Experimental Study on the Properties of Autoclave Curing High-Strength Concrete According to CaO/SiO2 Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
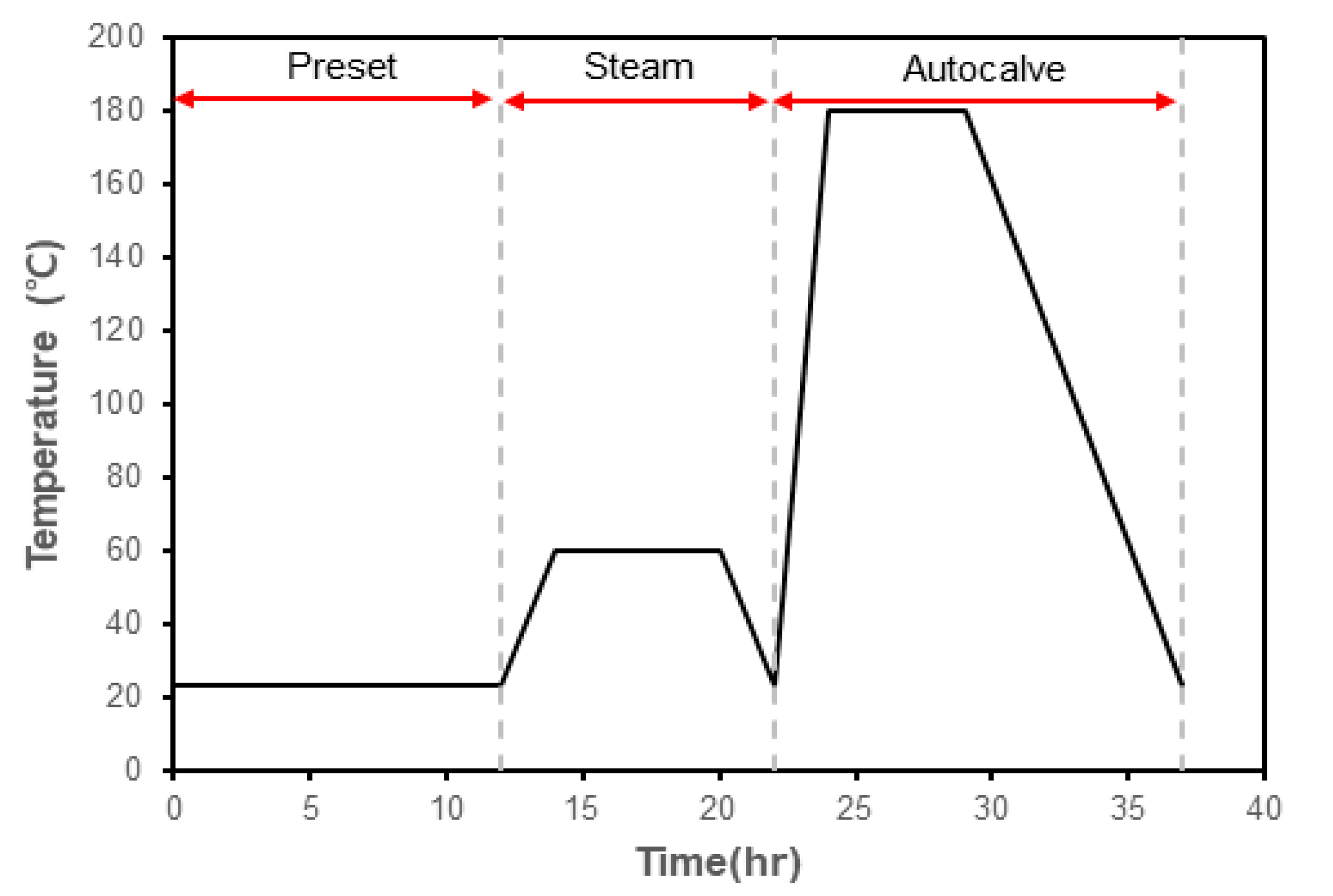
2.2. Methods
3. Results and Discussion
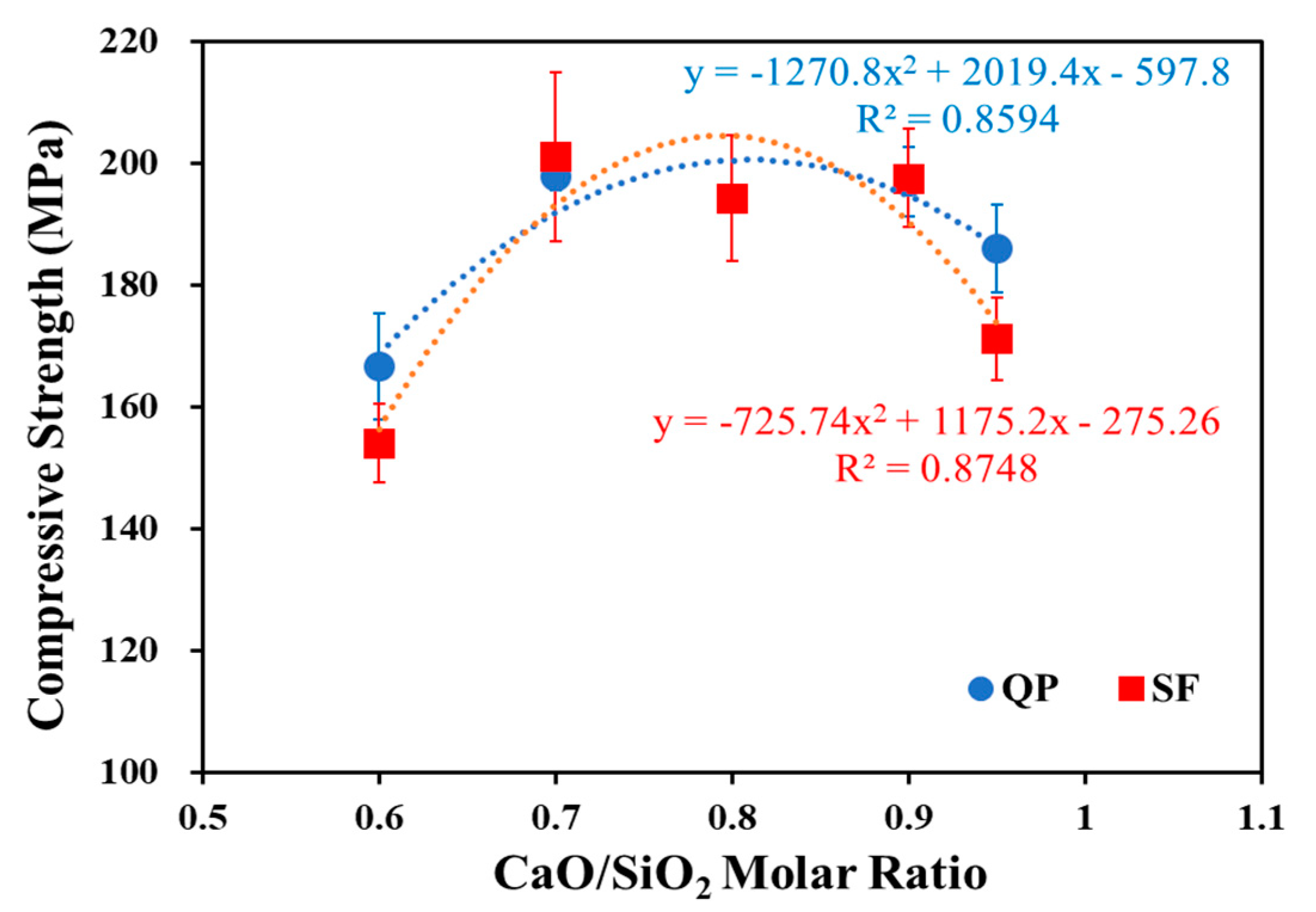
3.1. Compressive Strength
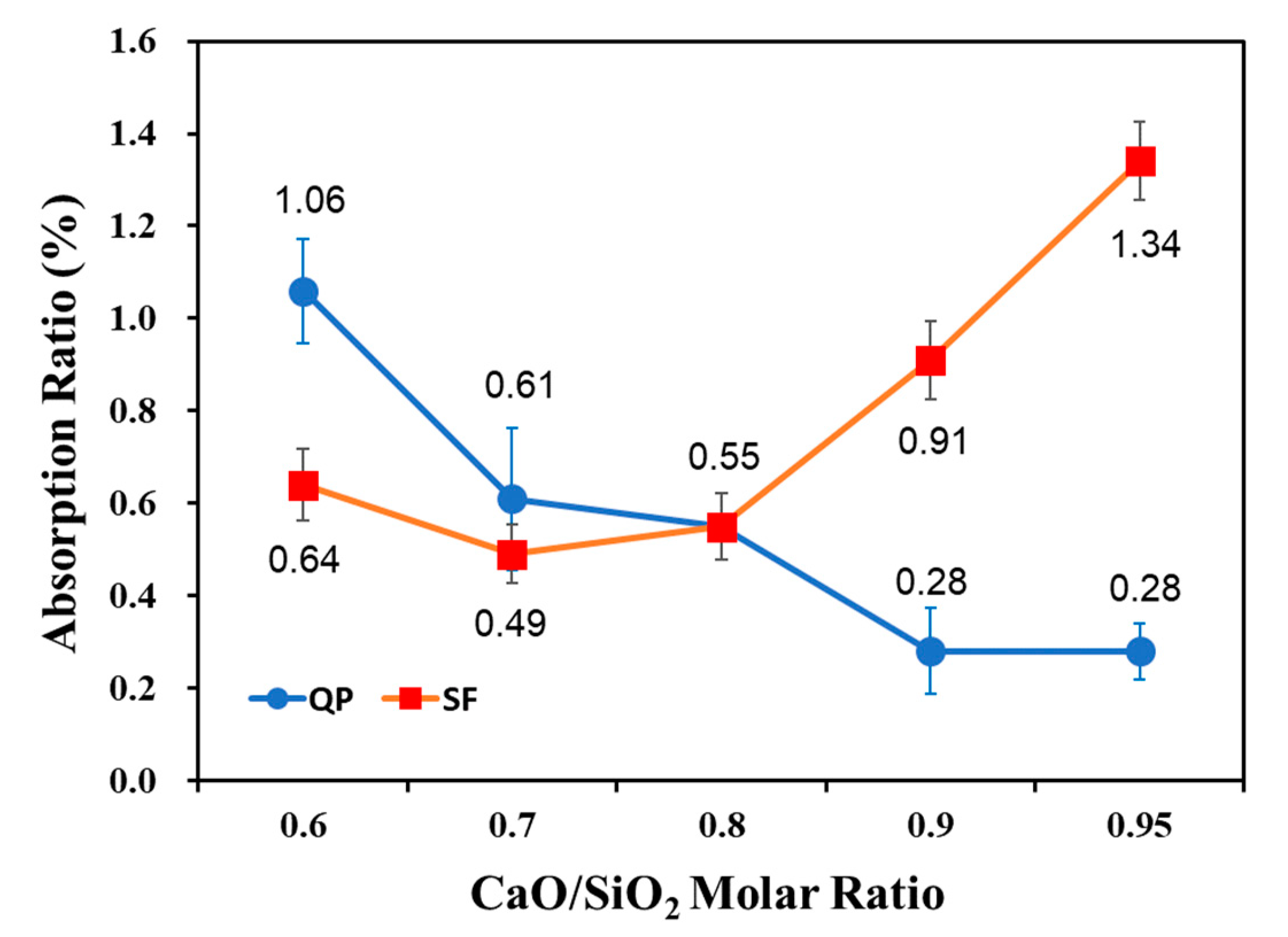
3.2. Absorption Rate
3.3. Thermogravimetric Analysis (TGA)
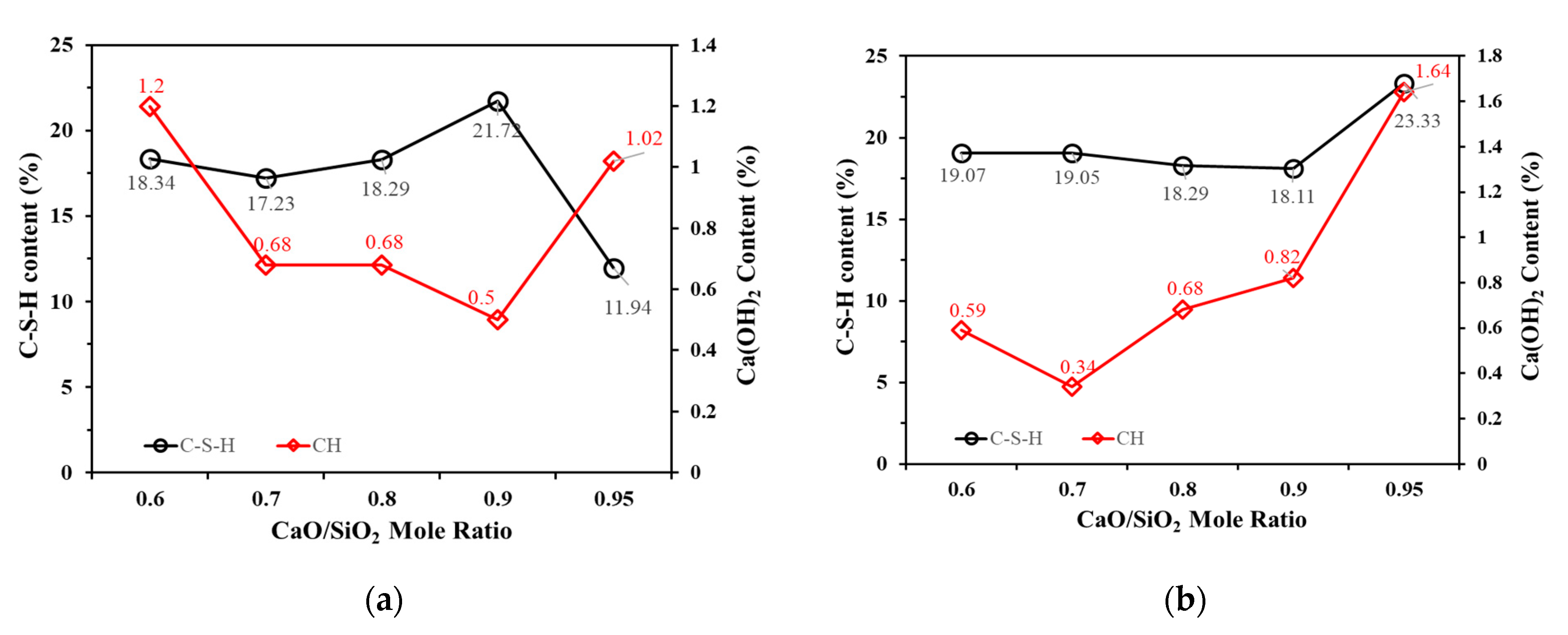
3.4. Mineral Analysis
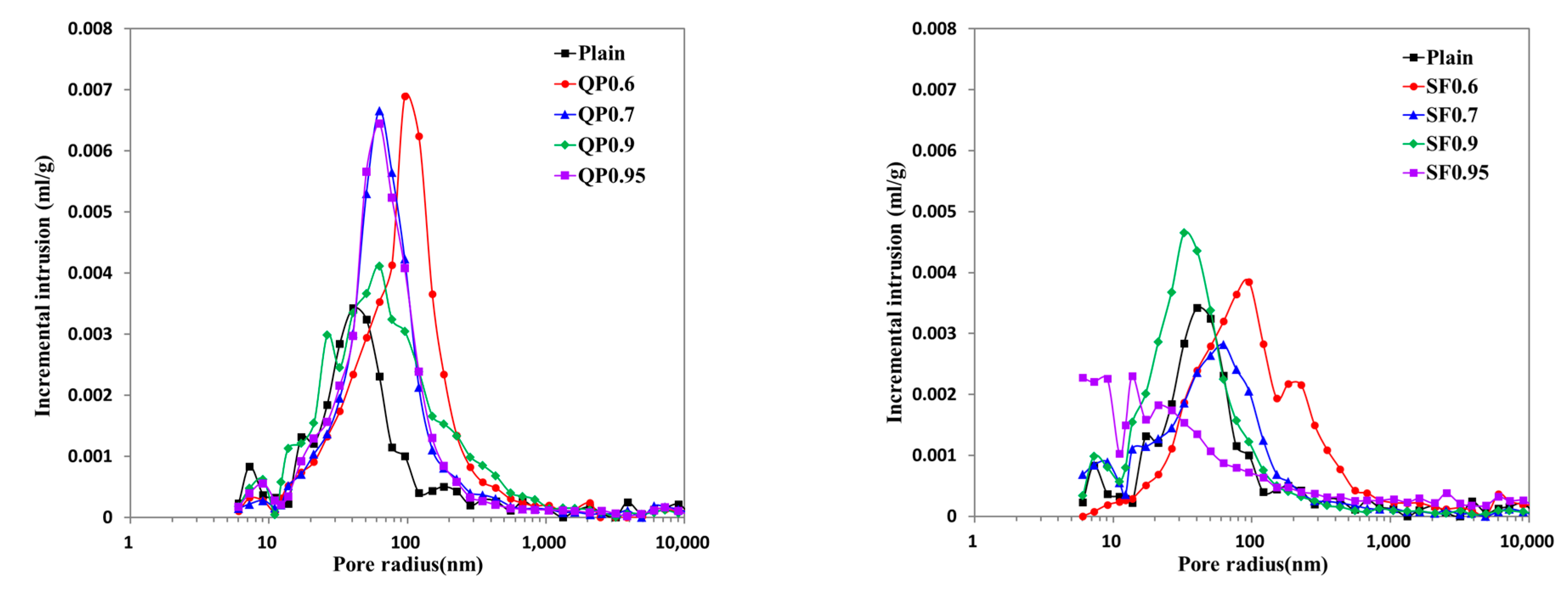
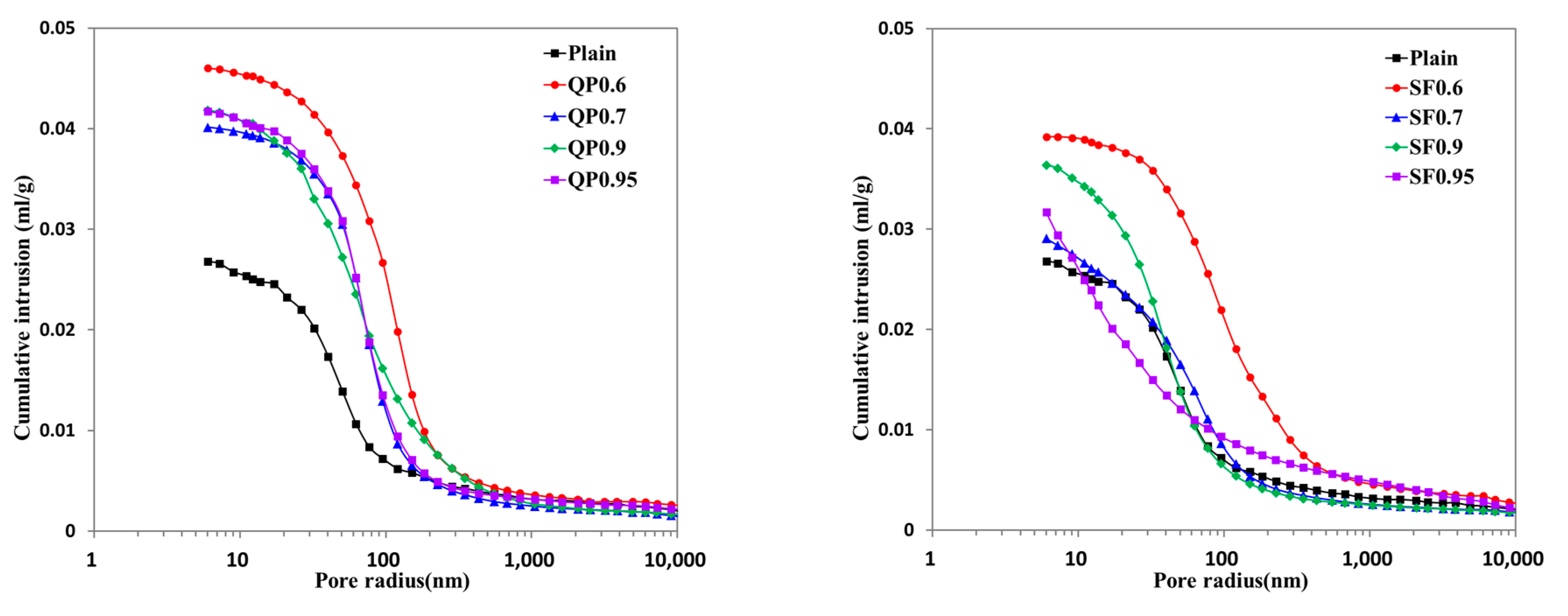
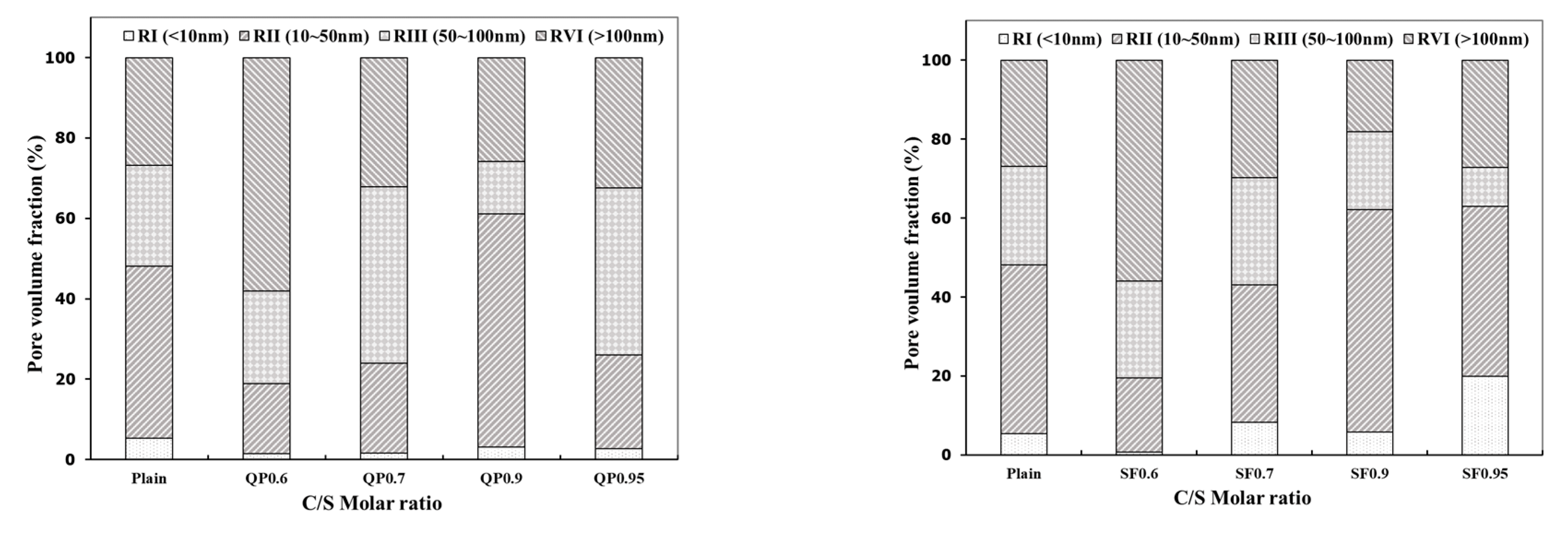
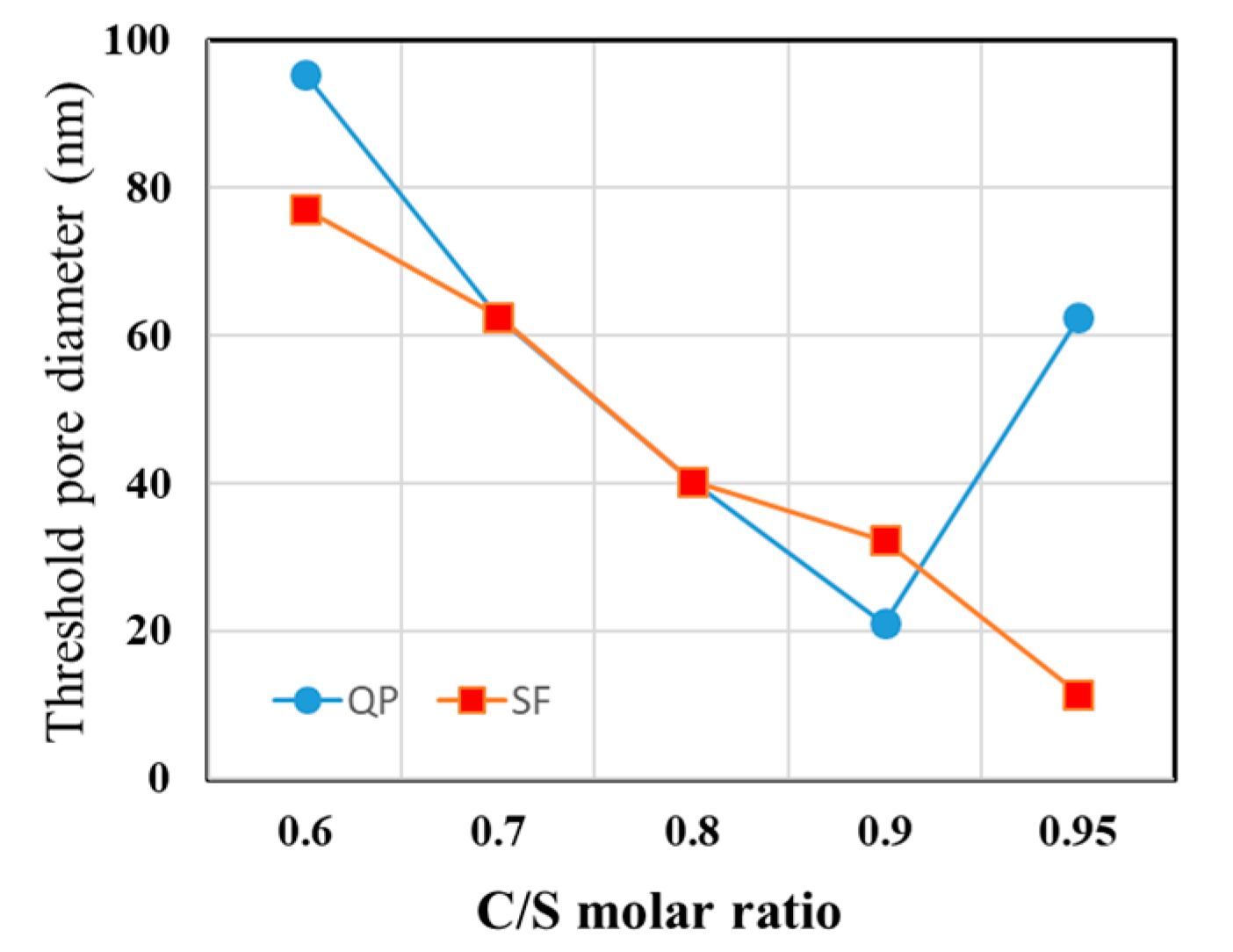
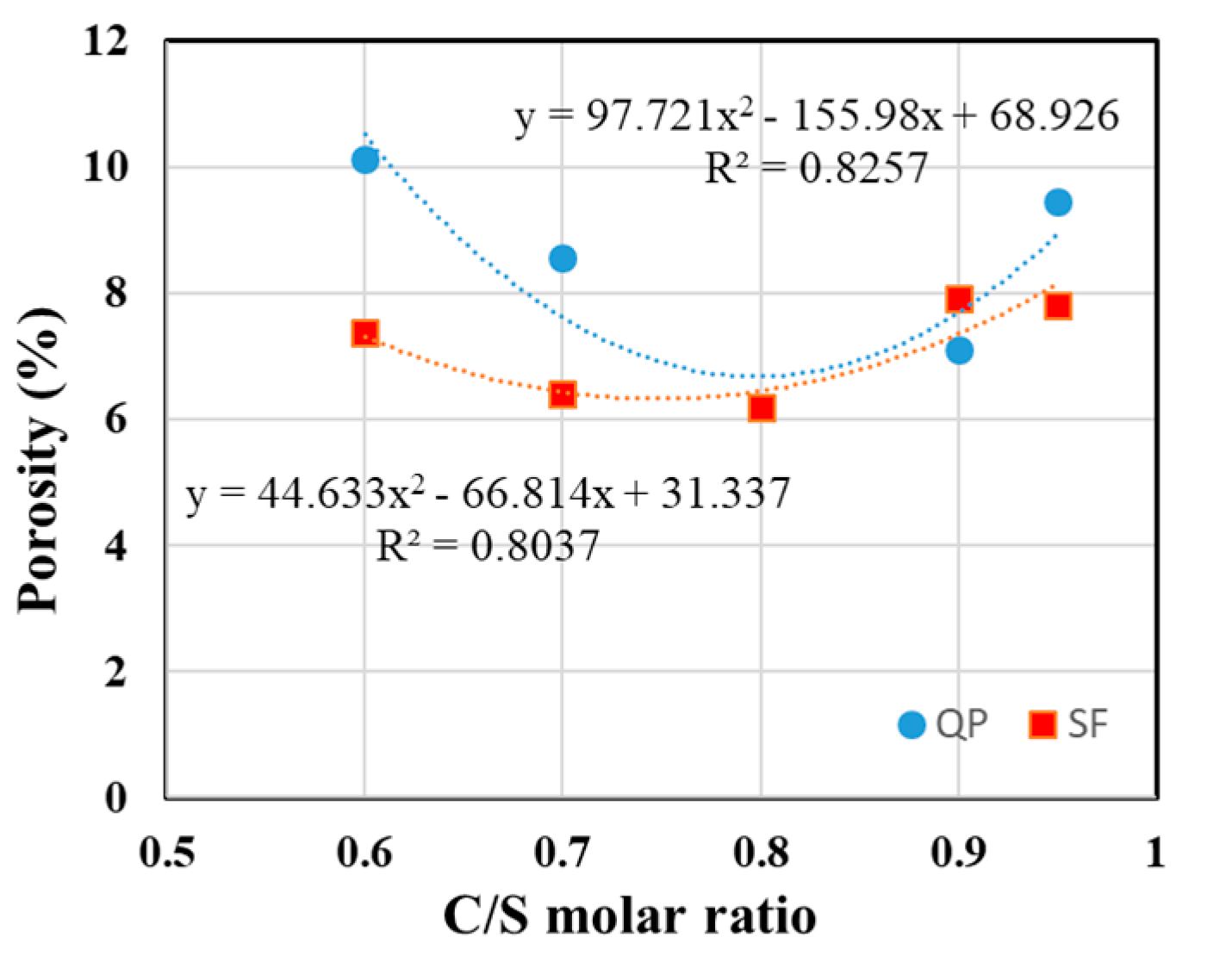
