | <0.001 |
| Number of cannulation attempts, ≤5/>5, n (%) | 316/87 (78.4/21.6) | 295/73 (80.2/19.8) | 21/14 (60/40) | 0.009 |
| Cannulation time: <5 min/≥5 min, n (%) | 337/66 (83.6/16.4) | 316/52 (85.9/14.1) | 21/14 (60/40) | <0.001 |
| Type of cannulation | ||||
| Wirsung cannulation, n (%) | 73 (18.1) | 60 (16.3) | 13 (37.1) | 0.005 |
| CBP cannulation CBP, n (%) | 365 (90.6) | 333 (90.5) | 32 (91.4) | 0.903 |
| Guide wire cannulation, n (%) | 363 (90.1) | 329 (89.4) | 34 (97.1) | 0.242 |
| SC cannulation, n (%) | 10 (2.5) | 9 (2.4) | 1 (2.9) | 0.675 |
| Selective cannulation, n (%) | 346 (85.9) | 316 (85.9) | 30 (85.7) | 0.819 |
| Sphincterotomy | ||||
| Without sphincterotomy, n (%) | 91 (22.9) | 88 (23.9) | 3 (8.6) | 0.021 |
| Partial biliary sphincterotomy, n (%) | 134 (33.3) | 120 (32.3) | 14 (40) | 0.053 |
| Total biliary sphincterotomy, n (%) | 178 (44.2) | 160 (43.5) | 18 (51.4) | 0.089 |
| Needle knife precut, n (%) | 25 (6.2) | 17 (4.6) | 8 (22.9) | 0.002 |
| Needle lnife fistulotomy, n (%) | 4 (1) | 4 (1.1) | 0 (0) | 0.163 |
| Trans-pancreatic, n (%) | 10 (2.5) | 9 (2.4) | 1 (2.9) | 0.841 |
| SIRS | 66 (16.4) | 54 (14.7) | 12 (34.3) | 0.002 |
| With suspicion of organ dysfunction (qSOFA) | 48 (11.9) | 39 (10.6) | 9 (25.7) | 0.008 |
| qSOFA 1 | 27 (6.7) | 23 (6.3) | 4 (11.4) | 0.017 |
| qSOFA 2 | 12 (3) | 9 (2.5) | 3 (8.6) | 0.018 |
| qSOFA 3 | 9 (2.2) | 7 (1.9) | 2 (5.7) | 0.014 |
| Organ dysfunction | 40 (9.9) | 32 (8.7) | 8 (22.9) | 0.007 |
| one dysfunction | 35 (8.7) | 29 (7.9) | 6 (17.1) | <0.001 |
| two dysfunctions | 3 (0.7) | 3 (0.8) | 0 (0) | 0.154 |
| three dysfunctions | 2 (0.5) | 0 (0) | 2 (5.7) | 0.003 |
| Duration of hospitalization, days, median (quartile) | 11 (7–17) | 11 (7–17) | 13 (10–22) | 0.007 |