3.5. Micropore Characteristics
3.6. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binici, H.; Aksogan, O.; Shah, T. Investigation of fibre reinforced mud brick as a building material. Constr. Build. Mater. 2005, 19, 313–318. [Google Scholar] [CrossRef]
- Rodrigues, R.; de Brito, J.; Sardinha, M. Mechanical properties of structural concrete containing very fine aggregates from marble cutting sludge. Constr. Build. Mater. 2015, 77, 349–356. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, P.; Wu, B.; Chang, S. A study on the color change benefits of sustainable green building materials. Constr. Build. Mater. 2015, 83, 1–6. [Google Scholar] [CrossRef]
- Ribeiro, C.E.G.; Rodriguez, R.J.S.; de Carvalho, E.A. Microstructure and mechanical properties of artificial marble. Constr. Build. Mater. 2017, 149, 149–155. [Google Scholar] [CrossRef]
- da Cunha Demartini, T.J.; Rodríguez, R.J.S.; Silva, F.S. Physical and mechanical evaluation of artificial marble produced with dolomitic marble residue processed by diamond-plated bladed gang-saws. J. Mater. Res. Techno. 2018, 7, 308–313. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Zhou, J. Experimental study and analytical model for pore structure of hydrated cement paste. Appl. Clay Sci. 2014, 101, 159–167. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials, 4th ed.; ProtoView; McGraw-Hill Education: New York, NY, USA, 2014; Volume 1. [Google Scholar]
- Birchall, J.D.; Howard, A.J.; Kendall, K. Flexural strength and porosity of cements. Nature 1981, 289, 388–390. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Recent developments in macro-defect-free (MDF) cements. Constr. Build. Mater. 2009, 23, 1761–1767. [Google Scholar] [CrossRef]
- Drabik, M.; Mojumdar, S.C.; Slade, R.C.T. Prospects of novel macro-defect-free cements for the new millennium. Ceramics 2002, 46, 68–73. [Google Scholar]
- Rodrigues, F.A.; Joekes, I. Macro-defect free cements: A new approach. Cem. Concr. Res. 1998, 28, 877–885. [Google Scholar] [CrossRef]
- Eker, H.; Bascetin, A. Influence of silica fume on mechanical property of cemented paste backfill. Constr. Build. Mater. 2022, 317, 126089. [Google Scholar] [CrossRef]
- Tuylu, S. Investigation of the effect of using different fly ash on the mechanical properties in cemented paste backfill. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2022, 37, 620–627. [Google Scholar] [CrossRef]
- Goñi, S.; Frías, M.; de la Villa, R.V.; Vegas, I. Decalcification of activated paper sludge—Fly ash-portland cement blended pastes in pure water. Cem. Concr. Compos. 2013, 40, 1–6. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Jin, M.; Liu, J.; Zhang, J.; Huang, J.; Lu, C.; Zeng, H.; Wang, J.; Zhao, H.; et al. Influences of leaching on the composition, structure and morphology of calcium silicate hydrate (C–S–H) with different Ca/Si ratios. J. Build. Eng. 2022, 58, 105017. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nonat, A. Calcium silicate hydrates: Solid and liquid phase composition. Cem. Concr. Res. 2015, 78, 57–70. [Google Scholar] [CrossRef]
- ASTM C150; Standard Specification for Portland Cement. American Society of Testing and Materials: West Conshohocken, PA, USA, 2012.
- KS F 2405; Standard Test Method for Compressive Strength of Concrete. Korea Standard Committee: Seoul, Republic of Korea, 2017.