| Patients (n = 403) | Complications | PEP | Bleeding | Infection | Perforation |
|---|---|---|---|---|---|
| n (%) | 77 (19.11) | 35 (8.68) | 12 (2.98) | 36 (8.93) | 3 (0.74) |
| PEP | Bleeding | Infection | Total | |||
|---|---|---|---|---|---|---|
| Absent | Present | |||||
| Absent | Absent | 327 | 81.14% | 31 | 7.69% | 358 |
| Present | 9 | 2.23% | 1 | 0.25% | 10 | |
| Present | Absent | 29 | 7.20% | 4 | 0.99% | 33 |
| Present | 2 | 0.50% | 0 | 0.00% | 2 | |
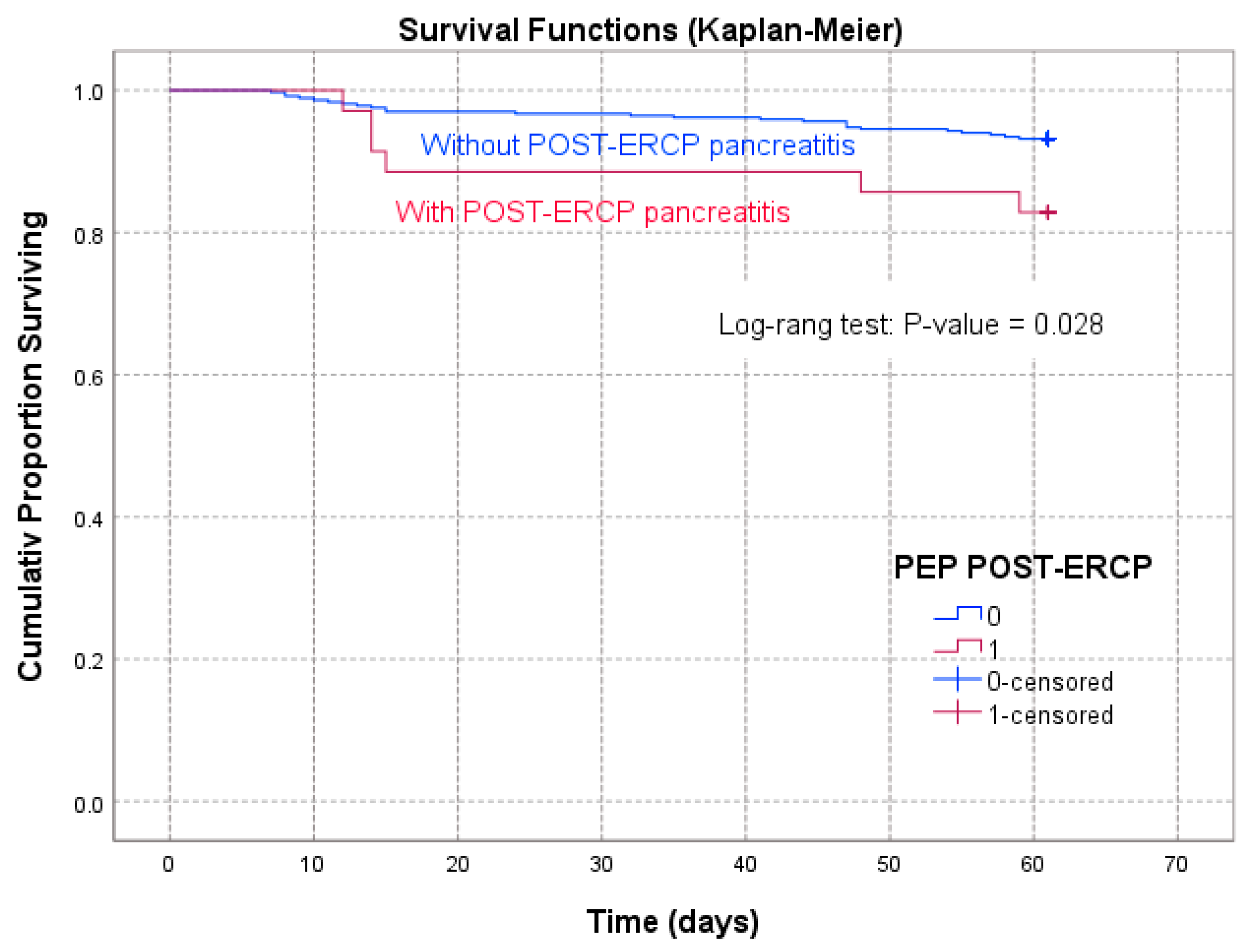
| Time Interval of Follow-Up | PEP Patients | Non-PEP Patients |
|---|---|---|
| (Days) | Survival Rate (%) | |
| 7 | 100 | 100 |
| 13 | 97.1429 | 98.0978 |
| 19 | 88.5714 | 97.0109 |
| 25 | 88.5714 | 96.7391 |
| 31 | 88.5714 | 96.7391 |
| 37 | 88.5714 | 96.1957 |
| 43 | 88.5714 | 95.9239 |
| 49 | 85.7143 | 94.5652 |
| 55 | 85.7143 | 94.2935 |
| 61 | 82.8572 | 93.2065 |
| Log-rank test | WW = −3.422; Sum = 30.853; Var = 2.4529 Test = −2.18516 | p = 0.02888 |
| PEP Present | |||||
|---|---|---|---|---|---|
| AUC | Standard Error | p-Value | AUC—95% CI | ||
| Lower Limit | Upper Limit | ||||
| Organ dysfunction | |||||
| SIRS | 0.926 | 0.044 | 0.000 | 0.839 | 1.000 |
| SOFA | 0.993 | 0.010 | 0.000 | 0.973 | 1.000 |
| Survival | |||||
| SIRS | 0.796 | 0.101 | 0.024 | 0.598 | 0.994 |
| SOFA | 0.885 | 0.097 | 0.003 | 0.696 | 1.000 |
| Length of stay | |||||
| SIRS | 0.506 | 0.113 | 0.955 | 0.285 | 0.728 |
| SOFA | 0.466 | 0.112 | 0.763 | 0.247 | 0.685 |
| PEP absent | |||||
| Organ dysfunction | |||||
| SIRS | 0.916 | 0.031 | 0.000 | 0.855 | 0.977 |
| SOFA | 0.977 | 0.018 | 0.000 | 0.941 | 1.000 |
| Survival | |||||
| SIRS | 0.808 | 0.054 | 0.000 | 0.702 | 0.913 |
| SOFA | 0.860 | 0.052 | 0.000 | 0.759 | 0.962 |
| Length of stay | |||||
| SIRS | 0.558 | 0.030 | 0.053 | 0.500 | 0.617 |