- Kumar, R.; Bhattacharjee, B. Study on some factors affecting the results in the use of MIP method in concrete research. Cem. Concr. Res. 2003, 33, 417–424. [Google Scholar] [CrossRef]
- Shi, D.; Winslow, D.N. Contact angle and damage during mercury intrusion into cement paste. Cem. Concr. Res. 1985, 15, 645–654. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, D.; Zhang, D.; Yan, C.; Zhang, J. Crystal transformation of calcium silicate minerals synthesized by calcium silicate slag and silica fume with increase of C/S molar ratio. J. Mater. Res. Technol. 2021, 15, 4185–4192. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Xu, L.; Zhu, Z.; Zhou, Y.; Pan, F.; Wu, K. Insight into the local C–S–H structure and its evolution mechanism controlled by curing regime and Ca/Si ratio. Constr. Build. Mater. 2022, 333, 127388. [Google Scholar] [CrossRef]
- Alhozaimy, A.; Fares, G.; Al-Negheimish, A.; Jaafar, M.S. The autoclaved concrete industry: An easy-to-follow method for optimization and testing. Constr. Build. Mater. 2013, 49, 184–193. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, W.; Xing, X.; Wu, M. Quantitative evaluation of hydrated cement modified by silica fume using QXRD, 27Al MAS NMR, TG–DSC and selective dissolution techniques. Constr. Build. Mater. 2012, 36, 925–932. [Google Scholar] [CrossRef]
- Wang, S.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.; Lecomte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.; Wu, Y.; Zhou, Y.; Tang, S. Hydration, shrinkage, pore structure and fractal dimension of silica fume modified low heat portland cement-based materials. Constr. Build. Mater. 2021, 272, 121952. [Google Scholar] [CrossRef]
- Wongkeo, W.; Thongsanitgarn, P.; Poon, C.; Chaipanich, A. Heat of hydration of cement pastes containing high-volume fly ash and silica fume. J. Therm. Anal. Calorim. 2019, 138, 2065–2075. [Google Scholar] [CrossRef]
- Shang, C.; Wu, C.; Wang, J.; Lu, L.; Fu, Q.; Zhang, Y.; Song, X. Investigating mechanical properties and microstructure of reactive powder concrete blended with sulfoaluminate cement. Case Stud. Constr. Mater. 2022, 17, e01552. [Google Scholar] [CrossRef]
- Kostakis, G. Characterization of the fly ashes from the lignite burning power plants of northern greece based on their quantitative mineralogical composition. J. Hazard. Mater. 2009, 166, 972–977. [Google Scholar] [CrossRef]
- Sakai, E.; Miyahara, S.; Ohsawa, S.; Lee, S.; Daimon, M. Hydration of fly ash cement. Cem. Concr. Res. 2005, 35, 1135–1140. [Google Scholar] [CrossRef]
- Liu, Z.; Winslow, D. Sub-distributions of pore size: A new approach to correlate pore structure with permeability. Cem. Concr. Res. 1995, 25, 769–778. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, Y.; Zhang, N.; Hu, S.; Yang, F.; Lu, L.; Wang, J. Durability and mechanism of high-salt resistance concrete exposed to sewage-contaminated seawater. Constr. Build. Mater. 2020, 257, 119534. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-chong, T.; Dangla, P. Pore structure characterization of cement pastes blended with high-volume fly-ash. Cem. Concr. Res. 2012, 42, 194–204. [Google Scholar] [CrossRef]
- Choi, Y.C.; Kim, J.; Choi, S. Mercury intrusion porosimetry characterization of micropore structures of high-strength cement pastes incorporating high volume ground granulated blast-furnace slag. Constr. Build. Mater. 2017, 137, 96–103. [Google Scholar] [CrossRef]
- Brue, F.; Davy, C.A.; Skoczylas, F.; Burlion, N.; Bourbon, X. Effect of temperature on the water retention properties of two high performance concretes. Cem. Concr. Res. 2012, 42, 384–396. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Surface fractal analysis of pore structure of high-volume fly-ash cement pastes. Appl. Surf. Sci. 2010, 257, 762–768. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S. A fractal-based approach for reconstructing pore structures of GGBFS-blended cement pastes. Constr. Build. Mater. 2020, 265, 120350. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Zhou, J. Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 2013, 40, 869. [Google Scholar] [CrossRef]
- Zhao, D.; Khoshnazar, R. Hydration and microstructural development of calcined clay cement paste in the presence of calcium-silicate-hydrate (C–S–H) seed. Cem. Concr. Compos. 2021, 122, 104162. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Nocuń-Wczelik, W. Effect of some inorganic admixtures on the formation and properties of calcium silicate hydrates produced in hydrothermal conditions. Cem. Concr. Res. 1997, 27, 83–92. [Google Scholar] [CrossRef]
- Black, L.; Garbev, K.; Stemmermann, P.; Hallam, K.R.; Allen, G.C. Characterisation of crystalline C–S–H phases by X-ray photoelectron spectroscopy. Cem. Concr. Res. 2003, 33, 899–911. [Google Scholar] [CrossRef]
- Siauciunas, R.; Baltakys, K. Formation of gyrolite during hydrothermal synthesis in the mixtures of CaO and amorphous SiO2 or quartz. Cem. Concr. Res. 2004, 34, 2029–2036. [Google Scholar] [CrossRef]

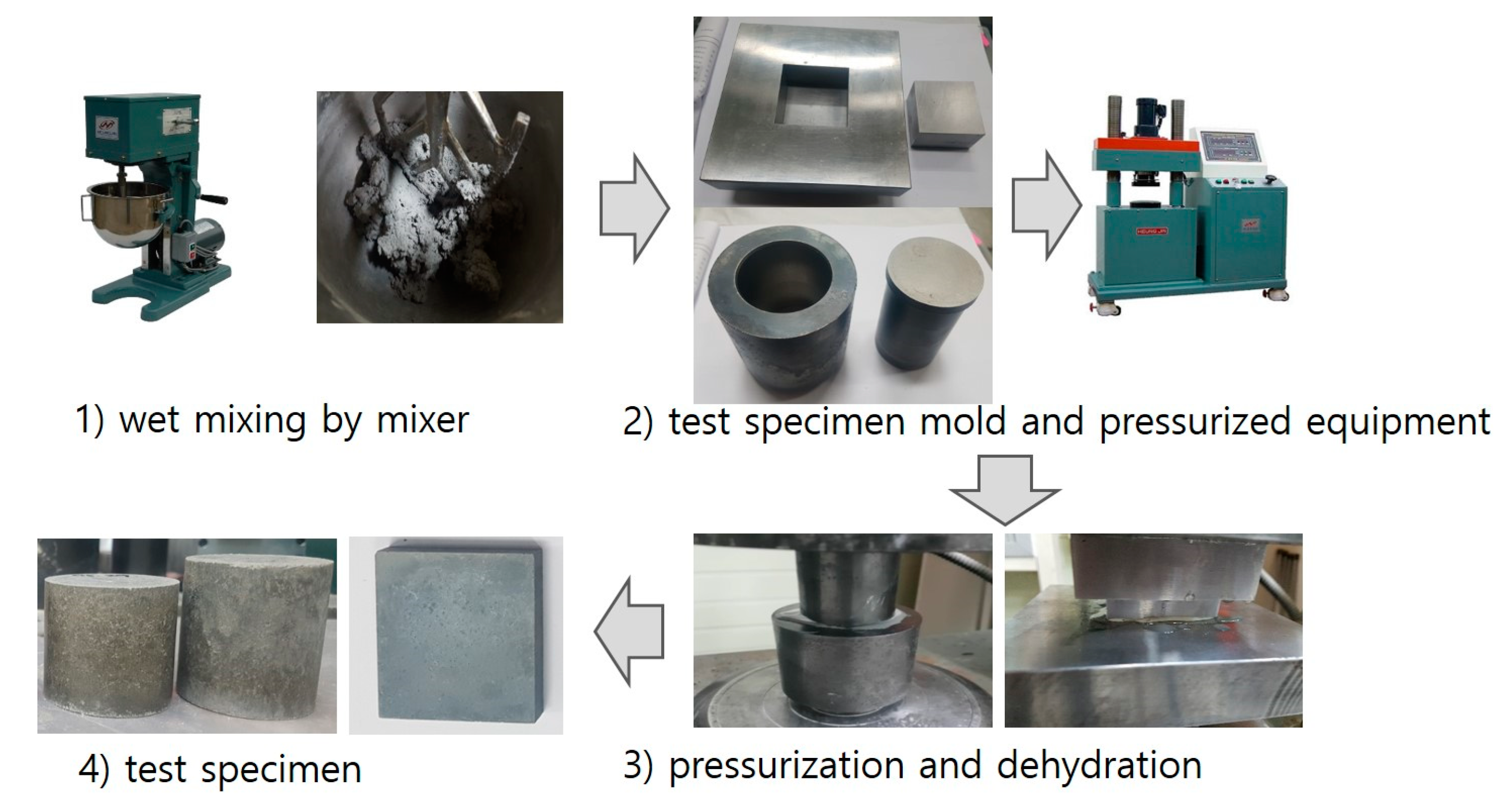




| Chemical Properties (%) | Physical Properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | SO3 | MgO | Fe2O3 | K2O | etc. | Sum | Fineness (cm2/g) | Specific Gravity | |
| OPC | 72.70 | 17.20 | 4.00 | 3.56 | 1.35 | 0.34 | 0.11 | 0.74 | 100.0 | 3400 | 3.14 |
| QP | 0.02 | 98.00 | 1.45 | 0.01 | 0.04 | 0.13 | 0.31 | 0.04 | 100.0 | 6000 | 2.6 |
| SF | 0.12 | 97.10 | 0.12 | 1.73 | 0.14 | 0.08 | 0.47 | 0.24 | 99.9 | 200,000 | 2.2 |
| Physical Properties | Chemical Properties | |||||
|---|---|---|---|---|---|---|
| Appearance | Moisture (wt.%) | Color (ΔE) | Specific gravity | SiO2 (%) | Fe2O3 (ppm) | |
| Quartz | Good | 0.04 | 0.83 | 2.63 | 99.2 | 90 |
| Type of Binder | C/S Molar Ratio | Test Items |
|---|---|---|
| QP SF | 0.6 0.7 0.9 0.95 |
|
| ID | W/B (%) | C/S Ratio | SP (wt.%) | Total Weight (kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| W | OPC | SF | QP | Q1 (0.1–0.3) | Q2 (0.3–0.7) | |||||
| Plain (C/S = 0.8) | 19.8 | 0.79 | 124.1 | 341.7 | 34.2 | 250.0 | 200.0 | 50.0 | 1.0 | 993.9 |
| QP0.6 | 18.9 | 0.60 | 118 | 291.1 | 34.2 | 300.6 | ||||
| QP0.7 | 18.9 | 0.70 | 319.1 | 272.6 | ||||||
| QP0.9 | 18.9 | 0.90 | 366.3 | 225.4 | ||||||
| QP0.95 | 18.9 | 0.95 | 375.7 | 216.0 | ||||||
| SF0.6 | 18.9 | 0.60 | 290.6 | 85.3 | 250.0 | |||||
| SF0.7 | 18.9 | 0.70 | 318.9 | 57.0 | ||||||
| SF0.9 | 18.9 | 0.90 | 366.4 | 9.5 | ||||||
| SF0.95 | 18.9 | 0.95 | 375.9 | 0.0 | ||||||
| Plain | QP0.9 | SF0.9 | ||||
|---|---|---|---|---|---|---|
| Ca | Si | Ca | Si | Ca | Si | |
| Point 1 | 32.2 | 17.91 | 21.07 | 25.55 | 16.31 | 29.72 |
| Point 2 | 12.48 | 34.02 | 12.54 | 14.13 | 20.15 | 31.15 |
| Point 3 | 18.8 | 12.56 | 17.7 | 18.33 | 7.17 | 61.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ra, J.; Shin, S.; Kim, J. Experimental Study on the Properties of Autoclave Curing High-Strength Concrete According to CaO/SiO2 Ratio. Appl. Sci. 2023, 13, 6190. https://doi.org/10.3390/app13106190
Ra J, Shin S, Kim J. Experimental Study on the Properties of Autoclave Curing High-Strength Concrete According to CaO/SiO2 Ratio. Applied Sciences. 2023; 13(10):6190. https://doi.org/10.3390/app13106190
Chicago/Turabian StyleRa, Jeongmin, Sangchul Shin, and Jinman Kim. 2023. "Experimental Study on the Properties of Autoclave Curing High-Strength Concrete According to CaO/SiO2 Ratio" Applied Sciences 13, no. 10: 6190. https://doi.org/10.3390/app13106190
APA StyleRa, J., Shin, S., & Kim, J. (2023). Experimental Study on the Properties of Autoclave Curing High-Strength Concrete According to CaO/SiO2 Ratio. Applied Sciences, 13(10), 6190. https://doi.org/10.3390/app13106190