| SOFA | 0.546 | 0.030 | 0.131 | 0.487 | 0.604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, G.G.; Timofte, O.; Gilca-Blanariu, G.-E.; Sfarti, C.; Diaconescu, S.; Gimiga, N.; Antighin, S.P.; Sandu, I.; Sandru, V.; Trifan, A.; et al. Predicting Hospitalization, Organ Dysfunction, and Mortality in Post-Endoscopic Retrograde Cholangiopancreatography Acute Pancreatitis: Are SIRS and qSOFA Reliable Tools? Appl. Sci. 2023, 13, 6650. https://doi.org/10.3390/app13116650
Balan GG, Timofte O, Gilca-Blanariu G-E, Sfarti C, Diaconescu S, Gimiga N, Antighin SP, Sandu I, Sandru V, Trifan A, et al. Predicting Hospitalization, Organ Dysfunction, and Mortality in Post-Endoscopic Retrograde Cholangiopancreatography Acute Pancreatitis: Are SIRS and qSOFA Reliable Tools? Applied Sciences. 2023; 13(11):6650. https://doi.org/10.3390/app13116650
Chicago/Turabian StyleBalan, Gheorghe Gh., Oana Timofte, Georgiana-Emmanuela Gilca-Blanariu, Catalin Sfarti, Smaranda Diaconescu, Nicoleta Gimiga, Simona Petronela Antighin, Ion Sandu, Vasile Sandru, Anca Trifan, and et al. 2023. "Predicting Hospitalization, Organ Dysfunction, and Mortality in Post-Endoscopic Retrograde Cholangiopancreatography Acute Pancreatitis: Are SIRS and qSOFA Reliable Tools?" Applied Sciences 13, no. 11: 6650. https://doi.org/10.3390/app13116650
APA StyleBalan, G. G., Timofte, O., Gilca-Blanariu, G.-E., Sfarti, C., Diaconescu, S., Gimiga, N., Antighin, S. P., Sandu, I., Sandru, V., Trifan, A., Moscalu, M., & Stefanescu, G. (2023). Predicting Hospitalization, Organ Dysfunction, and Mortality in Post-Endoscopic Retrograde Cholangiopancreatography Acute Pancreatitis: Are SIRS and qSOFA Reliable Tools? Applied Sciences, 13(11), 6650. https://doi.org/10.3390/app13116650










